Abstract
Background
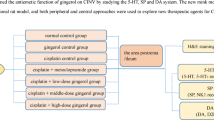
Zingiber officinale (ZO, family Zingiberaceae) has been reported for its antiemetic activity against cancer chemotherapy induced emesis in animal models and in clinics. Current study was designed to investigate ZO for potential usefulness against cisplatin induced vomiting in pigeon and its effects on central and peripheral neurotransmitters involved in the act of vomiting.
Methods
Zingiber officinale acetone fraction (ZO-ActFr) was investigated for attenuation of emesis induced by cisplatin in healthy pigeons. Neurotransmitters DA, 5HT and their metabolites DOPAC, HVA and 5HIAA were analyzed using High Performance Liquid Chromatography system coupled with electrochemical detector in area postrema, brain stem and intestine. Antiemetic effect of ZO-ActFr was correlated with central and intestinal neurotransmitters levels in pigeon.
Results
Cisplatin (7 mg/kg i.v.) induced emesis without lethality upto the observation period. ZO-ActFr (25, 50 & 100 mg/kg) attenuated cisplatin induced emesis ~ 44.18%, 58.13% (P < 0.05) and 27.9%, respectively; the reference drug, metoclopramide (MCP; 30 mg/kg), produced ~ 48.83% reduction (P < 0.05). ZO-ActFr reduced (P < 0.05 - 0.001) 5-hydroxytryptamine (5HT) concentration in the area postrema, brain stem and intestine at 3rd hour of cisplatin administration, while at the 18th hour ZO treatments attenuated the dopamine upsurge (P < 0.001) caused by cisplatin in the area postrema and 5HT concentration (P < 0.01 - 0.001) in the brain stem and intestine. ZO treatments alone did not altered the basal neurotransmitters and their metabolites in the brain areas and intestine.
Conclusion
The behavioral study verify the antiemetic profile of ZO against cisplatin induced emesis in the pigeon, where central and peripheral neural evidences advocate the involvement of serotonergic mechanism at initial time point (3rd hr), while the later time point (18th hr) is associated with serotonergic and dopaminergic component in the mediation of its antiemetic effect.
Similar content being viewed by others
Background
Cytotoxic agents like cisplatin and cyclophosphamide are having the side effects of nausea and vomiting most feared by patients undergoing cancer chemotherapy [1]. The D2 receptor blocker “metoclopramide” was found to be effective against Chemotherapy Induced Vomiting (CIV) at higher doses, where the anti-emetic effect is reported to be mediated through antagonism of 5-hydroxy tryptamine type 3 (5HT3) receptors [2,3]. These findings of 5HT3 mediated anti-emetic effect of metoclopramide led to the discovery of 5HT3 receptor antagonists.
The failure of single anti-emetic agent for the control of CIV is steering the etiology to be multifactorial, and there are evidences for the involvement of many neurotransmitter systems including serotonergic [4,5], dopaminergic [6,7] and neurokininergic [8,9] systems acting in emetic circuitry at different time pointes. In the past decades, major advances have been made in the understanding of the neuro-pharmacology of the emetic pathways.
The neurotransmitter “Serotonin” (5HT) is the primary culprit in the initiation of vomiting response especially considering CIV [10]. Upto 95% of 5HT is present in the enterochromaffin (EC) cells in the gastrointestinal mucosa along with substance P [11,12], which is released by the noxious stimulus caused by Highly Emetogenic Chemotherapy (HEC) agents like cyclophosphamide and cisplatin [5,13]. Furthermore, in human and animal studies, there are evidences for the increased level of 5-Hydroxy Indole Acetic Acid (5HIAA, urine) [14,15], 5HT in the intestinal mucosa (ileal segment), Tryptophan Hydroxylase (TPH, ileum), Aromatic L-amino Decarboxylase (AADC, ileum) [16] and in the brain stem following cisplatin treatment, while a decrease in Monoamine Oxidase (MAO, ileum) has also been reported [16]. This enhancement in 5HT biosynthesis and reduction in degradation ultimately lead to the upsurge of serotonin which is imperative in the mediation of vomiting act.
