Abstract
Purpose
Early evaluation of the efficacy of first-line chemotherapy combined with bevacizumab in patients with colorectal cancer liver metastasis (CRLM) remains challenging. This study used 2-month post-chemotherapy spectral computed tomography (CT) to predict the overall survival (OS) and response of CRLM patients with bevacizumab-containing therapy.
Method
This retrospective analysis was performed in 104 patients with pathologically confirmed CRLM between April 2017 and October 2021. Patients were treated with 5-fluorouracil, leucovorin, oxaliplatin or irinotecan with bevacizumab. Portal venous phase spectral CT was performed on the target liver lesion within 2 months of commencing chemotherapy to demonstrate the iodine concentration (IoD) of the target liver lesion. The patients were classified as responders (R +) or non-responders (R −) according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 at 6 months. Multivariate analysis was performed to determine the relationships of the spectral CT parameters, tumor markers, morphology of target lesions with OS and response. The differences in portal venous phase spectral CT parameters between the R + and R − groups were analyzed. Receiver operating characteristic (ROC) curves were used to evaluate the predictive power of spectral CT parameters.
Results
Of the 104 patients (mean age ± standard deviation: 57.73 years ± 12.56; 60 men) evaluated, 28 (26.9%) were classified as R + . Cox multivariate analysis identified the iodine concentration (hazard ratio [HR]: 1.238; 95% confidence interval [95% CI]: 1.089–1.408; P < 0.001), baseline tumor longest diameter (BLD) (HR: 1.022; 95% CI: 1.005–1.038, P = 0.010), higher baseline CEA (HR: 1.670; 95% CI: 1.016–2.745, P = 0.043), K-RAS mutation (HR: 2.027; 95% CI: 1.192–3.449; P = 0.009), and metachronous liver metastasis (HR: 1.877; 95% CI: 1.179–2.988; P = 0.008) as independent risk factors for patient OS. Logistic multivariate analysis identified the IoD (Odds Ratio [OR]: 2.243; 95% CI: 1.405–4.098; P = 0.002) and clinical N stage of the primary tumor (OR: 4.998; 95% CI: 1.210–25.345; P = 0.035) as independent predictor of R + . Using IoD cutoff values of 4.75 (100ug/cm3) the area under the ROC curve was 0.916, sensitivity and specificity were 80.3% and 96.4%, respectively.
Conclusions
Spectral CT IoD can predict the OS and response of patients with CRLM after 2 months of treatment with bevacizumab-containing therapy.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most common cancer globally and has a high mortality rate [1]. Approximately 30–50% of patients develop CRC liver metastasis (CRLM) during the course of the disease [2]. Liver resection is the primary curative treatment option for single CRLM, with a 5-year survival of 20–50% [2, 3]. However, most patients with CRLM require first-line chemotherapy including 5-fluorouracil, leucovorin, and oxaliplatin or irinotecan (FOLFOXIRI) [3, 4]. The use of targeted therapies, such as anti-vascular endothelial growth factor (bevacizumab) and anti-epidermal growth factor receptor (cetuximab or panitumumab) monoclonal antibodies, has resulted in increased survival compared with first-line chemotherapy [5,6,7]. Early assessment of the efficacy of bevacizumab chemotherapy can be helpful in clinical treatment decision making.
Assessment of the response to chemotherapy is based on size criteria, the most common being the Response Evaluation Criteria in Solid Tumors (RECIST) [8]. However, the size-based RECIST v1.1 may not be suitable for early evaluation of the efficacy of bevacizumab for CRLM treatment due to the cytostatic mechanism of bevacizumab [9,10,11]. In 2009, Chun et al. [9] proposed morphological assessment criteria for CRLM to assess the response to antiangiogenic therapy in the preoperative setting, which they validated in a nonsurgical patient cohort. Subsequent studies also showed that these criteria were associated with improved long-term prognosis [10, 12, 13]. According to this criterion, based on CRLM changing from heterogeneous masses with ill-defined margins into homogeneous hypoattenuating lesions with sharp borders after bevacizumab treatment is an important basis for response therapy. Besides, m-RECIT criteria is often used to evaluate the efficacy of targeted therapy for liver tumors by assessing the enhancement area in the arterial phase of the lesion. However, the reproducibility and applicability of these criteria derived from naked-eye observations are still limited [11]. In addition, the radiological features of the morphological criteria need to be verified in studies from other centers. As a new imaging analysis method, radiomics has been used to evaluate the effects of treatment on CRLM [11, 14]. However, this method has limited applicability in clinical practice as it is time consuming, labor intensive, and poorly reproducible.
