Abstract
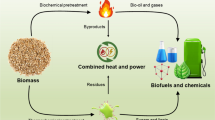
Biodiesel, one of the most important sources of renewable energy, is produced in large quantities around the world; however, its production generates different kinds of residues and by-products which raise economic and environmental concerns. This review presents a compilation of the data on current state of transformation of residues and by-products of biodiesel industry into products that are suitable for bio-refining. The review has analyzed glycerol, biodiesel washing wastewaters, and solid residues. The technologies were described and the most significant experimental results and variables were summarized to allow researchers an easy access to this information.
Similar content being viewed by others
Background
The extensive use of fossil fuels has generated environmental issues, such as global warming and atmospheric pollution (Siles et al. 2010). The replacement of fossil fuels for renewable biofuels is a necessity. The employment of biofuels from biological feedstocks is a significant option because they can be generated sustainably from sunlight, carbon dioxide, and water (Juang et al. 2011). Biodiesel is one of the most prominent biofuels in the world. Biodiesel is the name given to the fatty alkyl esters produced after transesterification of fatty acids using methanol or ethanol in the presence of a catalyst such as sodium hydroxide. To produce biodiesel, oils from different sources can be used. They are obtained from plants oil (palm, soy bean, sunflower, etc.) or animal fats (chicken, beef, and pork). Because biodiesel can be produced using different types of plant or animal oil, its production can be developed in most places around the world. The traditional technology is fully developed and technologically accessible. For example, in 2010, 19 billion L of biodiesel were produced worldwide (Global Renewable Fuels Alliance 2012) and its production has been increasing constantly over the last few years (Rossi et al. 2011).
The production of biodiesel in large quantities also results in the generation of abundant quantities of residues or by-products which cannot be utilized in biodiesel production process (Eliche-Quesada et al. 2012). Therefore, new applications to treat and use these compounds have become an important topic. In biodiesel, the most significant residues and by-products are glycerol, biodiesel washing wastewaters, methanol, and solid residues (Varanda et al. 2011). Glycerol is the by-product that generates the largest interest, because it can involve the largest revenue for the biodiesel industry. Glycerol is the main by-product from biodiesel transesterification (Nitayavardhana and Khanal 2011; Ethier et al. 2011); by 2016, the worldwide waste glycerol production is expected to reach 4 billion gallons (Yang et al. 2012). However, the glycerol produced from biodiesel has low quality and low price ($0.05 per pound) and its purification for further applications is economically unviable (Nitayavardhana and Khanal 2011). The largest residue produced by the biodiesel industry is the biodiesel washing wastewaters from the biodiesel washing process. The projected production of biodiesel for 2016 is 37 billion gallons, this amount of biodiesel will produce 43 billion gallons of wastewaters (Yang et al. 2012; Siles et al. 2011). This large amount of residues is related with disposal and environmental issues, because these wastewaters have a high organic load that does not allow its direct disposal into the sewage system (Rattanapan et al. 2011). Solid by-products are the residues that include pressed seed cakes, spent earth, and agricultural wastes. The biological solid residues are generally used in compost or for animal feeding; however, these compounds can increase the profitability of the biodiesel industry if they can be utilized as a substrate for the production of chemical compounds or energy.
The aim of this review was to summarize the current state of art in the transformation of the most significant biodiesel by-products and residues.
Biodiesel by-products and residues
Glycerol
1,2,3 propanetriol or commonly named as glycerol, glycerin, or glycerine is an organic compound with three carbons and three hydroxyl groups. Glycerol is mainly produced by saponification process and is widely utilized in pharmaceutical and food industry (Ethier et al. 2011). Besides the saponification process, glycerol is an abundant by-product of biodiesel production process. Crude glycerol is the name given to the glycerol produced during biodiesel production (Dobroth et al. 2011; Ethier et al. 2011) and is a by-product of transesterification reaction that takes place between the fatty acids with methanol or ethanol to produce methyl esters. Crude glycerol represents about 10 % of the product output in biodiesel production (Sousa et al. 2012); in fact, 1 kg of crude glycerol is produced per 12.6 L of biodiesel (Dobroth et al. 2011). Crude glycerol is considered as a residue because residual ethanol or methanol, fatty acid ethyl (or methyl) esters, and residual fatty acids are mixed with it (Dobroth et al. 2011). These impurities can be removed from crude glycerol to produce a raw material for pharmaceutical and food industries. The crude glycerol purification and refining is very expensive to be sustainable in the biodiesel industry (Ethier et al. 2011; Sabourin-Provost and Hallenbeck 2009). Therefore, the utilization of crude glycerol is important for the biodiesel industry future. The application of crude glycerol can be grouped into bioproduct's production (Rossi et al. 2012; André et al. 2010; Abad and Turon 2012), renewable energy production (Yoon et al. 2010; Ngo et al. 2011), and wastewater applications (Siles López et al. 2009; Bodík et al. 2009).
Bioproducts
The transformation of glycerol into bioproducts is performed by physicochemical or microbial processes. Physicochemical process transforms glycerol, or it utilizes glycerol as a participant in the production of other bioproducts. Hydrothermal electrolysis transforms glycerol into lactic acid using aqueous alkaline conditions at high temperatures and pressures (Yuksel et al. 2011). In this case, a designed autoclave and a flow type reactor were evaluated. The autoclave achieved a glycerol conversion of 83 %, whereas the flow type reactor accomplished a 75 % (Yuksel et al. 2011). Other waste glycerol uses are the production of castor oil glycerides and polyols. Glycerolysis of castor oil methyl esters and waste glycerol generated castor oil monoglycerides (50.4 %) and diglycerides (35 %) which can be employed in the plastic industries (Echeverri et al. 2013). On the other hand, polyols were produced from a sequential two-step liquefaction of lignocellulosic material using crude glycerol as liquefaction solvent. In this process, acid and base liquefactions promoted the esterification reaction between free fatty acids and glycerol and the condensation of polyols. These biopolyols had similar properties as those of the petroleum-based polyols (Hu and Li 2014).