Dopamine (DA) is also among the several neurotransmitters, which theater its role in the genesis of vomiting, through selective activation of D2 receptors, localized in the limbic system, hypothalamus, amygdala and in the brain stem emetic circuitry [17,18]. Dopaminergic agonists like morphine and apomorphine has been reported to be emetic in a variety of species including dogs [19], ferrets [6,20] and human [21]. The emetic action of apomorphine and loperamide has been suggested to be mediated in the chemoreceptor trigger zone/area postrema through stimulation of dopamine receptors, as ablation of this area abolished the vomiting response [17,22].
Plants are proving themselves as important therapeutic entities that are economical, safe and readily available particularly in rural communities [23-26]. Zingiber officinale (Ginger) has been used as a natural herb in the treatment of vomiting for more than 2000 years in China and as common spice for cooking in Asian countries [27]. One of its therapeutic indications has always been in the treatment of nausea and vomiting, as its carminative, spasmolytic and aromatic properties suggest its direct effects on the gastrointestinal system. The major components present in ginger extract which is a mixture of homologues having 10, 20 and 14 carbon atoms in side chain that are designated as gingerols [28]. Gingerols, in particular 6-gingerol has been identified as the major active constituent responsible for its characteristic taste and is reported to enhance gastrointestinal motility and have capability to antagonize 5HT3 receptors in the gastrointestinal tract (GIT) [29], which supports its anti-emetic property.
Keeping in view the gastroprokinetic and 5HT3 receptor antagonist property, the present study was designed to screen the intrinsic anti-emetic activity of Zingiber officinale (ZO) against cisplatin induced emesis in pigeon. Furthermore, considering the relevance of serotonin and dopamine in emesis, this study was extended to evaluate the participation of these monoamine neurotransmitters and their metabolites in cisplatin induced vomiting, and to examine the impact of Zingiber officinale (ZO) on serotonin, dopamine and their metabolites centrally in specific brain areas involved in the act of vomiting and peripherally in the intestine in pigeon.
Methods
Animals
Pigeons of either sex (mixed breed, Department of Pharmacy, University of Peshawar, Pakistan) weighing between 250 - 350 g were used. They were housed in groups of eight at 22–26°C under a 12 h light/dark cycle and had free access to food (locally available food; Millet + Wheat) and water before and during experimentation. All the experimental procedures were approved by the Ethical Committee of the Department of Pharmacy, University of Peshawar (Ref. No 5/EC/Pharm; Dated: June 15, 2011) and are in accordance with the UK Animal Scientific Procedure Act, 1986.
Drugs and chemicals
High Performance Liquid Chromatography (HPLC) grade acetonitrile, methanol, 1-octane sulphonic acid sodium salt (Fisher scientific U.K.), sodium dihydrogen orthophosphate and ethylene diamine tetra acetic acid (EDTA) were purchased from the Merck local distributor in Peshawar, Pakistan. Noradrenaline, DOPAC, Dopamine, 5HIAA, HVA, serotonin were from Acros organics, Belgium. Cisplatin was from Korea United Pharm. Inc. (Korea) and was dissolved in normal saline at 65 - 70°C. Metoclopramide (MCP) was purchased in solution from GlaxoSmithKline (GSK Pakistan Ltd.). Acetone was from Haq Chemicals Peshawar (Pakistan). The plant rhizome were purchased from a local market at Mardan and was authenticated by Prof. Dr. Muhammad Ibrar, Department of botany, University of Peshawar.
Extraction of Zingiber officinale
A total of 0.5 kg of fresh ginger was bought from the central vegetable market in Mardan, Pakistan. A sample was deposited at the Herbarium of the Department of Botany, The University of Peshawar, Peshawar, with the voucher number (voucher No 20017 - pup). Ginger was washed for any contaminants and then sliced to expose the inner part. It was then soaked in 2 L of acetone and kept for a total of 3 days, thrice. The combined filtrate was concentrated in a rotary evaporator to obtain a thick extract with a yield of 4.72% (Figure 1).
Drug formulation
Cisplatin was dissolved in normal saline by heating up to 60°C, cooled upto 40 - 45°C and immediately administered. The acetone fraction of Zingiber officinale was dissolved in normal saline by gentle agitation and sonicated to get clear solution and administered.
Drug administration
Intravenous and intramuscular routes were used for drug administration via brachial wing vein and chest muscle, respectively. Immediately, after the last injection, the animals were put back in the specially designed confining/observation cages and the number of emesis episodes and latency to first vomit were recorded for 24 h. ZO-ActFr and MCP or respective vehicles, were administered 30 minutes before cisplatin administration. At the end of experiment, body weight loss was calculated. Subsequently, the animals were decapitated to terminate the experiment.