Several quantitative imaging methods, including diffusion-weighted magnetic resonance imaging and F18-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT), have shown good performance in the early response evaluation of chemotherapy for CRLM [15,16,17,18]. However, because patients with CRLM require multiple imaging examinations, the high cost of MRI and PET/CT precludes their use in most patients with CRLM. As a new imaging modality, multiparametric spectral CT has been widely used to evaluate liver diseases [19]. Due to its multiparametric imaging characteristics, this method can evaluate tumor cell proliferation [20], microvessel density [21], and fiber structure [22], and it is widely used in clinical practice. In addition, bevacizumab does not lead to reduced size of the lesion in early post-chemotherapy phase but caused decreased iodine concentration. However, to our knowledge, there are no reports of the use of multiparametric spectral CT to assess the efficacy of first-line chemotherapy with bevacizumab-containing therapy in patients with CRLM.
This study was performed to explore the correlation between the overall survival (OS) of patients with CRLM and portal venous phase spectral CT analysis performed within 2 months of commencing first-line chemotherapy with bevacizumab-containing therapy. In addition, we examine whether portal venous phase spectral CT is useful for early identification of CRLM in patients showing a positive response to treatment.
Material and methods
Patients
The study was approved by the Ethics committee of Lanzhou University Second Hospital (2022A-298), and the requirement for informed consent was waived due to the retrospective nature of the study.
This retrospective analysis was performed in an initial cohort of 352 patients with pathologically confirmed CRLM between April 2017 and October 2021. These patients received first-line treatment with FOLFOXIRI and bevacizumab. The primary endpoint was OS, defined as the time from inclusion in the study to death or last follow-up. The date of the last follow-up was May 2022. All patients underwent contrast-enhanced CT of the abdomen at baseline and after 6 months of chemotherapy as part of their standard evaluation. Patients underwent at least one spectral CT scan (Time interval between treatment and spectral CT scan: 41.65 ± 6.88, day) between baseline and within 2 months of commencing chemotherapy. These patients were classified based on the RECIST v1.1 at 6 months (6-RECIST v1.1) as responders (R +), defined as a complete response or partial response in the target lesion, or as non-responders (R −), defined as progression, stable disease, or death within 6 months of follow-up.
The inclusion criteria were pathologically confirmed CRLM, at least one spectral CT examination within 2 months of starting chemotherapy, abdominal contrast-enhanced CT at baseline and 6 months after chemotherapy, and at least one target lesion > 1 cm at baseline. The exclusion criteria were spectral CT images that could not be analyzed, missing clinical tumor marker results before or during chemotherapy, unknown treatment options, combined transarterial chemoembolization and radiofrequency ablation, and lack of OS data. Finally, 104 patients with CRLM were included in the study. The patient recruitment flowchart is shown in Fig. 1. Radiological (T.L. and L.Y.) Follow-up was performed every 2–4 months with clinical examinations, including blood tests for the tumor markers carcinoembryonic antigen (CEA). Clinicopathological variables were retrieved and manually reviewed via electronic medical records.
CT imaging
Spectral CT was performed in gemstone spectral imaging mode using the 256-slice Revolution CT scanner or 128-slice Discovery 750HD CT scanner (GE Healthcare, Milwaukee, WI, USA). Baseline and 6-month contrast-enhanced CT was not always performed using the gemstone spectral imaging protocol. The spectral CT scanning parameters were as follows: flat sweep tube voltage, 120 kVp; automatic milliampere-seconds; tube current, 100- 600 mA; collimator width, 0.625 mm; rack speed, 0.6 s/rot; pitch, 0.983:1; and reconstruction layer thickness and layer spacing, 1.25 mm. During enhanced CT, iodixanol (320 mg I/mL) was injected through the anterior cubital vein using a high-pressure syringe at a flow rate of 3.5- 4.0 mL/s and dose of 1.0 mL/kg body weight. The trigger threshold for abdominal aorta monitoring was 100 Hounsfield units (HU). After triggering, the arterial, portal venous, and delay phase scan times were 5, 19, and 90 s, respectively. Moreover, 40% Asir-V iteration was used to reconstruct the portal venous phase spectral-enhanced images horizontally at the end of the scan.
Image reconstruction processed
40 keV, 50 keV, 60 keV and 70 keV mono energetic CT images were reconstructed. Then, the optimal contrast-to-noise ratio (CNR) was used to determine 70 keV mono energetic CT images as the optimal evaluation data set for target lesions.
