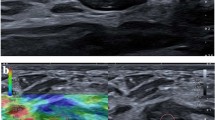
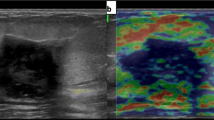
Abstract
Background
Elastography is an addition to grey-scale ultrasonic examination that has gained substantial traction within the last decade. Strain ratio (SR) has been incorporated as a semiquantitative measure within strain elastography, thus a potential imaging biomarker. The World Federation for Ultrasound in Medicine and Biology (WFUMB) published guidelines in 2015 for breast elastography. These guidelines acknowledge the marked variance in SR cut-off values used in differentiating benign from malignant lesions. The objective of this review was to include more recent evidence and seek to determine the optimal strain ratio cut off value for differentiating between benign and malignant breast lesions.
Methods
Comprehensive search of MEDLINE and Web of Science electronic databases with additional searches via Google Scholar and handsearching set from January 2000 to May 2020 was carried out. For retrieved studies, screening for eligibility, data extraction and analysis was done as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Diagnostic Test Accuracy (PRISMA-DTA) Statement guidelines of 2018. Quality and risk of bias assessment of the studies were performed using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Results
A total of 424 articles, 412 from electronic database and 12 additional searches were retrieved and 65 studies were included in the narrative synthesis and subgroup analysis. The overall threshold effect indicated significant heterogeneity among the studies with Spearman correlation coefficient of Logit (TPR) vs Logit (FPR) at − 0.301, p-value = 0.015. A subgroup under machine model consisting seven studies with 783 patients and 844 lesions showed a favourable threshold, Spearman’s correlation coefficient,0.786 (p = 0.036).
Conclusion
From our review, currently the optimal breast SR cut-off point or value remains unresolved despite the WFUMB guidelines of 2015. Machine model as a possible contributor to cut-off value determination was suggested from this review which can be subjected to more industry and multi-center research determination.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Breast cancer is the leading type of cancer both in diagnosis and mortality among women globally [1]. It is also estimated that 627,000 women died from the same in 2018 [2]. Early and accurate diagnosis is essential for its management as well as for the overall well-being of the woman.
Cytology or histology (biopsy) are the definitive diagnostic approach methods. However, imaging has provided a pathway in the diagnosis that reduces unnecessary and invasive cytology or biopsy, a strategy adopted by many guidelines. Breast ultrasound is one of the diagnostic imaging methods with lexicons like BI-RADS being key in differentiating benign from malignant lesions. This has been reported to have a global pooled sensitivity and specificity of 80.1 and 88.4% respectively [3].
Elastography is an addition to grey-scale ultrasonic examination that has gained substantial traction within the last decade. This bases its function on the fact that tissue elasticity can be a predictor of malignancy. Benign lesions are mainly expected to be more elastic while their malignant counterparts will most likely be stiffer. Strain elastography is currently more available than shear wave elastography. Further, strain ratio (SR) has been incorporated as a semiquantitative measure, thus a potential imaging biomarker. The number of articles that are being published annually on breast ultrasound elastography indicate that it is an evolving field. A meta-analytic study of SR carried out in 2012 showed a wide range of cut-off value from 0.5 to 4.5 [4]. However, that review included only nine studies among the ones which were available by then. The World Federation for Ultrasound in Medicine and Biology (WFUMB) published guidelines in 2015 for breast elastography [5, 6]. These guidelines acknowledge the marked variance in SR cut-off values used in differentiating benign from malignant lesions. At the same time, through scanning of literature there are more research papers that have been published since then. For SR to be fully established as a potential imaging biomarker for differentiating between benign and malignant breast lesions a more optimal cut-off value needs to be deduced. It is for that reason that in this review, we intended to determine the most current status in resolving the cut-off value.
The objective of this review was to include more recent evidence and seek to determine the SR cut off value for differentiating between benign and malignant breast lesions.
Methods
This study’s protocol was not registered or shared with any organization other than the University of Pisa’s Department of Translational Research. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Diagnostic Test Accuracy (PRISMA-DTA) Statement guidelines of 2018 [7] in carrying out the study and disseminating our findings.
Search strategy
We did comprehensive search of MEDLINE and Web of Science electronic databases with additional searches via Google Scholar and handsearching mainly through references of articles that were retrieved. The period of search was set from January 2000 to May 2020. Studies of interest were the those that had a patient population of breast mass and/or breast cancer with an index test of ultrasound with elastography and strain ratio calculated. Our search was done using combination of headings and terms that included at least two key words. The key words were “breast mass”, “elastography”, “strain ratio”, “breast ultrasound”, “histology”, “biopsy”, “cytology”, “breast cancer” and “cut off value”. The term “strain ratio” had to appear in each combination set.
Study selection
Three researchers (TMM, AA and GM) reviewed the retrieved articles and reached consensus on eligible study criteria. The inclusion criteria for the studies were as follows: (a) Breast ultrasound strain elastography with SR calculation performed. (b) Setting where ultrasound elastography examination was done before a reference standard diagnostic test and treatment. (c) Acceptable standard reference test like biopsy or cytology results and/or relevant follow up results of BI-RADS III lesions. (d) Adequate data presented in a format that could lead to creating a diagnostic study 2 × 2 table. (e) Articles in English language. (f) Reporting threshold of 24 “yes” out of 30 responses to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) 2015 criteria [8].
