Abstract
Background
The Yangtze River floodplain provides important wintering habitats for Hooded Cranes (Grus monacha) in China. Fluctuations in the water level change foraging habitat and food availability, affecting their temporal-spatial patterns of foraging activities. It is of considerable importance to investigate the effect of these fluctuations on food availability for wintering Hooded Cranes and their foraging response to these changes. Understanding their behavior patterns is beneficial in protecting the wintering crane population and restoring their wintering habitats.
Methods
A field survey of the winter behavior of cranes was carried out at Shengjin Lake from November in 2013 to April in 2014. Habitat variables, as well as the spatial distribution and behavior patterns of wintering cranes at their foraging sites during five stages of water level fluctuation were collected. Based on this data we analyzed the relationship of foraging behavior relative to water level fluctuations and habitat types.
Results
The foraging habitats used by Hooded Cranes varied at the different water level stages. As the water level decreased, the use of meadows and mudflats increased. When the water dropped to its lowest level, the use by the Hooded Crane in the mudflats reached a peak. There were statistically significant differences in time budget in the three types of habitats over the five stages of the water level. In the mudflats, the foraging behavior and maintenance behavior varied significantly with the water level, while the alert behavior showed little variation. Analysis of a generalized linear model showed that the five water level stages and three habitat types had a significant effect on foraging behavior, while the combined effect of these two variables was significant on the foraging time budget and the length of foraging activity of the Hooded Crane.
Conclusions
With the decrease in the water level, the use of mudflats by Hooded Cranes increased correspondingly. Food availability in different habitats was affected by changes in the water level. The Hooded Crane adjusted its foraging patterns and made full use of the three available types of habitat in order to acquire enough food in response to fluctuations in the water level.
Similar content being viewed by others
Background
Seasonal variation in food availability plays an important role in population dynamics and adjustment in behavior patterns of many avian species, especially those of waterbirds [1]. Winter is a critical period for waterbirds with its considerable effect on their annual life cycle [2]. According to the optimal foraging theory, animals choose the highest efficiency and the most advantageous patches for foraging in order to minimize their cost and maximize their income in the form of energy [3]. Affected by environmental factors of wetlands, their habitat and variation in food availability, waterbirds have to change their wintering habitats and behavior patterns in order to obtain sufficient energy for wintering [1, 4]. For many waterbirds, fluctuations in water levels change bird-perched micro-habitats [5] and directly affect the length of exposure to and availability of food in these habitats [6, 7]. Their foraging activities are also affected by human disturbance [8, 9]. The ‘water recession’ model suggests that during the dry season, lowering of water levels and surface cover will aggregate food resources in small areas, which provide extremely efficient foraging sites. Waterbirds gather in areas of rich food resources to meet their energy demand by adjusting their behavior patterns as much as possible [1, 10–12]. Studies have shown that waterbirds have the ability to adapt to changes in water levels, food resources and environmental changes of wetlands [13, 14], but this ability is relatively limited [8].
The habitat of a dynamic wetland is a model ecosystem to observe varying behavioral responses to food limitation, because wetland-dependent species such as waterbirds must adjust to food resources that change daily [1]. Hydrological cycles and microtopography determine the spatial and temporal availability of aquatic food resources and the rate of replenishment of foraging patches after they are depleted [12]. Food abundance in this system is largely affected by the duration of marsh inundation, vegetation structure and nutrient levels. Food supply becomes available to wading birds when changes in the water level result in the aggregation of food and interacts with the vegetation to provide an open foraging habitat [1, 15]. This changes the use of different habitats for waterbirds and limits their foraging behavior. Division of food resources leads waterbirds to take various strategies in different types of habitat [16]. In addition to biological factors, abiotic environmental factors may also strongly affect foraging and time budgets [16], e.g., temperature, wind and precipitation probably increase the need for temperature regulation to adjust foraging activities [17, 18].