Images and clinical information analysis
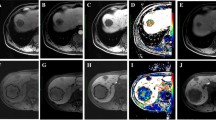
Portal venous phase CT is currently the internationally recommended standard imaging technique for follow-up of patients with liver metastasis [11]. Therefore, our analysis of target lesions was based on CT images of the portal venous phase. All abdominal CT scans were reviewed retrospectively and independently by experienced radiologists (T.L., L.Y., Y.X., and M.Y.Y. with 5, 6, 7, and 9 years of experience in the field of abdominal imaging, respectively) blinded to the clinical information of CRLM. Two radiologists (Y.X. and M.Y.Y.) independently analyzed the spectral CT images scanned within 2 months of starting treatment and recorded the morphological and spectral CT parameters of the target lesions. The patients’ tumor markers were documented using the hospital’s Picture Archiving and Communication System (PACS). The energy spectral parameters were averaged. Discrepancies between the radiologists were resolved by consensus review.
Two of the radiologists (T.L. and L.Y.) independently measured the longest diameter (LD) of the target lesion on contrast-enhanced CT at baseline, at spectral scan and at 6 months. In addition, the tumor marker results at baseline, at spectral scan and 6 months were recorded according to the hospital’s PACS. The LD of the target lesion was taken as the average of two radiologists' measurements. One month later, the baseline and 6-month contrast-enhanced CT images from 30 patients were randomly selected, and two radiologists again measured the LD of the target lesions, and the intra- and interobserver reliabilities of the measurements were calculated.
Qualitative review
According to the classification criteria of Chun et al. [9], we classified the morphological criteria of the target lesions as clear or unclear tumor borders and as homogeneous or heterogeneous. We also described the shape of the lesions (round or lobulated) based on the morphology of the target lesions [23]. Morphological classification criteria were assessed on spectral CT images within 2 months of commencing therapy. Liver oligometastasis, defined as the number of metastases between 1 and 5. The LD of the target lesions was measured on spectral CT images according to RECIST v1.1 (2-RECIST v1.1) to allow earlier identification of R + and R − .
Quantitative review
A region of interest (ROI) with an area of approximately 30 -100 mm2 avoiding obvious blood vessels and cystic/necrotic structures was delineated on the target lesion on portal venous phase spectral CT images. Portal venous phase spectral CT analysis was performed using the ROI of the target lesion, including the spectral CT iodine concentration (IoD), spectral curve slope (CS), and normalized IoD (NIoD). The target lesion CS = (CT40keV- CT70keV) / (70- 40), NIoD = IoD / Aorta IoD (aortic ROI 30 mm2). CEA of patients before and after treatment were obtained from electronic medical records. IoD, CEA, CS, and baseline longest diameter (BLD) of the target lesion were assessed as categorical variables using the cut-off values of the survival analysis, in which patients with the greatest reductions (Low) were compared with the remaining patients (High).
According to RECIST v1.1, patients were defined as R + if no target lesion was visible, or a partial response with a > 30% decrease in the target lesion size was observed. R − was defined as disease progression, > 20% increase in target liver lesion size, or stable disease [8]. To facilitate the application of RECIST v1.1, the LD of the target lesion at baseline and 6 months were classified as R + or R − using an online tool (https://www.radiologytutor.com/index.php/ cases/oncol/139-recist). The R + and R − classifications at 6 months, corresponding to the time point used in the literature, were defined as 6-RECIST v1.1 [11].
Statistical analysis
Continuous variables are expressed as the mean with standard deviation or median with interquartile range (IQR). Categorical variables are expressed as numbers with percentages. Data were tested for normality using Q-Q plots and the Kolmogorov–Smirnov test. Quantitative variables were compared using Student’s t test for normally distributed data or the Mann–Whitney U test for nonnormally distributed data. Qualitative variables were compared using the χ2 or Fisher’s exact test. Inter-reader agreement was analyzed using weighted k statistics for qualitative variables and the Spearman correlation coefficient for quantitative variables. A kappa coefficient or correlation coefficient (r) > 0.75 was considered to indicate good intra- or interobserver agreement, respectively.