Anti-emetic assay
On the day of experiment, the pigeons were placed in individual cages specially designed for video observation. After cisplatin (7 mg/kg) [30] administration at t = 0 [31] the behavior of the pigeon was recorded for 24 h. Food and water were available during the observation period and each animal was used only once. The response with or without oral expulsion was considered as one vomiting episode [32]. A vomiting episode was considered completed when the pigeon adapted relaxed posture.
Tissue sampling for neurotransmitters analysis
At the end of each experiment, animals were decapitated and the brain areas and intestinal samples 5 – 6 cm from the pylorus were rapidly collected and placed on a cold ice plate (0°C). The dissection of brain parts was carried out according to the atlas of Karten and Hodos [33] and Henri M. Duvernoy [34]. The collected brain tissue samples were first cleared of vessels and meninges, while fecal matter and mesenteries were carefully removed from intestinal samples, were weighed and stored at - 80°C until analysis.
Determination of neurotransmitters and their metabolites
Tissue samples were homogenized in cold 0.2% perchloric acid (PCA) at 5000 rpm with the help of Teflon glass homogenizer (Wise stir HS 30 E). After centrifugation (Centurion UK) at 12000 g/min (4°C) and filtered through a 0.45 micron filter. Neurotransmitters and their metabolites were analyzed using High Performance Liquid Chromatography system (HPLC, Shimadzu, Japan) coupled with Electrochemical Detection (ECD, ESA Coulochem III model 5300), a pump (model LC-20AT), and an analytical column (Teknokroma 3 × 150, 3um). Electrodes 1 and 2 of the analytical cell were set at + 200 and – 200 mV respectively, with a sensitivity of 2 mA, while the guard cell (model 5020) potential was set at 500 mV. The mobile phase consisted of 94 mM sodium dihydrogen orthophosphate, 40 mM Citric acid, 2.3 mM sodium 1-octane sulphonic acid, 50 uM EDTA, and 10% acetonitrile (pH 3). The flow rate was maintained at 0.6 mL/min. The limit of detection was 11 pg for each of the neurotransmitter and their metabolites except for HVA which was 19 pg. The standards used were noradrenaline hydrochloride (NA), 3, 4-dihydroxyphenylacetic acid (DOPAC), dopamine hydrochloride (DA), 5-hydroxyindole-3-acetic acid (5HIAA), Homovanillic acid (HVA) and serotonin (5HT) [35].
Statistical analysis
The differences between means were evaluated using a one way analysis of variance (ANOVA) followed by Dunnett or Tukey multiple comparison tests. P < 0.05 was considered as statistically significant. The animals which showed complete suppression of R + V were not included in statistical analysis for latency. Data represent the mean ± s.e.m. unless otherwise indicated.
Results
Effect of ZO-ActFr or MCP on cisplatin induced Retching plus Vomiting (R + V)
Zingiber officinale acetone fraction (ZO-ActFr) at the dose of 50 mg/kg provided maximum protection against the R + V episodes which was ~ 58.13% (18 ± 4.2 episodes) (P < 0.05) as compared to cisplatin control. The attenuation with the 25 & 100 mg doses observed was 44.18% (24 ± 4.1 episodes) and 27.9% (31 ± 5.6 episodes) respectively, but the suppression was found to be statistically non-significant (Table 1). The standard MCP reduced the R + V episodes ~ 48.83% (22 ± 4.3 episodes) (P < 0.05) and significantly increased (P < 0.01) the latency time as compared to cisplatin control.
In this study the standard MCP and the treatments failed to provide complete vomiting suppression, as all the animals tested showed the vomiting response. ZO-ActFr 25 & 50 mg provided protection upto 16 hr and 12 hr, respectively, while standard MCP was also found effective upto 12 hr of observation period (Figure 2).
The effect ofZingiber officinaleacetone fraction (ZO-ActFr; 25, 50 & 100 mg/kg) and standard metoclopramide (MCP; 30 mg/kg) on cisplatin induced Retching plus Vomiting (R + V) during a 24 hr observation period. Each bar represents the mean ± s.e.m of R + V episodes occurring during 4 hr periods (n = 6–8).