The shallow lake wetlands along with the middle and lower Yangtze River flood plain are wetlands of international importance [19]. They contain abundant aquatic resources and are important stopover and wintering grounds for large numbers of East Asian-Australasian migratory waterbirds, including several endangered species [20]. The unique hydrological processes of the river, connecting shallow lakes, bring improved productivity and enable the accumulation of rich nutrients [21]. After the water recedes, the roots of submerged and hygrophilous plants, exposed in the riparian zone, will attract large numbers of waterbirds [22]. In recent years, these wetlands have become exposed to rapid exploitation. Seine fishery and the construction of water conservation projects have changed natural hydrological fluctuations [23, 24]. Aquatic vegetation resources have decreased sharply, especially submerged vegetation [25]. These changes have severely disrupted the ecological process of some wetlands [22]. The habitat types and food availability have changed significantly for wintering waterbirds. Due to the degradation of the original foraging habitat, waterbirds have to turn to artificial habitats for food, such as paddy fields, resulting in changes in behavior patterns in order to adapt to the new environment [26, 27]. The existing studies showed that the behavior of wading birds is responsive to spatial and temporal variations in the quality, quantity and availability of their food resources [28, 29]. Foraging selection patterns can be used to assess the effects of these transient conditions.
The Hooded Crane (Grus monacha) is a large migrating wader, declared a vulnerable species by the IUCN and a national grade I protected bird in China. Its global population consists of about 11,600 birds [30]. Breeding sites are located in the eastern part of Siberia and extend to China’s Lesser Khingan mountains and wintering sites for the cranes in Japan, South Korea and the middle and lower reaches of the Yangtze River in China [23, 31]. Most of the wintering population of the Hooded Crane in China assembles in the inland lakes of the Yangtze River flood plain. The food resources and their availability affect the habitat use and behavior patterns of these cranes, which have now chosen rice paddies instead of mudflats as their foraging habitat [32]. The driver of the changes in their foraging strategy merits further study. In our investigation we pay particular attention to hydrological changes and their effect on the habitat use and foraging activities of these cranes. Our aim is to shed light into: (1) the characteristics of habitat selection by the wintering Hooded Crane during different water level periods and (2) the changes in the behavior patterns of these cranes and the relationship between foraging behavior and water levels.
Methods
Study area
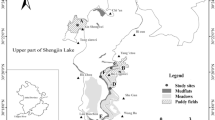
The study area was located at Shengjin Lake (30.25°–30.50°N, 116.92°–117.25°E), a shallow lake connected to the Yangtze River on the East Asian-Australasian flyway [33–35]. The region belongs to a humid subtropical monsoon climate, with an average annual temperature of 16.1°C and annual precipitation of 1,600 mm. There is a clear separation between the rainy and dry seasons. The rainy season is from April to the following October, when the water level reaches 17.6 m. The dry season usually lasts from November to March in the following year, with the water level fluctuating between 8 and 11 m. Because of the dam at the end of the upstream Shengjin Lake, the water level is maintained, in the early winter, at around 11 m or lower till late December. In the middle of January, the water drops to its lowest level, 8.18 m (Fig. 1). This decrease leads to the exposure of large areas of tidal flats, providing suitable habitats for wintering waterbirds. The submerged macrophytes, such as Carex tristachya, Phalaris arundinacaea and Polygonum cripolitanumare, successively exposed in the bare substrates of the lake bottom during the water recession in autumn and winter, attract a large number of waterbirds to winter here [36, 37]. In recent years, however, rapid economic development in this wetland area has led to changes in natural hydrological fluctuation [23, 24], causing a sharp decline in aquatic vegetation [25]. The habitat availability has changed significantly, forcing wintering waterbirds to abandon their original foraging habitat that has been degraded and turn to the rice paddies to obtain a sufficient amount of food.
Stages of the water level
The water level in upstream Shengjin Lake is controlled by a dam at Shengjin Lake bridge and the Huangpen sluice. Depending to the way the water level rises upstream at the lake, we defined five stages of the water level during the wintering period (November 2013 to early April 2014): (a) a stable stage, from early November to late December when, due to the interception of the dam at Shengjin Lake bridge, the water level was kept at 11.04 m; (b) a receding stage, from late December to early January: after the dam was opened, the water level dropped rapidly to 9 m; (c) a drought stage, from early January to the end of January, when the water level fluctuated at a stable level between 8 and 9 m; (d) an early recovery stage, from early February to mid-March, when the water level began to rebound, reaching 9–10 m; (e) a late recovery stage, from late March to early April, when the Hooded Cranes gradually flew away and the water level continued to rebound to 11 m.