Microbial transformation has been carried out for the production of oxalic and docosahexaenoic acid (Ethier et al. 2011), polymer-related molecules [polyhydroxybutyrate (PHB) and 1,3-propanediol] (Dobroth et al. 2011), surfactants (Sousa et al. 2012), and animal feed (Nitayavardhana and Khanal 2011). Table 1 shows the microbial transformation methods. To increase the concentration of bioproducts from crude glycerol, different strategies have been used. The most significant ones are genetic engineering, media and environmental condition's optimization. Genetic engineering has been employed to improve synthesis of different types of products. These improvements include genes insertion that allows consumption of crude glycerol and/or the production of different types of molecules. Escherichia coli was genetically modified by inserting the aldehyde reductase and aldehyde dehydrogenase genes, these genes improved the generation of poly 3-hydroxybutyrate (Shah et al. 2014). Corynebacterium glutamicum was genetically modified to increase the concentration of different types of amino acids (Meiswinkel et al. 2013). Likewise, free fatty acid concentration was improved by using an E. coli with a modified OPLlTE gen. This modification increased free fatty acids by 15.8 fold compared to the control (Lee et al. 2014). Recombinant engineering is an interesting option to improve the production of biobased products from waste glycerol. The cost of manipulating and maintaining these microorganisms needs to be considered. This technology improves the up-stream process; however, the principal cost in biotechnological processes is related with the recovery and purification costs. The developments in genetic engineering should be focused in improving the recovery and purification of these bioproducts. The most important modification to the culture media was nitrogen concentration. The change in nitrogen concentration increased the quantity of 1,3-propanediols and docosahexaenoic acid (Sabourin-Provost and Hallenbeck 2009; Chi et al. 2007). Bioproduct's microbial processes have been evaluated using different types of reactors configurations. Packed bed reactor was employed in the production of 1,3-propanediol (Casali et al. 2012), whereas stirred tank reactors have been operated for docosahexaenoic acid (Ethier et al. 2011) 1,3 propanediol (Rossi et al. 2012) and polyhydroxyalkanoates (PHA) (Dobroth et al. 2011). Fed-batch configuration produced dihydroxyacetone in quantities (2–5 fold) greater than batch culture (Liu et al. 2013b). Microfiltration was coupled to a batch reactor to improve the production of 1,3-propanodiol by C. butyricum. This modification achieved a larger yield and rate than the batch reactor without microfiltration (Szymanowska-Powałowska and Leja 2014).
In the last few years, the microbiological transformation of crude glycerol has become an interesting option to utilize crude glycerol and to increase the efficiency of biodiesel production process. Today, an industrial process using crude glycerol does not exist; however, several products have been generated from glycerol using biological transformations. As an example, an estimate of 19 tonnes per year of bioplastics (PHB) can be produced from a biodiesel plant of 38 million L per year (Dobroth et al. 2011). The potential for bioproduct from waste glycerol is great; however, the industrial recovery of these bioproducts is a major issue. Downstream processing is expensive, especially for products that are in low concentrations. In those cases, the bioproducts need to have a high value, therefore, the capital and production costs can be recuperated by the process. The future industrialization of bioproducts from glycerol is dependent of the economic aspects related with the advances in product recovery. Until these developments are not available, the industrial production of biobased product from waste glycerol is distant.
Renewable energy production
Renewable energy can be produced from crude glycerol using thermochemical or biological processes. The principal thermochemical processes include pyrolysis and gasification, whereas the most important biological processes are biological fuel cells, hydrogen generation, and anaerobic digestion (biomethane production).
Thermal conversion
Thermal conversions (gasification and pyrolysis) and biomass fuel cells are the two physicochemical processes used to generate energy from crude glycerol. Gasification and pyrolysis are performed at high temperatures (>300 °C) in an atmosphere which lacks oxygen or has a small quantity of it. These processes generate a gas phase (synthesis gas or Syngas), liquid phase (bio-oil or bio-liquor), and a solid phase (biochar). Each phase percentage is dependent of the reaction conditions. In these processes, the most important conditions are temperature, pressure, residence time, air to O2 ratio, and catalyst. Table 2 shows the thermal processes used in the transformation of crude glycerol into biofuels.
Pyrolysis studies have been performed using crude glycerol as an auxiliary compound to pyrolyze different types of feedstocks. The mixture of crude glycerol and swine manure improved the quality of bio-oil and its distillated fractions compared with the pyrolysis of swine manure alone (Cheng et al. 2014a). The addition of glycerol has been found as a factor to improve hydrogen (H2) concentration in the Syngas. The addition of 20 % of glycerol to lignite generated H2 sixfold higher than lignite pyrolysis (Manara and Zabaniotou 2013). Similar to lignite, olive kernel pyrolysis exhibited an increment of 11.6 % in the concentration of H2 when 25 % of the crude glycerol was added (Skoulou et al. 2012). Besides the increment in H2 concentration, glycerol also increased the concentration of light hydrocarbons in Syngas. A mixture 1:1 or 3:1 of crude glycerol and corn straw produced a twofold increment in C4 and C5 hydrocarbons compared with corn straw pyrolysis (Delgado et al. 2013).
Crude glycerol gasification used traditional processes; however, microwave plasma gasification and Supercritical Water Gasification have also been employed to gasify crude glycerol. Traditional crude glycerol gasification is performed in a co-gasification process. Crude glycerol has been gasified along with olive kernel, hardwood chips, physic nut waste, and palm shell waste (Sricharoenchaikul and Atong 2012; Wei et al. 2011; Rattanapan et al. 2011; Skoulou and Zabaniotou 2013). The co-gasification of crude glycerol and biomass incremented Syngas yield, heating value, and H2 concentration (Sricharoenchaikul and Atong 2012; Wei et al. 2011; Rattanapan et al. 2011; Skoulou and Zabaniotou 2013). The Syngas produced from the crude glycerol co-gasification can be further employed to generate electricity or to produce chemicals or biofuels (Skoulou and Zabaniotou 2013). On the other hand, microwave plasma gasification is a technology that utilizes plasma flames produced by external electrical sources to gasify substrates. Crude glycerol microwave plasma gasification demonstrated an increment in gasification efficiency and Syngas heating value (Yoon et al. 2013). Another type of gasification is supercritical water gasification. This technology utilizes supercritical water to improve the gasification efficiency. Supercritical water has the ability to act as an acid/base catalyst, dissolve non-polar organic compounds, and react with other compounds (Tapah et al. 2014). The application of supercritical water gasification in crude glycerol has been evaluated in the presence of KOH and Fe2O3–Cr2O3 as catalysts (Yang et al. 2013; Tapah et al. 2014). Catalytic supercritical water gasification improved the Syngas quality by reducing biochar impurities and increasing the amount of combustible gases, especially H2. However, KOH did not act as a catalyst; in fact, KOH acted as a reactant in the process and generated K2CO3 as the product (Yang et al. 2013).
Thermal conversion is an option for the transformation of glycerol into energy and other chemicals. In this type of process, crude glycerol is an interesting option as a co-substrate for gasification or pyrolysis processes. Therefore, the combination of a thermal conversion plant and biodiesel production can be an option for the biodiesel industry. The thermal conversion plant can use the agricultural residues (stems, leafs, stalks, husk, etc.) and the pressed seeds as main substrate, whereas waste glycerol can be employed as a co-substrate to increase the quality of the Syngas or the bio-oil produced. In this case, the viability will depend on the economic and logistic analyses for the thermal conversion plant.