Effect of ZO-ActFr or MCP on cisplatin induced jerks and weight loss
Zingiber officinale acetone fraction (ZO-ActFr) did not reduced jerking episodes at any dose treated and similarly the standard MCP also failed to reduce the jerking episodes as compared to cisplatin control. In cisplatin control group animals lost upto 16% of their starting body weight, while in treated groups only ZO-ActFr at the dose of 25 mg attenuated the weight loss significantly (P ˂ 0.05), while with other dose treatments the reduction observed was statistically non-significant (Table 1).
Effect of standard MCP or ZO-ActFr on basal level of neurotransmitters and their metabolites in the brain areas and small intestine
The standard MCP treatment reduced the concentration of 5HIAA in the areas of AP and BS with significance of (P < 0.05) and (P < 0.001), respectively as compared to basal level. In addition, the decrease in the concentration of HVA was also observed in the AP (P < 0.05) with respect to basal HVA concentration (Table 2). ZO-ActFr (50 mg/kg) did not altered the basal neurotransmitter level except a decrease in the concentration of 5HIAA in the brain area of BS, where the difference was found to be statistically significant (P < 0.05) as compared to basal level (Table 2).
Effect of standard MCP or ZO-ActFr on the level of neurotransmitters and their metabolites in the brain areas and small intestine at 3rd hour of cisplatin administration
Cisplatin treatment significantly increased (P < 0.001) the concentration of 5-hydroxy tryptamine (5HT) in the brain stem (BS) and intestine as compared to basal level, while a non-significant increase was observed in the area postrema (AP; Table 3). The treatment with standard MCP (30 mg/kg) failed to change the concentration of NA, DOPAC, DA, 5HIAA and HVA in all the brain areas (AP & BS) and intestine in comparison to saline control, but reduced the concentration of 5HT in the BS and intestine significantly (P < 0.001) as compared to cisplatin control (Table 3). In addition to its inhibitory effects on 5HT, MCP also decreased 5HIAA concentration in both the brain areas (AP & BS) and intestine significantly (P < 0.01-0.001, Table 3). ZO-ActFr (50 mg/kg) reduced the level of 5HIAA (P < 0.001) and 5HT (P < 0.05 - 0.001) in the brain (AP & BS) and intestine as compared to cisplatin control. No significant alteration was seen in NA, DOPAC, DA and HVA in the brain (AP & BS) and intestine (Table 3).
Effect of standard MCP or ZO-ActFr on the level of neurotransmitters and their metabolites in the brain areas and small intestine at 18th hour of cisplatin administration
Cisplatin increased the level of dopamine significantly (P < 0.001) in the AP, while a non-significant trend towards increase was observed in the areas of BS and intestine. 5HT concentrations were also raised in the brain area of BS (P < 0.05) and intestine (P < 0.001), without effecting the levels of NA, DOPAC, 5HIAA, HVA in the areas of BS and intestine and 5HT in the AP (Table 4). Treatment with standard metoclopramide (MCP; 30 mg/kg) significantly decreased the upsurge of DA at the brain area of AP (P < 0.001) and BS (P < 0.01). Furthermore a decrease in the concentration of 5HIAA and 5HT was also observed in the brain area of AP (P < 0.05 – 0.01) and intestine (P < 0.001) as compared to cisplatin control (Table 4). Pre-treatment with ZO-ActFr at (50 mg/kg) reduced the contents of DA in the brain area of AP (P < 0.001) and 5HT in the areas of BS and intestine (P < 0.001) as compared to cisplatin control (Table 4).