Habitat surveys
Based on the differences in vegetation and micro-geomorphological features, foraging habitats are distinguished into three types, i.e., mudflats, meadows and paddy fields. Within each stage of the water level, 30 quadrats of 0.5 m × 0.5 m with a depth of 0.15 m were arranged at the foraging range of the cranes [38], located with a handheld global positioning system (GPS, eTrex 30, Garmin, China). The recorded habitat variables consisted of food types, their abundance and soil hardness [38, 39]. In each quadrat, we collected all the tubers and roots of the aquatic vegetation, the entrails of mollusks and rice grains within 15 cm under the foraging site, the typical limit of foraging depth for crane species [40]. The samples were sent to the laboratory and dried at 60°C in an oven (YHG-9050A; Derip, Suzhou, China), for a period of 72 h or less, to a constant weight, which represented the dry weight (g). We defined the ratio of the dry weight of prey resources in a quadrat (0.25 m2) as prey abundance (g m−2). In order to ensure the independence of data samples, the distance between two adjacent quadrats was ≥50 m [16].
Bird surveys
Each week we used a direct counting method to record the number of crane flocks, as well as the number of individual birds in each flock. We reached their feeding grounds at 7:00 in the morning of each survey day. Once we found a group of cranes, the entire visible sites were clockwise scanned using a spotter telescope (Swarovski, 30 × 60). The number of foraging cranes in each habitat, habitat type and meeting time were recorded. In order to reduce the effect of severe weather on our observations, the survey was postponed to the next day on days with strong winds, thick fogs or heavy snows [35, 40].
Behavioral observations
Field observations were carried out on sunny days from November, 2013 to April, 2014. During field work, focal sampling was conducted with binoculars (8 × 42) or a telescope (20–60 × 63) to record behavior from 07:00 to 17:00 [41]. At the beginning of each focal observation, date, time, location and habitat type (paddy field, meadow, mudflat) were recorded. The object of observation was usually selected as one adult in a group (≥3). All observations were performed from a relatively remote location to avoid the effect of the investigators on the behavior of the crane [42]. A voice recorder (DVR-990, JNN) was used to record its behavior for 20 min unless we lost sight of our focal bird. Referring to Zhou et al. [35], the ethogram comprised the following types of behavior: foraging, alertness, maintenance, resting, locomotion and social behavior. As well, we calculated foraging success by counting the number of successful strikes during a foraging bout.
Data analysis
The relative abundance of Hooded Cranes in each habitat patch was estimated using the percentage of the number of cranes in each habitat as a proportion of the total number of cranes in all habitats during a specific period [16, 43]. The survey results from each 20 min period were used as an independent sample from which we calculated the foraging frequency (changes in behavior per unit of time), length of foraging time (the total time of foraging bouts) and foraging time budget [26, 42, 44]. Foraging success was quantified by analyzing the recordings of foraging birds and budgeting their time in foraging bouts [4].
The Kruskal–Wallis H test and Mann–Whitney U test were used to test the difference of relative abundance and time budgets in three habitats at the five stages of water level. In addition, the effect of fluctuations in the water level and habitat type on foraging behavior (foraging time budget, length of time in foraging, foraging success and foraging frequency) were analyzed using a generalized linear regression model. All analyses were performed using PASW Statistics 18 (IBM Inc., 2009). A significance level of 0.05 (p) was used for all statistical tests, with means expressed as mean ± SE.
Results
Foraging habitat use
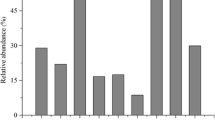
A total of 302 Hooded Cranes wintered at Shengjin Lake and their use of this habitat showed significant differences over the five stages of water level fluctuation (Fig. 2). The abundance of food resources in the meadow habitat was the highest, which showed significant differences over the five water level stages (χ 2 = 18.179, p = 0.001). The food resources in the mudflat habitat were the least, but still, significant differences (χ 2 = 12.538, p = 0.006) were found with fluctuations in the water level. In the paddy fields, the abundance of rice showed a declining trend, but the differences were not significant (χ 2 = 5.570, p = 0.234). As the water level receded, the number of cranes in the meadows and mudflats gradually increased. The number of cranes in the mudflats reached a peak during the drought stage. As the water level rose again, the cranes gradually reduced their use of the mudflats. At the late recovery stage, they abandoned the paddy fields and their use of the meadows and mudflats increased, with the meadows becoming the main foraging habitat. The number of cranes in the mudflats showed significant differences during the various stages of the water level (Z = 10.767, p < 0.05). The use of meadows increased during the entire period of changes in the water level, however without any significant difference. During these stages, the use of paddy fields showed a decline.