Data extraction and quality assessment
A data extraction plan was executed in which a total of 31 variables were identified within the realms of participants (patients with breast mass or masses), index test (SR), reference standard (pathological diagnosis) and target condition (malignancy). In addition, general variables of the studies, that is author, year of publication, journal and country were also included.
Quality assessment of the studies was performed using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [9]. This entailed assessment of risk of bias as well as the applicability. The observations of the assessment were presented both in tabular and graphic formats.
For each eligible study, a 2 × 2 table depicting true positives, false positives, false negatives and true negatives was constructed. The principal diagnostic accuracy measures were sensitivity and specificity per lesion.
Data synthesis and statistical analyses
Primary characteristics of the studies were entered as variables using MS Excel® worksheets.
Descriptive statistics from the diagnostic accuracy 2 × 2 tables of each individual study were computed using MedCalc® [10] and Meta-Disc [11] statistical software calculators. The measures were sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR).
Forest plots of sensitivity and specificity were deduced using Stata® statistical software [12]. A summary receiver operating characteristic (SROC) curve plot for sensitivity against 1-specificty (false positive rate) was also done using the Stata® software.
Heterogeneity and threshold effect were assessed in several stages as per standard recommendations [13] . First, visual examinations of the coupled forest plots of sensitivity and specificity as well as the SROC plot were done. Secondly, a Spearman’s correlation was calculated for sensitivity vs false positive rate. Lastly, the Cochran’s Q and Higgin’s (I2) statistics (though not much weight was given to them) were deduced during the derivation of the forest plots.
Qualitative (narrative) synthesis was done following failure to resolve heterogeneities of most of the studies through meta-regression and subgroup analysis of the studies. The subgroup variables were according to participants characteristics (age, sex and global region), index test (various strain ratio measurement methods) and reference standard approach methods.
Publication bias was interrogated using Funnel plot and Egger’s regression test of DORs against their standards errors (se) with the help of Stata® software.
Meta-analysis was carried out on a single subgroup that showed favourable threshold effects during the sub-group analysis process using Stata® software. A hierarchical summary receiver operating characteristic (HSROC) curve was constructed with the summary points displayed. Youden index was calculated and optimal cut-off strain ratio value for this subgroup derived.
Results
Study selection
A flow chart representing the study selection process is as shown in Fig. 1. A total of 424 articles, 412 from electronic database and 12 additional searches were retrieved. Duplicated articles were 31 and following title and abstract screening, 321 were found not to be related to breast strain elastography. Therefore, 72 full articles that met eligibility criteria were accessed. These had also scored at least 24 out of 30 points in the STARD checklist. Seven of the studies did not have enough data to provide a 2 × 2 table for diagnostic accuracy. Hence, 65 studies were included in the narrative synthesis and subgroup analysis. Meta-analysis was only feasible in one of the subgroups consisting seven studies as the rest had significant between study heterogeneities and unfavourable threshold effects that could not be resolved.
Study characteristics
An overview of the study characteristics is as shown in Table 1. The total number of lesions included in this systematic review was 11,759 but the total number of patients could not be computed as two authors had only provided the number of lesions in their studies. Malignant and benign lesions were 4897 (41.64%) and 6862 (58.46%) respectively. The combined age range documented was 12 to 93 years with a mean of 56.44 years. In terms of sex, 38 (58.46%) studies included only female patients, three (4.62%) had a mixed male and female population while 24 (36.92%) were not clear on this variable.
Study distribution among continents was as follows: Asia 40 (61.54%), Europe 17 (26.15%), Africa five (7.69%), S. America two (3.08%) and N. America one (1.54%). Out of the 65 studies, their design was prospective in 51 (78.46%), retrospective 13 (20%) and one (1.54%) unclear. Regarding the year of publication, the earliest was 2007 and the latest 2020. Out of the 65 studies, 44 (67.69%) were published after 2015.
The setting in all studies was uniform in that outpatient imaging before pathologic diagnosis or treatment was the recruitment point for the participants. There were 56 (86.15%) studies that recruited only solid masses while three (4.62%) had mixed cystic and solid masses. The rest, six (9.23%) did not state the mass consistency.
Assessment of SR was carried out in comparison with other modalities in 62 (95.38%) of the studies. These were combined B-mode ultrasound (BUS) plus elastography score (ES) in 34 (52.31%), BUS eight (12.31%), ES eight (12.31%), shear wave elastography (SWE) seven (10.77%), automated strain ratio (ASR) two (3.08%), mammography one (1.54%) and magnetic resonance imaging (MRI) one (1.54%). The remaining four studies reported only results of strain elastography.
Table 1 further summarizes several variables that were derived to depict individual study characteristics. The variables included were study author identifier, continent, study design, sample size (number of lesions), setting, index test (SR measurement method), reference standard (pathological diagnosis) approach and target condition definition. A full reference to the 31 variables derived during this review is also found as an MS Excel® sheet A within the additional files.
During the reading of the eligible articles, we discovered that some had reported more than one SR measurement method for their studies. For example, an article could be having different arms that assessed the diagnostic accuracy of SR through different reference tissue points like fat-to-lesion ratio (FLR), glandular-to-lesion ratio (GLR) or even a combination of both. Thus, two or more studies would be documented from such articles, if a clear 2 × 2 table was deduced for each arm. Examples to demonstrate this include Chee 2019, Graziano 2017, Jung Park 2016, Park 2020, Yoon 2017, Zhang 2020 and Zhou 2014. These are denoted in various figures and tables with a numerical value after the author’s name.
