Abstract
Climate change and the unsustainability of fossil fuels are calling for cleaner energies such as methanol as a fuel. Methanol is one of the simplest molecules for energy storage and is utilized to generate a wide range of products. Since methanol can be produced from biomass, numerous countries could produce and utilize biomethanol. Here, we review methanol production processes, techno-economy, and environmental viability. Lignocellulosic biomass with a high cellulose and hemicellulose content is highly suitable for gasification-based biomethanol production. Compared to fossil fuels, the combustion of biomethanol reduces nitrogen oxide emissions by up to 80%, carbon dioxide emissions by up to 95%, and eliminates sulphur oxide emission. The cost and yield of biomethanol largely depend on feedstock characteristics, initial investment, and plant location. The use of biomethanol as complementary fuel with diesel, natural gas, and dimethyl ether is beneficial in terms of fuel economy, thermal efficiency, and reduction in greenhouse gas emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive use of non-renewable fuel-energy sources continues to negatively impact the environment due to their association with greenhouse gas emissions (Chen et al. 2022). While there are several contributors, the transportation industry is one of the largest, accounting for about 23% of carbon dioxide emissions (Osman et al. 2021a). By 2030, the overall energy requirements for transportation are projected to increase by 80%, accompanied by a proportional increase in carbon dioxide emissions (Saboori et al. 2014; Leduc et al. 2010). Concurrently, it is widely acknowledged that rebalancing carbon dioxide emissions with natural or engineered removal processes, i.e. carbon sequestration, is necessary for societal well-being. Fuels derived from renewable sources are among the numerous options for supporting a transition to a carbon–neutral or negative economy (Olabi 2012). These fuels can be used as drop-in replacements or in blends to reduce the need for fossil alternatives and promote sustainable emphasis on the use of renewable energy, such as biofuels (Gahleitner 2013; Li et al. 2019; Zheng et al. 2017; Zhang et al. 2005; Asadieraghi et al. 2014), which offer significant advantages over wind and solar power in terms of energy density, storage, and intermittency (Kumar and Shukla 2016; Dincer 2000).
Efficient utilization of biomass resources can reduce greenhouse gas emissions, and by 2050, bioenergy demand is expected to contribute 120–155 exajoules per year (IPCC 2011). Moreover, lignocellulosic biomass is a valuable alternative energy source as it is relatively carbon dioxide neutral (Hill et al. 2006; Babu 2008; Huber et al. 2006; Pham et al. 2014; Zhang et al. 2010). One of the best ways to produce biofuels that can partially replace fossil fuels is thermochemical conversion of such biomass (Rodionova et al. 2017), with the resulting products also complementing or supporting the production of other biofuels like ethanol (Hasegawa et al. 2010; Lee et al. 2018; Karagoz et al. 2019; Taghizadeh-Alisaraei et al. 2019; Soam et al. 2016), methanol (Roode-Gutzmer et al. 2019; Carvalho et al. 2018a; Su et al. 2019; Taher and Chandran 2013; Geng et al. 2014), biodiesel (Ogunkunle and Ahmed 2019; Torres et al. 2017; Verma and Sharma 2016) and dimethyl ether (Parvez et al. 2018; Shi et al. 2018; Grové et al. 2018). Recent advancements in the biofuels include the production of gasoline via biomass gasification, electricity generation using microalgae, use of biodiesel, bioethanol, and biomethanol for energy generation and transportation (Tsita et al. 2019; Osman et al. 2021b).
Gasification (Zhang et al. 2010; Asadieraghi and Wan Daud 2015) does not directly result in the production of advanced liquid biofuels. Instead, these are produced via a syngas (synthesis gas, a mixture of hydrogen, carbon dioxide, carbon monoxide, and methane) intermediate (Miao et al. 2014; Mahinpey and Gomez 2016; Maity 2015; McKendry 2002). Since 2001, investigations in biomass gasification have increased in both the industrial and academic sectors due to its potential benefits (Sikarwar et al. 2016), particularly as it is considered a more environment-friendly process with lower overall greenhouse effect gas emissions (Pauls et al. 2016). It has also been found to be one of the most efficient and economically viable ways for waste lignocellulosic biomass utilization (Mahinpey and Gomez 2016; Ellabban et al. 2014; Sikarwar et al. 2017). The process itself is defined as the production of fuel gas by thermochemical conversion of biomass in a high-temperature oxygen-depleted atmosphere. Apart from key product syngas, it also results in biochar which has been widely researched for several applications, including heat and power generation, carbon sequestration, fertilizing, and various adsorption applications (Osman et al. 2022a; Fawzy et al. 2021). The resulting syngas has a high calorific value (Higman and van der Burgt 2008) with a favourable hydrogen fraction and acts as an important source for producing power and biochemical fuels (Parthasarathy and Narayanan 2014). It can be used to produce different valuable fuels, including methanol, hydrogen, and diesel (Carvalho et al. 2018a; Kruse 2009; Tijmensen et al. 2002; Holmgren et al. 2012) or burnt directly to generate electricity with a Brayton–Rankine combined cycle (Ståhl and Neergaard 1998; Kirubakaran et al. 2009).