Use of habitat type by Hooded Cranes under different water level features. This chart demonstrates the population dynamics of the Hooded Crane over five water level stages at three types of habitat. Changes in water level affect the spatial distribution of Hooded Cranes. The a, b, c, d, and e represent the five water level stages.
Activity time budget
There were significant differences in activity time budget for Hooded Cranes during different stages of water level fluctuation. In mudflat, foraging behavior and maintenance were significantly different at the five stages of water level fluctuation (χ 2 = 17.328, p < 0.05; χ 2 = 14.273, p < 0.01), while the alert and other behavior showed no significant difference. At the same time, the alert time in the mudflats was the highest of the three habitats (Table 1). Specifically, in the meadows the foraging behavior of the cranes increased gradually, but without any significant difference. During the entire period of water level fluctuations, the foraging time of the cranes in the paddy fields showed no significant difference (χ 2 = 3.391, p > 0.05),but was higher than in the mudflats. The alert time in the paddy fields decreased, with a slightly increase during the early recovery stage but again showed no significant difference (χ 2 = 6.485, p > 0.05).
Temporal-spatial changes in foraging activities
In the three habitat types, only the foraging behavior in the mudflats showed significant differences during the various water level stages. The foraging strategies of the cranes were considerably affected by fluctuations in the water level, changing the length of time in foraging, foraging frequency, foraging time budget and especially their foraging success (Table 2). The type of habitat showed an extremely marked effect on their foraging time budget and foraging success, but less on the length spent on foraging. Moreover, the combined action of habitat type and water level fluctuation had a significant effect on foraging time, but did not affect the foraging frequency nor foraging success.
Discussion
Effect of water level fluctuation on foraging habitat use
There are many factors that can affect the habitat selection of waterbirds. Consequently, understanding the role of fluctuation in water levels in wetlands has become even more crucial, especially with current concerns about global climate change [38, 45, 46]. Seasonality and the extent of these fluctuations affect habitat features such as water depth, food availability and degree of isolation from human disturbance, creating a mosaic that changes over time and space [47–49]. A mudflat is a habitat where food availability changes considerably with the water level. When the water level declines, the area of mudflats becomes larger, especially in the shallow lake wetlands along with the floodplains of the middle and lower Yangtze River. On the other hand, when the water level rises, waterbirds will be faced with a reduction of habitat area and food availability, as well as with an increase in human interference [50]. According to the ‘water recession’ model, lowering water levels and receding surface cover tend to concentrate food in small areas which provide extremely efficient foraging sites [11], thereby meeting the food demands of more waterbirds. Recently, aquatic vegetation resources in Shengjin Lake decreased sharply, especially the submerged vegetation [25]. Although more mudflats will be exposed with a falling water level, the available food resources for the cranes have not increased significantly, thus failing to provide an adequate food supply. All the same, the number of Hooded Cranes wintering in the mudflats has increased considerably. Although the mudflats failed to provide sufficient food, the Hooded Crane has not yet given up this optimal foraging habitat. This is mainly because human disturbance is lower in the mudflats and declining water levels provide for a wider range of habitats. The strategy of seasonal movement allows cranes to use the resources available over all of the wetlands during the course of the wintering period [51].
Effect of water level fluctuation on activity time budget
The foraging strategy of predators often conforms to the principle of optimal foraging theory [52–54]. The daily food requirements of birds depend on time budget, activity level, assimilation efficiency and the amount of energy contained in food [6]. Time-activity budgets of waterbirds in the winter could provide data on differential habitat use, evaluate responses of waterbirds to environmental conditions, make inferences about energy budgets and compare populations at different stages during the winter [55, 56]. To study the behavioral patterns and time budgets for understanding the changes of habitat use, energy requirement and seasonal variation is of great importance [42].
