Abstract
Background
A high level of comorbidity at dialysis initiation is associated with an increased risk of death. However, contemporary assessments of the validity and prognostic value of comorbidity indices are lacking.
Objectives
To assess the validity of two comorbidity indices and to determine if a high degree of comorbidity is associated with mortality among dialysis patients.
Design
Cohort study.
Setting
QEII Health Sciences Centre (Halifax, Nova Scotia, Canada).
Patients
Incident, chronic dialysis patients between 01 Jan 2006 and 01 Jul 2013.
Measurements
Exposure: The Charlson Comorbidity Index (CCI) and End-Stage Renal Disease Comorbidity Index (ESRD-CI) were used to classify individual comorbid conditions into an overall score. Comorbidities were classified using patient charts and electronic records.
Outcome: All-cause mortality.
Confounders: Patient demographics, dialysis access, cause of ESRD and baseline laboratory data.
Methods
Regression coefficients were estimated on the CCI and ESRD-CI. Discrimination for death was assessed using Harrell’s c-index. Adjusted Cox proportional hazard models were used to calculate relative hazards and 95 % confidence intervals for each category of the CCI and ESRD-CI.
Results
The cohort consisted of 771 ESRD patients from 01 Jan 2006 to 01 Jul 2013. Most were male (62 %) and Caucasian (91 %). The cohort had a high proportion of diabetes (48 %), history of previous myocardial infarction (31 %) and heart failure (22 %). Regression coefficients on the CCI and ESRD-CI were 0.55 and 0.52, respectively. The c-index, for the prediction of death, was 0.61 for the CCI and 0.63 for the ESRD-CI. ESRD-CI scores of 4, 5 and ≥6 were associated with a similar mortality risk (adjusted relative hazard of 1.95, 1.89 and 1.99, respectively). There was a small increased mortality risk for CCI scores of 4, 5 and ≥6 (adjusted relative hazard of 1.86, 2.38 and 2.71, respectively).
Limitations
Classification of comorbidities for each patient was determined by clinical impression.
Conclusions
The CCI and ESRD-CI have a limited ability to discriminate mortality risk for incident dialysis patients. Acknowledging the frequency with which they are used, this study emphasizes the need to re-examine the usefulness of previously derived comorbidity indices in contemporary dialysis cohorts.
Résumé
Contexte
Un taux élevé de comorbidité en début de dialyse est lié à un risque accru de mort. Toutefois, rares sont les évaluations récentes de la validité et de la valeur pronostique des indices de comorbidité.
Objectifs
Vérifier la validité de deux indices de comorbidité et déterminer la relation entre un taux élevé de comorbidité et le taux de mortalité chez les patients dialysés.
Type d’étude
Étude de cohorte.
Contexte
Centre des sciences de la santé QEII (Halifax, Nouvelle-Écosse, Canada).
Participants
Patients incidents en dialyse chronique du 1er janv. 2006 au 1er juil. 2013.
Mesures
Exposition: L'indice de comorbidité Charlson (CCI) et l'indice de comorbidité au stade terminal d'insuffisance rénale (ESRD-CI) ont servi à la mesure de la comorbidité, à la lumière des fiches et des dossiers électroniques des patients.
Résultat: Taux de mortalité, toutes causes confondues.
Facteurs de confusion: caractéristiques sociodémographiques des patients, accès à la dialyse, cause de l'insuffisance rénale terminale (IRT) et données de référence du laboratoire.
Méthodes
On a procédé à l'estimation des coefficients de régression du CCI et de l'ESRD-CI, puis à l'évaluation du seuil de mortalité à l'aide de l'indice C de Harrell. On a enfin utilisé des modèles des risques proportionnels de Cox ajustés afin de calculer les risques relatifs et les intervalles de confiance à 95 % pour chaque catégorie du CCI et de l'ESRD-CI.