Still, some studies did not specify the specific reference points for their measurements. FLR alone was the most used method by 38 studies (58.46%) while GLR alone was applied by five (7.69%) and a combination of both recorded in 14 (21.54%) studies. The remaining eight studies (12.31%) did not report their reference point within the scanned breast. Only four (6.15%) studies reported the lesion depth measurements. Further, 47 (72.31%) studies performed a single point SR measurement, while 10 (15.38%) did multiple points with a mean value calculated. The rest of the studies did not specify on the number of points that were used during the SR measurement.
The machine models that were used in the studies were by the following manufacturers: Hitachi 23 (35.38%), Toshiba 11 (16.92%), Philips seven (10.77%), GE Healthcare six (9.23%), Siemens four (6.15%), Samsung six (9.23%), Mindray three (4.62%) and Esaote MyLab two (3.08%). Three studies did not report the machine model that they used. There were 50 studies that reported their imaging frequency used and in the higher value, the range was between 6.5 to 15 MHz.
Experience of operators was reported in 35 (53.85%) of the studies and it ranged from 0 to 20 years.
For the size of lesions, 21 (32.31%) studies had their ranges and mean. In this subgroup, the range was from 0.1 to 13.0 cm in their longest diameter with cumulative mean diameter 1.74 cm.
Blinding to the index test while reading the reference standard was clearly stated in 59 (90.77%) studies while one reported non-blinding out of its retrospective design. The other five (7.69%) were unclear on the same.
Performance of two or more interobserver variability assessment was reported in 15 (23.08%) of the studies. Agreement was quantified and qualified in eight of the studies spanning from fair, moderate, good and excellent. The other seven just reported non-quantified agreement between the observers.
The reference standard was purely based on pathological diagnosis in majority of cases and a few incorporated follow up of indeterminate (BI-RADS III) lesions. Further, the approach to pathological diagnosis involved core biopsy, cytology and surgical (excisional biopsy or cure-intent lumpectomy) specimens. Most of the studies, 39 (60%) had a mixed approach to the reference standard. Purely core biopsy and surgical excisions were applied in 20 (30.77%) and two (3.08%) of studies, respectively. In four studies (6.15%) the reference standard approach was not stated.
All the studies had clear definition of the target condition, easily dichotomized from the pathological diagnosis. On one hand malignant lesion that would require definite aggressive treatment or intervention is defined. In the same vein, a benign lesion that would be left alone or treated based on patient symptomatology was also defined.
Timing between the index test and reference standard within six-month period was clearly reported in 26 (40%) studies while it was unclear in the rest of studies.
Risk of bias and applicability
Results of the QUADAS 2 tool assessment are presented in Fig. 2. A representative table A for the individual study derivatives for the same is attached within the additional files. The papers accessed for full article reading were of moderate to high quality. Within the risk of bias 94.25, 71.43, 90.00 and 41.43% of the studies had low-risk score for patient selection, index test, standard reference and flow and timing, respectively. The main observation within the flow and timing realm, was that 58.57% of the studies had a good flow but did not succinctly indicate the time between the index test and the reference test. We interpreted this as unclear disease progression bias risk, at least theoretically.
Graphic presentation of the QUADAS-2 tool assessment results. Most of the papers were of moderate to high quality. The main observation is that 41 studies (58.58%) had unclear information on the time between the index test and reference standard but the flow was acceptable in that imaging was done before pathological diagnosis
For the applicability, in other words the generalizability of the study results, over 90% of the studies returned low-risk assessment results within the realms of the patient selection, index test and reference test. The rest were of unclear bias for example some studies subjected a specific subset of solid breast lesions to strain elastography as a problem-solving tool. Other bias points where when the point of reference in strain ratio measurement was not well stated or multiple reference standards were mentioned. As such the risks of spectrum effect, analysis bias and differential verification bias respectively were queried in this minority of studies.
Results of individual studies
The detailed report of each study’s diagnostic performance in terms of 2 × 2 table derivatives such as sensitivity, specificity and odds ratios are presented in sheet A within additional files. The sensitivity values ranged from 26.47 to 96.67% and for specificity from 37.5 to 99.96%. This information is summarily presented in coupled forest plots as shown in Fig. 3. For better visualization with ability to magnify the same images are within additional files as an MS Excel® sheet B.
Index test positivity was determined using a cut-off value of the SR. In 62 (95.38%) of the studies this was derived from a ROC curve with a Youden index measure. Two (3.08%) studies were not clear on how they derived their cut-off values, while one (1.54%) study reported a predetermined vendor cut-off value.
Heterogeneity and threshold effect
Visual inspection of the coupled forest plots of sensitivity and specificity does not reveal a smooth funnel shaped arrangement expected in good degree of homogeneity [16, 17]. This is as shown in Fig. 3 while individual forest plots for sensitivity and specificity can be accessed as figures A and B within additional files. The overall threshold effect as calculated using the Spearman correlation coefficient of Logit (TPR) vs Logit (FPR) was − 0.301 p-value = 0.015. A SROC plane that can be accessed within additional files as figure C, also visually demonstrates the heterogeneity. Cochran’s Q statistic values for sensitivity and specificity were 492.96 (p = 0.000) and 647.10 (p = 0.000) respectively and Higgin’s (I2) were 87.0 and 90.1% respectively.
Synthesis of results and sub-group meta-analysis
Having established significant heterogeneity between the 65 studies, it was not possible to proceed to a pooled establishment of sensitivity and specificity. We delved into assessing the heterogeneities further by performing subgroup analyses. We took 13 key covariates related to participants, index test, reference standard and target condition. These were derived from the characteristics of studies worksheet. The same can be accessed within additional files as table B.