Wetland degradation leads to changes in the optimal wintering habitat of Hooded Cranes and interferes with their behavior pattern [57]. Waterbirds can respond in their behavior to spatial and temporal variations in the quality, quantity and availability of food resources [29]. A habitat use study of the Hooded Crane in Chongming Dongtan suggests that a mudflat is its optimal foraging habitat [58]. In our present study, we found that the area of mudflats exposed in Shengjin Lake increased when the water level decreased. During this process, foraging time did not increase much, whereas maintenance behavior clearly increased. This suggests that the Hooded Crane did not give up its original habitat, but shifted its foraging strategy to adapt to this relatively degraded habitat. The degradation of natural wetlands has forced the cranes to shift their foraging habitats to artificial wetlands, thus leading to a reallocation of time budgets in order to adapt to the new habitat [26, 27]. Paddy field and meadow habitats with stronger human disturbance were originally unsuitable habitat types, but now these have become the main foraging habitats of the Hooded Crane in the lakes of the Yangtze River floodplain in Anhui Province. Simultaneously, mudflats have become the main stopover for cranes foraging [32]. As water level rose again, food resources could not be obtained and waterbirds opted for other habitats for foraging [7]. The Hooded Cranes turned to meadows, increasing the foraging time to get sufficient energy. Accordingly, changing the behavior pattern is a strategy to adapt in the face of the collapse of food resources. Their behavior of changing the types of foraging habitat is consistent with optimal foraging theory.
Effect of water levels on foraging frequency and success rate
Hydrological cycles and micro-topography determine the spatial and temporal availability of aquatic food and the rate of replenishment of foraging patches after they are depleted [1]. Predilection of habitat type and water depth and the shift of diet for waterbirds all can be caused by the availability of food resources [1]. Foraging frequency, length of time spent on foraging and foraging success can reflect the effect of water level fluctuations and the foraging efficiency of waterbirds [56]. The stages of the water level have significantly impacted the foraging behavior of the Hooded Crane. Fluctuating water levels change the area of the exposed habitats as well as the soil hardness, which causes changes in food availability [59]. Accordingly, cranes adjust their foraging time budget to different water levels, responding to the changes in food resource abundance and availability and thus gain a greater energy intake with relatively less effort [60]. The foraging frequency of cranes was affected by the various types of food and interference in different habitats. Human disturbance is higher in paddy fields, but food resources are richer than in other habitats. This allowed the cranes the highest foraging frequency and foraging success rate. They foraged here during their foraging peak, then flew back to the habitats with lower human disturbance such as the mudflats. The behavior rhythm of cranes has clear spatial heterogeneity [61]. The foraging behavior of our cranes showed temporal and spatial differences, caused by a combination of changes in the water level and habitat type. They did not forage in a single habitat, but allocated their foraging times to different habitats. This is probably because the type and density of food resources in the various habitats are not the same. The Hooded Cranes avoid excessive use of a single type of habitat and balance their nutritional intake by foraging a variety of food resources [16, 62]. Therefore, in order to gain higher foraging yield, the cranes adapted to the changes in food resource availability caused by water levels and habitat type by altering their foraging strategy.
Importance of water management for crane conservation
The normal water level fluctuations of lake wetlands play a key role in maintaining waterbird communities [63]. Understanding the impact of changing water levels on waterbirds is of great importance for the restoration of their wintering habitats and for the protection of bird populations [64]. For the last years, the over-exploitation of wetland has led to a decline in wetland areas, thus reducing the habitat available to cranes [65]. Water conservation projects are implemented for the sake of human demands such as control of the water level, shipping and aquaculture. But these projects often cause changes in the natural hydrology rhythm. Long periods of high water levels will cause a reduction in food resources on mudflats, while rapidly decreasing water levels will accelerate the drying of mudflats. Both conditions can reduce the availability of food resource on mudflats and affect the population of wintering cranes. It is critical to enhance the lake management and ecological restoration. The dam built under the Shengjin Lake bridge should be opened as early as possible to prevent the upstream of Shengjin Lake remaining at high water levels all the time. In this way, the tidal flats can be exposed, providing sufficient habitats for cranes and other waterbirds. More research is needed for determining the response patterns among water levels, habitats and wintering cranes. It is beneficial for wetlands and waterbirds to conduct long-term effective monitoring of water levels, waterbirds and other ecological characteristics.
Conclusions
Our study has shown that with the decrease of water levels, the use of mudflats by Hooded Cranes increased correspondingly. Food availability in the various habitats was affected by fluctuations in the water level. The Hooded Crane reacted by adjusting its behavior patterns and made full use of the three types of habitat in order to obtain a sufficient amount of food in response to the fluctuations in the water level.