As a gasifying agent, air, steam, oxygen, or carbon dioxide can be utilized; however, the air is typically chosen due to its low cost, resulting in reduced lower heating value syngas. By decreasing tar and char yields and increasing hydrogen yields, product gas quality can be enhanced through steam gasification (Pala et al. 2017). The process comprises three main steps, viz. (a) pyrolysis, in which the biomass decomposes to tar, gas, and char, (b) oxidation process, in which large amounts of heat are released to help drive the endothermic processes, and (c) gasification, where syngas is produced (Alipour Moghadam et al. 2014). A number of different catalysts are often used to enhance the gasification reactions and suppress undesired compounds, including weak acid alkali metal salts such as potassium carbonate, sodium carbonate, potassium sulphide, and sodium sulphide. The primary components of syngas produced from biomass gasification are hydrogen and carbon monoxide, but methane, carbon dioxide, water vapour, and nitrogen with different contaminants like ammonia, tars, and hydrogen sulphide are also present (Abdoulmoumine et al. 2014). In general, the quality of the gasification product and contaminants is highly dependent on the feedstock properties, catalyst types, gasifying agent, and reactor conditions (Sikarwar et al. 2016; Farzad et al. 2016).
Under high temperature and pressure conditions (250–300 °C and 5–15 MPa), and with the help of an appropriate catalyst, the syngas can be converted to methanol (Yang and Ge 2016). Methanol or methyl alcohol, or wood alcohol, is the simplest alcohol which is widely utilized as an extremely versatile chemical in different industrial applications. Methanol is one of the ‘mega’ industrial platform chemicals, and worldwide, about 90 methanol production plants produce approximately 110 million metric tons of methanol annually. This huge demand and low margins necessitate large plants to co-locate these low-cost and abundant carbon resources such as coal and natural gas. However, different feedstocks like biomass, biogas, or organic municipal waste can be used to produce biomethanol (van Kasteren 2016), i.e. methanol derived from organic materials. Other options would be the hydrogenation of captured carbon dioxide hydrogen produced from renewable energy (Osman et al. 2022b). This may or may not be classified as biomethanol, depending on the carbon dioxide or hydrogen source.
Biomethanol can be utilized as a possible energy source and has numerous benefits, including high octane number (87–110) (Osman et al. 2021c), low flammability, high performance, and low emissions. It is chemically indistinguishable from fossil-based methanol and, as such, is fully miscible with water, conventional methanol, petrol, and different organic compounds (Singh et al. 2014). It finds its usefulness in several ways, viz. (a) as a substitute fuel to gasoline in internal combustion engines; (b) as a replacement to diesel, through biodiesel or dimethyl ether production; (c) in methanol-fuelled vehicles or in hybrid automobiles; (d) for electricity generation through a gas turbine or fuel cell; and (e) also as a household fuel source (Morone and Cottoni 2016; Demirbas 2008; Bergins et al. 2016).
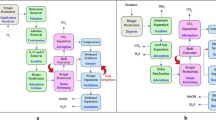
Dimethyl ether, which can be produced from methanol or syngas, is another potential future fuel (Osman and Abu-Dahrieh 2018; Osman et al. 2012). Dimethyl ether has a high cetane number (> 55), low emission, fuel flexibility; these factors make dimethyl ether a potential useful fuel for industrial and automotive applications (Osman et al. 2017). Figure 1 shows an overview of different processes involved in a gasification-based methanol generation process. Several studies have been found regarding different methanol/biomethanol production techniques but were seen to lack clarity regarding emissions, utilization and feedstock materials. A requirement for a review study of the techno-economic viability of methanol/dimethyl ether as a future generation fuel, different production processes with all possible feedstocks, dual-fuel utilization in different sectors were found.
Production of methanol by gasification. A gasifier can produce raw syngas from a variety of biomass feedstocks, including briquette, forest residue, wood, crop residue, and waste biomass. Raw syngas is passed through cyclone, cooler, and scrubber units to produce clean syngas, which is then passed to the methanol synthesis reactor. After condensation and separation of gases, methanol is synthesized from its elemental components. This methanol can be utilized in transportation, energy generation, industrial applications, and the production of additional fuels and chemicals
Here we review overall methanol production and utilization scenario in recent times. This review mainly focuses on the overall production processes available for producing methanol from different possible feedstocks considering both practical experimental and modelling studies in recent times. The techno-economic and environmental effects of production and utilization of methanol/dimethyl ether in different sectors were also discussed. The possibility of dual-fuel application of methanol/dimethyl ether alongside other renewable energies and the emission characteristics have also been reviewed to minimize fossil fuel use and improve the environmental outcomes.
Methods of methanol production
Production of methanol by gasification
The specifications of a biomethanol generation plant are almost similar to that of a coal gasification-based methanol production. The main processes followed by fossil-based methanol production plant include different processes: (a) gasification, (b) gas cleaning, (c) hydrocarbon reforming, (d) water–gas shift reaction, (e) hydrogen addition and/or carbon dioxide removal, and (f) synthesis of methanol and filtration (Galindo Cifre and Badr 2007; Hamelinck and Faaij 2002).
Biomethanol production primarily from biomass sources needs raw material pretreatment (drying and chipping). Then, the processed biomass is gasified to produce syngas. To optimize the hydrogen and carbon monoxide formation and reduce the unwanted water and carbon dioxide amount, oxygen supply is limited during feedstock heating, which is above 700 °C. Removal of the contaminants and impurities is performed before passing the product gas through several conditioning steps for composition optimization. Syngas conditioning is mainly done to produce syngas with at least double hydrogen molecules than carbon monoxide molecules. The optimal hydrogen-to-carbon monoxide, H2/CO, ratio depends on the initial syngas composition and hydrogen availability. There are different ways to alter the concentrations of hydrogen and carbon monoxide as described below (Galindo Cifre and Badr, 2007; Mignard and Pritchard, 2008; Specht et al. 1999).