Résultats
La cohorte comprenait 771 patients en IRT du 1er janv. 2006 au 1er juil. 2013. La plupart étaient des hommes (62 %) de race blanche (91 %). On y trouvait une proportion élevée de diabète (48 %), d'infarctus du myocarde antérieur (31 %), et d'insuffisance cardiaque (22 %). Les coefficients de régression du CCI et de l'ESRD-CI indiquaient 0,55 et 0,52, respectivement. L'indice C du risque de décès était de 0,61 pour le CCI et de 0,63 pour l'ESRD-CI. Pour ce dernier indice, des valeurs de 4, 5 et 6 ou plus étaient liées à un risque de mortalité équivalent (risque relatif ajusté de 1,95, de 1,89 et de 1,99, respectivement). On a noté une légère augmentation du risque de mortalité pour les valeurs du CCI de 4, 5 et 6 ou plus (risque relatif ajusté de 1,86, de 2,38 et de 2,71, respectivement).
Limites
Le classement des comorbidités de chaque patient était déterminé par opinion clinique.
Conclusions
Le CCI et l'ESRD-CI sont limités en ce qui a trait à la capacité de déterminer le risque de mortalité chez une population incidente dialysée. En regard de la fréquence d’utilisation de la dialyse, la présente étude souligne le besoin de réévaluer l'utilité des indices de comorbidité précédemment dérivés des récentes cohortes dialysées.
Similar content being viewed by others
What was known before
The Charlson Comorbidity Index (CCI) and End-Stage Renal Disease Comorbidity Index (ESRD-CI) are commonly used in studies of dialysis patients, but assessments of their validity are lacking.
What this adds
Both indices had a limited ability to discriminate mortality risk in this study emphasizing that they may not be the best method of risk adjustment in contemporary dialysis cohorts.
Introduction
In patients with end-stage renal disease (ESRD), the presence of comorbid conditions has been shown to have a negative impact on survival [1–3]. A commonly used approach for summating individual conditions into an overall “score” of comorbidity for risk stratification is calculation of the Charlson Comorbidity Index (CCI) [4]. The CCI was initially derived in a cohort of 559 patients, and tested in a second cohort of 680 patients followed for 10 years [4]. In the original study, 19 comorbid conditions were evaluated in a Cox proportional hazards model. Point scores were assigned to each comorbidities hazard ratio depending on the value. The sum of the points equalled a given individuals’ overall CCI score. A higher CCI score is associated with an increased mortality risk in ESRD patients [5–12], however the CCI does have limitations when applied to ESRD patients. The inclusion of renal disease as one of the component comorbidities is redundant and medical advances since the development of the CCI have changed the prognosis of some of the individual comorbid conditions within the index [13].
There have been a number of additional comorbidity indices that have been created for ESRD patients [1, 14–16], including the End Stage Renal Disease Comorbidity Index (ESRD-CI) which avoids some of the limitations associated with the original CCI [15]. Having been designed as an adaptation of the CCI, the ESRD-CI was developed in a cohort of 237 incident dialysis patients [15]. 15 of the 19 conditions in the CCI were evaluated in a multivariable Cox survival analysis. Similar to the CCI, point scores were assigned to each condition’s hazard ratio and summed for each individual in the derivation cohort. In the model derivation study the ESRD-CI had slightly better performance characteristics compared to the CCI in the tested population (c-statistic of 0.73 versus 0.72) [15].
While both the CCI and ESRD-CI are frequently used for risk adjustment in studies of dialysis patients [17–22], only a few studies have attempted to validate either index [15, 23, 24]. In addition, these validation studies have limitations including incomplete inclusion of all necessary comorbid conditions, and validation techniques that are not specific to time-to-event analyses [15, 23, 24]. Finally, validation in a more recent era-cohort (acknowledging that patient characteristics, disease prevalence and outcome after dialysis initiation may differ from those in older cohorts) has not been conducted in many studies. A lack of validity/limited prognostic ability of either index will emphasize the need to re-examine the usefulness of previously derived comorbidity indices in contemporary dialysis cohorts.
Therefore, the purpose of this study was to assess the validity of the CCI and ESRD-CI in a contemporary cohort of ESRD patients, and to determine if a high degree of comorbidity was independently associated with mortality. We hypothesized that both indices would have a reduced level of discrimination compared to the derivation studies, but that increased comorbidity burden would be associated with a higher risk of death for dialysis patients.