Microbial fuel cells
Microbial fuel cell is other approximation to physicochemical energy generation. This process employs microbial strains to catalyze the oxidation of organic or inorganic matter for electricity generation (Feng et al. 2011). Feng et al. (2011) found that it is possible to produce electricity using crude glycerol as substrate for microbial fuel cells. In their research, a maximum cathode power density of 2110 mW/m2 was obtained from the heat-treated anode of the microbial fuel cell with biodiesel waste medium (Feng et al. 2011).
Hydrogen generation
Hydrogen is the most studied biofuel from crude glycerol. Hydrogen is produced by dark fermentation and photofermentation processes (Rossi et al. 2011; Ghosh et al. 2012b). Table 3 describes the different processes used in hydrogen production from crude glycerol.
Dark fermentation employs anaerobic or facultative microorganisms in a process similar to anaerobic digestion. This fermentation has been improved by modifying the microorganisms, the culture media, or the reactor conditions. The bacteria applied normally came from methanogenic fermentation including several types of hydrogenic bacteria (Rossi et al. 2011); however, the strains have been improved using molecular techniques (transgenic strains) (Gonzalez et al. 2008) or selection techniques (eco-biotechnological) (Varrone et al. 2013b), and these methodologies have allowed the increment of H2 generation and glycerol consumption. In dark fermentation, culture medium has achieved improvements in the hydrogen production. Rossi et al. (2011) found that biomass pretreatment before glycerol conversion improved the hydrogen yield by fivefold. This process reduced the methanogenic bacteria in the sludge which was added as the inoculum (Rossi et al. 2011). Ngo et al. (2011) improved the hydrogen yield (1.55–2 fold) compared with the un-supplemented culture by using a buffer solution (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and N2 sparging conditions (Ngo et al. 2011). Similarly, Sarma et al. (2013a) found that slaughterhouse liquid waste, brewery waste biomass, and urea increased the H2 generation by Enterobacter aerogenes NRRL B 407 from 18 to 38 % (Sarma et al. 2013a). The addition of KH2PO4 and NH4Cl maximized the hydrogen (0.27 mol/mol) and ethanol (0.63 mol/mol) yields from crude glycerol using Klebsiella sp. TR17 (Chookaew et al. 2014b). The modification of the growth media can be introduced by reducing the inhibitor concentrations. Methanol and saponified-free fatty acids (SFFA) were found as inhibitors of hydrogen production by Enterobacter aerogenes NRRL B 407 (Sarma et al. 2013b). The SFFA was also found as the most important inhibitor. The removal of this inhibitor was evaluated using salting out and MgCl2 reactions. Salting out removed 42 % of SFFA; however, it also decreased the carbon/nitrogen ratio, producing an inhibition of the H2 production. On contrary, addition of MgCl2 transformed SFFA into its inactive form (scum), increased H2 cumulative production (34.70 %), and glycerol utilization (2.5-fold) (Sarma et al. 2014). The reactor type and configuration have been evaluated to increase the H2 production from glycerol. The employment of a continuous reactor in the culture of Clostridium pasteurianum augmented the production of H2 compared to the batch reactor (Lo et al. 2013). The immobilization of Klebsiella sp. TR17 in an upflow anaerobic sludge blanket (UASB) reactors increased the H2 yield and hydraulic retention time (Chookaew et al. 2014a).
Photofermentation is the bio-hydrogen production from organic matter in the presence of light. Photofermentation is normally performed using organic acids; however, some purple non-sulfur photosynthetic bacteria can also transform glycerol into bio-hydrogen directly (Ghosh et al. 2012a; Sabourin-Provost and Hallenbeck 2009). Rhodopseudomonas palustris, a purple non-sulfur photosynthetic bacterium, incremented the hydrogen yield 6 times compared with Enterobacter aerogenes or E. coli (Ghosh et al. 2012b). Rhodopseudomonas palustris increased hydrogen production in cultures with high nitrogen concentration (Sabourin-Provost and Hallenbeck 2009) and by optimizing crude glycerol concentration (30 mM), glutamate concentration (4.5 mM), and light intensity (175 W/m2) (Ghosh et al. 2012a). Similar to dark fermentation, photofermentation is highly inhibited by SFFA. Pott et al. (2013) demonstrated that SFFA is the most important inhibitor in crude glycerol, generating a reduction in the growth rate of R. palustris (Pott et al. 2013). To reduce the SFFA concentration in the crude glycerol, Pott et al. (2014) evaluated different techniques (ethanol and activated carbon, pH adjustment, solvent extraction, and precipitation of the fatty acids with calcium) to reduce SFFA concentration. The best two treatments included pH adjustment and SFFA precipitation with calcium salts. These two treatments (23–27 ml/g/h) generated similar hydrogen yields as highly purified glycerol (29 ml/g/h) (Pott et al. 2014). Besides the large hydrogen yields, photofermentation also needs to improve light use by R. palustris and this raises the need to develop economic hydrogen permeable photobioreactors (Ghosh et al. 2012a; Sabourin-Provost and Hallenbeck 2009).
Anaerobic digestion
In anaerobic digestion, crude glycerol is employed as the principal carbon source (Hutňan et al. 2013) or as a complementary carbon source (Siles López et al. 2009) to improve denitrification processes (Bodík et al. 2009) or methane generation (Álvarez et al. 2010). Table 4 shows the details of the crude glycerol anaerobic digestion. Hutňan et al. (2013) evaluated the efficiency of using crude glycerol in an UASB reactor. They improve the methane yield by diluting previously acidified crude glycerol, these modifications generated high chemical oxygen demand (COD) removal, and methane production (Hutňan et al. 2013). In the work of Siles López et al. 2009, the stoichiometry reaction for crude glycerol was found: C3H8O3 + aNH3 → bCH4 + cCO2 + dC5H7NO2 + eNH4HCO3, where the values of a, b, c, d, and e were 0.663, 1.648, 0.526, 0.041, and 0.622 mol, respectively (Siles López et al. 2009). The evaluation of anaerobic treatment of crude glycerol was made using pretreated glycerol (distilled and acidified) and granular or non-granular sludge. In this, work the highest methane yield was obtained by granular sludge-distilled glycerol; even so, granular sludge and acidified glycerol achieved the highest glycerol degradation (100 %) and an appreciable methane yield (Siles López et al. 2009).