-
(1)
If the crude syngas contains methane and other light hydrocarbons in small amounts, then catalytic steam reforming at high temperature or auto thermal reforming is used to reform methane into carbon dioxide, carbon monoxide and hydrogen (Abdi et al. 2017; Borole and Greig 2019) as shown in Eqs. (1) and (2).
$${\text{CH}}_{{4}} + {\text{ H}}_{{2}} {\text{O }} \leftrightarrow {\text{ CO }} + {\text{ 3 H}}_{{2}} \quad \Delta {\text{H}}_{{{25}^\circ {\text{C}}}} = { 2}0{\text{6 kJ}}/{\text{mol}}$$(1)$${\text{CH}}_{{4}} + {\text{ 2 H}}_{{2}} {\text{O }} \leftrightarrow {\text{ CO}}_{{2}} + {\text{4 H}}_{{2}} \quad \Delta {\text{H}}_{{{25}^\circ {\text{C}}}} = {\text{ 165 kJ}}/{\text{mol}}$$(2) -
(2)
For optimal methanol synthesis, the early hydrogen concentration of the syngas is usually very low. Equation (3) shows the water–gas shift reaction (WGSR), which is used to reduce the carbon monoxide concentration and increase hydrogen share, producing carbon dioxide and hydrogen from water and carbon monoxide. Carbon dioxide can also be produced directly from flue gases of other processes through amine chemical absorption. Some more carbon dioxide elimination processes include liquid adsorption, cryogenic separation, and membrane permeation (Songolzadeh et al. 2014; Olah et al. 2009; Carvalho et al. 2018b).
$${\text{CO }} + {\text{ H}}_{{2}} {\text{O }} \leftrightarrow {\text{ CO}}_{{2}} + {\text{ H}}_{{2}} \quad \Delta {\text{H}}_{{{25}^\circ {\text{C}}}} = - { 41} {\text{kJ}}/{\text{mol}}$$(3) -
(3)
A separate hydrogen production setup, using water electrolysis or methane steam reforming, can supply the hydrogen to syngas produced. Despite the high cost involved, the water electrolysis process is highly effective, with the product oxygen being used for gasification partial oxidation, replacing the necessity for air/oxygen production from air separation. Using renewable electricity in the water electrolysis step makes the whole process more environment-friendly (Olah et al. 2009; Clausen et al. 2010). However, even if the amount of oxygen needed for gasification is provided by water electrolysis, the cogenerated amount of hydrogen in the process cannot fulfil the need for optimal stoichiometry for methanol synthesis.
$${\text{H}}_{{2}} {\text{O }} + {\text{ electricity }} \to {\text{H}}_{{2}} + {\text{ O}}_{{2}}$$(4)$${\text{CO }} + {\text{ 2 H}}_{{2}} \leftrightarrow {\text{ CH}}_{{3}} {\text{OH}}\quad \Delta {\text{H}}_{{{25}^\circ {\text{C}}}} = \, - { 9}0.{\text{5 kJ}}/{\text{mol}}$$(5)$${\text{CO}}_{{2}} + {\text{ 3 H}}_{{2}} \leftrightarrow {\text{ CH}}_{{3}} {\text{OH }} + {\text{ H}}_{{2}} {\text{O}}\quad \Delta {\text{H}}_{{{25}^\circ {\text{C}}}} = \, - { 49}.{\text{5 kJ}}/{\text{mol}}$$(6)
Therefore, for optimized syngas conditioning, the carbon dioxide removal process is needed with the water–gas shift reaction process (Hamelinck and Faaij, 2002). After completion of conditioning, through catalytic synthesis with catalysts like chromium oxide, zinc oxide, or copper oxide, the syngas is converted into methanol (Yang and Ge, 2016; Specht et al. 1999; Minteer 2011; Lange 2001; Mao et al. 2009; Riaz et al. 2013), as shown by Eqs. (5) and (6). Carbon dioxide addition in the carbon monoxide/hydrogen feed was found to increase the yield of methanol (Saito et al. 1996). A distillation process is also used to remove water generated during methanol synthesis (Speight 2014).
Apart from gasification, methanol can be produced from many different processes using different feedstocks, which are discussed in this section. Although every process has its own significance, the highest biomethanol yield was seen in the case of gasification (Sindhu et al. 2019; Ganesh and Banerjee, 2001; Speight 2011).
Partial oxidation of methane
Partial oxidation of methane is the most commonly used process of methanol production, first studied by W.A Bone in around 1935 (Bone 1935), gaining much popularity thereafter.
Equation (7) shows the POM reaction carried out in high pressure (0.5–15 MPa) environment with catalysis for a high yield of desired products (Gesser et al. 1985). Gesser et al. (1985) and Arutyunovet al. (1996) studied the effect of different parameters, reaction mechanisms, and selectivity of methanol production through partial oxidation of methane. Nozaki et al. (2011) reported a 30% conversion efficiency of CH4 to methanol with 40% selectivity.
Photocatalytic conversion
In photocatalytic conversion, photogenerated hydroxyl radicals generate methyl radicals from methane (Fig. 2), and these radicals produce methanol and hydrogen reacting with additional water molecules (Yang et al. 2014). Hameed et al. studied the effect of impregnation of tungsten trioxide with silver on the methanol production from methane using 355 mm ultraviolet light, where enhancement in hydroxyl radical formation, as well as improvement in conversion efficiency, was observed (Hameed et al. 2014). Taylor and Noceti studied the hydroxyl radical generation process using visual light at 1 MPa, and 94 °C, methanol (per g catalyst per hr) of 1.3 g for steady-state mode, and 43 g with hydrogen peroxide addition was produced (Taylor and Noceti 2000). López-Martín et al. investigated the photochemical methanol production from methane with hydrogen peroxide aqueous solution, which was found to have higher yields compared to other reported photocatalytic approaches (López-Martín et al. 2017) (Table 1).