Methods
Population
We conducted a cohort study of incident, adult (≥18 years) chronic dialysis patients in a large tertiary care institute between 1 Jan 2006 and 01 Jul 2013. Follow-up for patients began at the initial start date for their dialysis.
Exposure definition
Comorbidity data was collected at the start of dialysis in all patients in a prospective manner, using documentation in patient charts (dating back to the first nephrology visit) and electronic records by the patients’ primary nephrologist. Comorbidities were subsequently verified in all patients by two nephrologists (K.T. and B.K.) and one nephrology trainee (T.A.). All 19 individual comorbid conditions in the CCI were collected at the time of dialysis initiation and scored according to the CCI derivation study. ESRD-CI scores were retrospectively calculated by re-scoring the comorbidities comprising the ESRD-CI based on the derivation paper. ESRD-CI scores were analyzed as ordinal variables and after categorization into six groups to be consistent with the derivation paper (using scores of 0/1, 2, 3, 4, 5 and ≥6) [15]. The CCI was analyzed both as an ordinal variable and in categories (2, 3, 4, 5 and ≥6). Since all patients in our dataset had ESRD, the lowest possible CCI score was 2.
In addition to the CCI and ESRD-CI scores, demographic data (age, race, gender), dialysis access (central venous catheter or arteriovenous fistula) type of dialysis modality (peritoneal or hemodialysis), cause of ESRD (diabetes, glomerulonephritis, hypertension, other) and baseline laboratory data (hemoglobin, phosphate, estimated glomerular filtration rate and albumin) were collected on all patients at the start of dialysis using a combination of electronic records and paper chart review.
Outcome
The primary outcome was all cause mortality after dialysis initiation. Administrative censoring was imposed on 01 Jan 2014. Patient survival was censored at the date of transplantation.
Statistical analysis
Descriptive statistics for baseline characteristics of the study cohort were reported as counts with proportions, mean with standard deviation and median with interquartile range for categorical, normally distributed continuous and non-normally distributed continuous variables, respectively.
External validation of the CCI and ESRD-CI followed the methods previously described by Royston et al. based on availability of data in the original derivation studies [25]:
-
1.
Regression coefficients were estimated on the CCI and ESRD-CI (defined as the precise CCI and precise ESRD-CI).
-
2.
Regression coefficients were also estimated on the categorical CCI (2, 3, 4, 5 and 6+) and ESRD-CI (0/1, 2, 3, 4, 5 and 6+) as a secondary analysis (defined as the categorical CCI and categorical ESRD-CI).
-
3.
Discrimination (defined as the level of concordance between the risk predicted by a model and the rate of events experienced [25]) was assessed for the precise and categorical CCI as well as the precise and categorical ESRD-CI using Harrell’s c-index. Harrell’s c-index assesses the fraction of all possible pairings of patients in which the predictions and outcomes are concordant [26]. Scores range from 0.5 (no discrimination), to 1.0 (perfect discrimination). As a reference, the CHADS2 score for atrial fibrillation stroke risk has a reported c-index of 0.683 [27].
-
4.
Kaplan-Meier Survival curves were plotted for each category of CCI/ESRD-CI and discrimination was also visually assessed according to the ordering and separation of the curves.
Cox proportional hazard models were used to calculated relative hazards and 95 % confidence intervals for each category of the CCI and ESRD-CI. Proportionality of hazards was assessed using Schoenfeld Residuals. Multivariable models included variables based on clinical judgment and those derived from the literature as being associated with mortality in studies of dialysis patients including age [28], gender [29], Caucasian versus non-Caucasian race [28, 30], dialysis modality [31], cause of ESRD, albumin [32], hemoglobin [33], phosphate [34, 35] and modification of diet in renal disease (MDRD) estimated glomerular filtration rate [36]. A two sided P value of <0.05 was the threshold for statistical significance. Approval to conduct this study was granted by our institutional research ethics board (Nova Scotia Health Authority, CDHA-RS/2014-288). All analyses were conducted using Stata version 12.0, College Station, TX, USA.
