Abstract
Background
Rumex species are traditionally used for the treatment of neurological disorders including headache, migraine, depression, paralysis etc. Several species have been scientifically validated for antioxidant and anticholinestrase potentials. This study aims to investigate Rumex hastatus D. Don crude methanolic extract, subsequent fractions, saponins and flavonoids for acetylcholinestrase, butyrylcholinestrase inhibition and diverse antioxidant activities to validate its folkloric uses in neurological disorders. Rumex hastatus crude methanolic extract (Rh. Cr), subsequent fractions; n-hexane (Rh. Hex), chloroform (Rh. Chf), ethyl acetate (Rh. EtAc), aqueous fraction (Rh. Aq), crude saponins (Rh. Sp) and flavonoids (Rh. Fl) were investigated against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) at various concentrations (125, 250, 500, 1000 μg/mL) using Ellman’s spectrophotometric analysis. Antioxidant potentials of Rh. Sp and Rh. Fl were evaluated using DPPH, H2O2 and ABTS free radical scavenging assays at 62.5, 125, 250, 500, 1000 μg/mL.
Results
All the test samples showed concentration dependent cholinesterase inhibition and radicals scavenging activity. The AChE inhibition potential of Rh. Sp and Rh. Fl were most prominent i.e., 81.67 ± 0.88 and 91.62 ± 1.67 at highest concentration with IC50 135 and 20 μg/mL respectively. All the subsequent fractions exhibited moderate to high AChE inhibition i.e., Rh. Cr, Rh. Hex, Rh. Chf, Rh. EtAc and Rh. Aq showed IC50 218, 1420, 75, 115 and 1210 μg/mL respectively. Similarly, against BChE various plant extracts i.e., Rh. Sp, Rh. Fl, Rh. Cr, Rh. Hex, Rh. Chf, Rh. EtAc and Rh. Aq resulted IC50 165, 175, 265, 890, 92, 115 and 220 μg/mL respectively. In DPPH free radical scavenging assay, Rh. Sp and Rh. Fl showed comparable results with the positive control i.e., 63.34 ± 0.98 and 76.93 ± 1.13% scavenging at 1 mg/mL concentration (IC50 312 and 104 μg/mL) respectively. The percent ABTS radical scavenging potential exhibited by Rh. Sp and Rh. Fl (1000 μg/mL) were 82.58 ± 0.52 and 88.25 ± 0.67 with IC50 18 and 9 μg/mL respectively. Similarly in H2O2 scavenging assay, the Rh. Sp and Rh. Fl exhibited IC50 175 and 275 μg/mL respectively.
Conclusion
The strong anticholinesterase and antioxidant activities of Rh. Sp, Rh. Fl and various fractions of R. hastatus support the purported ethnomedicinal uses and recommend R. hastatus as a possible remedy for the treatment of AD and neurodegenerative disorders.
Similar content being viewed by others
Background
According to WHO report 2001, round the world 25% individuals suffer from mental/neurological disorders in their life time [1]. The most common neurological disorders are epilepsy, headache, migraine, susto (fright), madness, numbness, insomnia, stress, depression, alzeimer’s disease, parkinson’s disease, anxiety etc [2,3]. In developed countries the treatment of these diseases is achieved by proper preventative measures, rehabilitation and improved monitoring of patients. But in developing and underdeveloped countries, the patients suffering from different diseases primarily rely on traditional practitioners and herbal medicine. The traditional use of herbal medicine is as old as the history of man. Even today the herbal medicine is utilized in sizable portion of world’s population. As far as the use of traditional medicine for the neurological diseases are concerned, the scientific validation of various medicinal plants supports their traditional uses and have led to development of novel drugs [4,5]. Large numbers of natural bioactive compounds have been derived from various plants species based on their traditional knowledge. For example the Ginkgo biloba was traditionally perceived as memory enhancer and was considered as anti-ageing which was scientifically verified but later on it was also confirmed that it is effective in the treatment of mild to moderate alzeimer’s disease [6-8]. Similarly galanthamine a drug used against AD that is derived from Galanthus nivalis is also the outcome of traditional knowledge. It has been reported in detail that Galanthamine basically increases acetylcholine level in synapsis by inhibiting the acetylcholinesterase responsible for the breakdown of acetylcholine. AD occurs as a result of decreased cholinergic transmission, increased oxidative stress, increased inflammatory condition, increased β-amyloid formation, decreased nerve growth factors etc [9]. But for the treatment of AD only cholinesterase inhibitors are considered primarily. The other factors responsible for AD are not emphasized as the cholinergic transmission, yet they are having ample importance.