References
Beerens JM, Gawlik DE, Herring G, Cook MI (2011) Dynamic habitat selection by two wading bird speices with divergent foraging strategies in a seasonally fluctuation wetland. Auk 128(4):651–662
Marra PP, Hobson KA, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable carbon isotopes. Science 282:1884–1886
May R, Mclean A (2010) Theoretical ecology. Oxford University Press, Oxford
Kuwae T, Miyoshi E, Sassa S, Watabe Y (2010) Foraging mode shift in varying environmental conditions by dunlin Calidris alpina. Mar Ecol Prog Ser 406:281–289
Farago S, Hangya K (2012) Effects of water level on waterbird abundance and diversity along the middle section of the Danube River. Hydrobiologia 697(1):15–21
Kushlan JA (1978) Nonrigorous foraging by robbing egrets. Ecology 59(4):649–653
Maheswaran G, Rahmani AR (2001) Effects of water level changes and wading bird abundance on the foraging behavior of black necked storks Ephippiorhynchus asiaticus in Dudwa National Park, India. J Biosci 26(3):373–382
Lantz SM, Gawlik DE, Cook MI (2010) The effects of water depth and submerged aquatic vegetation on the selection of foraging habitat and foraging success of wading birds. Condor 112(3):460–469
Master TL, Lesier JK, Bennett KA (2005) Patch selection by Snowy Egrets. Colon Waterbird 28:220–224
Lewis TL, Esler D, Boyd WS (2008) Foraging behavior of Surf Scoters (Melanitta perspicillata) and White-Winged Scoters (M. fusca) in relation to clam density: inferring food availability and habitat quality. Auk 125(1):149–157
Loftus WF, Eklund AM (1994) Long-term dynamics of an Everglades small-fish assemblage. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. CRC Press, Boca Raton, FL, pp 461–483
Russell GJ, Bass OL Jr, Pimm SL (2002) The effect of hydrological patterns and breeding-season flooding on the numbers and distribution of wading birds in Everglades National Park. Anim Conserv 5:185–199
Baschuk MS, Koper N, Wrubleski DA, Goldsborough G (2012) Effects of water depth, cover and food resources on habitat use of marsh birds and waterfowl in boreal wetlands of Manitoba, Canada. Waterbirds 35(1):44–55
Holm TE, Clausen P (2006) Effects of water level management on autumn staging waterbird and macrophyte diversity in three Danish coastal lagoons. Biodiv Conserv 15:4399–4423
Bancroft GT, Gawlik DE, Rutchey K (2002) Distribution of wading birds relative to vegetation and water depths in the northern Everglades of Florida, USA. Waterbirds 25:265–277
Jing K, Ma ZJ, Li B, Li JH, Chen JK (2007) Foraging strategies involved in habitat use of shorebirds at the intertidal area of Chongming Dongtan, China. Ecol Res 22:559–570
Beauchamp G (2006) Spatial, temporal and weather factors influencing the foraging behavior of migrating Semipalmated Sandpipers. Waterbirds 29(2):221–225
Pienkowski MW (1983) Surface activity of some intertidal invertebrates in relation to temperature and the foraging behaviour of their shorebird predators. Mar Ecol Prog Ser 11:141–150
Zhao F, Zhou L, Xu W (2013) Habitat utilization and resource partitioning of wintering Hooded Cranes and three goose species at Shengjin Lake. Chin Birds 4:281–290
Cao L, Barter M, Zhao MJ, Meng HX, Zhang Y (2011) A systematic scheme for monitoring waterbird populations at Shengjin Lake, China: methodology and preliminary results. Chin Birds 2:1–17
Liu ZY, Xu WB, Wang QS, Shi KC, Xu JS, Yu GQ (2001) Environmental carrying capacity for over wintering Hooded Cranes in Shengjin Lake. Resour Envir Yangtze Basin 10(5):454–459
Cao L, Fox AD (2009) Birds and people both depend on China’s wetlands. Nature 460:173
BirdLife International (2001) Threatened birds of Asia: the BirdLife international red data book. BirdLife International, Cambridge, pp 1174–1179
Yang GS, Ma GH, Zhang L, Jiang JH, Yao SC, Zhang M et al (2010) Lake status, major problems and protection strategy in China. J Lake Sci 22(6):799–810