Crude glycerol has been used principally as a co-substrate for different types of compounds such as sewage sludge, manure, and food wastes. Large concentration of crude glycerol has been inhibitory in the anaerobic digestion of sewage sludge; in fact, the recommended concentration is around 0.6–3 %. However, the crude glycerol addition increased the methane yield around 2–4 times compared with the control (Fountoulakis et al. 2010; Athanasoulia et al. 2014; Nghiem et al. 2014). Similar to sewage sludge, pig manure anaerobic digestion was improved by the addition of crude glycerol. Alvarez et al. (2010) found that methane generation can be maximized by anaerobic co-digestion of crude glycerol with pig manure and fish wastes. The highest methane production rate (16.4 L CH4/kg COD/day) was reached mixing 89 % of pig manure, 4 % of fish wastes, and 8 % of crude glycerin (Álvarez et al. 2010). Likewise, Astals et al. 2013 incremented the methane generation in 180 % by adding 3 % crude glycerol (Astals et al. 2013). Crude glycerol has been identified as a sustainable carbon source for denitrification processes. In the work of Bodik et al. (2009), the addition of crude glycerol into a denitrification process increased almost 2.5 fold the denitrification efficiency with a reduction of 5 ppm NO3/100 L of crude glycerol (Bodík et al. 2009). Crude glycerol anaerobic digestion manages large volumes, produces energy, and has a low price (Bodík et al. 2009). However, anaerobic digestion did not produce products with higher value.
Anaerobic digestion is an option to utilize waste glycerol and produce energy. Similar to thermal conversion, glycerol is not used as a main substrate; however, it can be sold or supplied to other anaerobic digestion plants or the biodiesel plant that can include anaerobic digestion reactors to treat the organic wastes produced from the biodiesel production. Anaerobic digestion is a more developed and less expensive technique than thermal conversion.
Biodiesel washing wastewaters
After the transesterification reaction, the methyl esters are separated from glycerol and the biodiesel is subjected to purification process to remove impurities (Suehara et al. 2005). The principal impurities are the remaining oil and methanol, residual catalyst, soap, and glycerin (Phukingngam et al. 2011). Impurities removal consumes approximately 20 L of water to 100 L of biodiesel (Suehara et al. 2005); this process is repeated 2–5 times depending on the amount of impurities present in the methyl esters (Phukingngam et al. 2011). At the end, the washing process generates between 20–120 L of wastewater from 100 L of biodiesel produced (Rattanapan et al. 2011). Biodiesel washing wastewaters (BWW) are alkaline (pH ≈ 9), have high chemical oxygen demand (COD) (60,000–545,000 mg/L), and grease and oil (G&O) (7000–44,300 mg/L) (Rattanapan et al. 2011). However, these wastewaters will have low nitrogen and phosphorus compounds, which make their biological treatment a challenging topic (Suehara et al. 2005; Rattanapan et al. 2011; Phukingngam et al. 2011). Biodiesel washing wastewaters need to be treated before being released into the environment because, without any such treatment, the existing compounds can produce drainage plugging, and decrease biological activity of the sewage treatment plants (Suehara et al. 2005). The general treatment methodology utilized over this residue starts with an initial pretreatment for G&O reduction followed by a treatment for COD reduction. However, in recent years, the application of adsorption technologies has modified the BWW treatment. Table 5 summarizes the treatment and pretreatment technologies used for washing wastewaters.
BWW pretreatment avoids COD degradation inhibition (Phukingngam et al. 2011). The pretreatment technologies are chemical or physicochemical. The chemical pretreatment utilizes strong acids to remove G&O (Ngamlerdpokin et al. 2011). In this pretreatment, the protons from the acid produce a coalescence effect over the oil drops making them to move until they reach upper part of the solution (Rattanapan et al. 2011). Additionally, protons can neutralize residual alkali catalyst in biodiesel wastewater and substitute the Na atom in soap molecules formed in the transesterification reaction (Ngamlerdpokin et al. 2011). Strong acids such as H2S04, HCl, and HNO3 have been employed in these pretreatments; however, the best results were obtained by H2S04 with a pH of 2 or lower (Rattanapan et al. 2011; Ngamlerdpokin et al. 2011). The most used physicochemical pretreatment is electrocoagulation. In this pretreatment, the wastewater is placed in a reactor which produces an electric field that generates flocculating agents (aluminum related) capable of removing the G&O or COD particles (Chavalparit and Ongwandee 2009).
After pretreatment, BWW have been treated using anaerobic digestion or coagulation agents. Coagulation is a physicochemical treatment that reduces G&O or COD concentrations in the BWW. The principal types are electrochemical and chemical coagulation (Ngamlerdpokin et al. 2011). Chemical coagulation utilizes an external coagulant agent to bond different particles of G&O or COD in small groups, which can be easily removed by the addition of a flocculating agent (Ngamlerdpokin et al. 2011). On the contrary, electrochemical coagulation utilizes an electrical field to generate its own coagulant agent. In both coagulations, the most common flocculating agents produced or introduced are aluminum related. Aluminum compounds are recognized by its best properties in coagulation and flocculation processes (Siles et al. 2011). The most important factors in electrocoagulation are voltage, pH, and reaction time. In chemical coagulation, the important factors are concentration, pH, and reaction time (Ngamlerdpokin et al. 2011; Rattanapan et al. 2011). Coagulations have showed efficient removal of G&O when they are utilized as pretreatment or treatment agents. However, coagulation only could achieve substantial COD removal when it is performed after a previous pretreatment (Chavalparit and Ongwandee 2009; Jaruwat et al. 2010).
Biodiesel washing wastewater anaerobic treatment is another option besides coagulation. Similar to coagulation, anaerobic digestion needs to pretreat BWW to reduce G&O and COD (Bezerra et al. 2011). Pretreatment processes allowed efficient COD and G&O reduction using anaerobic treatment; nevertheless, high concentrations of some chemicals from the pretreatment can be harmful for the anaerobic process (Siles et al. 2011). In addition to COD and G&O removal, anaerobic process produces considerable amount of methane, with removals around 278–305 mL CH4/g COD removed, which is about 88 % of the possible methane yield from COD (Siles et al. 2011; Bezerra et al. 2011).
In the last few years, BWW has been treated using adsorption and membrane technology to reduce the COD and G&O concentrations. Shirazi et al. 2013 evaluated electrospun microporous membranes before and after surface modification to reduce the COD from BWW. The modified membrane achieved the largest reduction in COD (75 %). This technology did not use any type of pretreatment to reduce COD or G&O concentration, which is an advantage over the other BWW treatments (Shirazi et al. 2013). Similar to membranes, chitosan adsorption is another alternative for treating BWW. Chitosan flakes considerably reduce the concentrations of COD (90 %) and G&O (67 %), at 3.5 g/L chitosan, pH 4, and 3 h of incubation (Pitakpoolsil and Hunsom 2013). To improve this treatment, four successive treatments were tested. This modification enhanced BOD, COD, and G&O removal up to 93.6, 97.6, and 95.8 %, respectively (Pitakpoolsil and Hunsom 2014).