Results
Baseline characteristics
The cohort consisted of 771 ESRD patients from 01 Jan 2006 to 01 Jul 2013. Baseline characteristics of the cohort are noted in Table 1. The majority of patients were male (62 %) and Caucasian (91 %). Common comorbidities included diabetes (48 %), previous myocardial infarction (31 %), congestive heart failure (22 %) and peripheral vascular disease (20 %). The median CCI score was 4 (Q1-Q3: 3–6), and the median ESRD-CI Score was 2 (Q1-Q3: 0–4).
Validation
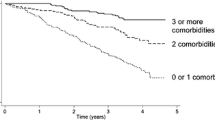
Cox regression on the precise CCI and ESRD-CI revealed coefficients of 0.55 (SE 0.08) and 0.52 (SE 0.07), respectively (Table 2). Cox regression on the categorical CCI and ESRD-CI revealed similar coefficients (0.56 and 0.52, respectively). The c-index was 0.61 (SE 0.02) for both the precise and categorical CCI, and 0.63 (SE 0.02) and 0.62 (SE 0.02) for the precise and categorical ESRD-CI (Table 2). Kaplan-Meier survival curves for each ESRD-CI score cut-off are displayed in Fig. 1 (Log-rank P < 0.001). There was separation of the curves for patients with a high versus low score (6+ versus 0/1 or 2). However, discordance was observed for patients with an intermediate score. A similar finding was noted for the CCI, however, there was slightly more separation between the curves in an incremental fashion based on score (Fig. 2).
Mortality
Over 1796.6 patient years at risk, there were 311 deaths. The distribution of deaths stratified by CCI and ESRD-CI scores are graphically displayed in Fig. 3. There was a rise in the number of deaths and fall in the number of patients that received a kidney transplant with each ESRD-CI score cut-off. In an unadjusted Cox survival analysis, relative to patients with an ESRD-CI score of ≤1, those with scores of ≥6 had a mortality HR of 2.64 (95 % CI 1.91 to 3.65, p < 0.001, Table 3). A similar mortality HR was observed for patients with CCI scores of ≥6 compared to 2 (HR 2.91, 95 % CI 2.04 to 4.15, p < 0.001). After multivariable adjustment, there was attenuation in the HR. Similar HR’s were noted for patients with scores of 4–6 for the ESRD-CI. For the CCI, there was separation in the HR’s for scores of 4–6 however, confidence intervals overlapped (Table 3).
Discussion
In this cohort study we evaluated the ability of the CCI and ESRD-CI to predict mortality in a population of incident dialysis patients. The association between comorbidity and mortality in our dataset was not as strong as in the derivation cohorts. In addition although higher comorbidity burden using the CCI and ESRD-CI was associated with mortality after multivariable adjustment, there was not a large separation in mortality risk when evaluating incremental changes in comorbidity scores. We can speculate that the limited utility of the CCI and ESRD-CI is due to several potential underlying reasons; limited generalizability of both indices, variability in comorbidity classification and comorbidity prevalence within either cohort, limitations with respect to derivation (including statistical over-fitting) and limited utility of comorbidity indices in general.
Limited generalizability may explain the observation of only partial validity. The CCI and ESRD-CI used a relatively small population from a single geographical area and validation in this study occurred in a separate single geographical area. Differences in the determinants of health in two communities may contribute to the different outcomes of two dialysis patients with similar comorbidities. Social determinants of health, in particular, have been shown to impact mortality rates in patients with ESRD [37–39]. More recently, frailty has been shown to be an important prognostic factor for incident dialysis patients [40]. Therefore, prediction models that incorporate clinical, demographic and social factors as well as assessments of frailty may be more applicable to ESRD cohorts [41]. An attempt at further validation of this index in a national or international sample of patients from a number of different centers in different geographic regions might help to clarify its generalizability and provide a clearer picture of its clinical utility.
