Abstract
Purpose
To evaluate the performance of hepatobiliary MRI parameters as predictors of clinical response to chemotherapy in patients with initially unresectable colorectal cancer liver metastases (CRLM).
Methods
Eighty-five patients with initially unresectable CRLM were retrospectively enrolled from two hospitals and scanned using gadobenate dimeglumine-enhanced MRI before treatment. Therapy response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Conventional parameters (i.e., signal intensity [SI]) and radiomics features of portal venous phase (PVP) and hepatobiliary phase (HBP) images were analyzed between the responders and non-responders. Next, the combined model was constructed, and the area under the receiver operating characteristic (ROC) curve (AUC) was calculated. The relationship between the combined model and progression-free survival (PFS) was analyzed using Cox regression.
Results
Of the 85 patients from two hospitals, 42 were in the response group, and 43 were in the non-response group. Upon conducting five-fold cross-validation, the normalized relative enhancement (NRE) of CRLM during the PVP yielded an AUC of 0.625. Additionally, a radiomics feature derived from the tumor area in the HBP achieved an AUC of 0.698, while a separate feature extracted from the peritumoral region in the HBP recorded an AUC of 0.709. The model that integrated these three features outperformed the individual features, achieving an AUC of 0.818. Furthermore, the combined model exhibited a significant correlation with PFS (P < 0.001).
Conclusion
The combined model, based on baseline hepatobiliary MRI, aids in predicting chemotherapeutic response and PFS in patients with initially unresectable CRLM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) ranks as the third most prevalent malignancy globally, and the prognosis for CRC patients is significantly influenced by numerous factors, with colorectal cancer liver metastasis (CRLM) being one of the most crucial [1]. CRLM is a leading cause of mortality in patients with CRC. Roughly 50% of CRC patients will eventually develop liver metastases, with about 25% being diagnosed at the time of their initial presentation and an additional 25% developing CRLM after the resection of the primary tumor [2]. Surgical removal of metastases is the sole curative option for CRLM, yet it remains a viable option for only about 20% of CRLM patients [3]. Consequently, a substantial portion of individuals with CRLM undergo chemotherapy as part of their treatment regimen.
Systemic chemotherapy is a treatment approach for initially unresectable CRLM, aimed at reducing the burden of liver metastases to potentially allow for future surgical intervention, while also providing palliative care and improving survival [2, 4]. Given the heterogeneity of CRLM, first-line chemotherapy regimens often fail, resulting in approximately 50% of patients not responding well to treatment and potentially even progressing [5, 6]. Accurate biomarkers of the response to therapy are essential to ensure that patients are not exposed to potentially toxic side effects without any therapeutic benefit. Furthermore, the early identification of non-responders allows for timely transition to alternative treatment regimens.
Magnetic resonance imaging (MRI) plays a crucial role in the diagnosis and management of liver diseases, particularly in the detection and follow-up of CRLM [7, 8]. Additionally, MRI is increasingly recognized as a promising tool for evaluating therapeutic response in CRLM, as it provides both diagnostic and prognostic information during a single examination. Hepatobiliary MRI, which assesses CRLM during the hepatobiliary phase (HBP), is a potent modality that provides excellent contrast between lesions and liver tissue, thereby enhancing lesion detection rates and potentially offering benefits for evaluating the efficacy of chemotherapy [9,10,11]. However, relying solely on the measurement of signal intensity (SI) of CRLM during HBP is insufficient for capturing the comprehensive information about the heterogeneity of CRLM [9].
Recently, radiomics has emerged as a capability that can process and interpret information in medical images that is imperceptible to the human eye [12]. Several studies have employed MRI-based radiomics to predict therapeutic efficacy and prognosis in patients with CRLM [13,14,15,16], suggesting that conventional MRI-based radiomics is a promising tool for such assessments. However, these studies have not focused on hepatobiliary MRI, and the correlation between its parameters and therapeutic response is not well understood.
Therefore, we aimed to develop an accurate predictive model that combines various parameters from baseline hepatobiliary MRI, encompassing both conventional and radiomic features, to predict chemotherapeutic response and prognosis in patients with initially unresectable CRLM.