From our findings of the subgroup analyses, all covariates had heterogeneities that could not be resolved except for two that had favourable threshold effects. These were within the machine model and combination of SR with other imaging mode subgroups. The first subgroup had seven studies and the latter four studies. The four studies reported results of SR without BUS, ES or any other imaging modality. These had a borderline Spearman’s correlation coefficient of 0.600 for sensitivity vs specificity-1. A positive Spearman’s correlation coefficient value of 0.6 and above is recommended for consideration in including diagnostic accuracy studies for meta-analysis [71]. Besides, we did not consider this subgroup as a candidate for meta-analysis since the studies were too few. Again, it cannot produce a scientific basis that measurement of SR without combining with other methods would lead to a more accurate result.
Under machine manufacturer models covariate, we discovered one manufacturer model that produced a favourable threshold effect. This subgroup had seven studies whose sensitivity vs specificity-1 Spearman’s correlation coefficient was 0.786 (p = 0.036). For exploratory purposes, we carried a meta-analysis on this sub-group which consisted of 783 patients and 844 lesions. The results are presented as a hierarchical summary receiver operating characteristic curve (HSROC) in Fig. 4. Sensitivity and specificity were 0.86 and 0.74 respectively at the summary point. Using the calculated Youden index value of 0.57 the optimal cut-off value was 2.81.
Publication bias
No significant publication bias was demonstrated for the 65 studies as well as for the seven that went to the sub-group meta-analysis. The funnel plots of the DORs are as shown in Fig. 5. Egger’s test was at p-value of 1.00 for the entire group and 0.44 for the meta-analytic subgroup.
Additional analysis
The results of all covariate subgroup analysis demonstrating the heterogeneity factors are presented in extensive table B within additional files for reference of interest.
Discussion
Summary of evidence
Measurable values, either quantitative or semiquantitative in diagnostic radiology are potential imaging biomarkers that can be defined by cut-off values [72]. Breast elastography SR measurement is a semiquantitative method that we sought to establish its cut-off value for benign and malignant lesion diagnosis. From our findings during this review, breast SR measurement is still laden with multiple heterogeneities. These lie within the whole range of assessment involving the patient selection, index test, reference standard and flow and timing. The WFUMB guidelines of 2015 for breast elastography acknowledged widely varied SR cut-off values [6]. From our systematic review, nothing has changed since then.
From our results, the points of reference when carrying out the SR measurement are quite varied. This is despite the WFUMB guidelines advocating for FLR. Some researchers have been non-discriminatory on either fibro-glandular tissue or subcutaneous fat. Still, others do the ratios referencing on GLR purely. Some studies have been carried out to determine the difference of diagnostic performance dependent on FLR or GLR. We came across three studies that compared the diagnostic performance between the two points of reference by Chee et al. [19], Graziano et al. [27] and Zhou et al. [70]. They all concluded that FLR had better diagnostic performance than GLR. For general clinical application, the point of reference may have its challenges, since breast tissue composition is varied. Some breasts will be mainly fibro-glandular (dense breasts) and others almost purely fatty [73, 74]. Sorting out this point of reference heterogeneity for clinical application due to breast tissue composition requires more investigation.
Purely simple cysts are well diagnosed on B-mode ultrasound with clear designation of BI-RADS II category. Qualitative elastography also gives two characteristic appearances, the bullseye artefact or the blue-green-red (BGR) sign to cysts [75, 76]. The diagnostic value of performing SR measurements on a purely simple cyst lesion may be unnecessary when looking for an imaging biomarker that differentiates benign from malignant lesions. Benchmarking with the WFUMB guidelines, there is no clarity on the issue of SR measurement on simple cysts.
Based on its imaging functionality, SR cannot be purely used on its own without looking at the BUS and colour coded elastograms that give the elastographic score (ES). For that reason, it would be clinically meaningless to report any favourable threshold effects generated from studies that only report SR diagnostic performance.
The small subgroup of seven studies in our review that went to meta-analysis was under a particular manufacturer model. This raises a possibility that manufacturer model may influence SR values. We strongly recommend that this needs to be taken with a lot of caution as the rest of the seven machine model subgroups did not reproduce the same favourable threshold effects. Having a machine predetermined cut-off value at this stage must depend on more robust evidence. In fact, the only study in this review that reported using a vendor machine predetermined cut-off value fell in a subgroup that did not produce favourable threshold effects. Furthermore, there are pieces of literature that indicate quantified variance for in vitro and clinical applications of SR measurement [77, 78]. We still propose more research on the manufacturer model calibration aspects.
Operator dependency in ultrasound is a fact that may affect semiquantitative procedures like SR [78]. It is not an easily quantifiable entity even though we explored through some surrogates like experience of operators as well as studies that conducted interobserver variability assessment. None of those on their own could produce a favourable threshold effect.
Our review had two limitations. First, we could not have access to Embase, one of the most renown electronic databases due to lack of institutional accessibility at the time of conducting our search. Despite this we did the best in the search as per published evidence as we accessed three of four major databases good enough for a systematic review [79]. On this front, we remain open to more forthcoming evidence concerning our topic in the future. Another limitation is that efforts to get more information from some authors whom we contacted for certain clarifications were not replied to.