The ethnomedicinal uses of flora make it easy to know the latent potentials of a specific plant specie. R. hastatus, one of the members of polygonaceae has wide variety of traditional uses, for examples, it is commonly used as laxative, alterative, tonic, in rheumatism [10], in skin diseases, piles, bilious complaints and lungs bleeding [11]. The juice of R. hastatus is used to treat blood pressure [12], in tonsillitis and sore throat [13], as flavoring agent, carminative and diuretic [14]. Similarly it has also been reported to be used traditionally against giddiness and insanity [15]. As far as other species of Rumex are concerned, majority of them are traditionally being used for various neurological disorders i.e., R. acetosa is traditionally used in paralysis [16], R. abyssinicus is used for the treatment of migraine, headache, rabies [17], R. tuberosus is used as tension regulator [18] and R. nepalensis, R. maderensis are also used in headache [19,20]. Moreover the R. patientia has been scientifically confirmed to improve memory and passive avoidance learning [21]. Antioxidant activities of several species of Rumex have been reported while the anticholinesterase activities are still to be scientifically confirmed [22,23]. Almost all the species of a specific genus resembles considerably due to genome similarity among the species of the same genus [24]. As discussed earlier, the species of Rumex are traditionally being used in paralysis, headache and other CNS disorders so it provide an ethnomedicinal evidence for the presence of cholinergic transmission enhancing principles in this genus. Based on the previous literature and ethnomedicinal uses, this investigational study is designed to uncover the anticholinesterase and antioxidant potentials of various samples of R. hastatus and to provide a bridge between the traditional and scientific knowledge.
Results
Phytochemical screening and extraction yields
The preliminary phytochemical screening of R. hastatus confirmed the presence of alkaloids, saponins, anthraquinone glycoside, tannins and flavonoids. The extraction yield of Rh. Sp and Rh. Fl of R. hastatus was 6.5 and 8% respectively.
Acetylcholinesterase inhibition assay
The most prominent AChE inhibition of various samples of R. hastatus was recorded for Rh. Sp and Rh. Fl. The percent AChE inhibition observed for Rh. Sp were 81.67 ± 0.88, 72.87 ± 1.27, 60.95 ± 2.01 and 49.08 ± 1.04 at the concentration of 1000, 500, 250 and 125 μg/mL respectively with IC50135 μg/mL, while the Rh. Fl displayed 91.62 ± 1.67, 84.76 ± 0.61, 81.03 ± 0.86 and 67.70 ± 0.92% AChE inhibition at afore mentioned concentrations with IC50 20 μg/mL. Among the resultant fractions the Rh. Chf revealed good results as shown in Table 1. All the fractions showed concentration dependent activity. The IC50 calculated for Rh. Cr, Rh. Hex, Rh. Chf, Rh. EtAc and Rh. Aq were 218, 1420, 75, 115 and 1210 μg/mL respectively, in which Rh. Chf was observed as most potent.