Materials and methods
Patients
This retrospective study was approved by the local Institutional Review Board (ID: 2023-121), and the requirement for informed consent was waived due to the retrospective nature of the study. The study included 85 patients with confirmed CRLM from two hospitals, with data collected between January 2018 and March 2024. The inclusion criteria were as follows: (1) pathologically confirmed colorectal adenocarcinoma; (2) baseline hepatobiliary MRI and follow-up CT/MRI were acquired; (3) the diameter of measurable CRLM was larger than 1 cm; and (4) CRLMs were deemed unresectable at diagnosis. The exclusion criteria included: (1) the absence of the Response Evaluation Criteria in Solid Tumors (RECIST) evaluation; (2) presence of other tumors during the same period; and (3) poor quality of MR images. A total of 65 patients were enrolled from the hospital 1, while an additional 20 patients were included from the hospital 2. A flow diagram summarizing the study period and exclusion procedures is shown in Fig. 1.
All available clinical data, including sex, age, primary tumor location, whether the CRLM was metachronous or synchronous, chemotherapy regimen, targeted therapy, RECIST evaluation, chemotherapeutic response, and progression-free survival (PFS), were obtained and analyzed. These data are presented in Table 1.
Response evaluation and prognosis
Most patients in our study (75/85, or 88%) received standard first-line chemotherapy regimens tailored to their individual conditions, including the XELOX, FOLFOX, and FOLFIRI regimens. Response evaluation was conducted using CT or MRI scanning, in accordance with RECIST Version 1.1, the most widely accepted criteria for assessing tumor response to treatment [17, 18]. These criteria, which are based on tumor size measurement, are commonly used due to their ease of acquisition with the simple imaging modalities. The application of these criteria is relatively complex [17], and this section provides the definitions used to determine objective tumor response for target lesions: (1) complete response (CR): disappearance of all target lesions. (2) partial response (PR): at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. (3) progressive disease (PD): at least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study. In addition to the relative increase of 20%, and the appearance of one or more new lesions is also considered progression. (4) stable disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Imaging examinations were chosen based on their proximity to the time of treatment changes, such as the cessation of chemotherapy, switching to other regimens, resection, or ablation, to conduct RECIST evaluation. Patients achieving CR or PR were assigned to the response group, while those with SD or PD were assigned to the non-response group. PFS was calculated from the start of chemotherapy until the time of progression as determined by CT/MRI follow-up.
MRI technique and image analysis
The main types of MRI scanners used in the study included GE SIGNA MR380, GE SIGNA Architect, and SIEMENS MAGNETOM Vida. The transverse T1-weighted volume interpolated breath-hold examination (T1-VIBE) sequence or liver acquisition with volume acceleration (LAVA) sequence was acquired before and after gadobenate dimeglumine (Gd-BOPTA) injection in the arterial phase (50 s), and PVP (80 s). All patients rested for 1 h. After that, HBP (90 min) imaging was acquired. Gd-BOPTA was administered at a dose of 0.05 mmol/kg body weight.
The regions of interest (ROIs) for conventional parameters were determined by two diagnostic radiologists (with 5 and 17 years of experience in radiology) who were blinded to the chemotherapy response. The mean apparent diffusion coefficient (ADC) values of the largest CRLM were recorded by selecting random regions (whole lesion and solid component) on the ADC map. The signal intensities (SI) in the T1-weighted pre-contrast phase, PVP, and HBP were measured by drawing ROIs on the CRLM, normal-appearing liver parenchyma, and renal cortex. The relative signal intensity difference (RSID) was calculated by using formula \(RSID = SI({\text{liver}}) - SI(CRLM)\). The normalized relative enhancement (NRE) was calculated by using formula \(NRE = (SI_{LC}/SI_{RC} - SI_{L0}/SI_{R0})/(SI_{L0}/SI_{R0})\), where SIL0 and SIR0 were signal intensities of liver metastases and renal cortex on T1-weighted pre-contrast images, and SILC and SIRC were signal intensities of liver metastases and renal cortex on T1-weighted contrast images, respectively.