There were notable characteristic differences comparing patients in our dataset and the dataset of the original studies, which may explain the reduced discrimination. Our cohort was in a more recent era (2006–2013), and the relative impact of some comorbid conditions may have changed [42–45]. The mortality risk in our cohort was higher than either derivation cohort [15] however; certain comorbidities such as chronic lung disease (16.7 % vs. 27.4 %) and neoplasia (6.5 % vs. 12.2 %) were less common in our cohort [15]. Furthermore, there was a reduced prevalence of overall comorbidity compared to the derivation cohorts. This reduced burden of disease might have affected the predictive value of the individual comorbidities comprising both indices. Alternatively, under-reporting of individual health conditions in each cohort may have explained the observed differences. For example, if comorbidity in our validation cohort was under-reported, despite a “falsely low” burden of comorbidity patients would have continued to have a high mortality rate. This in turn would have reduced the discrimination in our dataset.
The derivation of the CCI or ESRD-CI may have also impacted its validation. The linear predictor from a Cox model is ideal for developing a prognostic index. The linear predictor is described as the weighted sum of the variables in the model, where the regression coefficients are the weights [25]. In the development of the CCI, (which was replicated in the derivation of the ESRD-CI to maintain consistency) scoring weights were assigned to each HR derived from the Cox model [4, 15]. This may lead to over-weighting of conditions with high hazard ratios but limited precision and marked variability around the estimate.
While there are limitations with the comorbidity indices, it is important to acknowledge that both indices did have some validity. It is not unexpected that a higher level of comorbidity would be associated with a higher risk of death among dialysis patients, however most evaluations of comorbidity scores look at short term mortality or use validation techniques that do not incorporate survival time [15, 19, 23, 24]. Discrimination was lower in our validation dataset, however, the relative hazard for death was proportional across the index and the mortality association using either index persisted despite a relatively long duration of follow-up. Furthermore, it is not uncommon for validation studies to identify some reduction in predictive value [46] In addition there are other features of these indices that make them valuable. Both are intuitive; comorbid conditions that would be expected to confer a higher hazard for mortality are weighted more heavily. The major exception to this would be HIV (a component of the original CCI) a condition with a lower contemporary mortality rate [42]. Another advantage is that both indices draw on clinical data that is often present in patient charts, making them practical tools that do not necessarily require other diagnostic testing or laboratory investigation. Finally, the association with mortality persisted in our study even after multivariable adjustment for known predictors of mortality among dialysis patients.
It is important to note that there are limitations of comorbidity indices in general that warrant consideration. Most indices do not fully take into account the stage of progression, severity or proximity of the comorbid condition in relation to dialysis initiation [1, 14–16]. Furthermore, accumulation of comorbidity that often accompanies the early period after dialysis initiation [47] is not typically included in comorbidity indices. Global scoring systems that incorporate all prior comorbidities are easier to calculate and extract from patient records and facilitate ease of clinical application at point-of-care. More novel indices such as the Adult Comorbidity Evaluation-27, which takes into account the proximity and severity of the comorbidity, have been shown to out perform the CCI at predicting mortality [48, 49] and may be better suited for dialysis cohorts. Additionally, simple prognostic models that include the “surprise question” have also been shown to accurately predict survival for patients receiving hemodialysis [50].
This study utilized a substantial Canadian cohort and a long follow up time while leveraging electronic medical records to ensure a high quality and robust dataset. Additionally validation of the index was performed utilizing stringent methodology [25]. Examining the adjusted association between the CCI and ESRD-CI and mortality was enhanced by the relatively large number of outcomes.
There are, however, limitations to this study. The classification of comorbidities for each patient was determined by clinical impression (based on documentation in paper chart and electronic records). This introduces the possibility for misclassification bias. The derivation cohort(s) would have also been affected by misclassification bias, potentially compounding the observed differences in disease prevalence and prognostic utility. Our ability to completely validate either index was limited by the level of information provided in the original studies. In particular, an assessment of calibration (the ability of the index to assign the correct event probability at any relevant follow-up time and every level of predicted risk [25]) could not be performed.
Conclusion
The CCI and ESRD-CI had a limited ability to discriminate risk of death for incident dialysis patients in a contemporary Canadian cohort. Although a higher comorbidity burden was associated with mortality, incremental increases in index scores did not considerably change the risk of death.