Fox AD, Cao L, Zhang Y, Barter M, Zhao MJ, Meng FJ et al (2011) Declines in the tuber-feeding waterbird guild at Shengjin Lake National Nature Reserve, China—a barometer of submerged macrophyte collapse. Aquatic Conserv Mar Freshw Ecosyst 21:82–91
Li ZQ, Wang Z, Ge C (2013) Time budgets of wintering Red-Crowned Cranes: effects of habitat, age and family size. Wetlands 33:227–232
Wood C, Qiao Y, Li P, Ding P, Lu BZ, Xi YM (2010) Implications of rice agriculture for wild birds in China. Waterbirds 33(Sp1):30–43
Aviles JM (2003) Time budget and habitat use of the Common Crane wintering in dehesas of southwestern Spain. Can J Zool 81:1233–1238
Erwin RM (1983) Feeding habitats of nesting wading birds: spatial use and social influences. Auk 100:960–970
BirdLife International (2012) Grus monacha. The IUCN red list of threatened species. Version 2015.2. http://www.iucnredlist.org. Accessed 2 Aug 2015
Jiao SW, Guo YM, Falk H, Lei GC (2014) Nest-site selection analysis of Hooded Crane (Grus monacha) in Northeastern China based on a multivariate ensemble model. Zoolog Sci 31:430–437
Luo ZJ (2012) Habitat selection of Hooded Crane (Grus monacha) wintering at the lakes of Yangtze River Floodplain in Anhui Province. Master’s Thesis. Anhui University
Fox AD, Hearn RD, Cao L (2008) Preliminary observations of diurnal feeding patterns of swan geese (Anser cygnoides) using two different habitats at Shengjin Lake, Anhui Province, China. Wildfowl 58:20–30
James H (2000) Migratory stopover and wintering locations in eastern China used by White-naped Cranes Grus vipio and Hooded Cranes G. monacha as determined by satellite tracking. Forktail 16:93–99
Zhou B, Zhou LZ, Chen JY, Cheng YQ, Xu WB (2010) Diurnal time-activity budgets of wintering Hooded cranes (Grus monacha) in Shengjin Lake, China. Waterbirds 33(1):110–115
Xu LL, Xu WB, Sun QY, Zhou ZZ, Shen J, Zhao XX (2008) Flora and vegetation in Shengjin Lake. J Wuhan Botan Res 27(3):264–270
Yang XL (2011) Research on the numbers, distribution, feeding behavior and diet of Great White Fronted Geese at Shengjin Lake, the national natural reserves in Anhui Province. Master’s Thesis. University of Science and Technology of China
Ma ZJ, Li B, Jing K, Zhao B, Tang SM, Chen JK (2003) Effects of tidewater on the feeding ecology of Hooded Crane (Grus monacha) and conservation of their wintering habitats at ChongmingDongtan, China. Ecol Res 18:321–329
Zhao C, Li YH, Hu J, Yao G, He Q, Li HY et al (2012) Feeding sites selection of long-billed plover in winter at the middle reaches of Jialing River. Sichuan J Zool 31(1):22–26
Jia YF (2013) Impact of water level fluctuation on Siberian Crane and other wintering waterbirds in Poyang Lake. PhD Thesis. Beijing Forestry University, Beijing
Lamoot I (2004) Foraging behaviour and habitat use of large herbivores in a coastal dune landscape. PhD Thesis. Universiteit Gent, Gent
Azevedo CS, Ferraz JB, Tinoco HP, Young RJ, Rodrigues M (2010) Time-activity budget of greater rheas (Rhea Americana, Aves) on a human-disturbed area: the role of habitat, time of the day, season and group size. Acta Ethol 13:109–117
Gyimesi A, Franken MS, Feige N, Nolet BA (2012) Human disturbance of Bewick’s Swans is reflected in giving-up net energy intake rate, but not in giving-up food density. Ibis 154(4):781–790
Lafever KE (2006) Spatial and temporal winter territory use and behavioral responses of Whooping Cranes to human activities. Doctoral Dissertation. Texas A&M University
Coops H, Beklioglu M, Crisman TL (2003) The role of water-level fluctuations in shallow lake ecosystems—workshop conclusions. Hydrobiologia 506–509:23–27
Wantzen KM, Rothhaupt KO, Mortl M, Cantonati M, Toth LG, Fischer P (2008) Ecological effects of water-level fluctuations in lakes: an urgent issue. Hydrobiologia 613:1–4