Butyrylcholinesterase inhibition assay
In BChE inhibition assay, Rh. Fl and Rh. Sp excelled among all the test samples. The percent BChE inhibition calculated for Rh. Sp were 77.52 ± 3.28, 63.93 ± 0.67, 61.83 ± 1.21 and 42.22 ± 1.28 at 1000, 500, 250 and 125 μg/mL respectively with IC50 165 μg/mL. Rh. Fl displayed 79.16 ± 0.86, 77.98 ± 0.72, 59.65 ± 0.98 and 41.93 ± 1.01% BChE inhibition at afore mentioned concentrations with IC50 175 μg/mL. Similarly all the fractions showed moderate to high activity and the IC50 calculated for Rh. Cr, Rh. Hex, Rh. Chf, Rh. EtAc and Rh. Aq were 265, 890, 92, 115 and 220 μg/mL respectively against BChE as shown in the Table 1. Briefly the BChE inhibition observed for each plant samples were dose dependent and the results of Rh. Fl and Rh. Sp were almost comparable with the positive control.
DPPH radical scavenging effect
The antioxidant activity of Rh. Sp and Rh. Fl against DPPH free radicals displayed a dose dependent response. The DPPH scavenging potential of Rh. Fl was comparable with ascorbic acid while the Rh. Sp displayed a moderate antioxidant activity in comparison with the positive control. The percent radical scavenging potentials of Rh. Sp were 63.34 ± 0.98, 56.32 ± 1.06, 48.05 ± 0.75, 44.70 ± 1.25 and 38.74 ± 0.68 at the concentrations of 1000, 500, 250, 125 and 62.5 μg/mL respectively with IC50 312 μg/mL. The Rh. Fl demonstrated 76.93 ± 1.13, 64.74 ± 1.29, 61.42 ± 0.57, 52.34 ± 1.01 and 46.73 ± 0.78% radicals scavenging potentials at 1000, 500, 250, 125 and 62.5 μg/mL respectively with IC50 104 μg/mL as shown in the Table 2.
H2O2 scavenging effect
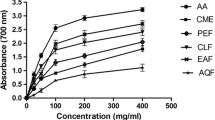
The antioxidant activity of R. hastatus against hydrogen peroxide revealed good results for Rh. Sp. The Rh. Fl demonstrated good antioxidant activity with IC50 275 μg/mL, which was comparable with the positive control. The percent hydrogen peroxide scavenging potentials exhibited by Rh. Sp were 29.13 ± 0.20, 37.85 ± 0.97, 44.82 ± 0.82, 56.90 ± 0.78 and 65.70 ± 1.25 at the concentrations of 62.5, 125, 250, 500 and 1000 μg/mL respectively with the IC50 of 175 μg/mL. At the highest concentration, i.e., 1000 μg/mL the Rh. Fl and positive control exhibited 54.34 ± 1.01 and 54.17 ± 1.69% inhibitions respectively while the Rh. Sp demonstrated 65.70 ± 1.25% antioxidant potentials as shown in Figure 1.
ABTS radical scavenging effect
The ABTS radical scavenging activity of Rh. Sp and Rh. Fl of R. hastatus was more prominent in comparison to other antioxidant assays. Both the plant samples exhibited very low IC50 values in this activity. The Rh. Fl displayed 88.25 ± 0.67, 82.23 ± 0.39, 74.92 ± 1.08, 70.53 ± 0.90 and 61.70 ± 1.60% ABTS radical scavenging potential at the concentrations of 1000, 500, 250, 125 and 62.5 μg/mL respectively with the IC50 of 9 μg/mL. The Rh. Sp showed the IC50 value of 18 μg/mL, which was larger (less potent) than those of Rh. Fl (IC50 = 9 μg/mL) and positive control (IC50 = 5 μg/mL). Briefly the ABTS radical scavenging activity of samples of R. hastatus revealed remarkable results with dose dependent response as shown in the Figure 2.