Univariate Cox regression analysis was performed to assess the associations of tumor markers, morphology, and spectral CT parameters with OS as the endpoint. Multicollinearity between independent variables was evaluated through the calculation of the variance inflation factor (VIF). Kaplan–Meier survival curves and the log-rank test were used to test the associations between outcomes with IoD, CS, CEA, and BLD. Factors with P < 0.05 in the univariate analysis were included in the multivariate Cox analysis, and the results are expressed as hazard ratios (HR) with 95% confidence intervals (CI). Variables with a VIF ≥ 10 were excluded in order to avoid multicollinearity. The associations of clinical factors, morphology, and spectral CT parameters with respond as the endpoint were assessed by Logistic regression, and the results are expressed as odds ratios (OR) with 95% CI. Receiver operating characteristic (ROC) analyses of the spectral CT parameters significant in the multivariate analysis were performed. The Yuden index was used to calculate cutoff, sensitivity, and specificity values of the parameters with an area under curve (AUC). All statistical analyses were performed using R (version 4.1.2; https://www.r-project.org/), and P < 0.05 was taken to indicate statistical significance.
Results
Study cohort
The study population consisted of 104 patients with CRLM with a median age of 57.73 (range, 31- 87) years, of whom 42.31% (44/104) were female; 51.00% (53/104) of the primary tumors were rectal cancer. According to the 6-RECIST v1.1, 26.92% (28/104) patients were classified as showing a R + . The total duration of survival follow-up in this study was 48 months. After a median follow-up of 24.0 (range, 9- 42) months, 11.54% (12/104) of the patients were still alive. Baseline data are presented in Table 1.
Univariate and multivariate associations of baseline data and spectral CT parameters with OS
Univariate Cox analysis showed that IoD, CS, baseline CEA, BLD, histologic grade of the primary tumor, K-RAS mutation, clinical T stage and N stage of the primary tumor, and metachronous liver metastasis were associated with OS. Patients with CRLM with longer OS had lower IoD [< 5.93 (100ug/cm3)] and CS (< 0.37), as well as lower baseline CEA (< 31.7 ng/mL) and BLD (< 25.77 mm) in Fig. 2.
NIOD was excluded from the multivariate Cox analysis (VIF = 13.328). Multivariate Cox analysis showed that the IoD (hazard ratio [HR]: 1.238; 95% confidence interval [95% CI]: 1.089–1.408; P < 0.001), BLD (HR: 1.022; 95% CI: 1.005–1.038, P = 0.010), higher baseline CEA (HR: 1.670; 95% CI: 1.016–2.745, P = 0.043), K-RAS mutation (HR: 2.027; 95% CI: 1.192–3.449; P = 0.009), and metachronous liver metastasis (HR: 1.877; 95% CI: 1.179–2.988; P = 0.008) were independent risk factors for OS in patients with CRLM, Table 2, Fig. 3.
Univariate and multivariate associations of baseline data and spectral CT parameters with R −
Single factor analysis showed that IoD, CS, CEA, K-RAS mutation, and clinical T stage and N stage of the primary tumor were associated with R − , Table 3. Multivariate logistic analysis showed that IoD (OR: 2.243; 95% CI: 1.405–4.098; P = 0.002) and clinical N stage of the primary tumor (OR: 4.998; 95% CI: 1.210–25.345; P = 0.035) was an independent predictor of R − in patients with CRLM, Table 4.
All 104 CRLM patients were divided into R + (n = 28) and R − (n = 76) groups. Comparison of spectral CT parameters revealed significant differences between the two groups in IOD, CS, and baseline CEA. The R + group had lower IoD, CS and baseline CEA early during the treatment process, Figs. 4, and 5. Using IoD cutoff values of 4.75 (100ug/cm3), the AUC was 0.916, sensitivity and specificity were 80.3% and 96.4%, positive and negative predictive value were 0.984 and 0.643, respectively, Fig. 5 D.
Discussion
Early evaluation of the efficacy of first-line chemotherapy combined with bevacizumab in patients with CRLM is critical for improving patient outcomes. The 2-month portal venous phase spectral CT analysis for early quantitative assessment of the efficacy of FOLFOXIRI with bevacizumab in patients with CRLM showed that IoD was an independent predictor of R + . IoD, baseline CEA, BLD, K-RAS mutation, and metachronous liver metastasis were independent risk factors for OS after treatment for CRLM. However, based on 2-RECIST v1.1, there were no significant associations with OS in patients with CRLM. In addition, IoD had good discriminative performance for R − defined by 6-RECIST v1.1, with AUC of 0.916, sensitivity and specificity were 80.3% and 96.4%, positive and negative predictive value were 0.984 and 0.643, respectively. These observations indicated that IoD on portal venous phase spectral CT can reflect treatment efficacy in patients with CRLM administered FOLFOXIRI combined with bevacizumab within 2 months of commencing treatment.