Synthesis of methanol via photocatalysis and carbon dioxide captured from flue gas. Oxygen and hydrogen are separated from water through photocatalysis, and hydrogen reacts with flue gas-captured carbon dioxide to produce methanol. The methanol produced can be utilized in various energy and other applications
Gondal et al. used a semiconductor photocatalyst to produce methanol from methane by generating hydroxyl radicals with 355 mm ultraviolet light at room temperature (Gondal et al. 2004). They found maximum methane to methanol conversion selectivity of 20%, 29%, and 21% with nickel oxide, tungsten trioxide, and titanium dioxide, respectively. Adekoya et al. did a study to analyse the effect of process parameters in the methanol production process from the reduction of carbon dioxide using a photocatalytic process (Adekoya et al. 2019). Trudewind et al. found lower environmental impacts for photocatalytic methane production compared to photocatalytic methanol production as it is more energy-intensive. This process was found unsuitable in dry regions because of the high-water consumption (Trudewind et al. 2014).
Biological conversion
The biological conversion of methane to methanol is another favourable approach to methanol production. Two groups of bacteria: (a) ammonia-oxidizing bacteria and (b) methanotrophic bacteria are only useful for carbon–hydrogen bond activation in methane. Ammonia is used by ammonia-oxidizing bacteria as a source of energy and produces methanol by partial oxidation of methane (Taher and Chandran 2013). All methanotrophic bacteria use methane monooxygenase to produce methanol by activating methane. Methane is the sole source of energy and carbon for methanotrophic bacteria (Han et al. 2013).
Ge et al. discussed the mechanism, performance, and generic performance modification of conversion of methane into methanol by three microorganism groups (acetogens, methanotrophs, and ammonia-oxidizing bacteria) (Ge et al. 2014). Taher and Chanadran developed a biological process utilizing the ammonia-oxidizing bacteria to produce methanol from methane. The metabolic flexibility of this process to produce methanol from methane was found to be potentially advantageous for the wastewater treatment plant to reduce part of their methanol costs (Taher and Chandran 2013). Su et al. produced a novel continuous process to produce methanol from methane using nitrifying activated sludge, which was efficient in improving wastewater treatment plan efficiency (Su et al. 2019).
Indirect conversion
Another well-known way of methanol production is biogas reforming, followed by methanol synthesis. Effective use of various catalysts like Cu/ZnO/Al2O3, Pd/CeO2, Cu/ZrO2, Mo(CO)6, ZrO2/CuZnO, Cu/CeO2, and Ni/Mo for methanol synthesis have been found (Cai et al. 1997; Lee et al. 2019; van de Water et al. 2018; Barrault and Probst 1991; Jali et al. 2011; Ma et al. 2008). Santos et al. studied methanol generation from different biogas sources: palm oil effluent, corn cobs, landfill, and sorghum fermentation using mathematical modelling and simulation (Santos et al. 2018). Palm oil biogas was the most beneficial, and the landfill gas turned out to be the lowest methanol-producing source (Santos et al. 2018). Ghosh et al. performed methanol synthesis from biogas produced from wastewater sludge, dairy, food, and animal wastes. The use of Cu/Zn/Al/Zr catalyst and steam reforming resulted in a highest of 2100 tons/day methanol production from a 1.8 × 106 Nm3/year biogas input (Ghosh et al. 2019).
Patel et al. also found that regulation of gas composition and hydrogen supplementation can increase the methanol production from biogas (Patel et al. 2016). Jadav et al. discussed the reaction pathway to produce methanol by catalytic hydrogeneration using flue gas captured carbon dioxide and hydrogen from water electrolysis. CuO/ZrO2/ZnO catalysts made in a novel way were found to show good performance in methanol production (Jadhav et al. 2014). Methanol production from hydrogen and carbon dioxide is seen as environmentally and economically beneficial when the utilization of carbon dioxide is more than that of the hydrogen manufacturing process (Raudaskoski et al. 2009). Atsonios et al. studied the economic performance of the methanol production process with various designs and operating aspects of industrially captured carbon dioxide. Methanol generation from the flue gas captured carbon dioxide technique (Fig. 2) was about 2.5 times more than the conventional price (Atsonios et al. 2016).
Anicic et al. performed an economically and energy-efficiency analysis on two methanol production technologies, using carbon dioxide and hydrogen as basic feedstock. The former technology produces methanol from direct carbon dioxide synthesis, and the latter technology involves two steps: (a) carbon monoxide production from carbon dioxide (reverse water–gas shift reaction) and (b) methanol production (Anicic et al. 2014). Boretti studied the carbon dioxide recycling process produced from fossil fuel combustion using renewable hydrogen into methanol (Boretti 2013). Gupta et al. studied the reduction in sulphur oxide and nitrogen oxide emissions with the methanol production in integrated gasification combined cycle plant. Chemical production of hydrogen, ethanol, methanol, and dimethyl ether can also be produced from synthetic gas composed of hydrogen and carbon monoxide (Gupta et al. 2010). Figure 3 shows the methanol production through catalytic conversion by both the traditional route and catalytic hydrogenation.
A Methanol production via the conventional syngas and a Cu/ZnO catalyst. At 850 °C, methane from natural gas and water molecules are reacted over a Ni/Al2O3 catalyst. Then, carbon monoxide (CO) and water react under high pressure with a Cu/ZnO catalyst to produce methanol. B Production of methanol by catalytic hydrogenation of carbon dioxide over a Pd/Zn catalyst. Carbon dioxide and water undergo a low-temperature, low-pressure reaction with a Pd/Zn catalyst to produce methanol
This section explains the two available methanol production processes, including their parameters and benefits. Various studies were discovered discussing various positive outcomes in methanol production using these various methods. All of the variables that influence methanol production have also been discussed. Gasification can be used to produce methanol from both renewable and nonrenewable feedstocks, despite the difficulty of determining which process is superior. Gasification has several advantages over other methods.