Discussions
Acetylcholine, an extensively distributed neurotransmitter especially in the central nervous system has a key role in transmission of signal across the synapses, cerebral blood flow, cognitive performance and coordination. The decreased level of acetylcholine within the nervous system of the body may be due to reduced acetyltransferase or increased level of AChE. These two factors are correlated with dementia and AD. The hydrolysis of acetylcholine is decreased by inhibiting AChE in the brain [25,26]. There are numerous evidences which support the deteriorating action of free radicals, which can lead to cognitive ageing and CNS disorders [27]. The age related brain performance can be easily ameliorated by the consumption of fruits and vegetables rich in flavonoids, which possess the ability to neutralize the free radicals [28]. It has already been reported that the cognitive performance can be significantly improved by taking diet rich in antioxidant species especially the flavonoids [29,30]. It is believed that the natural products are safe as compared to synthetic compounds [31]. Due to great interest towards the natural products, the scientists are trying to investigate more and more plants for the presence of effective compounds, which may cure a specific disease. The presence of thousands of flavonoids has been confirmed in more than 3000 plant species. Flavonoids have been reported to be significantly effective in cholinesterase inhibition, i.e., Leufolins A and B (flavonoids) isolated from Leucas urticifolia have been scientifically verified to possess strong BChE inhibitory potential [32]. It is also obvious of our current investigations that Rh.Fl possess comparable anticholinesterase potential with the positive control (galantamine). Similarly the saponins are also prominent secondary metabolites, which have been verified to possess various beneficial pharmacological potentials. For instant, the saponins isolated from traditional Chinese medicines have demonstrated excellent antioxidant activity [33]. As obvious of the current investigational data, the Rh.Sp demonstrated good antioxidant and significant anticholinesterase potential. Similarly the antioxidant potential of Rh.Sp against ABTS was comparable to the positive control and Rh.Fl as shown in the Figure 2. The current investigation and the literature review intimates the presence of anticholinesterase compounds in Rh.Sp and Rh.Fl of R. hastatus just like the saponins (Bacosides) of Bacopa monnieri and the flavonoids (ginkgoflavonglycosides) of Ginkgo biloba [34]. Among the subsequent fractions the excellent activity of Rh.Chf and Rh.EtAc represents that the saponins and flavonoids may be concentrated in the said fractions. The previously reported data also goes parallel with our current investigation i.e., in the current study the Rh.Fl has shown significant antioxidant potential and in the previous reports, the fractions (chloroform, ethyl acetate and butonal) having high quantity of total phenolics and total flavonoids content had shown significant antioxidant activity as reported by Sehreen et al and Afzal et al [35,36]. Likewise several other plant species have been reported to possess multiple pharmacological activities [37-39].
Conclusions
The thorough literature survey and the results of our current investigation support the potential role of R. hastatus in the treatment of nervous disorders. Similarly, based on the significant enzymes (AChE, BChE) inhibition and radicals scavenging demonstrations of various samples of R. hastatus, it may be inferred that the Rh. Fl and Rh. Sp of R. hastatus may be the best sources of antioxidant and anticholinesterase compounds. The bioguided isolation of R. hastatus is in progress in our laboratory, which may lead to new anti-Alzheimer’s disease and anti-ageing candidates.
Methods
Plant collection, extraction and fractionation
The whole plant of R. hastatus was collected from the hilly area of Gorha Gat nearby University of Malakand, Chakdara (Lower dir) KPK, Pakistan. The plant was identified by plant taxonomist Ali Hazrat and deposited with voucher number (1015SJ) in the herbarium, Department of Botany, Shaheed Benazir Bhutto University, Sheringal (Dir Upper) KPK, Pakistan. All the extra particles (sand and dust) were thoroughly removed from the plant and was scattered on neat paper in a room protected from sunlight and properly shade dried for 20 days. The paper was changed every day to avoid fungal growth. After shade drying the plant material was converted into coarse particles using cutter mill. The powdered plant sample (4 kg approximately) was subjected to maceration in 80% methanol and filtered after 15 days using muslin cloth. The filtrate was concentrated using rotary evaporator and then solidified using water bath at 40°C yielding 300 g of crude methanolic extract. The crude methanolic extract obtained (260 g) was subject to fractionation using successive solvent-solvent extraction process starting from n-hexane, chloroform, ethyl acetate and water yielding 30, 22, 53 and 140 g of Rh. Hex, Rh. Chf, Rh. EtAc and Rh. Aq respectively [40].