Previous studies [24, 25] showed that the K-RAS mutations are negatively associated with OS in CRLM patients. Our results also found that K-RAS mutation was an independent predictor of OS after first-line chemotherapy combined with bevacizumab in patients with CRLM. The reason could be the difference in response to systemic chemotherapy according to the K-RAS mutation status. In the context of systemic chemotherapy, Zimmitti G et al. [26] investigated the association between RAS mutational status and response to preoperative chemotherapy in patients with CRLM. They revealed that RAS mutations were significantly associated with minor pathological and suboptimal morphological responses. Other studies also demonstrated that K-RAS mutations were significantly associated with minor response to chemotherapy in patients with CRLM, and that RAS mutation status may serve as a biomarker for response to chemotherapy [27, 28]. In addition, higher baseline CEA (≥ 31.7 ng/mL), BLD, and metachronous liver metastasis were independent risk factors for OS after FOLFOXIRI combined with bevacizumab for CRLM. We believe that the size of liver metastases at baseline affects the penetration of chemotherapy drugs into the tumor. Previous studies have also shown that the size of liver metastases at baseline and the size of metastases at the first follow-up are important prognostic predictors of CRLM [29, 30]. Therefore, systemic therapy should be combined with transcatheter arterial chemoembolization or radiofrequency ablation to improve the long-term survival of patients with large baseline (≥ 25.77 mm) liver metastases [30]. Furthermore, univariate analysis showed that the T stage and N stage of the primary tumor, and histologic grade of the primary tumor were associated with the OS of CRLM, in line with several previous studies in CRLM [31, 32].
Compared with traditional mixed-energy CT, spectral CT has diverse imaging parameters, including base material and single-energy CT imaging. Previous studies showed that the IoD can reflect the tumor microvessel density [22, 33]. Tumor microvessels lack the normal vascular wall composition, resulting in retention or extravasation of iodine in the tumor. Drljevic-Nielsen et al. [34] used the dual-energy CT IoD for early prediction of the efficacy of targeted therapy for metastatic renal cell carcinoma. IoD at baseline and after 1 month of chemotherapy were independent risk factors for outcomes in patients with mRCC. In addition, Luo et al. [22] also reported that the NIoD can predict early recurrence of hepatocellular carcinoma after surgery. Histopathological analysis showed that the NIoD was positively correlated with microvessel density in hepatocellular carcinoma. Therefore, the difference in the NIoD reflects treatment efficacy. In the present study, IoD within 2 months of treatment were also independent risk factors for OS in patients with CRLM. In addition, there was an association between lower CS and OS with CRLM in univariate analysis. We believe that the higher IOD [≥ 4.75 (100ug/cm3)] in CRLM after FOLFOXIRI combined with bevacizumab indicates that the microvascular structure of the tumor is not effectively inhibited or destroyed. Therefore, IoD was found to be an independent predictor of CRLM by multivariate logistic regression. However, some of the previous studies have suggested that baseline and post-treatment CT parameters do not predict survival outcomes in patients with CRLM [35, 36]. Our findings suggest that spectral CT parameters may be a useful biomarker for noninvasively predicting early efficacy response and survival outcomes in patients with CRLM.
This study had some limitations. First, as this was a retrospective study with a small sample size from a single center, the dynamic changes in multiparametric spectral CT parameters before and after chemotherapy for CRLM could not be determined. Second, there may have been differences in the treatment options among patients, but FOLFIRI or FOLFOX with bevacizumab is recommended as a first-line chemotherapy regimen for CRLM in previous studies [37]. Third, as only a small number of patients in our cohort underwent surgical resection of liver metastasis after chemotherapy, histopathological analysis of liver metastasis was not included in this study. However, this appears to be important based on previous studies of the HGP of liver metastasis [38]. Finally, we used spectral CT only in the portal venous phase and acquired spectral CT parameters for only a single target lesion. Further prospective studies are required including baseline spectral CT in patients with CRLM to further validate the correlations of the spectral CT parameters of multiple target lesions with survival outcomes and early response.
Conclusions
In conclusion, spectral CT IoD can predict the OS and responder of patients with CRLM after 2 months of treatment with bevacizumab-containing therapy. At the same time, baseline CEA, BLD, K-RAS mutation, and metachronous liver metastasis were independent risk factors for OS after treatment for CRLM.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the.corresponding author on reasonable request.