Radiomics analysis
The volumes of interest (VOI) for the largest CRLM were meticulously delineated slice by slice by a radiologist with 5 years of experience in abdominal MRI, encompassing both the PVP and HBP VOIs. Then, the software (Multimodal Research Platform V2.5.1, https://keyan.deepwise.com) automatically expanded the VOI outward by 3 mm to create an annular VOI of peritumoral area, as depicted in Fig. 2. The PVP and HBP images were resampled to a pixel spacing of 1.0 mm in three anatomical directions. Subsequently, several filters were applied to preprocess the original images. A total of 1786 features, including first-order statistics, shape features, and texture features, were extracted from each VOI using Pyradiomics (version 2.2.0). We then selected radiomics features using correlation analysis and an F-test. A model combining conventional MRI parameters and radiomics features was constructed using ten algorithms, followed by fivefold cross-validation. The performance of the combined model was compared to that of the single parameters.
Cox analysis and subgroup analysis
The predictive value of the combined model for PFS was assessed using Cox regression, followed by a Kaplan–Meier (KM) survival analysis. For subgroup analysis, we compared the performance of the combined model in various subgroups: those with and without targeted therapy, and those receiving the XELOX regimen versus other chemotherapy regimens.
Statistical analysis
The statistical analyses were performed using SPSS Statistics (version 21.0) and Medcalc (version 15.2.2). The graphs were generated using Medcalc. Categorical variables were analyzed using chi-square test or Fisher’s exact test, and continuous variables were analyzed using the independent t-test or Mann‒Whitney U test. The areas under the curve (AUCs) of the parameters and model were compared using DeLong test. A P value < 0.05 was considered significant.
Results
Clinical features and prognosis
As shown in Table 1, this study included 85 patients with initially unresectable CRLM. Of these patients, 42 (49%) were in the response group and 43 (51%) were in the non-response group. Figures 3 and 4 present the PVP and HBP images from two cases: one demonstrating PR to treatment and the other showing SD. There were 54 cases of CRLM derived from colon cancer, accounting for approximately 64% of all CRLM cases. Only 12 cases of CRLM occurred after the removal of the primary tumor. About 49 patients with CRLM received chemotherapy with the XELOX regimen. Targeted therapy was combined with chemotherapy in 40 patients. There was no statistically significant difference in these indicators between the response group and the non-response group. The median time to disease progression in these 85 CRLM patients was 4 months.
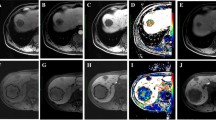
A patient with CRLM exhibited a partial response to chemotherapy. The CRLM was initially indicated by white arrows in baseline PVP (a) and HBP (b) images. After 4 months of chemotherapy, the CRLM was reduced in size, as indicated by blue arrows in both the post-chemotherapy PVP (c) and HBP (d) images. CRLM colorectal cancer liver metastasis, PVP portal venous phase, HBP hepatobiliary phase
A patient with CRLM showed stable disease after undergoing chemotherapy. The CRLM was initially indicated by white arrows in baseline PVP (a) and HBP (b) images. After 2 months of chemotherapy, the CRLM did not significantly change in size, as indicated by blue arrows in both the post-chemotherapy PVP (c) and HBP (d) images. CRLM colorectal cancer liver metastasis, PVP portal venous phase, HBP hepatobiliary phase
Conventional MRI parameters
There were statistically significant differences in RSID and NRE of CRLM between the response and non-response groups, with the non-responders exhibiting higher RSID and lower NRE for the whole lesion in the PVP compared to the responders (P = 0.033 and 0.018), as shown in Table 2. The remaining conventional MRI parameters lacked statistical significance between the two groups.
Radiomics analysis
A total of 4 features were selected, as shown in Table 3. There were statistically significant differences in these four features between the response and non-response groups.
We conducted correlation analyses on the features with P < 0.05 in Tables 2 and 3. If a correlation was found between two features, the one with lower performance for predicting chemotherapeutic response was excluded from the modeling. Finally, we incorporated NRE of CRLM in the PVP images and the two HBP radiomics features into a model. Linear SVC achieved the highest five-fold cross-validated AUC of 0.818 among the ten algorithms, with a sensitivity of 79.1% and a specificity of 71.4% (Table 4). The AUC of the combined model was higher than that of the single parameters, with statistically significant differences observed between the combined model and NRE_PVP (P = 0.002) as well as gradient_glcm_correlation_HBP (P = 0.010) (Fig. 5a).