Discussion
In this study the extract of Zingiber officinale (ZO) was screened against cisplatin induced Retching plus Vomiting (R + V) in pigeons. Zingiber officinale (ZO; family Zingiberaceae) commonly known as ginger; the plant rhizome and is distributed and cultivated in Pakistan, India, Malaysia, China, Taiwan and Bangladesh. The standardization of ginger extract is reporting the quantities of gingerols in concentration ~ 60 mg/g of extract [36]. In our study, we used acetone fraction of ZO based on the study of Sharma and Co-workers [25], where acetone fraction is reported to be more effective to attenuate cisplatin induced emesis in dogs. Ginger have been screened in postoperative nausea and vomiting in clinics and is found to be superior to placebo and equally effective as metoclopramide [29]. In this study, the dose of 50 mg was found to be highly effective in attenuating cisplatin induced R + V, nonetheless longer but moderate protection i.e. upto 16 hr was observed with 25 mg dose. There are several lines of evidences explaining the anti-emetic effect of ginger; in animal models ginger is shown to enhance gastrointestinal transport i.e. having gastroprokinetic properties. Furthermore, ginger is having anti-hydroxytryptamine activity in the isolated ileal segments [37] as galanolactone, one of the component of ginger, has been proved to be a competitive antagonist at ileal 5HT3 receptors. Thus anti-emetic effects could be brought about by antagonism at 5HT3 receptors in the gastrointestinal tract [37,38]. Furthermore, ginger has also been found to be having inhibitory action on substance P and the expression of NK1 receptors [27]. Our results of current study are indicative of promising anti-emetic activity of ginger acetone extract against cisplatin induced vomiting in the pigeon. Ginger acetone extract was selected to find out its intrinsic antiemetic activity in line with its impact on central and peripheral neurotransmitters (and metabolites) involved in the act of vomiting, because the said fraction was found to be more effective to attenuate cisplatin induced vomiting in dogs as reported by Sharma and co-workers [25].
Metoclopramide (MCP), which is a clinically relevant anti-emetic with dopamine and 5-HT3 receptor antagonist properties [39] was used as a positive control. The dose of MCP that we selected is higher than required to antagonize cisplatin induced emesis in other species [40], and was based on a previous study in the pigeon showing activity against reserpine-induced emesis [2]. The metoclopramide was selected as standard because of the intrinsic emetic activity of 5HT3 receptor antagonists in pigeon (unpublished data).
As evident from the literature, neurochemical mediators of various types are responsible in vomiting circuits for CIV, especially serotonin (5HT), dopamine, substance P and prostaglandins are postulated to contribute in its genesis [6,26,41,42]. The emetogenic anti-cancer agent cisplatin induces biphasic vomiting both in human [43] and in other vomiting species [27,44-46]. Neural analysis of cisplatin control group in pigeon model indicated the upsurge of serotonin in the brain areas of BS and intestine at acute time point (03 hr) suggesting the neurotransmitter serotonin (5HT) as the triggering mediator for acute phase response in pigeons. The increase in 5HT concentration in our study is in line with the previous findings in animal models where 5HT3 receptor antagonists (ondansetron, granisetron, palonosetron etc.) are found to be effective against acute phase of CIV [10]. Furthermore, at delayed time point (18 hr) in pigeon the increase in the concentration of dopamine at the level of AP and serotonin in the brain area of BS and in the intestine is indicating the differential involvement of neurotransmitters at this time point; as CIV is thought to be a multifactorial phenomenon and both the phases of vomiting are mechanistically different [46], that’s why the prophylactic and intermittent administration of single antiemetic agent fails to provide complete control against cisplatin induced vomiting in clinics.
The pungent constituents collectively known as gingerols have been reported to be having inhibitory effects on the upsurge of substance P both centrally and peripherally and NK1 receptors expression in the vomit model of mink [27]. In our study, ZO-ActFr (50 mg) provided maximum protection against cisplatin induced R + V in pigeons, where the protection observed was ~ 58.13%. ZO-ActFr (25 mg) also resulted in the decrease in jerks (indicative of vomiting intensity) and reduced the weight loss significantly. The reduction in jerks and weight loss are the secondary parameters supporting the antiemetic profile of ginger acetone extract. Furthermore, the impact of ZO-ActFr on the major neurotransmitter mediators including dopamine and serotonin is encouraging and is in support for its anti-emetic effect where ZO-ActFr (50 mg) decreased the concentration of 5HT and 5HIAA in the brain areas (AP & BS) and intestine at acute time point (3 hr). Moreover, at delayed time point (18 hr) resulted in the decreased concentration of dopamine in the brain area of AP, while 5HT decrease was observed at the level of BS and in the intestine.
Conclusions
In conclusion, ZO acetone fraction attenuated cisplatin induced vomiting in the pigeon which is mediated by both central and peripheral anti-serotonergic and anti-dopaminergic components in a blended manner at the two different time points. At the acute time point (3rd hr), dominantly the anti-serotonergic effects were seen centrally in the area postrema and brain stem as well as peripherally at the level of intestine, while at the delayed time point (18th hr) anti-serotonergic effects were observed centrally in the brain stem and peripherally in the intestine where centrally in the area postrema there was anti-dopaminergic aftermath.