Abbreviations
- CRLM:
-
Colorectal cancer liver metastasis
- IoD:
-
Iodine concentration
- NIoD:
-
Normalized iodine concentration
- OS:
-
Overall survival
- RECIST v1.1:
-
Response Evaluation Criteria in Solid Tumors Version 1.1
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- R + :
-
Responders
- R − :
-
Non-responders
References
Siegel RL, Miller KD. Jemal A (2019) Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, D’Angelica MI, Endo I, Parks RW, Doyle M, de Santibanes E, Pawlik TM. Liver metastases Nat Rev Dis Primers. 2021;7(1):27. https://doi.org/10.1038/s41572-021-00261-6.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T, Group EG-ITC, Cancer Research UK, Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft O, Australasian Gastro-Intestinal Trials G, Federation Francophone de Cancerologie D (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial Lancet Oncol 14 (12):1208-1215. https://doi.org/10.1016/S1470-2045(13)70447-9
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, Park JO, Kim SY, Kim TY, Kim JH, Ahn JB, Lim SB, Yu CS, Kim JC, Yun SH, Kim JH, Park JH, Park HC, Jung KH, Kim TW. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15(11):1245–53. https://doi.org/10.1016/S1470-2045(14)70377-8.
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. https://doi.org/10.1200/JCO.2007.14.9930.
Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, Lasserre S, Hermann F, Waterkamp D, Adam R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–8. https://doi.org/10.1093/annonc/mdu580.
Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, Bordonaro R, Latiano TP, Tamburini E, Santini D, Passardi A, Marmorino F, Grande R, Aprile G, Zaniboni A, Murgioni S, Granetto C, Buonadonna A, Moretto R, Corallo S, Cordio S, Antonuzzo L, Tomasello G, Masi G, Ronzoni M, Di Donato S, Carlomagno C, Clavarezza M, Ritorto G, Mambrini A, Roselli M, Cupini S, Mammoliti S, Fenocchio E, Corgna E, Zagonel V, Fontanini G, Ugolini C, Boni L, Falcone A, Investigators GF. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507. https://doi.org/10.1016/S1470-2045(19)30862-9.
Eisenhauer EA, Therasse P, Bogaerts J, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H, Charnsangavej C, Loyer EM. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338–44. https://doi.org/10.1001/jama.2009.1755.
Mazard T, Boonsirikamchai P, Overman MJ, Asran MA, Choi H, Herron D, Eng C, Maru DM, Ychou M, Vauthey JN, Loyer EM, Kopetz S. Comparison of early radiological predictors of outcome in patients with colorectal cancer with unresectable hepatic metastases treated with bevacizumab. Gut. 2018;67(6):1095–102. https://doi.org/10.1136/gutjnl-2017-313786.
Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P, Bennouna J, Ghiringhelli F, Barbier E, Boige V, Taieb J, Bouche O, Francois E, Phelip JM, Borel C, Faroux R, Seitz JF, Jacquot S, Ben Abdelghani M, Khemissa-Akouz F, Genet D, Jouve JL, Rinaldi Y, Desseigne F, Texereau P, Suc E, Lepage C, Aparicio T, Hoeffel C, Investigators P, Investigators P. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69(3):531–9. https://doi.org/10.1136/gutjnl-2018-316407.
Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA, Charnsangavej C, Aloia TA, Vauthey JN. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30(36):4566–72. https://doi.org/10.1200/JCO.2012.45.2854.
Yoshita H, Hosokawa A, Ueda A, Ando T, Kajiura S, Kato H, Kawabe H, Tomizawa G, Horikawa N, Yabuhita K, Note M, Sugiyama T. Predictive value of optimal morphologic response to first-line chemotherapy in patients with colorectal liver metastases. Digestion. 2014;89(1):43–8. https://doi.org/10.1159/000356218.
Latacz E, van Dam PJ, Vanhove C, Llado L, Descamps B, Ruiz N, Joye I, Grunhagen D, Van Laere S, Dirix P, Mollevi DG, Verhoef C, Dirix L, Vermeulen P. Can medical imaging identify the histopathological growth patterns of liver metastases? Semin Cancer Biol. 2021;71:33–41. https://doi.org/10.1016/j.semcancer.2020.07.002.
Vriens D, van Laarhoven HW, van Asten JJ, Krabbe PF, Visser EP, Heerschap A, Punt CJ, de Geus-Oei LF, Oyen WJ. Chemotherapy response monitoring of colorectal liver metastases by dynamic Gd-DTPA-enhanced MRI perfusion parameters and 18F-FDG PET metabolic rate. J Nucl Med. 2009;50(11):1777–84. https://doi.org/10.2967/jnumed.109.064790.