Extraction of crude saponins
For the extraction of Rh. Sp from R. hastatus, 20 g powdered plant was added to 100mL ethanol (20%). The sample was heated at 55°C in water bath for four hours with continuous stirring. After four hours the sample obtained was filtered and the residue having greenish color was re-extracted with 200 mL of 20% ethanol. The sample after extraction was heated until a concentrated volume of 40 mL was obtained. Then it was transferred into a separating funnel and 20 mL of diethyl ether was added to it. After vigorous shaking, the separating funnel was put in a stand to get two layered sample. The lower layer was collected, which was aqueous layer while the upper diethyl ether layer was discarded. The aqueous layer obtained was diluted with 60 mL of n-butanol and the combined n-butanol extract was washed with 10 mL of 5% sodium chloride solution. The final solution obtained was kept in a hot water bath until complete evaporation and the Rh. Sp obtained were dried in an oven yielding 1.3 g of Rh. Sp [41].
Extraction of flavonoids
For the extraction of flavonoids from R. hastatus, the procedure of Harborne was followed [42]. Powdered plant sample weighing 20 g was heated at 50°C in 200 mL of 2M HCl under reflux for half an hour. It was cooled and filtered using whatman No.42 filter paper. The filtrate was treated with equal volume of ethyl acetate. The Rh.Fl present in the extract were precipitated, which were recovered with the help of weighed filter paper. The weight of Rh.Fl obtained was 1.6 g (8%) [43].
Preliminary phytochemical screening
The preliminary phytochemical screening of the R. hastatus was carried out to determine the existence of alkaloids, saponins, anthraquinone glycoside, tannins and flavonoids [44].
Anticholinesterase assays
Chemicals required
Electric eel acetylcholinesterase (type-VI-S, Sigma-Aldrich USA), Aquine butyrylcholinesterase (Sigma-Aldrich USA), Acetylthiocholine Iodide (Sigma-Aldrich UK), Butyrylthiocholin iodide (Sigma-Aldrich Switzerland), 5,5-dithio-bis-nitrobenzoic acid (DTNB) (Sigma-Aldrich Germany), Potassium phosphate buffer (pH 8.0), Galantamine from Lycoris Sp. (Sigma-Aldrich France).
Spectroscopic analysis
AChE and BChE inhibition was assessed spectrophotometrically for Rh. Fl and Rh. Sp using Acetylthiocholine iodide and Butyrylthiocholine iodide as substrate following the method of Ellman [45]. In this method, 5 μL of AChE (0.03 U/mL) and BChE (0.01 U/mL) were taken in a cuvette and 205 μL of plant samples (125–1000 μg/mL) were added to them. DTNB (5 μL) was added to the mixture, transferred to a water bath having temperature of 30°C and incubated for 15 minutes. Substrates having volume of 5 μL were added to the mixture to start the reaction. The reaction mixture was analyzed at 412 nm using a double beam spectrophotometer. Absorption was recorded for 4 minutes. The formation of yellow color indicated the formation of 5-thio-2-nitrobenzoate anion as a result of the reaction between thiocholines and DTNB. To check the non-enzymatic hydrolysis of substrate, white assay was carried out without enzymes and plant samples. The reaction mixture containing all the components excluding plant sample was taken as control. Percent enzyme activity and percent inhibition were figured out as follows.
(Where V denotes rate of reaction in the present of inhibitor and Vmax denotes rate of reaction without inhibitor).