References
Khan IH, Catto GR, Edward N, Fleming LW, Henderson IS, MacLeod AM. Influence of coexisting disease on survival on renal-replacement therapy. Lancet. 1993;341(8842):415–8.
Keane WF, Collins AJ. Influence of co-morbidity on mortality and morbidity in patients treated with hemodialysis. Am J Kidney Dis. 1994;24(6):1010–8.
Miskulin D, Bragg-Gresham J, Gillespie BW, Tentori F, Pisoni RL, Tighiouart H, et al. Key comorbid conditions that are predictive of survival among hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(11):1818–26.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108(8):609–13.
Chae JW, Song CS, Kim H, Lee KB, Seo BS, Kim DI. Prediction of mortality in patients undergoing maintenance hemodialysis by Charlson Comorbidity Index using ICD-10 database. Nephron Clin Pract. 2011;117(4):c379–84.
Fried L, Bernardini J, Piraino B. Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis. 2001;37(2):337–42.
Fried L, Bernardini J, Piraino B. Comparison of the Charlson Comorbidity Index and the Davies score as a predictor of outcomes in PD patients. Perit Dial Int. 2003;23(6):568–73.
Lin YT, Wu PH, Kuo MC, Lin MY, Lee TC, Chiu YW, et al. High cost and low survival rate in high comorbidity incident elderly hemodialysis patients. PLoS One. 2013;8(9), e75318.
Poses RM, McClish DK, Smith WR, Bekes C, Scott WE. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol. 1996;49(7):743–7.
Rattanasompattikul M, Feroze U, Molnar MZ, Dukkipati R, Kovesdy CP, Nissenson AR, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol. 2012;44(6):1813–23.
Wu PH, Lin YT, Lee TC, Lin MY, Kuo MC, Chiu YW, et al. Predicting mortality of incident dialysis patients in Taiwan--a longitudinal population-based study. PLoS One. 2013;8(4), e61930.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am J Epidemiol. 2011;173(6):676–82.
Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17(6):1085–92.
Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42(1):125–32.
Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2009;77(2):141–51.
Chang JH, Rim MY, Sung J, Ko KP, Kim DK, Jung JY, et al. Early start of dialysis has no survival benefit in end-stage renal disease patients. J Korean Med Sci. 2012;27(10):1177–81.
Erickson KF, Tan KB, Winkelmayer WC, Chertow GM, Bhattacharya J. Variation in nephrologist visits to patients on hemodialysis across dialysis facilities and geographic locations. Clin J Am Soc Nephrol. 2013;8(6):987–94.
Lee S, Ryu JH, Kim H, Kim KH, Ahn HS, Hann HJ, et al. An Assessment of Survival among Korean Elderly Patients Initiating Dialysis: A National Population-Based Study. PLoS One. 2014;9(1), e86776.
Chang TI, Kim YL, Kim H, Ryu GW, Kang EW, Park JT, et al. Hyponatremia as a predictor of mortality in peritoneal dialysis patients. PLoS One. 2014;9(10), e111373.
Shih CJ, Chen YT, Ou SM, Yang WC, Kuo SC, Tarng DC. The impact of dialysis therapy on older patients with advanced chronic kidney disease: a nationwide population-based study. BMC Med. 2014;12(1):169.
Brunelli SM, Blanchette CM, Claxton AJ, Roy D, Rossetti S, Gutierrez B. End-stage renal disease in autosomal dominant polycystic kidney disease: a comparison of dialysis-related utilization and costs with other chronic kidney diseases. Clinicoecon Outcomes Res. 2015;7:65–72.
Fernandez Lucas M, Teruel JL, Zamora J, Lopez Mateos M, Rivera M, Ortuno J. A Mediterranean age-comorbidity prognostic index for survival in dialysis populations. J Nephrol. 2007;20(6):696–702.
Marinovich S, Lavorato C, Morinigo C, Celia E, Bisignano L, Soratti M, et al. A new prognostic index for one-year survival in incident hemodialysis patients. Int J Artif Organs. 2010;33(10):689–99.