Bolduc F, Afton AD (2008) Monitoring waterbird abundance in wetlands: the importance of controlling results for variation in water depth. Ecol Model 216(3):402–408
Dimalexis A, Pyrovetsi M (1997) Effect of water level fluctuations on wading bird foraging habitat use at an Irrigatio Reservoir, Lake Kerkini, Greece. Colon Waterbird 20(2):244–252
Rajpar MN, Zakaria M (2011) Effects of water level fluctuation on waterbirds distribution and aquatic vegetation composition at natura wetland reserve, Peninsular Malaysia. ISRN Ecol 2011:1–13
Li YK, Shan JH, Ma JZ, Miu LJ, Li J, Yuan FK et al (2014) The effects of climate and water level fluctuation on the wintering population of Oriental White Stork. Chin J Ecol 33(4):1061–1067
Kushlan JA (1986) Responses of wading birds to seasonally fluctuating water levels: strategies and their limits. Colon Waterbird 2(9):155–162
Krebs JR (1978) Optimal foraging: decision rules for predators. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell, Oxford, pp 23–63
Macarthur R, Recher H, Cody M (1966) On the relation between habitat selection and species diversity. Amer Natur 100:319–332
Stephens DW (1986) Foraging theory. Princeton University Press, Princeton
Paulus SL (1988) Time-activity budgets of nonbreeding Anatidae: a review. In: Weller MW (ed) Waterfowl in winter. University of Minnesota Press, Minneapolis, pp 135–152
Woodin MC, Michot TC (2006) Foraging behavior of redheads (Aythya americana) wintering in Texas and Louisiana. Hydrobiologia 567:129–141
Lee SD, Jabtonski PG, Higuchi H (2007) Winter foraging of threatened cranes in the Demilitarized Zone of Korea: behavioral evidence for the conservation importance of unplowed rice fields. Biol Conserv 138:286–289
Jing K, Tang SM, Chen JK, Ma ZJ (2002) Primary research on the characteristics of feeding sites of Grus monacha in the east tide flat of Chongming. Zool Res 23(1):84–88
Riis T, Hawes I (2002) Relationships between water level fluctuations and vegetation diversity in shallow water of New Zealand lakes. Aquat Bot 74:133–148
Zheng M (2014) The effects of food resource dynamics on foraging behaviors of wintering Hooded Cranes (Grus monacha) over spatio-temporal scales. Master’s Thesis. Anhui University
Aborn DA (2010) Behavior and habitat use of Greater Sandhill Cranes wintering in east Tennessee. Crane Workshop 11:9–14
Slobodkin LB (1974) Prudent predation does not require group selection. Amer Natur 108:665–678
Coops H, Hosper SH (2002) Water-level management as a tool for the restoration of Shallow Lake in the Netherlands. Lake Reserv Manag 18(4):293–298
Gawlik DE (2006) The role of wildlife science in wetland ecosystem restoration: lessons from the Everglades. Ecol Eng 26:70–83
Zhu WZ, Zhou LZ (2010) Biodoversity and conservation in Anqing floodplain wetlands. Hefei University of Technology Press, Hefei
Authors’ contributions
DZ and LZ conceived and designed the experiments. DZ performed the experiments, DZ and LZ analyzed the data. LZ contributed the reagents/materials/analysis tools and DZ and LZ wrote the paper. LZ liaised with nature reserve authorities and obtained province guidance and permission for the field work. YS participated in the field work. All authors read and approved the final manuscript.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant no. 31172117, 31472020) and the Graduate Student Innovation Research Projects of Anhui University (YQH100269). We express appreciation to Dr. Chunlin Li for his helpful comments and suggestions on our study and the staff of the Shengjin Lake National N. R. for their helps in the field work.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, D., Zhou, L. & Song, Y. Effect of water level fluctuations on temporal-spatial patterns of foraging activities by the wintering Hooded Crane (Grus monacha). Avian Res 6, 16 (2015). https://doi.org/10.1186/s40657-015-0026-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40657-015-0026-x