Cox analysis and subgroup analysis
The predictive value of the combined model was an independent risk factor for the PFS of CRLM patients, with a hazard ratio of 2.550 and a P-value of < 0.001. The relationship between the combined model and PFS was analyzed by KM analysis, as shown in Fig. 5b.
As illustrated in Table 5, subgroup analyses revealed no statistically significant difference in the performance (AUC) of the combined model between patients with targeted therapy and those without. Similarly, the performance was not significantly different between patients treated with the XELOX regimen and those on alternative chemotherapy regimens. Despite the lack of significant differences, the combined model exhibited superior performance in the patients undergoing XELOX regimen without the addition of targeted therapy.
Discussion
This study demonstrated that baseline hepatobiliary MRI proved effective in predicting chemotherapeutic response of initially unresectable CRLM patients, with an AUC of 0.818. Furthermore, the integrated model can serve as a simple and feasible method to predict PFS of CRLM patients in advance.
Gd-BOPTA is taken up into hepatocytes and subsequently excreted into the biliary system [19]. The number and activity of organic anion transporting polypeptides decrease in CRLM, leading to reduced uptake of Gd-BOPTA [20, 21]. Now hepatobiliary MRI is widely used in the clinical diagnosis and efficacy evaluation of CRLMs. Its main advantages include improving the detection rate and characterization of lesions on HBP imaging, thereby enhancing the confidence of radiologists in diagnosis [21].
In our study, the radiomics features from both the tumor and peritumoral area in the HBP might be useful in evaluating the response to chemotherapy in CRLM. Previously published reports have evaluated responses to chemotherapy in CRLMs using conventional MRI or CT texture analyses, with the highest AUC of 0.82, which was similar to our results [22, 23]. Dohan et al. demonstrated that a radiomics score from contrast-enhanced CT could predict overall survival (OS) and identify good responders in patients with CRLMs [24]. However, some results are controversial. Lee et al. suggested that texture analysis could not predict the prognosis of liver metastasis [25]. Our study indicated that radiomics analysis of baseline hepatobiliary MRI can predict the PFS of CRLM patients.
In the study by Hosseini-Nik et al. [9], NRE and RSID after chemotherapy were found to help assess the efficacy of treatment. However, unlike that study, we used baseline hepatobiliary MRI before chemotherapy, allowing for the earlier identification of patients who do not respond to chemotherapy, so that alternative treatments can be considered. Our research analyzed various conventional parameters, in which NRE_PVP was found to help differentiate the responders from non-responders, with a relatively low AUC of 0.625. This suggests that conventional parameters have limited efficacy in assessing the heterogeneity of CRLM.
Therefore, we analyzed the heterogeneity of CRLM using radiomics. The largest CRLM was selected for analysis, while other CRLMs were ignored, due to the high biological similarity among different liver metastases in the same patient, as well as their similar responses to the same chemotherapy regimen. This practice may introduce some selection bias. The radiomics features included in our model contained only the features of HBP, indicating that HBP images have unique advantages in evaluating the heterogeneity of CRLM.
Pathologically, peritumoral parenchyma is representative of cancerous heterogeneity, and the crucial information can be indicated by changes in the area surrounding tumors, such as biological aggressiveness [26]; thus, accurate evaluation of the neighboring tissue around tumors may also be useful in predicting treatment response and prognosis. In our model, a peritumoral feature from HBP imaging was also included, which improved the efficacy of the combined model.
For the prognostic assessment of CRLM patients, we found that the combined model, based on the baseline hepatobiliary MRI, helped predict the prognosis of patients. We used PFS instead of OS because hepatobiliary MRI is a new technology, and the proportion of patients who have reached the endpoint of death in our hospital is currently small.
It should be noted that some patients in this study received chemotherapy regimen XELOX, while others received other chemotherapy regimens, and some patients were treated with targeted therapy in combination, while others were not. Therefore, we conducted subgroup analyses and found no significant differences in predictive power (AUC) of the combined model between the subgroups.