Royston P, Altman D. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13(1):33.
Harrell Jr FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247(18):2543–6.
Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS(2) versus CHA(2)DS(2)-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol. 2013;10(3):258–66.
Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. Jama. 2011;306(6):620–6.
Depner T, Daugirdas J, Greene T, Allon M, Beck G, Chumlea C, et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int. 2004;65(4):1386–94.
Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting Survival Differences by Race and Ethnicity among Hemodialysis Patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol. 2006;17(10):2910–8.
McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between Dialysis Modality and Mortality. J Am Soc Nephrol. 2009;20(1):155–63.
de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127–35.
Brattich M. Morbidity and mortality in patients on dialysis: the impact of hemoglobin levels. Nephrol Nurs J. 2006;33(1):64–7. 90; quiz 68–69.
Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, et al. Serum Phosphate Levels and Mortality Risk among People with Chronic Kidney Disease. J Am Soc Nephrol. 2005;16(2):520–8.
Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, et al. Serum Phosphate and Mortality in Patients with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2010;5(12):2251–7.
Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887–96.
Agodoa L, Eggers P. Racial and Ethnic Disparities in End-Stage Kidney Failure—Survival Paradoxes in African-Americans. Semin Dial. 2007;20(6):577–85.
Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O'Hare AM. Geography Matters: Relationships among Urban Residential Segregation, Dialysis Facilities, and Patient Outcomes. Ann Intern Med. 2007;146(7):493–501.
Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol. 2013;24(2):293–301.
Swidler M. Considerations in Starting a Patient with Advanced Frailty on Dialysis: Complex Biology Meets Challenging Ethics. Clin J Am Soc Nephrol. 2013;8(8):1421–8.
Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of Frailty among Dialysis Patients. J Am Soc Nephrol. 2007;18(11):2960–7.
Zavascki AP, Fuchs SC. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol. 2007;60(9):867–8.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
Puymirat E, Simon T, Steg P, et al. Association of changes in clinical characteristics and management with improvement in survival among patients with st-elevation myocardial infarction. Jama. 2012;308(10):998–1006.
Andresdottir G, Jensen ML, Carstensen B, Parving H-H, Hovind P, Hansen TW, et al. Improved prognosis of diabetic nephropathy in type 1 diabetes. Kidney Int. 2015;87(2):417–26.
Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19(4):453–73.
Miskulin DC, Meyer KB, Martin AA, Fink NE, Coresh J, Powe NR, et al. Comorbidity and its change predict survival in incident dialysis patients. Am J Kidney Dis. 2003;41(1):149–61.
Soares M, Salluh JF, Ferreira C, Luiz R, Spector N, Rocco J. Impact of two different comorbidity measures on the 6-month mortality of critically ill cancer patients. Intensive Care Med. 2005;31(3):408–15.
Mayr R, May M, Martini T, Lodde M, Pycha A, Comploj E, et al. Predictive capacity of four comorbidity indices estimating perioperative mortality after radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2012;110(6b):E222–7.
Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting Six-Month Mortality for Patients Who Are on Maintenance Hemodialysis. Clin J Am Soc Nephrol. 2010;5(1):72–9.
Acknowledgements
None to Declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Financial Competing Interests: No authors received reimbursements, fees, funding or salary from an organization that could gain or lose financially from the publication of this manuscript.
Other Financial Competing Interests: Steven Soroka received support for research and honorarium from Amgen and Otsukta for the past 3 years. Karthik Tennankore has received unrestricted grant support for quality improvement projects from Amgen, Canada.
Non-financial competing interests: The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed to the conception and design of the study. KT, BK SS and TA were involved in data acquisition. JPR and KT did the statistical analysis. All authors contributed to the interpretation of data. AG drafted the original manuscript and all authors helped with revisions for intellectual content. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gomez, A.T., Kiberd, B.A., Royston, J.P. et al. Comorbidity burden at dialysis initiation and mortality: A cohort study. Can J Kidney Health Dis 2, 34 (2015). https://doi.org/10.1186/s40697-015-0068-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40697-015-0068-3