References
Hesketh P, Grunberg SMG RJ, Warr DG, Roila F, De Wit R, Chawla SP, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112–9.
Coronas R, Pitarch L, Mallol J. Blockade of reserpine emesis in pigeons by metoclopramide. Eur J Pharmacol. 1975;32(2):380–2.
Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol. 2012;88(3):497–9.
Higgins G, Kilpatrick G, Bunce K, Jones B, Tyers M. 5HT3 receptor antagonists injected into the area postrema inhibit cisplatin induced emesis in the ferret. Br J Pharmacol. 2012;97(1):247–55.
Perciedu Sert N, Rudd J, Apfel C, Andrews PLR. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT3 receptor antagonists. Cancer Chemother Pharmacol. 2011;67(3):667–86.
Osinski MU, ME Miller LN, Seifert TS, TK Nakane M, Cox B, Brioni J, et al. Dopamine D2, but not D4, receptor agonists are emetogenic in ferrets. Pharmacol Biochem Behav. 2005;81(1):211–9.
Darmani N, Crim J. Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav. 2005;80(1):35–44.
Grelot LD J, Esteve EF A, Bianchi AL, Sheldrick RLG, Gardner CJ, Ward P. Potent inhibition of both the acute and delayed emetic responses to cisplatin in piglets treated with GR205171, a novel highly selective tachykinin NK1 receptor antagonist. Br J Pharmacol. 2009;124(8):1643–50.
Tanihata S, Oda S, Kakuta S, Uchiyama T. Antiemetic effect of a tachykinin NK1 receptor antagonist GR205171 on cisplatin-induced early and delayed emesis in the pigeon. Eur J Pharmacol. 2003;461(2–3):197–206.
Grunberg SM, Koeller JM. Palonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesis. Expert Opin Pharmacother. 2003;4(12):2297–303.
Minami M, Endo TH M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99(2):149–65.
Diemunsch P, Grelot L. Potential of substance P antagonists as antiemetics. Drugs. 2000;60(3):533–46.
Wolff MC, Leander JD. Effects of a 5-HT1A receptor agonist on acute and delayed cyclophosphamide-induced vomiting. Eur J Pharmacol. 1997;340(2–3):217–20.
Cubeddu L, O'Connor D, Hoffmann I, Parmer R. Plasma chromogranin A marks emesis and serotonin release associated with dacarbazine and nitrogen mustard but not with cyclophosphamide-based chemotherapies. Br J Cancer. 1995;72(4):1033–8.
Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy. Predictive models for evaluation of new compounds. Drugs. 1997;53(2):206–34.
Endo T, Takahashi M, Minami M, Yoshioka M, Saito H, Parvez S. Effects of anticancer drugs on enzyme activities and serotonin release from ileal tissue in ferrets. Biogenic Amines. 1993;9(5–6):479–89.
Yoshikawa T, Yoshida N, Hosoki K. Involvement of dopamine D3 receptors in the area postrema in R (+) 7-OH-DPAT induced emesis in the ferret. Eur J Pharmacol. 1996;301(1):143–9.
Darmani N, Zhao W, Ahmad B. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew). J Neural Transm. 1999;106(11):1045–61.
Foss JF, Yuan CS, Roizen MF, Goldberg LI. Prevention of apomorphine-or cisplatin-induced emesis in the dog by a combination of methylnaltrexone and morphine. Cancer Chemother Pharmacol. 1998;42(4):287–91.
Osinski MA, Seifert TR, Shaughnessy TK, Gintant GA, Cox BF. Emetic liability testing in ferrets. Current Protocols Pharmacol. 2003;5:31–5.
Schofferman JA. A clinical comparison of syrup of ipecac and apomorphine use in adults. J Am College Emergency Physicians. 1976;5(1):22–5.
Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15(4):301–20.
Zia-Ul-Haq M, Shahid SA, Muhammed S, Qayum M, Khan I, Ahmad S. Antimalarial, antiemetic and antidiabetic potential of Grewia aslatica L. leaves. J Med Plant Res. 2012;6(16):3087–92.
Darmani N. Delta (9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24(2):198–203.
Sharma S, Kochupillai V, Gupta S, Seth S, Gupta Y. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol. 1997;57(2):93–6.