There are certain limitations to this study. First, due to the retrospective design, some biases may exist, but they were within controllable limits. Second, the sample size was relatively small. Third, only cross-validation was performed. In the future, it will be necessary to collect data from more diverse regions to further enhance the generalizability of the model.
Conclusion
The combined model based on baseline hepatobiliary MRI helps in predicting the chemotherapeutic response and PFS in patients with initially unresectable CRLM.
Data availability
No datasets were generated or analysed during the current study.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 71:209-249. https://doi.org/10.3322/caac.21660.
van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH (2015) Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 32:457-65. https://doi.org/10.1007/s10585-015-9719-0.
Adam R, Kitano Y (2019) Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 3:50-56. https://doi.org/10.1002/ags3.12227.
Adam R, de Gramont A, Figueras J, et al. (2015) Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 41:729-41. https://doi.org/10.1016/j.ctrv.2015.06.006.
Zhou BY, Liu H, Pu YY, et al. (2024) Quantitative analysis of pre-treatment dynamic contrast-enhanced ultrasound for assessing the response of colorectal liver metastases to chemotherapy plus targeted therapy: a dual-institutional study. Abdom Radiol (NY). 49:414-424. https://doi.org/10.1007/s00261-023-04055-0.
Bolhuis K, Kos M, van Oijen MGH, Swijnenburg RJ, Punt CJA (2020) Conversion strategies with chemotherapy plus targeted agents for colorectal cancer liver-only metastases: A systematic review. Eur J Cancer. 141:225-238. https://doi.org/10.1016/j.ejca.2020.09.037.
Cai Q, Mao Y, Dai S, Gao F, Xiao Q, Hu W, Qin T, Yang Q, Li Z, Cai D, Zhong ME, Ding K, Wu XJ, Zhang R (2022) The growth pattern of liver metastases on MRI predicts early recurrence in patients with colorectal cancer: a multicenter study. Eur Radiol. 32:7872-7882. https://doi.org/10.1007/s00330-022-08774-8.
Tsili AC, Alexiou G, Naka C, Argyropoulou MI (2021) Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: a meta-analysis. Acta Radiol. 62:302-312. https://doi.org/10.1177/0284185120925481.
Hosseini-Nik H, Fischer SE, Moulton CA, Karbhase G, Menezes RJ, Gallinger S, Jhaveri KS (2016) Diffusion-weighted and hepatobiliary phase gadoxetic acid-enhanced quantitative MR imaging for identification of complete pathologic response in colorectal liver metastases after preoperative chemotherapy. Abdom Radiol (NY). 41:231-8. https://doi.org/10.1007/s00261-015-0572-z.
Zhang L, Yu X, Huo L, Lu L, Pan X, Jia N, Fan X, Morana G, Grazioli L, Schneider G (2019) Detection of liver metastases on gadobenate dimeglumine-enhanced MRI: systematic review, meta-analysis, and similarities with gadoxetate-enhanced MRI. Eur Radiol. 29:5205-5216. https://doi.org/10.1007/s00330-019-06110-1.
Canellas R, Patel MJ, Agarwal S, Sahani DV (2019) Lesion detection performance of an abbreviated gadoxetic acid-enhanced MRI protocol for colorectal liver metastasis surveillance. Eur Radiol. 29:5852-5860. https://doi.org/10.1007/s00330-019-06113-y.
Mayerhoefer ME, Materka A, Langs G, et al. (2020) Introduction to radiomics.J Nucl Med. 61:488-495. https://doi.org/10.2967/jnumed.118.222893.
Rompianesi G, Pegoraro F, Ceresa CD, Montalti R, Troisi RI (2022) Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J Gastroenterol. 28:108-122. https://doi.org/10.3748/wjg.v28.i1.108.
Granata V, Fusco R, De Muzio F, Cutolo C, Mattace Raso M, Gabelloni M, Avallone A, Ottaiano A, Tatangelo F, Brunese MC, Miele V, Izzo F, Petrillo A (2022) Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics (Basel). 12(5):1115. https://doi.org/10.3390/diagnostics12051115.