Biomethanol production
Biomethanol was generated with many biomass feedstocks through thermochemical and biochemical pathways. As discussed in the previous section, high carbon materials such as waste, biomass, coal, or even carbon dioxide can be used as raw materials for methanol production.
Shamsul et al. studied different types of biomass feedstocks and thermochemical processes used to produce biomethanol: including pyrolysis, gasification, and liquefaction, among which lignocellulosic biomass was found to be one of the effective sources for gasification as well as biomethanol production (Shamsul et al. 2014). Sikarwar et al. discussed the effectiveness of biomass gasification with forest biomass because of their high cellulose and lignocellulosic contents (Sikarwar et al. 2016). Table 2 depicts different biomasses which can be used for biomethanol production with their respective cellulose hemicellulose and lignin contents. This also gives an idea about the status of different lignocellulosic biomass used so far for the methanol production study.
Some potential energy crops for biomethanol production are maize, beets, sugarcane, sweet sorghum or yam (Singh et al. 2014). Nakagawa et al. discussed the use of different biomass resources like Japanese cedar bark and sawdust, salix, waste wood, sorghum, chipped Japanese larch, straw, forage grasses, rice husk, bamboo, trees, and crop residues to produce biomethanol and found that lignocellulosic biomass resources like wood materials and rice bran can provide a 55% methanol production. However, a much lower yield of 36% and 39% was found with rice straw and rice husks, respectively (Nakagawa et al. 2007). Many Nigerian biofuels projects utilized 1st-generation biomass feedstocks, mainly food crops. Ben-Iwo et al. discussed the potential of using the agricultural, urban, forest, and other wastes as biomass resources available in Nigeria to fulfil its biofuel demand and for maximizing the use of its natural assets (Ben-Iwo et al. 2016).
Carvalho et al. studied the techno-economic feasibility and advantages of catalytic synthesis of methanol via gasification of lignin and forest residues (Carvalho et al. 2017). Suntana et al. found a possibility of 86% of Indonesian village electrification with the help of methanol production from forest biomass, thereby using it in a fuel cell (Suntana et al. 2009). Xiao et al. performed an analytical study of the energy, environmental and economic performance of biomethanol production with rice straw in China (Xiao et al. 2009). According to Kumar et al., India has surplus biomass availability of about 500 million metric tons/year with a power generation capacity is 17,500 megawatts. They predicted a 50% growth in biomass power production by 2023 and an increase of up to 55 gigawatt electricity of installed biomass power capacity by 2020 (Kumar et al. 2015). Methanol production from biogas as a primary feedstock with steam reforming and catalytic synthesis processes was also found in many pieces of literature (Santos et al. 2018; Ghosh et al. 2019; Patel et al. 2016; Vita et al. 2018).
Several studies used Aspen Plus modelling and discussed the production of methanol from syngas through the methanol synthesis process (Arteaga-Pérez et al. 2016; Liu et al. 2016; Puig-Gamero et al. 2018; Yin et al. 2005). A techno-economic Aspen Plus modelling-based study of methanol production with a biomass feed of 15 kg/s was found to give good methanol yield results (Bai et al. 2015). Yang et al. used biomass as the main feedstock to design and model a theoretical, highly efficient biomass-to-methanol process (Yang et al. 2018). Similarly, AlNouss et al. discussed a novel poly-generation system for producing power, high-quality urea, methanol, and Fisher–Tropsch liquids considering Qatar’s different biomass feedstock (AlNouss et al. 2019). Carvalho et al. used an Aspen Plus simulation model with a thermodynamic equilibrium model to study the downstream production process of biomethanol from catalytic biomass gasification of various solid feedstocks (Carvalho et al. 2017).
Methanol production based on carbon capture and utilization was considered in many different studies (Bonfim-Rocha et al. 2018; Van-Dal and Bouallou, 2013). Rodionova et al. studied the production of biomethanol from microalgae such as Spirulina sp. by gasification (Rodionova et al. 2017). Using 50% nonrecycled plastics with 50% biomass as a feedstock, Citua et al. produced a syngas mixture with a 1.21 hydrogen-to-carbon ratio and 219.1 Btu/cf higher heating value. The methanol yield from 50% biomass-50% nonrecycled plastics as feedstock was 1.44 times compared to 100% biomass (Ciuta et al. 2018). AlNouss et al. used sewage sludge, date pits, and manure as feedstocks to optimize the gasification process for biomethanol production (AlNouss et al. 2020).
This section discusses various feedstocks used to produce biomethanol, as well as the parameters and processes employed. Gasification was the most important process for producing syngas from various biomass feedstocks. The ratio of hydrogen to carbon and the temperature of the produced syngas significantly affects the quality of biomethanol. Comparisons have also been made between the characteristics of various feedstocks and their respective methanol yields and regions of study (Table 3).
Parametric evaluation of methanol production
The techno-economic viability of the methanol production, yield, and production efficiency largely depends on the optimization of different process parameters discussed in this section. Shamsul et al. (2014) discussed the potential of biomethanol as a renewable energy source by considering the world demand, power density, economic assessment, and possible applications. Effects of different process parameters, like heating rate, temperature, catalyst types, particle size, main conversion process, and residence time for high yield of biomethanol, were also studied. They mentioned that improvements in methanol synthesis were possible by improving the efficiency of electrolysis and renewable electricity.