DPPH radical scavenging assay
The method of Brand-Williams et al. [46] with some modifications was followed for the DPPH assay. DPPH (24 mg) was dissolved in 100 mL of methanol to get DPPH solution. The stock solutions of plant samples having concentrations of 1 mg/mL were prepared in methanol and then diluted to the concentrations of 500, 250, 125, 62.5 μg/mL. 0.1 mL diluted solution of each sample was mixed with 3 mL of DPPH solution in methanol. The solution was incubated at 23°C for 30 minutes and then the absorbance was measured at 517 nm. For positive control ascorbic acid was used. Each concentration was taken in triplicate and the data obtained was presented as mean ± S.E.M. The percent radical scavenging activity was calculated using the following equation:
Hydrogen peroxide scavenging assay
The hydrogen peroxide scavenging activity of samples was conducted following the procedure of Ruch et al [47]. Hydrogen peroxide solution (2 mM) was prepared in 50 mM phosphate buffer having the pH of 7.4. Various plant samples having volume of 0.1 mL were taken in test tubes and their volumes were made 0.4 mL by addition of 50mM phosphate buffer. Hydrogen peroxide solution (0.6 mL) was added to it and vertexed. After 10 minutes the absorbance of each sample was measured at 230nm against the blank. The hydrogen peroxide scavenging activity was measured using the following equation:
ABTS free radical scavenging assay
Antioxidant potentials of Rh. Sp and Rh. Fl were assessed using the free radicals of 2, 2-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid (ABTS) [48]. Solutions of ABTS 7 mM and potassium persulfate 2.45 mM were prepared and thoroughly mixed. The solution was kept overnight in dark to produce free radicals. After incubation the absorbance of ABTS solution was adjusted to 0.7 at 745 nm by the addition of 50% methanol. Then 300 μl of test samples were taken and 3 mL of ABTS solution was added to it. The solution obtained was transferred to cuvette and absorbance of was measured using a double beam spectrophotometer for six minutes. For positive control Ascorbic acid was used. The data was recorded in triplicate and percent ABTS free radicals scavenging activity was calculated as follows:
Estimation of IC50 values
Concentrations of test samples, which inhibited substrate hydrolysis (AChE and BChE) by 50% (IC50). Radical scavenging activity was determined by a linear regression analysis among the percent inhibition against the test samples concentrations via MS Excel program.
Statistical data analysis
All the tests were performed in triplicate and values were expressed as means ± S.E.M. Significance between radical scavenging activity and test samples were analyzed using Mann-Whitney U test. Group comparison was performed by Student’s t-test in which the P < 0.05 were considered significant.
References
WHO, Salud OMdl. The World Health Report 2001: Mental Health: New Understanding. New Hope: World Health Organization Geneva; 2001.
Bourbonnas-Spear N, Awad R, Maquin P, Cal V, Vindas PS, Poveda L, et al. Plant use by the Q’eqchi’Maya of Belize in ethnopsychiatry and neurological pathology. Eco Bot. 2005;59(4):326–36.
Aarsland D, Beyer MK, Kurz MW. Dementia in Parkinson’s disease. Curr Opin Neurol. 2008;21(6):676–82.
Sheng-Ji P. Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharm Biol. 2001;39(s1):74–9.
Ullah I, Subhan F, Ayaz M, Shah R, Ali G, Haq IU, et al. Anti-emetic mechanisms of zingiber officinale against cisplatin induced emesis in the pigeon; behavioral and neurochemical correlates. BMC Complement Altern Med. 2015;15(1):34.
Burkard G, Lehrl S. Verhältnis von Demenzen vom Multiinfarkt-und vom Alzheimertyp in ärztlichen Praxen. Münch Med Wschr. 1991;133 Suppl 1:38–43.
Kanowski S, Herrmann W, Stephan K, Wierich W, Hörr R. Proof of efficacy of the Ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry. 1996;29(02):47–56.
Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. Jama. 1997;278(16):1327–32.
Perry EK, Pickering AT, Wang WW, Houghton P, Perry NS. Medicinal plants and Alzheimer’s disease: Integrating ethnobotanical and contemporary scientific evidence. J Altern Complement Med. 1998;4(4):419–28.