Anaerobic process and coagulation have similitudes in the degradation level; both processes achieved values up to 85 %. The duration of the process is variable; anaerobic treatment is a slow process which takes 30–40 days (Bezerra et al. 2011); however, coagulation takes only 20 min to 1 h (Ngamlerdpokin et al. 2011). Anaerobic process and membrane technologies have a reduced cost compared with coagulation; the first ones do not have any additional price and anaerobic digestion has the advantage of producing energy from methane which could be sold or utilized in the biodiesel plant. On the other hand, coagulation processes need the presence of coagulants and large quantities of energy to run the reactors; these factors eventually increase the process costs.
Treatment of other biodiesel residues
Besides glycerin and BWW, biodiesel production produces other residues. These are produced in lower quantities or have characteristics allowing the utilization of conventional treatment techniques. Methanol is one of these residues, and it can be easily recovered using distillation techniques. Methanol recovery systems such as flash distillation (Wang et al. 2011) or vacuum distillation (Varanda et al. 2011) are available in the market. These techniques remove the methanol efficiently without affecting the transesterification process because of the differences between the boiling points in their components (Varanda et al. 2011).
Pressed seed cakes, spent earth, and agricultural wastes from biodiesel crops are also residues from the biodiesel production. Pressed seed cakes are the seed biomass leftover from oil extraction. This solid residue is normally used as an organic amendment, animal feed, or is composted (Varanda et al. 2011). Recently, seed cakes have been evaluated for hydrogen generation. Lopes et al. 2015 used dark fermentation to produce hydrogen from pretreated and untreated jatropha seed cake. The highest specific bio-hydrogen yield (68.2 mL H2/g VS) was achieved using 2.5 g VS of untreated jatropha seed cake; however, the largest cumulative concentration was achieved with 10 g VS (Lopes et al. 2015). Likewise, Panagiotopoulos et al. (2013) evaluated dark fermentation of cotton-seed cake. Caldicellulosiruptor saccharolyticus produced significantly higher hydrogen yield (84–112 %) than the control. However, hydrogen was only generated when the cotton-seed cake was pretreated with NaOH (10 %) (Panagiotopoulos et al. 2013). In a different approach, seed cakes have been used in solid-state fermentation to produce edible fungus and enzymes (Veerabhadrappa et al. 2014; da Luz et al. 2013). da Luz et al. (2013) described the production of the edible fungus Pleurotus ostreatus from jatropha seed cake; this fungal strain degraded the antinutritional factors in the jatropha seed cake (da Luz et al. 2013). Similarly, Veerabhadrappa et al. (2014) reported proteases and lipases from Aspergillus versicolor CJS-98 in the solid-state fermentation of jatropha seed cake. The fermentation of jatropha seed cake with medium supplementation (maltose and peptone 2 %), 40 % moisture, and 25 °C produced maximum amount of proteases (3366 U/g) and lipases (1288 U/g) and removed jatropha seed cake anti-nutrients (Veerabhadrappa et al. 2014). Pressed seed cakes and agricultural wastes from biodiesel crops have also been studied for activated carbon production (Foo and Hameed 2009; Nunes et al. 2009). Activated carbons from biofuel solid residues are produced via thermochemical conversion, in a two-step process: first, the removal of residual oil and second, the thermochemical activation using temperatures between 600 and 800 °C under constant N2 flow (Nunes et al. 2009). From the biofuel agricultural residues, it is possible to produce low-cost activated carbons with wide superficial area and good uptake capacity; however, more research is needed to develop an activated carbon which can be applied in water purification or wastewater treatments (Foo and Hameed 2009).
Bentonites soaked in biodiesel also called spent earth are a residue produced from biodiesel polishing process. In this step, saturated bentonites with a biodiesel concentration of 20–50 % are obtained. The oil in the bentonites can be treated by chemical extraction; similar to biodiesel production from vegetable oil. Besides that, this residue has been evaluated to produce environmentally friendly, low-cost, and lightweight construction materials. In the work of Eliche-Quesada et al. (2012), glycerin and spent earth were mixed with clay to produce lightweight bricks (Eliche-Quesada et al. 2012). The addition of oil containing bentonites (at 15 %) into the clay generated ceramic insulation bricks with reduced thermal conductivity and reduced energy costs (Eliche-Quesada et al. 2012).
Summary
Biodiesel is one of the most important sources of renewable energy; however, biodiesel production is associated with by-products and residues generation. Numerous techniques have been developed to utilize or to treat biodiesel by-products or residues. These techniques have shown that the above residues can be used to generate bioproducts and renewable energy. These processes and techniques can also reduce environmental pollution and increase the opportunities to generate additional income or reduce the costs of production of biodiesel. Invention of advanced technologies to deal with these residues could also make the biodiesel production process more sustainable and competitive against fossil fuels.
Abbreviations
- BWW:
-
biodiesel washing wastewaters
- COD:
-
chemical oxygen demand
- G&O:
-
grease and oil
- PHA:
-
polyhydroxyalkanoates
- PHB:
-
polyhydroxybutyrate
- SFFA:
-
saponified-free fatty acids
- TVS:
-
total volatile solids
- UASB:
-
upflow anaerobic sludge blanket
- VS:
-
volatile solids
References
Abad S, Turon X (2012) Valorization of biodiesel derived glycerol as a carbon source to obtain added-value metabolites: focus on polyunsaturated fatty acids. Biotechnol Adv 30(3):733–741
Álvarez JA, Otero L, Lema JM (2010) A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour Technol 101(4):1153–1158. doi:10.1016/j.biortech.2009.09.061
André A, Diamantopoulou P, Philippoussis A et al (2010) Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind Crops Prod 31(2):407–416. doi:10.1016/j.indcrop.2009.12.011