Conclusions
From our review, currently the optimal breast SR cut-off point or value remains unresolved despite the WFUMB guidelines of 2015. Machine model possibility as a contributor to cut-off value determination was suggested from this review which can be subjected to more industry and multi-center research determination.
Availability of data and materials
Raw data of variables obtained from individual studies is found within the additional (supplementary) files herein attached. Any further information on datasets can be provided by the corresponding author on considerable request.
Abbreviations
- ASR:
-
Automated strain ratio
- BI-RADS:
-
Breast Imaging-Reporting and Data System
- BUS:
-
B-mode ultrasound
- DOR:
-
Diagnostic odds ratio
- ES:
-
Elastography score
- FLR:
-
Fat-to-lesion ratio
- FPR:
-
False positive rate
- GLR:
-
Glandular-to-lesion ratio
- HSROC:
-
Hierarchical summary receiver operating characteristic
- MRI:
-
Magnetic resonance imaging
- NLR:
-
Negative likelihood ratio
- PLR:
-
Positive likelihood ratio
- PRISMA-DTA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Diagnostic Test Accuracy
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
- STARD:
-
Standards for Reporting of Diagnostic Accuracy Studies
- SR:
-
Strain ratio
- SROC:
-
Summary receiver operating characteristic
- TPR:
-
True positive rate
- WFUMB:
-
World Federation for Ultrasound in Medicine and Biology
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin. 2020 Jul;70(4):313]. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
World Health Organization (WHO). Breast cancer. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/. Accessed on 27 Aug 2020.
Sood R, Rositch AF, Shakoor D, et al. Ultrasound for breast cancer detection globally: a systematic review and meta-analysis. J Glob Oncol. 2019;5:1–17. https://doi.org/10.1200/JGO.19.00127.
Sadigh G, Carlos RC, Neal CH, Dwamena BA. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat. 2012;134(3):923–31. https://doi.org/10.1007/s10549-012-2020-x.
Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126–47. https://doi.org/10.1016/j.ultrasmedbio.2015.03.009.
Barr RG, Nakashima K, Amy D, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: breast. Ultrasound Med Biol. 2015;41(5):1148–60. https://doi.org/10.1016/j.ultrasmedbio.2015.03.008.
McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96. https://doi.org/10.1001/jama.2017.19163 PMID: 29362800.
Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. https://doi.org/10.1136/bmj.h5527 Published 2015 Oct 28.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
MedCalc Statistical Software version 19.4.1. Ostend: MedCalc Software Ltd; 2020. https://www.medcalc.org.
Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp LLC; 2019.
Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-Part I. General guidance and tips. Korean J Radiol. 2015;16(6):1175–87. https://doi.org/10.3348/kjr.2015.16.6.1175.
Ahmed AT. Diagnostic utility of strain and shear wave ultrasound elastography in differentiation of benign and malignant solid breast lesions. Egypt J Radiol Nucl Med. 2020;51:70. https://doi.org/10.1186/s43055-020-00181-7.
Alhabshi SM, Rahmat K, Abdul Halim N, Aziz S, Radhika S, Gan GC, et al. Semi-quantitative and qualitative assessment of breast ultrasound elastography in differentiating between malignant and benign lesions. Ultrasound Med Biol. 2013;39(4):568–78. https://doi.org/10.1016/j.ultrasmedbio.2012.10.016 Epub 2013 Feb 4. PMID: 23384468.
Arslan S, Öncü F, Eryılmaz MA, Durmaz MS, Altunkeser A, Ünlü Y. Advantages of b-mode ultrasound combined with strain elastography in differentiation of idiopathic granulomatous mastitis from malignant breast lesions. Turk J Med Sci. 2018;48(1):16–23. https://doi.org/10.3906/sag-1708-34 PMID: 29479939.
Balçık A, Polat AV, Bayrak İK, Polat AK. Efficacy of sonoelastography in distinguishing benign from malignant breast masses. J Breast Health. 2016;12(1):37–43. https://doi.org/10.5152/tjbh.2015.2843 Published 2016 Jan 1.
Bojanic K, Katavic N, Smolic M, et al. Implementation of elastography score and strain ratio in combination with B-mode ultrasound avoids unnecessary biopsies of breast lesions. Ultrasound Med Biol. 2017;43(4):804–16. https://doi.org/10.1016/j.ultrasmedbio.2016.11.019.
Chee C, Lombardo P, Schneider M, Danovani R. Comparison of the fat-to-lesion strain ratio and the gland-to-lesion strain ratio with controlled precompression in characterizing indeterminate and suspicious breast lesions on ultrasound imaging. J Ultrasound Med. 2019;38(12):3257–66. https://doi.org/10.1002/jum.15037.
Cheng R, Li J, Ji L, Liu H, Zhu L. Comparison of the diagnostic efficacy between ultrasound elastography and magnetic resonance imaging for breast masses. Exp Ther Med. 2018;15(3):2519–24. https://doi.org/10.3892/etm.2017.5674.
Cho N, Moon WK, Kim HY, Chang JM, Park SH, Lyou CY. Sonoelastographic strain index for differentiation of benign and malignant nonpalpable breast masses. J Ultrasound Med. 2010;29(1):1–7. https://doi.org/10.7863/jum.2010.29.1.1.
Dawood MA, Ibrahim NMA, Elsaeed HH, Hegazy NG. Diagnostic performance of sonoelastographic Tsukuba score and strain ratio in evaluation of breast masses. Egypt J Radiol Nucl Med. 2018;49(1):265–71. https://doi.org/10.1016/j.ejrnm.2017.10.00.