Manenti et al. studied the thermal conversion ways of lignocellulosic residuals into biosyngas and then into biomethanol (heterogeneously catalysed synthesis) with the help of mathematical modelling. They also graphically represented the water-cooled/gas-cooled length ratio and shell temperature effect on total biomethanol yield. The optimum length ratio was 1.121, with a shell temperature of 247 °C and additional biomethanol synthesis of 0.4 mol % (Manenti et al. 2015). Kasmuri et al. analysed the influence of various proportions of biomass raw materials (i.e. moisture content, volatile matter content, ash and fixed carbon content) for higher biomethanol yield. They discussed the effects of proximate analysis, particle sizes and energy efficiency for the maximum yield of biomethanol. Sugarcane bagasse of particle size range of 5–10 mm was selected as feedstock for the pyrolysis process and achieved 2.41 weight % of biomethanol yield was achieved with 6% of energy (Kasmuri et al. 2016). Citua et al. mentioned a 44% increase in methanol production by increasing the nonrecycled plastics from 15–50% because of an increase in the hydrogen-to-carbon ratio in the feedstock (Ciuta et al. 2018). Anicic et al. found that the electricity cost had the highest impact on methanol production as hydrogen was generated from water electrolysis (Anicic et al. 2014). Direct methanol synthesis was found to have higher energy and economic efficiency. The carbon dioxide capture-unit location influenced the inlet gas production and the final methanol price (Atsonios et al. 2016).
Liu et al. (2016) studied the influence of process parameters like biomass particle size, steam-to-biomass ratio, gasification temperature and equivalence ratio on methanol yield. It was observed that a higher equivalence ratio corresponds to a lower yield of methanol and higher temperature contributes to higher methanol yield. Yang et al. found 18.5 mol/kg dry biomass improvement in the yield of methanol by adding carbon dioxide and steam into the gasification process and applying a slurry phase synthesis reactor. The steam-to-biomass ratio mentioned in their study was 0.35–0.45 with 0.45–0.55 carbon dioxide-to-biomass ratio (Yang et al. 2018). Yin et al. mentioned that the operating parameters affected the selectivity and yield of methanol produced from biosyngas. Methanol yield was dependent on the hydrogen-to-the carbon dioxide-to-carbon monoxide ratio, more influenced by (carbon monoxide + carbon dioxide) ratio and methanol selectivity. The yield of methanol from catalytic gasification was more than the air steam gasification, but in the case of selectivity of methanol, it was found to be the reverse. Partial carbon dioxide removal was found to improve methanol yield and selectivity (Yin et al. 2005). Zhang et al. observed a maximum yield of methanol of 12.19 mol/(kg biomass (dry and ash-free)) with 750 °C operating gasification temperature and pressure approaching ambient value, maintaining a steam-to-biomass ratio of 0.4–0.5. The use of interconnected fluidized bed technology also increased the methanol yield by increasing steam/biomass ratio, gasifier temperature, and hydrogen content and decreasing carbon dioxide and carbon monoxide (Zhang et al. 2009).
This section attempts to summarize the literature concerning the biomethanol production process's various parameters. The characteristics of the biomass feedstock were found to have a significant effect on the final biomethanol yield. Forest biomass with a high cellulose and hemicellulose content was found to be ideal for gasification-based biomethanol production (Sikarwar et al. 2016). The steam-to-biomass ratio in the production process also affected the biomethanol yield.
Techno-economic analysis of methanol utilization
Modelling-based approach
Regarding the production of biomethanol, different researchers used modelling-based analysis techniques to determine the possible outcomes with different resources and parameters. The techno-economic analysis of production and utilization of biomethanol was mainly done with Aspen Plus model simulations, thereby comparing the results with other available literature. Table 4 shows the parameters associated with different modelling analyses of the methanol production process.
The use of Aspen Plus for analysis of the thermo-economic performance of a proposed solar-driven biomass gasification polygeneration process for generating electricity and methanol was reported by Bai et al. Biomass gasification was conducted in a temperature range of 727–1227 °C. The syngas' ideal hydrogen-to-carbon monoxide molar ratio for the methanol synthesis was 1.43–1.89. The proposed system achieved the highest energy and exergy efficiency of 56.09% and 54.86%, respectively (Bai et al. 2015). Arteaga-Pérez et al. developed a comprehensive simulation modelling to show that synthesis gas can produce synthetic natural gas and methanol with a gasification temperature of 800–850 °C and an equivalence ratio of 0.25. The gasifier's energy efficiency was 74%, and the overall methanol and synthetic natural gas production was reported as 0.59 kg methanol/kg dry biomass and 0.33 kg synthetic natural gas/kg dry biomass, respectively (Arteaga-Pérez et al. 2016). Liu et al. also discussed the production of methanol from biosyngas using Aspen Plus model. For better accuracy of the results, they divided the circulating fluidized bed gasifier reactor axially into two parts: a dilute upper region and a lower density region. The model prediction results were also observed to be in good agreement compared with other literature (Liu et al. 2016).