Astals S, Nolla-Ardèvol V, Mata-Alvarez J (2013) Thermophilic co-digestion of pig manure and crude glycerol: process performance and digestate stability. J Biotechnol 166(3):97–104. doi:10.1016/j.jbiotec.2013.05.004
Athanasoulia E, Melidis P, Aivasidis A (2014) Co-digestion of sewage sludge and crude glycerol from biodiesel production. Renew Energy 62:73–78. doi:10.1016/j.renene.2013.06.040
Bezerra R, Rodrigues J, Ratusznei S et al (2011) Effect of organic load on the performance and methane production of an AnSBBR treating effluent from biodiesel production. Appl Biochem Biotechnol 165(1):347–368. doi:10.1007/s12010-011-9255-6
Bodík I, Blšťáková A, Sedláček S et al (2009) Biodiesel waste as source of organic carbon for municipal WWTP denitrification. Bioresour Technol 100(8):2452–2456. doi:10.1016/j.biortech.2008.11.050
Casali S, Gungormusler M, Bertin L et al (2012) Development of a biofilm technology for the production of 1,3-propanediol (1,3-PDO) from crude glycerol. Biochem Eng J 64:84–90. doi:10.1016/j.bej.2011.11.012
Chavalparit O, Ongwandee M (2009) Optimizing electrocoagulation process for the treatment of biodiesel wastewater using response surface methodology. J Environ Sci 21(11):1491–1496. doi:10.1016/S1001-0742(08)62445-6
Cheng D, Wang L, Shahbazi A et al (2014a) Catalytic cracking of crude bio-oil from glycerol-assisted liquefaction of swine manure. Energy Convers Manag 87:378–384. doi:10.1016/j.enconman.2014.06.084
Cheng D, Wang L, Shahbazi A et al (2014b) Characterization of the physical and chemical properties of the distillate fractions of crude bio-oil produced by the glycerol-assisted liquefaction of swine manure. Fuel 130:251–256. doi:10.1016/j.fuel.2014.04.022
Chi Z, Pyle D, Wen Z et al (2007) A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem 42(11):1537–1545. doi:10.1016/j.procbio.2007.08.008
Chookaew T, Prasertsan P, Ren ZJ (2014a) Two-stage conversion of crude glycerol to energy using dark fermentation linked with microbial fuel cell or microbial electrolysis cell. New Biotechnol 31(2):179–184
Chookaew T, O-Thong S, Prasertsan P (2014b) Statistical optimization of medium components affecting simultaneous fermentative hydrogen and ethanol production from crude glycerol by thermotolerant Klebsiella sp TR17. Int J Hydrogen Energy 39(2):751–760. doi:10.1016/j.ijhydene.2013.10.141
da Luz JMR, Paes SA, Torres DP et al (2013) Production of edible mushroom and degradation of antinutritional factors in jatropha biodiesel residues. LWT Food Sci Technol 50(2):575–580. doi:10.1016/j.lwt.2012.08.006
Delgado R, Rosas JG, Gómez N et al (2013) Energy valorisation of crude glycerol and corn straw by means of slow co-pyrolysis: production and characterisation of gas, char and bio-oil. Fuel 112:31–37. doi:10.1016/j.fuel.2013.05.005
Dobroth ZT, Hu S, Coats ER et al (2011) Polyhydroxybutyrate synthesis on biodiesel wastewater using mixed microbial consortia. Bioresour Technol 102(3):3352–3359. doi:10.1016/j.biortech.2010.11.053
Echeverri DA, Perez WA, Rios LA (2013) Synthesis of maleated-castor oil glycerides from biodiesel-derived crude glycerol. Ind Crops Prod 49:299–303. doi:10.1016/j.indcrop.2013.05.008
Eliche-Quesada D, Martínez-Martínez S, Pérez-Villarejo L et al (2012) Valorization of biodiesel production residues in making porous clay brick. Fuel Process Technol 103:166–173
Ethier S, Woisard K, Vaughan D et al (2011) Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour Technol 102(1):88–93. doi:10.1016/j.biortech.2010.05.021
Feng Y, Yang Q, Wang X et al (2011) Treatment of biodiesel production wastes with simultaneous electricity generation using a single-chamber microbial fuel cell. Bioresour Technol 102(1):411–415. doi:10.1016/j.biortech.2010.05.059
Feng X, Walker TH, Bridges WC et al (2014) Biomass and lipid production of Chlorella protothecoides under heterotrophic cultivation on a mixed waste substrate of brewer fermentation and crude glycerol. Bioresour Technol 166:17–23. doi:10.1016/j.biortech.2014.03.120
Foo KY, Hameed BH (2009) Utilization of biodiesel waste as a renewable resource for activated carbon: application to environmental problems. Renew Sustain Energy Rev 13(9):2495–2504. doi:10.1016/j.rser.2009.06.009
Fountoulakis M, Petousi I, Manios T (2010) Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manage 30(10):1849–1853
Ghosh D, Sobro IF, Hallenbeck PC (2012a) Stoichiometric conversion of biodiesel derived crude glycerol to hydrogen: response surface methodology study of the effects of light intensity and crude glycerol and glutamate concentration. Bioresour Technol 106:154–160. doi:10.1016/j.biortech.2011.12.021
Ghosh D, Tourigny A, Hallenbeck PC (2012b) Near stoichiometric reforming of biodiesel derived crude glycerol to hydrogen by photofermentation. Int J Hydrogen Energy 37(3):2273–2277. doi:10.1016/j.ijhydene.2011.11.011
Global Renewable Fuels Alliance. World ethanol production playing an increasing role in energy security. http://www.globalrfa.org/pr_021111.php. Accessed 04 Dec 2012
Gonzalez R, Murarka A, Dharmadi Y et al (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10(5):234–245. doi:10.1016/j.ymben.2008.05.001
Hu S, Li Y (2014) Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour Technol 161:410–415. doi:10.1016/j.biortech.2014.03.072
Hutňan M, Kolesárová N, Bodík I et al (2013) Long-term monodigestion of crude glycerol in a UASB reactor. Bioresour Technol 130:88–96. doi:10.1016/j.biortech.2012.12.003
Ito T, Nakashimada Y, Senba K et al (2005) Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J Biosci Bioeng 100(3):260–265. doi:10.1263/jbb.100.260
Jaruwat P, Kongjao S, Hunsom M (2010) Management of biodiesel wastewater by the combined processes of chemical recovery and electrochemical treatment. Energy Convers Manag 51(3):531–537. doi:10.1016/j.enconman.2009.10.018
Juang C, Whang L, Cheng H (2011) Evaluation of bioenergy recovery processes treating organic residues from ethanol fermentation process. Bioresour Technol 102(9):5394–5399. doi:10.1016/j.biortech.2010.10.069
Juszczyk P, Tomaszewska L, Kita A et al (2013) Biomass production by novel strains of Yarrowia lipolytica using raw glycerol, derived from biodiesel production. Bioresour Technol 137:124–131. doi:10.1016/j.biortech.2013.03.010
Khanna S, Goyal A, Moholkar VS (2013) Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel 112:557–561. doi:10.1016/j.fuel.2011.10.071