De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, Van de Wiele C, Troisi R, Libbrecht L, Laurent S, Geboes K, Peeters M. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer. 2012;106(12):1926–33. https://doi.org/10.1038/bjc.2012.184.
Liu LH, Zhou GF, Lv H, Wang ZC, Rao SX, Zeng MS. Identifying response in colorectal liver metastases treated with bevacizumab: development of RECIST by combining contrast-enhanced and diffusion-weighted MRI. Eur Radiol. 2021;31(8):5640–9. https://doi.org/10.1007/s00330-020-07647-2.
Boraschi P, Donati F, Cervelli R, Pacciardi F, Tarantini G, Castagna M, Urbani L, Lencioni R. Colorectal liver metastases: ADC as an imaging biomarker of tumor behavior and therapeutic response. Eur J Radiol. 2021;137:109609. https://doi.org/10.1016/j.ejrad.2021.109609.
Majeed NF, BraschiAmirfarzan M, Wald C, Wortman JR. Spectral detector CT applications in advanced liver imaging. Br J Radiol. 2021;94(1123):20201290. https://doi.org/10.1259/bjr.20201290.
Hellbach K, Sterzik A, Sommer W, Karpitschka M, Hummel N, Casuscelli J, Ingrisch M, Schlemmer M, Graser A, Staehler M. Dual energy CT allows for improved characterization of response to antiangiogenic treatment in patients with metastatic renal cell cancer. Eur Radiol. 2017;27(6):2532–7. https://doi.org/10.1007/s00330-016-4597-7.
Wang X, Liu D, Zeng X, Jiang S, Li L, Yu T, Zhang J. Dual-energy CT quantitative parameters for evaluating Immunohistochemical biomarkers of invasive breast cancer. Cancer Imaging. 2021;21(1):4. https://doi.org/10.1186/s40644-020-00370-7.
Luo N, Li W, Xie J, Fu D, Liu L, Huang X, Su D, Jin G. Preoperative normalized iodine concentration derived from spectral CT is correlated with early recurrence of hepatocellular carcinoma after curative resection. Eur Radiol. 2021;31(4):1872–82. https://doi.org/10.1007/s00330-020-07330-6.
Shi HY, Lu ZP, Li MN, Ge YQ, Jiang KR, Xu Q. Dual-Energy CT Iodine Concentration to Evaluate Postoperative Pancreatic Fistula after Pancreatoduodenectomy. Radiology. 2022;304(1):65–72. https://doi.org/10.1148/radiol.212173.
Sakai N, Furukawa K, Takayashiki T, Kuboki S, Takano S, Ohtsuka M. Differential effects of KRAS mutational status on long-term survival according to the timing of colorectal liver metastases. BMC Cancer. 2021;21(1):412. https://doi.org/10.1186/s12885-021-08144-5.
Tosi F, Magni E, Amatu A, Mauri G, Bencardino K, Truini M, Veronese S, De Carlis L, Ferrari G, Nichelatti M, Sartore-Bianchi A, Siena S. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin Colorectal Cancer. 2017;16(3):e153–63. https://doi.org/10.1016/j.clcc.2017.01.004.
Zimmitti G, Shindoh J, Mise Y, Kopetz S, Loyer EM, Andreou A, Cooper AB, Kaur H, Aloia TA, Maru DM, Vauthey JN. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(3):834–42. https://doi.org/10.1245/s10434-014-4042-6.
Garcia-Carbonero N, Martinez-Useros J, Li W, Orta A, Perez N, Carames C, Hernandez T, Moreno I, Serrano G, Garcia-Foncillas J (2020) KRAS and BRAF Mutations as Prognostic and Predictive Biomarkers for Standard Chemotherapy Response in Metastatic Colorectal Cancer: A Single Institutional Study. Cells 9 (1). https://doi.org/10.3390/cells9010219
Margonis GA, Amini N, Andreatos N, Sasaki K, McVey J, Mirza MB, Warner S, Buettner S, Barbon C, Wang J, Pulvirenti A, Angelou A, Kamphues C, Antoniou E, Pikoulis E, Pawlik TM, Kaczirek K, Poultsides G, Wagner D, Endo I, Imai K, Aucejo F, Kreis ME, Wolfgang CL, Weiss MJ. KRAS mutational status impacts pathologic response to pre-hepatectomy chemotherapy: a study from the International Genetic Consortium for Liver Metastases. HPB (Oxford). 2019;21(11):1527–34. https://doi.org/10.1016/j.hpb.2019.03.368.