Yin et al. performed a methanol synthesis process from biosyngas in a microreactor under high pressure using Cu/ZnO/Al2O3 catalyst. They configured and studied four different biosyngas models with various ratios of H2/CO/CO2/N2. They conducted a series of experiments under various pressures of 4.6, 3.6, and 2.6 MPa, within a 215–270 °C temperature range and with 4000–12,000 /h space velocity (Yin et al. 2005). Puig-Gamero et al. developed a simulation model to study the syngas–methanol synthesis obtained from the gasification of pine biomass. A temperature of 900 °C and a steam-to-biomass ratio of 0.9 were the best operational conditions for producing methanol. For the decomposition of tar use of dolomite as a catalyst was mentioned. They used a pressure swing adsorption process by which about 80% of the carbon dioxide and 95% of the methane were isolated. Moreover, for optimal methanol synthesis, a pressure of 55 atm and a temperature of 220 °C was found best. 30% reduction in the required carbon to burn was also achieved by recycling the waste stream to the combustion chamber (Puig-Gamero et al. 2018).
Ramachandran et al. developed a simulation model to produce biomethanol by replacing part of natural gas with pure glycerol (up to ∼54%) through syngas production at 900 °C temperature with steam-to-carbon ratio ∼3. The prices of the overall capital investment and feedstock (glycerol and natural gas) influenced the biomethanol production cost. They found biomethanol from a hybrid steam reforming process to be more economically attractive with natural gas of more than 0.5 USD/Nm3 or glycerol price of less than 100 USD/tonne (Balegedde Ramachandran et al. 2013). Bassani et al. proposed a novel methanol production process from coal gasification. They used Aspen HYSYS® for the process simulation and GASDS for the simulation of the coal gasifier. Their novel approach resulted in 388.54 kmol/h syngas production with 181.78 kmol/h methanol production, which was increased by 1.7% for both cases compared to the traditional process. The carbon dioxide emission was found to be 116.49 kmol/h which was about 2.5% lower than the traditional methanol production (Bassani et al. 2017). Milani et al. developed a natural gas-based methanol synthesis model with the help of an Aspen Plus V8.4 model to integrate carbon dioxide in a methanol synthesis plant based on natural gas. They mixed syngas with the carbon dioxide-rich stream (265.9 t/h) collected from a 660 MWel base power plant carbon capture process. This integration was advantageous in reducing methane uptake by 25.6% (reducing 116.1 mtons/h of methane consumption) and decreasing the overall carbon dioxide emissions by 21.9% (1783.4 \(\text t_{\text{CO}_2}/\text h\)) for both plants. The methanol production rate was 766 t/h with a methanol-to-methane equivalent ratio of 2.27 (Milani et al. 2015).
Qatar’s different biomass feedstock properties were studied by AlNouss et al. for producing power, high-quality urea, methanol, and Fisher–Tropsch liquids using the Aspen Plus model of a novel poly-generation system (AlNouss et al. 2019). The methanol production technique with a profit of 0.035 USD/kg input biomass was found to be the most economically feasible process pathway, while with a carbon dioxide reduction potential of 0.71 kg carbon dioxide/kg biomass feed, the urea process pathway was observed to have the lowest environmental effect. AlNouss et al. optimized the gasification process for different biomass feedstock (sewage sludge, date pits and manure), developing an Aspen Plus simulation model. The syngas was found to have maximum hydrogen content at around 850 °C and 1 bar, with a modified of 2.5 and an air-stream ratio of 0.6 (AlNouss et al. 2020). A highly efficient biomass-to-methanol process overall biomass consumption of 1.99 t/t methanol was found for their system with an exergy efficiency of 70% (Yang et al. 2018). The biomass-to-methanol process is more utilization efficient and environment-friendly as its water consumption and carbon dioxide emission were only 5.88 t/t methanol and 1.46 t/t methanol produced, respectively, which were found to be much smaller than the coal-to-methanol process. Pellegrini et al. (2011) studied the economic viability of a proposed combined energy-methanol plant using the Aspen HYSYS® process simulator. Although the combined production requires high capital cost, the payback period was less than 5 years.
Xiao et al. conducted a life cycle assessment to determine the effect of pollutant emissions in biomethanol production with an Aspen Plus simulation model of methanol production through fluidized bed gasification. The methanol yield was 0.308 kg/(kg rice straw) with a 42.7% energy efficiency. Considering a biomethanol plant with 50,000 tons of annual production, the total production cost of biomethanol was 387 USD/t, out of which 338.35 USD/t is the economic cost and 38.65 USD/t is the environmental cost. From the life cycle assessment, rice straw as a material for methanol production was found favourable for agricultural waste utilization and environmental improvization (Xiao et al. 2009). Zhang et al. studied the effects of process parameters, like steam/biomass ratio, gasification pressure and temperature, and temperature and pressure for liquefaction, on the methanol yield for calcium carbonate catalysis. The product gas was found to have a maximum hydrogen content of 82.14% at 700 °C gasification temperature by adding calcium carbonate to the biomass gasification process (Zhang et al. 2009).
Carvalho et al. studied the effect of catalysts on gasification reactions by determining methanol yields (on an energy basis), economic performance, energy efficiency and overall systems efficiency. Large-scale biomass gasification via alkali aided entrained flow process for methanol production (for a 99,423.75 USD/MWh selling price) was cost-competitive for other biofuels. The addition of lignin was also found beneficial but was economically viable for lignin prices below 27.58 USD/MWh (Carvalho et al. 2017). Phillips et al. studied the zeolite catalyst (ZSM-5) 's feasibility in producing gasoline from methanol using Aspen Plus. A metric tonne of dry biomass produced 229.9 L and 38.8 L of gasoline and liquefied petroleum gas, respectively, with a final price of 15.73 USD/GJ for gasoline and liquefied petroleum gas together. The gasoline and liquefied petroleum gas cost were found to be 0.52 USD/L and 0.40 USD/L, respectively (Phillips et al. 2011).