Lee S, Park S, Park C et al (2014) Enhanced free fatty acid production by codon-optimized Lactococcus lactis acyl-ACP thioesterase gene expression in Escherichia coli using crude glycerol. Enzyme Microb Technol 67:8–16. doi:10.1016/j.enzmictec.2014.08.004
Liu B, Christiansen K, Parnas R et al (2013a) Optimizing the production of hydrogen and 1,3-propanediol in anaerobic fermentation of biodiesel glycerol. Int J Hydrogen Energy 38(8):3196–3205. doi:10.1016/j.ijhydene.2012.12.135
Liu Y, Sun Y, Tan C et al (2013b) Efficient production of dihydroxyacetone from biodiesel-derived crude glycerol by newly isolated Gluconobacter frateurii. Bioresour Technol 142:384–389. doi:10.1016/j.biortech.2013.05.055
Lo Y, Chen X, Huang C et al (2013) Dark fermentative hydrogen production with crude glycerol from biodiesel industry using indigenous hydrogen-producing bacteria. Int J Hydrogen Energy 38(35):15815–15822. doi:10.1016/j.ijhydene.2013.05.083
Lopes SL, Fragoso R, Duarte E et al (2015) Bioconversion of Jatropha curcas seed cake to hydrogen by a strain of Enterobacter aerogenes. Fuel 139:715–719. doi:10.1016/j.fuel.2014.09.004
Manara P, Zabaniotou A (2013) Co-pyrolysis of biodiesel-derived glycerol with Greek lignite: a laboratory study. J Anal Appl Pyrolysis 100:166–172. doi:10.1016/j.jaap.2012.12.013
Martín MA, Fernández R, Serrano A et al (2013) Semi-continuous anaerobic co-digestion of orange peel waste and residual glycerol derived from biodiesel manufacturing. Waste Manage 33(7):1633–1639. doi:10.1016/j.wasman.2013.03.027
Meiswinkel TM, Rittmann D, Lindner SN et al (2013) Crude glycerol-based production of amino acids and putrescine by Corynebacterium glutamicum. Bioresour Technol 145:254–258. doi:10.1016/j.biortech.2013.02.053
Metsoviti M, Zeng A, Koutinas AA et al (2013) Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J Biotechnol 163(4):408–418. doi:10.1016/j.jbiotec.2012.11.018
Moita R, Freches A, Lemos PC (2014) Crude glycerol as feedstock for polyhydroxyalkanoates production by mixed microbial cultures. Water Res 58:9–20. doi:10.1016/j.watres.2014.03.066
Ngamlerdpokin K, Kumjadpai S, Chatanon P et al (2011) Remediation of biodiesel wastewater by chemical- and electro-coagulation: a comparative study. J Environ Manage 92(10):2454–2460. doi:10.1016/j.jenvman.2011.05.006
Nghiem LD, Nguyen TT, Manassa P et al (2014) Co-digestion of sewage sludge and crude glycerol for on-demand biogas production. Int Biodeterior Biodegrad 95(Part A(0)):160–166. doi:10.1016/j.ibiod.2014.04.023
Ngo TA, Kim M, Sim SJ (2011) High-yield biohydrogen production from biodiesel manufacturing waste by Thermotoga neapolitana. Int J Hydrogen Energy 36(10):5836–5842. doi:10.1016/j.ijhydene.2010.11.057
Nitayavardhana S, Khanal SK (2011) Biodiesel-derived crude glycerol bioconversion to animal feed: a sustainable option for a biodiesel refinery. Bioresour Technol 102(10):5808–5814. doi:10.1016/j.biortech.2011.02.058
Nunes AA, Franca AS, Oliveira LS (2009) Activated carbons from waste biomass: an alternative use for biodiesel production solid residues. Bioresour Technol 100(5):1786–1792. doi:10.1016/j.biortech.2008.09.032
Panagiotopoulos IA, Pasias S, Bakker RR et al (2013) Biodiesel and biohydrogen production from cotton-seed cake in a biorefinery concept. Bioresour Technol 136:78–86. doi:10.1016/j.biortech.2013.02.061
Pflügl S, Marx H, Mattanovich D et al (2014) Heading for an economic industrial upgrading of crude glycerol from biodiesel production to 1,3-propanediol by Lactobacillus diolivorans. Bioresour Technol 152:499–504. doi:10.1016/j.biortech.2013.11.041
Phukingngam D, Chavalparit O, Somchai D et al (2011) Anaerobic baffled reactor treatment of biodiesel-processing wastewater with high strength of methanol and glycerol: reactor performance and biogas production. Chem Pap 65(5):644–651. doi:10.2478/s11696-011-0061-y
Pitakpoolsil W, Hunsom M (2013) Adsorption of pollutants from biodiesel wastewater using chitosan flakes. J Taiwan Inst Chem Eng 44(6):963–971. doi:10.1016/j.jtice.2013.02.009
Pitakpoolsil W, Hunsom M (2014) Treatment of biodiesel wastewater by adsorption with commercial chitosan flakes: parameter optimization and process kinetics. J Environ Manage 133:284–292. doi:10.1016/j.jenvman.2013.12.019
Pott RWM, Howe CJ, Dennis JS (2013) Photofermentation of crude glycerol from biodiesel using Rhodopseudomonas palustris: comparison with organic acids and the identification of inhibitory compounds. Bioresour Technol 130:725–730. doi:10.1016/j.biortech.2012.11.126
Pott RWM, Howe CJ, Dennis JS (2014) The purification of crude glycerol derived from biodiesel manufacture and its use as a substrate by Rhodopseudomonas palustris to produce hydrogen. Bioresour Technol 152:464–470. doi:10.1016/j.biortech.2013.10.094
Rattanapan C, Sawain A, Suksaroj T et al (2011) Enhanced efficiency of dissolved air flotation for biodiesel wastewater treatment by acidification and coagulation processes. Desalination 280(1–3):370–377. doi:10.1016/j.desal.2011.07.018
Rossi DM, Berne da Costa J, Aquino de Souza E et al (2011) Comparison of different pretreatment methods for hydrogen production using environmental microbial consortia on residual glycerol from biodiesel. Int J Hydrogen Energy 36(8):4814–4819. doi:10.1016/j.ijhydene.2011.01.005
Rossi DM, da Costa JB, de Souza EA et al (2012) Bioconversion of residual glycerol from biodiesel synthesis into 1,3-propanediol and ethanol by isolated bacteria from environmental consortia. Renewable Energy 39(1):223–227. doi:10.1016/j.renene.2011.08.005
Ruhal R, Choudhury B (2012) Use of an osmotically sensitive mutant of Propionibacterium freudenreichii subspp. shermanii for the simultaneous productions of organic acids and trehalose from biodiesel waste based crude glycerol. Bioresour Technol 109:131–139. doi:10.1016/j.biortech.2012.01.039
Sabourin-Provost G, Hallenbeck PC (2009) High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresour Technol 100(14):3513–3517. doi:10.1016/j.biortech.2009.03.027
Sarma SJ, Brar SK, Le Bihan Y et al (2013a) Evaluation of different supplementary nutrients for enhanced biohydrogen production by Enterobacter aerogenes NRRL B 407 using waste derived crude glycerol. Int J Hydrogen Energy 38(5):2191–2198. doi:10.1016/j.ijhydene.2012.11.110
Sarma SJ, Dhillon GS, Brar SK et al (2013b) Investigation of the effect of different crude glycerol components on hydrogen production by Enterobacter aerogenes NRRL B-407. Renew Energy 60:566–571. doi:10.1016/j.renene.2013.06.007