Fausto A, Rubello D, Carboni A, Mastellari P, Chondrogiannis S, Volterrani L. Clinical value of relative quantification ultrasound elastography in characterizing breast tumors. Biomed Pharmacother. 2015;75:88–92. https://doi.org/10.1016/j.biopha.2015.08.029.
Fujioka T, Mori M, Kubota K, et al. Simultaneous comparison between strain and shear wave elastography of breast masses for the differentiation of benign and malignant lesions by qualitative and quantitative assessments. Breast Cancer. 2019;26(6):792–8. https://doi.org/10.1007/s12282-019-00985-0.
Gheonea IA, Stoica Z, Bondari S. Differential diagnosis of breast lesions using ultrasound elastography. Indian J Radiol Imaging. 2011;21(4):301–5. https://doi.org/10.4103/0971-3026.90697.
Gong X, Wang Y, Xu P. Application of real-time ultrasound elastography for differential diagnosis of breast tumors. J Ultrasound Med. 2013;32(12):2171–6. https://doi.org/10.7863/ultra.32.12.2171.
Graziano L, Bitencourt AG, Cohen MP, et al. Elastographic evaluation of indeterminate breast masses on ultrasound. Avaliação pela elastografia dos nódulos mamários indeterminados na ultrassonografia. Rev Bras Ginecol Obstet. 2017;39(2):72–9. https://doi.org/10.1055/s-0036-1597753.
Gürüf A, Öztürk M, Bayrak İK, Polat AV. Shear wave versus strain elastography in the differentiation of benign and malignant breast lesions. Turk J Med Sci. 2019;49(5):1509–17. https://doi.org/10.3906/sag-1905-15 Published 2019 Oct 24.
Hao Y, Ren G, Yang W, et al. Combination diagnosis with elastography strain ratio and molecular markers effectively improves the diagnosis rate of small breast cancer and lymph node metastasis. Quant Imaging Med Surg. 2020;10(3):678–91. https://doi.org/10.21037/qims.2020.02.14.
Jung HJ, Hahn SY, Choi HY, Park SH, Park HK. Breast sonographic elastography using an advanced breast tissue-specific imaging preset: initial clinical results. J Ultrasound Med. 2012;31(2):273–80. https://doi.org/10.7863/jum.2012.31.2.273.
Jung NY, Park CS, Kim SH, et al. Sonoelastographic strain ratio: how does the position of reference fat influence it? Jpn J Radiol. 2016;34(6):440–7. https://doi.org/10.1007/s11604-016-0543-5.
Khamis MEM, El-deen AMA, Ismail AAA. The diagnostic value of sonoelastographic strain ratio in discriminating malignant from benign solid breast masses. Egypt J Radiol Nucl Med. 2017;48:1149–57. https://doi.org/10.1016/j.ejrnm.2017.05.005.
Kim HJ, Kim SM, Kim B, et al. Comparison of strain and shear wave elastography for qualitative and quantitative assessment of breast masses in the same population. Sci Rep. 2018;8(1):6197. https://doi.org/10.1038/s41598-018-24377-0 Published 2018 Apr 18.
Kumm TR, Szabunio MM. Elastography for the characterization of breast lesions: initial clinical experience. Cancer Control. 2010;17(3):156–61. https://doi.org/10.1177/107327481001700303.
Lee JH, Kim SH, Kang BJ, et al. Role and clinical usefulness of elastography in small breast masses. Acad Radiol. 2011;18(1):74–80. https://doi.org/10.1016/j.acra.2010.07.014.
Li Q, Wang L, Wu H, et al. Controlled study of traditional ultrasound and ultrasound elastography on the diagnosis of breast masses. Ultrasound Q. 2015;31(4):250–4. https://doi.org/10.1097/RUQ.0000000000000195.
Liu XJ, Zhu Y, Liu PF, Xu YL. Elastography for breast cancer diagnosis: a useful tool for small and BI-RADS 4 lesions. Asian Pac J Cancer Prev. 2014;15(24):10739–43. https://doi.org/10.7314/apjcp.2014.15.24.10739.
Mansour SM, Omar OS. Elastography ultrasound and questionable breast lesions: does it count? Eur J Radiol. 2012;81(11):3234–44. https://doi.org/10.1016/j.ejrad.2012.04.021.
Menezes R, Sardessai S, Furtado R, Sardessai M. Correlation of strain elastography with conventional sonography and FNAC/Biopsy. J Clin Diagn Res. 2016;10(7):TC05–10. https://doi.org/10.7860/JCDR/2016/20239.8177.
Mu WJ, Zhong WJ, Yao JY, et al. Ultrasonic elastography research based on a multicenter study: adding strain ratio after 5-point scoring evaluation or not. PLoS One. 2016;11(2):e0148330. https://doi.org/10.1371/journal.pone.0148330 Published 2016 Feb 10.
Mutala TM, Ndaiga P, Aywak A. Comparison of qualitative and semiquantitative strain elastography in breast lesions for diagnostic accuracy. Cancer Imaging. 2016;16(1):12. https://doi.org/10.1186/s40644-016-0070-8 Published 2016 May 26.
Nakashima K, Mizutou A, Sakurai S. Auto strain ratio system for the quality control of breast strain elastography. J Med Ultrason (2001). 2018;45(2):261–8. https://doi.org/10.1007/s10396-017-0830-5.