Suzuki C, Blomqvist L, Sundin A, Jacobsson H, Bystrom P, Berglund A, Nygren P, Glimelius B. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol. 2012;23(4):948–54. https://doi.org/10.1093/annonc/mdr350.
Yang G, Wang G, Sun J, Xiong Y, Li W, Tang T, Li J. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: A systematic review and meta-analysis based on 22 studies. Int J Surg. 2021;87:105896. https://doi.org/10.1016/j.ijsu.2021.105896.
Nakanishi R, Oki E, Hasuda H, Sano E, Miyashita Y, Sakai A, Koga N, Kuriyama N, Nonaka K, Fujimoto Y, Jogo T, Hokonohara K, Hu Q, Hisamatsu Y, Ando K, Kimura Y, Yoshizumi T, Mori M. Radiomics Texture Analysis for the Identification of Colorectal Liver Metastases Sensitive to First-Line Oxaliplatin-Based Chemotherapy. Ann Surg Oncol. 2021;28(6):2975–85. https://doi.org/10.1245/s10434-020-09581-5.
Iwanicki-Caron I, Di Fiore F, Roque I, Astruc E, Stetiu M, Duclos A, Tougeron D, Saillard S, Thureau S, Benichou J, Paillot B, Basuyau JP, Michel P. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol. 2008;26(22):3681–6. https://doi.org/10.1200/JCO.2007.15.0904.
Lv P, Liu J, Yan X, Chai Y, Chen Y, Gao J, Pan Y, Li S, Guo H, Zhou Y. CT spectral imaging for monitoring the therapeutic efficacy of VEGF receptor kinase inhibitor AG-013736 in rabbit VX2 liver tumours. Eur Radiol. 2017;27(3):918–26. https://doi.org/10.1007/s00330-016-4458-4.
Drljevic-Nielsen A, Mains JR, Thorup K, Andersen MB, Rasmussen F, Donskov F. Early reduction in spectral dual-layer detector CT parameters as favorable imaging biomarkers in patients with metastatic renal cell carcinoma. Eur Radiol. 2022. https://doi.org/10.1007/s00330-022-08793-5.
Huellner MW, Hennedige TP, Winterhalder R, Zander T, Venkatesh SK, Yong WP, Soo RA, Seifert B, Treumann TC, Strobel K, Veit-Haibach P. Prognostic value of different CT measurements in early therapy response evaluation in patients with metastatic colorectal cancer. Cancer Imaging. 2012;12:212–24. https://doi.org/10.1102/1470-7330.2012.0021.
Lastoria S, Piccirillo MC, Caraco C, Nasti G, Aloj L, Arrichiello C, de Lutio di Castelguidone E, Tatangelo F, Ottaiano A, Iaffaioli RV, Izzo F, Romano G, Giordano P, Signoriello S, Gallo C, Perrone F,. Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med. 2013;54(12):2062–9. https://doi.org/10.2967/jnumed.113.119909.
Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–18. https://doi.org/10.1056/NEJMoa1403108.
Osawa G, Yoshimatsu K, Yokomizo H, Okayama S, Sagawa M, Naritaka Y. Correlation between response to chemotherapy with concomitant bevacizumab for hepatic metastasis of colorectal cancer and degree of enhancement using contrast-enhanced computed tomography. Cancer Chemother Pharmacol. 2013;72(1):209–15. https://doi.org/10.1007/s00280-013-2186-x.
Acknowledgements
Not applicable.
Funding
This study was supported by grants of the National Youth Science Foundation Project (82102151) and the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2021-ZD-01).
Author information
Authors and Affiliations
Contributions
Shenglin Li: Conceptualization, Validation, Formal analysis, Resources, Writing—original draft, Writing—review & editing. Long Yuan, Mengying Yue, Yuan Xu, Suwei Liu and Ting Lu: Formal analysis, Resources, Data curation, Follow up. Juan Deng, Qiu Sun, Xianwang Liu and Caiqiang Xue: Consult literatures, Statistical Analysis. Feng Wang, Xiaoqin Liu and Fengyan Wang: Spectral CT scan and data collection. Wenjuan Zhang and Junlin Zhou*: Conceptualization, Supervision, Project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by our institutional ethics committee (2022A-298), and the requirement for informed consent was waived due to the retrospective nature of the study. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Yuan, L., Yue, M. et al. Early evaluation of liver metastasis using spectral CT to predict outcome in patients with colorectal cancer treated with FOLFOXIRI and bevacizumab. Cancer Imaging 23, 30 (2023). https://doi.org/10.1186/s40644-023-00547-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-023-00547-w