Sarma SJ, Brar SK, Le Bihan Y et al (2014) Mitigation of the inhibitory effect of soap by magnesium salt treatment of crude glycerol– A novel approach for enhanced biohydrogen production from the biodiesel industry waste. Bioresour Technol 151:49–53. doi:10.1016/j.biortech.2013.10.042
Shah P, Chiu F, Lan JC (2014) Aerobic utilization of crude glycerol by recombinant Escherichia coli for simultaneous production of poly 3-hydroxybutyrate and bioethanol. J Biosci Bioeng 117(3):343–350. doi:10.1016/j.jbiosc.2013.08.018
Shirazi M, Kargari A, Bazgir S et al (2013) Characterization of electrospun polystyrene membrane for treatment of biodiesel’s water-washing effluent using atomic force microscopy. Desalination 329:1–8
Siles López JÁ, Santos MD, María de los Ángeles, Chica Pérez AF et al (2009) Anaerobic digestion of glycerol derived from biodiesel manufacturing. Bioresour Technol 100(23):5609–5615. doi:10.1016/j.biortech.2009.06.017
Siles JA, Martín MA, Chica AF et al (2010) Anaerobic co-digestion of glycerol and wastewater derived from biodiesel manufacturing. Bioresour Technol 101(16):6315–6321. doi:10.1016/j.biortech.2010.03.042
Siles JA, Gutiérrez MC, Martín MA et al (2011) Physical–chemical and biomethanization treatments of wastewater from biodiesel manufacturing. Bioresour Technol 102(10):6348–6351. doi:10.1016/j.biortech.2011.02.106
Skoulou VK, Zabaniotou AA (2013) Co-gasification of crude glycerol with lignocellulosic biomass for enhanced syngas production. J Anal Appl Pyrolysis 99:110–116. doi:10.1016/j.jaap.2012.10.015
Skoulou V, Manara P, Zabaniotou A (2012) H 2 enriched fuels from co-pyrolysis of crude glycerol with biomass. J Anal Appl Pyrolysis 97:198–204
Sousa M, Melo VMM, Rodrigues S et al (2012) Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst Eng 35(6):897–906
Sricharoenchaikul V, Atong D (2012) Fuel gas generation from thermochemical conversion of crude glycerol mixed with biomass wastes. Energy Procedia 14:1286–1291. doi:10.1016/j.egypro.2011.12.1090
Suehara K, Kawamoto Y, Fujii E et al (2005) Biological treatment of wastewater discharged from biodiesel fuel production plant with alkali-catalyzed transesterification. J Biosci Bioeng 100(4):437–442. doi:10.1263/jbb.100.437
Szymanowska-Powałowska D, Leja K (2014) An increasing of the efficiency of microbiological synthesis of 1,3-propanediol from crude glycerol by the concentration of biomass. EJB 17(2):72–78. doi:10.1016/j.ejbt.2013.12.010
Tapah BF, Santos RCD, Leeke GA (2014) Processing of glycerol under sub and supercritical water conditions. Renew Energy 62:353–361. doi:10.1016/j.renene.2013.07.027
Varanda MG, Pinto G, Martins F (2011) Life cycle analysis of biodiesel production. Fuel Process Technol 92(5):1087–1094. doi:10.1016/j.fuproc.2011.01.003
Varrone C, Liberatore R, Crescenzi T et al (2013a) The valorization of glycerol: economic assessment of an innovative process for the bioconversion of crude glycerol into ethanol and hydrogen. Appl Energy 105:349–357. doi:10.1016/j.apenergy.2013.01.015
Varrone C, Rosa S, Fiocchetti F et al (2013b) Enrichment of activated sludge for enhanced hydrogen production from crude glycerol. Int J Hydrogen Energy 38(3):1319–1331. doi:10.1016/j.ijhydene.2012.11.069
Veerabhadrappa MB, Shivakumar SB, Devappa S (2014) Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J Biosci Bioeng 117(2):208–214. doi:10.1016/j.jbiosc.2013.07.003
Verhoef S, Gao N, Ruijssenaars HJ et al (2014) Crude glycerol as feedstock for the sustainable production of p-hydroxybenzoate by Pseudomonas putida S12. New Biotechnol 31(1):114–119. doi:10.1016/j.nbt.2013.08.006
Wang C, Chen W, Wang W et al (2011) Experimental study on methanol recovery through flashing vaporation in continuous production of biodiesel via supercritical methanol. Energy Convers Manag 52(2):1454–1458. doi:10.1016/j.enconman.2010.10.008
Watanabe R, Tada C, Baba Y et al (2013) Enhancing methane production during the anaerobic digestion of crude glycerol using Japanese cedar charcoal. Bioresour Technol 150:387–392. doi:10.1016/j.biortech.2013.10.030
Wei L, Pordesimo LO, Haryanto A et al (2011) Co-gasification of hardwood chips and crude glycerol in a pilot scale downdraft gasifier. Bioresour Technol 102(10):6266–6272. doi:10.1016/j.biortech.2011.02.109
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol–a byproduct of biodiesel production. Biotechnol Biofuels 5(1):1
Yang F, Hanna MA, Marx DB et al (2013) Optimization of hydrogen production from supercritical water gasification of crude glycerol? Byproduct of biodiesel production. Int J Energy Res 37(13):1600–1609. doi:10.1002/er.2969
Yen H, Yang Y, Yu Y (2012) Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J Biosci Bioeng 114(4):453–456. doi:10.1016/j.jbiosc.2012.04.022
Yoon SJ, Choi Y, Son Y et al (2010) Gasification of biodiesel by-product with air or oxygen to make syngas. Bioresour Technol 101(4):1227–1232. doi:10.1016/j.biortech.2009.09.039
Yoon SJ, Yun YM, Seo MW et al (2013) Hydrogen and syngas production from glycerol through microwave plasma gasification. Int J Hydrogen Energy 38(34):14559–14567. doi:10.1016/j.ijhydene.2013.09.001
Yuksel A, Sasaki M, Goto M (2011) A new green technology: hydrothermal electrolysis for the treatment of biodiesel wastewater. Res Chem Intermed 37(2):131–143. doi:10.1007/s11164-011-0260-8
Zhou Y, Nie K, Zhang X et al (2014) Production of fumaric acid from biodiesel-derived crude glycerol by Rhizopus arrhizus. Bioresour Technol 163:48–53. doi:10.1016/j.biortech.2014.04.021
Authors’ contributions
JP was responsible of manuscript drafting and writing. SC was responsible of final manuscript approval. Both authors read and approved the final manuscript.
Acknowledgements
The Colombian government and the Fulbright organization (FULBRIGHT-COLCIENCIAS) are acknowledged for the financial support granted to Jersson Plácido in the USA.
Competing interests
The authors declare that they have no competing interests.
Funding
The Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS) and the Fulbright organization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Plácido, J., Capareda, S. Conversion of residues and by-products from the biodiesel industry into value-added products. Bioresour. Bioprocess. 3, 23 (2016). https://doi.org/10.1186/s40643-016-0100-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-016-0100-1