Ullah I, Subhan F, Rudd JA, Rauf K, Alam J, Shahid M, et al. Attenuation of cisplatin-induced emetogenesis by Standardized Bacopa monniera extracts in the Pigeon: behavioral and neurochemical correlations. Planta Medica. 2014;80(17):1569–79.
Qiu-hai Q, Wang Y, Wen-hui C, Zhi-hong Y, Zhan-tao L, Yao-xia W. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin Med J. 2010;123(4):478–84.
Tyler VE, Brady LR, Robbers JE. Phrmacognosy. 9th ed. Philadelphia: Lea and Febiger; 1988. p. 150.
Ernst E, Pittler M. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000;84(3):367–71.
Ullah I, Subhan F, Rauf K, Badshah A, Ali G. Role of gastrointestinal motility/gastric emptying in cisplatin-induced vomiting in pigeon. Afr J Pharma Pharmacol. 2012;6(35):2592–9.
Tanihata S, Igarashi H, Suzuki M, Uchiyama T. Cisplatin-induced early and delayed emesis in the pigeon. Br J Pharmacol. 2000;130(1):132–8.
Preziosi P, Amanto MD, Carmine RD, Martire M, Pozzoli G, Navarra P. the effects of 5-HT3 receptor antagonists on cisplatin-induced emesis in the pigeon. Eur J Pharmacol. 1992;221:343–50.
Karten H, Hodos W. A stereotaxic atlas of the brain of the pigeon (Columba livia). Baltimore: Johns Hopkins Press Baltimore; 1967.
Duvernoy H, Risold P. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev. 2007;56(1):119–47.
Rauf K, Subhan F, Sewell RDE: A Bacoside Containing Bacopa monnieri Extract Reduces Both Morphine Hyperactivity Plus the Elevated Striatal Dopamine and Serotonin Turnover. Phytotherapy Research 758–763 2011.
Rai S, Mukherjee K, Mal M, Wahile A, Saha BP, Mukherjee PK. Determination of 6-gingerol in ginger (Zingiber officinale) using high performance thin layer chromatography. J Sep Sci. 2006;29(15):2292–5.
Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530(1–2):136–43.
Yamahara J, Rong HQ, Iwamoto M, Kobayashi G, Matsuda H, Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytother Res. 1989;3(2):70–1.
Al-Zubaidy MHI, Mohammad FK. Metoclopramide-induced central nervous system depression in the chicken. BMC Vet Res. 2005;1(1):2–6.
Zhang F, Wang L, Yang ZH, Liu ZT, Yue W. Value of mink vomit model in study of anti-emetic drugs. World J Gastroenterol. 2006;12(8):1300–2.
Johnston KD, Lu Z, Rudd JA. Looking beyond 5-HT3 receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol. 2014;722:13–25.
Saito R, Takano Y, Kamiya H. Roles of substance P and NK1 receptor in the brainstem in the development of emesis. J Pharmacol Sci. 2003;91(2):87–94.
Hesketh P, Van Belle SA M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003;39(8):1074–80.
Navari R: Management of Chemotherapy-Induced Nausea and Vomiting: Focus on Newer Agents and New Uses for Older Agents. Drugs 2013.
De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R902–11.
Darmani NA, Crim JL, Janoyan JJ, Abad J, Ramirez J. A re-evaluation of the neurotransmitter basis of chemotherapy-induced immediate and delayed vomiting: evidence from the least shrew. Brain Res. 2009;1248:40–58.
Acknowledgments
We sincerely thank Higher Education Commission of Pakistan for sponsoring the studies. We are also thankful to Korea United Pharm. Inc. Korea for donating cisplatin active material for this study.
Funding
This research was supported by Higher Education Commission (HEC) of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IU initiated the experiments and standardized the procedures for assessment of anti-emetic activity with establishment of complete video recording setup at Bio-Assay laboratories Department of Pharmacy, University of Peshawar, Peshawar, Pakistan. FS guided the students as research supervisor and helped the student in planning and conduction of experiments throughout the project. MA helped in behavioral experiments and manuscript writing. RS, GA and SU helped in the manuscript write up and data interpretation. All authors have read and approved the final manuscript for publication.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ullah, I., Subhan, F., Ayaz, M. et al. Anti-emetic mechanisms of zingiber officinale against cisplatin induced emesis in the pigeon; behavioral and neurochemical correlates. BMC Complement Altern Med 15, 34 (2015). https://doi.org/10.1186/s12906-015-0556-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-015-0556-0