Chen J, Cheung HMC, Karanicolas PJ, Coburn NG, Martel G, Lee A, Patel C, Milot L, Martel AL (2023) A radiomic biomarker for prognosis of resected colorectal cancer liver metastases generalizes across MRI contrast agents. Front Oncol. 13:898854. https://doi.org/10.3389/fonc.2023.898854.
Su X, Zhang H, Wang Y (2023) A predictive model for early therapeutic efficacy of colorectal liver metastases using multimodal MRI data. J Xray Sci Technol. 31:357-372. https://doi.org/10.3233/XST-221317.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
Morse B, Jeong D, Ihnat G, Silva AC (2019) Pearls and pitfalls of response evaluation criteria in solid tumors (RECIST) v1.1 non-target lesion assessment. Abdom Radiol (NY). 44(2):766-774. https://doi.org/10.1007/s00261-018-1752-4.
Liu C, Shen Z, Ma H, Wang X, Wang X, Liu K, Wang X, Zhu Q, Zhao X (2022) Gd-BOPTA-enhanced hepatobiliary phase MR imaging can predict the prognosis of patients with acute-on-chronic liver failure. Eur Radiol. 32:3006-3015. https://doi.org/10.1007/s00330-021-08440-5.
Bonatti M, Valletta R, Avesani G, Lombardo F, Cannone F, Zamboni GA, Mansueto G, Manfredi R, Ferro F (2021) Liver enhancement during hepatobiliary phase after Gd-BOPTA administration: correlation with liver and renal function. Eur Radiol. 31:2490-2496. https://doi.org/10.1007/s00330-020-07279-6.
Brismar TB, Kartalis N, Kylander C, Albiin N (2012) MRI of colorectal cancer liver metastases: comparison of orally administered manganese with intravenously administered gadobenate dimeglumine. Eur Radiol. 22:633-41. https://doi.org/10.1007/s00330-011-2288-y.
Ha S, Lee CH, Kim BH, Park YS, Lee J, Choi JW, Kim KA, Park CM (2012) Paradoxical uptake of Gd-EOB-DTPA on the hepatobiliary phase in the evaluation of hepatic metastasis from breast cancer: is the "target sign" a common finding? Magn Reson Imaging. 30:1083-90. https://doi.org/10.1016/j.mri.2012.03.007.
Beckers RCJ, Trebeschi S, Maas M, Schnerr RS, Sijmons JML, Beets GL, Houwers JB, Beets-Tan RGH, Lambregts DMJ (2018) CT texture analysis in colorectal liver metastases and the surrounding liver parenchyma and its potential as an imaging biomarker of disease aggressiveness, response and survival. Eur J Radiol. 102:15-21. https://doi.org/10.1016/j.ejrad.2018.02.031.
Dohan A, Gallix B, Guiu B, et al. (2020) Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 69:531-539. https://doi.org/10.1136/gutjnl-2018-316407.
Lee SJ, Zea R, Kim DH, Lubner MG, Deming DA, Pickhardt PJ (2018) CT texture features of liver parenchyma for predicting development of metastatic disease and overall survival in patients with colorectal cancer. Eur Radiol. 28:1520-1528. https://doi.org/10.1007/s00330-017-5111-6.
Zhao Y, Zhang J, Wang N, Xu Q, Liu Y, Liu J, Zhang Q, Zhang X, Chen A, Chen L, Sheng L, Song Q, Wang F, Guo Y, Liu A (2023) Intratumoral and peritumoral radiomics based on contrast-enhanced MRI for preoperatively predicting treatment response of transarterial chemoembolization in hepatocellular carcinoma. BMC Cancer. 23:1026. https://doi.org/10.1186/s12885-023-11491-0.
Funding
This work was supported by the Natural Science Foundation of Sichuan Province from Science & Technology Department of Sichuan Province (Grant No. 2023NSFSC1718) and Foundation of Sichuan Provincial People’s Hospital (Grant No. 2022QN59).
Author information
Authors and Affiliations
Contributions
Y.C. and M.L. wrote the main manuscript text. T.L., Y.Z., H.L., and J.X. prepared tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Lu, T., Zhang, Y. et al. Baseline hepatobiliary MRI for predicting chemotherapeutic response and prognosis in initially unresectable colorectal cancer liver metastases. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04492-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04492-5