Özel D, Özel BD. Evaluating the role of strain ratio elastography in determining malignancy potential and calculating objective BIRADS US scores using ultrasonography and elastography features. Pol J Radiol. 2018;83:e268–74. https://doi.org/10.5114/pjr.2018.76790 Published 2018 Jun 8.
Ozsoy A, Acar D, Barca AN, et al. Diagnostic performance of real-time strain sonoelastography in BI-RADS 4 and 5 breast masses. Diagn Interv Imaging. 2016;97(9):883–9. https://doi.org/10.1016/j.diii.2016.04.004.
Parajuly SS, Lan PY, Yun MB, Gang YZ, Hua Z. Diagnostic potential of strain ratio measurement and a 5 point scoring method for detection of breast cancer: Chinese experience. Asian Pac J Cancer Prev. 2012;13(4):1447–52. https://doi.org/10.7314/apjcp.2012.13.4.1447.
Park HJ, Kim SM, Yun B, et al. Comparison of one- and two-region of interest strain elastography measurements in the differential diagnosis of breast masses. Korean J Radiol. 2020;21(4):431–41. https://doi.org/10.3348/kjr.2019.0479.
Rehman H, Raza S, Aziz S, Ahmad AM, Tahir S. Diagnostic accuracy of sonoelastography in the non-invasive diagnosis of malignant breast cancer compared to histopathology as a gold standard. J Coll Physicians Surg Pak. 2017;27(5):267–70.
Ricci P, Maggini E, Mancuso E, et al. Elastography in the diagnosis of breast lesions: comparison of different elastographic features. Acta Radiol. 2017;58(10):1189–97. https://doi.org/10.1177/0284185116687169.
Seo M, Ahn HS, Park SH, et al. Comparison and combination of strain and shear wave elastography of breast masses for differentiation of benign and malignant lesions by quantitative assessment: preliminary study. J Ultrasound Med. 2018;37(1):99–109. https://doi.org/10.1002/jum.14309.
Singla V, Prakash A, Prabhakar N, et al. Does shear wave elastography score over strain elastography in breast masses or vice versa? Curr Probl Diagn Radiol. 2020;49(2):96–101. https://doi.org/10.1067/j.cpradiol.2019.02.013.
Stachs A, Hartmann S, Stubert J, et al. Differentiating between malignant and benign breast masses: factors limiting sonoelastographic strain ratio. Ultraschall Med. 2013;34(2):131–6. https://doi.org/10.1055/s-0032-1313168.
Stoian D, Timar B, Craina M, Bernad E, Petre I, Craciunescu M. Qualitative strain elastography - strain ratio evaluation - an important tool in breast cancer diagnostic. Med Ultrason. 2016;18(2):195–200. https://doi.org/10.11152/mu.2013.2066.182.bcd.
Thomas A, Degenhardt F, Farrokh A, Wojcinski S, Slowinski T, Fischer T. Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol. 2010;17(5):558–63. https://doi.org/10.1016/j.acra.2009.12.006.
Türker MF, Tok Umay S, Akça T, Karabacak T, Esen K, Balcı Y, et al. Diagnostic value of ultrasound elastography characterization of solid breast lesions. JAREM. 2017;7:74–81. https://doi.org/10.5152/jarem.2017.124.
Ueno E, Tohno E, Morishima I, Umemoto T, Waki K. A preliminary prospective study to compare the diagnostic performance of assist strain ratio versus manual strain ratio. J Med Ultrason (2001). 2015;42(4):521–31. https://doi.org/10.1007/s10396-015-0633-5.
Ueno, E, Umemoto, T, Bando, H, Tohno, E, Waki, K, Matsumura, T. New quantitative method in breast elastography: Fat Lesion Ratio (FLR). Radiological Society of North America 2007 Scientific Assembly and Annual Meeting, November 25 - November 30, 2007, Chicago IL. http://archive.rsna.org/2007/5015476.html. Accessed 3 June 2020.
Wang Z, He Y, Liang X, Tang S, Guo H, Xiao Y, et al. Differential diagnostic value of ultrasound elasticity score and strain ratio in breast cancer. Int J Clin Exp Med. 2018;11(7):7018–28.
Yağcı B, Erdem Toslak I, Çekiç B, et al. Differentiation between idiopathic granulomatous mastitis and malignant breast lesions using strain ratio on ultrasonic elastography. Diagn Interv Imaging. 2017;98(10):685–91. https://doi.org/10.1016/j.diii.2017.06.009.
Yerli H, Yilmaz T, Kaskati T, Gulay H. Qualitative and semiquantitative evaluations of solid breast lesions by sonoelastography. J Ultrasound Med. 2011;30(2):179–86. https://doi.org/10.7863/jum.2011.30.2.179.
Yıldız MS, Goya C, Adin ME. Contribution of sonoelastography to diagnosis in distinguishing benign and malignant breast masses. J Ultrasound Med. 2020;39(7):1395–403. https://doi.org/10.1002/jum.15236.
Yılmaz E, Yılmaz A, Aslan A, Inan I, Evren MC, Tekesin K. Real-time elastography for differentiation of breast lesions. Pol J Radiol. 2017;82:664–9. https://doi.org/10.12659/PJR.902596 Published 2017 Nov 17.
Yoon JH, Song MK, Kim EK. Semi-quantitative strain ratio in the differential diagnosis of breast masses: measurements using one region-of-interest. Ultrasound Med Biol. 2016;42(8):1800–6. https://doi.org/10.1016/j.ultrasmedbio.2016.03.030.