Shinwari ZK, Gilani SS. Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (Northern Pakistan). J Ethnopharmacol. 2003;84(2):289–98.
Gorsi MS, Miraj S. Ethnomedicinal survey of plants of Khanabad village and its allied areas, district Gilgit. Asian J Plant Sci. 2002;1(5):604–15.
Manan Z, Razzaq A, Islam M. Diversity of medicinal plants in Wari subdivision district Upper Dir, Pakistan. Pak J Plant Sci (Pakistan). 2007;13(1):19–26.
Taylor R, Hudson J, Manandhar N, Towers G. Antiviral activities of medicinal plants of southern Nepal. J Ethnopharmacol. 1996;53(2):105–10.
Ullah A, Rashid A, Parveen SN. Medicinal plants used in the isolated region of Bumburet, Kalash valley, district Chitral, Pakistan. Pak J Weed Sci Res. 2014;20(3):359–73.
Pande P, Tiwari L, Pande H. Ethnoveterinary plants of Uttaranchal-A review. Ind J Trad Knowl. 2007;6(3):444–58.
Tita I, Mogosanu GD, Tita MG. Ethnobotanical inventory of medicinal plants from the South-West of Romania. Farmacia. 2009;57(2):141–56.
Awas T, Demissew S. Ethnobotanical study of medicinal plants in Kafficho people, southwestern Ethiopia. In: Proceedings of the 16th International Conference of Ethiopian Studies, ed. by Svein Ege, Harald Aspen, Birhanu Teferra and Shiferaw Bekele, Trondheim 2009;711–726.
Uysal İ, Onar S, Karabacak E, Çelik S. Ethnobotanical aspects of Kapıdağ Peninsula (Turkey). Biol Divers Conserv. 2010;3(3):15–22.
Ummara U, Bokhari TZ, Altaf A, Younis U, Dasti AA. Pharmacological Study of Shogran Valley Flora, Pakistan. Int J Sci Eng Res. 2013;4(9):1–9.
Tavares L, Carrilho D, Tyagi M, Barata D, Serra AT, Duarte CMM, et al. Antioxidant capacity of Macaronesian traditional medicinal plants. Molecules. 2010;15(4):2576–92.
Baluchnejadmojarad T, Roghani M. Chronic Rumex Patientia Seed Feeding Improves Passive Avoidance Learning and Memory in Streptozotocin-Diabetic Rats. Basic Clin Neurosci. 2010;1(4):53–6.
Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49(8):4083–9.
Kerem Z, Bilkis I, Flaishman MA, Sivan L. Antioxidant Activity and Inhibition of α-Glucosidase by trans-Resveratrol, Piceid, and a Novel trans-Stilbene from the Roots of Israeli Rumex bucephalophorus L. J Agric Food Chem. 2006;54(4):1243–7.
Peakall R, Gilmore S, Keys W, Morgante M, Rafalski A. Cross-species amplification of soybean (Glycine max) simple sequence repeats (SSRs) within the genus and other legume genera: implications for the transferability of SSRs in plants. Mol Biol Evol. 1998;15(10):1275–87.
Ogawa M, Iida Y, Nakagawa M, Kuge Y, Kawashima H, Tominaga A, et al. Change of central cholinergic receptors following lesions of nucleus basalis magnocellularis in rats: search for an imaging index suitable for the early detection of Alzheimer’s disease. Nuclear Med Biol. 2006;33(2):249–54.
Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm. 2006;113(11):1625–44.
Golden TR, Hinerfeld DA, Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1(2):117–23.
Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic Biol Med. 2004;37(11):1683–93.
Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, et al. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198(2):352–8.
Schroeter H, Spencer J, Rice-Evans C, Williams R. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J. 2001;358:547–57.
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20(12):522–31.
Noor A-t, Fatima I, Ahmad I, Malik A, Afza N, Iqbal L, et al. Leufolins A and B, potent butyrylcholinesterase-inhibiting flavonoid glucosides from Leucas urticifolia. Molecules. 2007;12(7):1447–54.
Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22(2):228–37.
Das A, Shanker G, Nath C, Pal R, Singh S, Singh HK. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol Biochem Behav. 2002;73(4):893–900.
Sahreen S, Khan MR, Khan RA. Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. Leaves. J Med Plants Res. 2011;5(13):2755–65.
Afzal S, Tabassum S, Gilani MA, Hussain N, Farooq R, Zahid S, et al. Total Phenolic Content, In Vitro Radical Scavenging And Antimicrobial Activities Of Whole Plant Rumex hastatus. Sci Int. 2014;26(2):721–7.
Shah S, Shah SMM, Ahmad Z, Yaseen M, Shah R, Sadiq A, et al. Phytochemicals, in vitro antioxidant, total phenolic contents and phytotoxic activity of Cornus macrophylla Wall bark collected from the North-West of Pakistan. Pak J Pharm Sci. 2015;28(1):23–8.
Shah SMM, Sadiq A, Shah SMH, Khan S. Extraction of saponins and toxicological profile of Teucrium stocksianum boiss extracts collected from District Swat, Pakistan. Biol Res. 2014;47(1):65.
Shah SMM, Ullah F, Shah SMH, Zahoor M, Sadiq A. Analysis of chemical constituents and antinociceptive potential of essential oil of Teucrium Stocksianum bioss collected from the North West of Pakistan. BMC Complement Altern Med. 2012;12(1):244.
Imran M, Ullah F, Sadiq A, Ayaz M, Ahmad S, Kamal Z, et al. Investigation Of Total Phenolic Contents, Antibacterial, Antifungal And Anthelmintic Potentials Of Crude Methanolic Extract, Subsequent Fractions And Crude Saponins Of Nonea micrantha Boiss. & Reut. Pharmacologyonline. 2014;3:26–31.
Ayaz M, Junaid M, Subhan F, Ullah F, Sadiq A, Ahmad S, et al. Heavy metals analysis, phytochemical, phytotoxic and anthelmintic investigations of crude methanolic extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complement Altern Med. 2014;14(1):465.
Harborne A. Phytochemical methods a guide to modern techniques of plant analysis. Netherlands: Springer; 1998.
Zeb A, Sadiq A, Ullah F, Ahmad S, Ayaz M. Investigations of anticholinestrase and antioxidant potentials of methanolic extract, subsequent fractions, crude saponins and flavonoids isolated from Isodon rugosus. Biol Res. 2015;47:76.
Zeb A, Sadiq A, Ullah F, Ahmad S, Ayaz M. Phytochemical and toxicological investigations of crude methanolic extracts, subsequent fractions and crude saponins of Isodon rugosus. Biol Res. 2014;47(1):57.
Ayaz M, Junaid M, Ahmed J, Ullah F, Sadiq A, Ahmad S, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complement Altern Med. 2014;14(1):145.
Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30.
Ruch RJ, Cheng S-j, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10(6):1003–8.
Re R, Pellegrini N, Proteggente A, Pannala A, Yong M, Rice-Evas C. Antioxidant activity applying an improved FBTS radical cation decolorization assay. Free Rad Biol Med. 1999;26(9/10):1231–7.
Acknowledgements
The author is grateful to the taxonomist for the identification of plant species and the head of Department of Pharmacy for the accomplishment of these activities.
Funding
The authors conducted the whole activities on their own behalf and no funding body was involved in the financial support of these activities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SA and MA carried out experimental work, data collection and evaluation, literature search and manuscript preparation. FU, AS and MI refined the manuscript for publication. All authors read and approved the final manuscript for publication.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ahmad, S., Ullah, F., Ayaz, M. et al. Antioxidant and anticholinesterase investigations of Rumex hastatus D. Don: potential effectiveness in oxidative stress and neurological disorders. biol res 48, 20 (2015). https://doi.org/10.1186/s40659-015-0010-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-015-0010-2