Yoon JH, Song MK, Kim EK. Semi-quantitative strain ratio determined using different measurement methods: comparison of strain ratio values and diagnostic performance using one- versus two-region-of-interest measurement. Ultrasound Med Biol. 2017;43(5):911–7. https://doi.org/10.1016/j.ultrasmedbio.2017.01.005.
You Y, Song Y, Li S, Ma Z, Bo H. Quantitative and qualitative evaluation of breast cancer prognosis: a sonographic elastography study. Med Sci Monit. 2019;25:9272–9. https://doi.org/10.12659/MSM.918806 Published 2019 Dec 5.
Youk JH, Son EJ, Gweon HM, Kim H, Park YJ, Kim JA. Comparison of strain and shear wave elastography for the differentiation of benign from malignant breast lesions, combined with B-mode ultrasonography: qualitative and quantitative assessments. Ultrasound Med Biol. 2014;40(10):2336–44. https://doi.org/10.1016/j.ultrasmedbio.2014.05.020.
Zhang L, Dong YJ, Zhou JQ, Jia XH, Li S, Zhan WW. Similar reproducibility for strain and shear wave elastography in breast mass evaluation: a prospective study using the same ultrasound system. Ultrasound Med Biol. 2020;46(4):981–91. https://doi.org/10.1016/j.ultrasmedbio.2019.12.017.
Zhao XB, Yao JY, Zhou XC, et al. Strain elastography: a valuable additional method to BI-RADS?. Strain Elastografie: Eine wertvolle Zusatzmethode für BI-RADS? Ultraschall Med. 2018;39(5):526–34. https://doi.org/10.1055/s-0043-115108.
Zhi H, Xiao XY, Yang HY, Ou B, Wen YL, Luo BM. Ultrasonic elastography in breast cancer diagnosis: strain ratio vs 5-point scale. Acad Radiol. 2010;17(10):1227–33. https://doi.org/10.1016/j.acra.2010.05.004.
Zhou J, Zhou C, Zhan W, Jia X, Dong Y, Yang Z. Elastography ultrasound for breast lesions: fat-to-lesion strain ratio vs gland-to-lesion strain ratio. Eur Radiol. 2014;24(12):3171–7. https://doi.org/10.1007/s00330-014-3366-8.
Zhou J, Zhan W, Dong Y, Yang Z, Zhou C. Stiffness of the surrounding tissue of breast lesions evaluated by ultrasound elastography. Eur Radiol. 2014;24(7):1659–67. https://doi.org/10.1007/s00330-014-3152-7.
Steinhauser S, Schumacher M, Rücker G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med Res Methodol. 2016;16:97. https://doi.org/10.1186/s12874-016-0196-1.
deSouza NM, Achten E, Alberich-Bayarri A, et al. Validated imaging biomarkers as decision-making tools in clinical trials and routine practice: current status and recommendations from the EIBALL* subcommittee of the European Society of Radiology (ESR). Insights Imaging. 2019;10:87. https://doi.org/10.1186/s13244-019-0764-0.
Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS® ultrasound. In: ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston: American College of Radiology; 2013.
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston: American College of Radiology; 2013.
Dietrich CF, Barr RG, Farrokh A, et al. Strain Elastography - How To Do It? Ultrasound Int Open. 2017;3(4):E137–49. https://doi.org/10.1055/s-0043-119412.
Barr RG. Future of breast elastography. Ultrasonography. 2019;38(2):93–105. https://doi.org/10.14366/usg.18053.
Havre, R.F.; Waage, J.E.R.; Mulabecirovic, A.; Gilja, O.H.; Nesje, L.B. Strain ratio as a quantification tool in strain imaging. Preprints 2018:2018040199. https://doi.org/10.20944/preprints201804.0199.v1.
Wing-Han Yuen Q, Zheng YP, Huang YP, et al. In-vitro strain and modulus measurements in porcine cervical lymph nodes. Open Biomed Eng J. 2011;5:39–46. https://doi.org/10.2174/1874120701105010039.
Bhatia KS, Lee YY, Yuen EH, Ahuja AT. Ultrasound elastography in the head and neck. Part I. Basic principles and practical aspects. Cancer Imaging. 2013;13(2):253–9. https://doi.org/10.1102/1470-7330.2013.0026 Published 2013 Jul 22.
Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):245. https://doi.org/10.1186/s13643-017-0644-y Published 2017 Dec 6.
Acknowledgements
The authors would like to sincerely acknowledge the assistance of the combined faculty of University of Pisa, Department of Translational Research and European Society of Oncologic Imaging for providing guidance and necessary critique during the study period. In particular we are grateful to Prof Mayerhoefer and Dr Damiano Caruso for their intensive role in this. We are also grateful to the library management at the University of Pisa for providing us with the electronic databases that made this work possible. Finally, much appreciation goes to the fellow faculty members at the Department of Diagnostic Imaging and Radiation Medicine, University of Nairobi, for their moral support during the study period.
Funding
None.
Author information
Authors and Affiliations
Contributions
TMM and EN contributed in the protocol development. TMM, AA and GNM contributed in eligibility and quality assessment of study articles. All authors participated in the manuscript preparation. All authors consented to the publication of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None of the authors declare conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mutala, T.M., Mwango, G.N., Aywak, A. et al. Determining the elastography strain ratio cut off value for differentiating benign from malignant breast lesions: systematic review and meta-analysis. Cancer Imaging 22, 12 (2022). https://doi.org/10.1186/s40644-022-00447-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-022-00447-5