Gasoline and butanol production by using methanol from sugarcane bagasse gasification was discussed by Michailos et al. They performed a thermo-economic and environmental analysis using Aspen Plus and MATLAB software. They found that gasoline from methanol was economically more viable, while low carbon dioxide emissions were seen for butanol productions (Michailos et al. 2016). Li et al. developed an Aspen Plus process simulator model to optimize hydrogen-to-carbon monoxide ratio production of methanol, which resulted in a reduction of 9% material input for co-feeding of natural gas and biomass compared to the individual system. With natural gas/biomass feed of 2, the highest value of 10% energy saving ratio was achieved. The methanol-to-power output ratio was 1.5 to 2.1 (Li et al. 2010). Iaquaniello et al. studied the environmental and economic feasibility of the waste-to-methanol process. They simulated the process using the PROII process simulator. The process involved high-temperature mixing refuse-derived fuels with oxygen for syngas and methanol production. For a waste-to-methanol production plant with a 300 t/d plant with a 29% return on investment, the estimated cost of production of biomethanol from refuse-derived fuels was about 122.5 USD/t. The waste-to-methanol process was found to produce methanol with a reduction in greenhouse gas emissions of about 40% and 30–35% compared to fossil–methanol and biomethanol, respectively (Iaquaniello et al. 2017).
Bonfim-Rocha et al. discussed the technical and economic performance of a new proposed biomethanol production scenario in Brazil using carbon dioxide from ethanol production plants. They developed an Aspen Plus® industrial methanol plant model and optimized it using MATLAB®. They found that 1136–1988 t/year methanol production was found possible with the cost of production (0.51–0.62) USD/kg with a carbon dioxide reduction potential of (−2198 to −1814) t/year (Bonfim-Rocha et al. 2018). Similarly, the methanol production process by flue gas carbon dioxide capture and absorption with the help of Aspen Plus was also reported by E. S. Van-Dal and C. Bouallou. The methanol plant provided 36% of the total thermal energy required for carbon dioxide capture. They found that carbon dioxide reduction potential of 1.6 t/t of methanol production (Van-Dal and Bouallou, 2013).
Zhang et al. discussed the influence of pressure (1–5 atm), temperature (400–1200 °C) and the ratio of methane-to-flue gas (0.4–1.0) on the quality of syngas production using an Aspen Plus model of the tri-reforming methanol production process. The optimum condition for maximum methanol production was 1 atm, 850 °C, with a methane-to-flue gas ratio of 0.4. 99% conversion of carbon dioxide and hydrogen-to-carbon dioxide ratio of 2 was achieved under the optimum condition (Zhang et al. 2013). Luu et al. studied methanol production based on carbon capture and utilization from a 265.9 t/h power plant. They proposed different methodologies for a comparative study of different parameters like: (A) energy intensity, (B) intensity of methane production, (C) intensity of carbon dioxide feed, and (D) intensity of carbon dioxide emission with an Aspen Plus model (Luu et al. 2015).
Soni et al. performed a simulation using AVL FIRE CFD software on a one-cylinder diesel engine (TV1 model) undertaking a two-stage emissions control strategy. Initially, low load mathematical simulation was done to find the ‘optimum blend’. In the second stage, where the influence of exhaust gas recirculation variation (10% and 20%), initial swirl ratio (1.0, 1.3, 1.6, and 2) and water addition (5%, 10%, and 15%) (under the same operating condition) in case of the optimum blend were studied for emission characteristics. They also tried to find the best emission reduction method by analysing the brake-specific fuel consumption (BSFC) and brake thermal efficiency for pure diesel, diesel–methanol fuel. D + M30 was considered as the optimum blend with a 30% increase in methanol, with a maximum carbon monoxide, hydrocarbon, and nitric oxide emission reduction of 58%, 65%, and 27%, respectively, achieved compared to pure diesel (Soni and Gupta 2016).
Zhou et al. studied the combustion emission characteristics and performance of a methanol–biodiesel dual-fuel reactivity-controlled compression ignition engine using three-dimensional numerical simulation. A good agreement was found for ignition delay, heat release rate, and cylinder pressure in and when the predictions of the developed mechanisms were compared with that of experimental results. At a 20% interval, the methanol input amount varied between 0 and 80%. With the increase of methanol, no tangible change was observed in nitrogen oxide emission under medium and high loads. However, emission was found to decrease remarkably at 10% load. They concluded that induction of premixed methanol resulted in stronger engine performance under medium and full loads, simultaneously reducing the ringing intensity and avoiding engine knocking (Zhou et al. 2015).
Rivera-Tinoco et al. studied a power-to-methanol technology's technical and economic feasibility with Aspen Plus V8.0 simulation model. It was found that the influence of life-span and capital expenditure on the methanol production cost was higher for the solid oxide electrolyser cell methanol process. Again, for the proton exchange membrane/methanol process, the predominance of operating expenditure on the methanol cost was observed. The production cost of methanol was 546.583 USD/tonne in the case of SOEC/methanol and 89.2115 USD/tonne for the proton exchange membrane/methanol process, which were 1.5 and 2.5 times that of the methanol market price, respectively (Rivera-Tinoco et al. 2016).
Experimental investigation
Different experimental studies have also been reported regarding the techno-economic feasibility of methanol production. This section contains studies related to techno-economic production utilization of methanol mainly via gasification (using biomass, black liquor, pyrolysis oil), pyrolysis, integrated biomass pyrolysis–gasification, and methanol synthesis (IBPGM) which have been discussed. In Table 4, different parameters related to the study of methanol production have also been summarized. The environmental concern and different mixed ways of methanol utilization can also be found in other works of the literature which are also discussed below.
Brigagão et al. investigated the technical and economic feasibility of three energy generation pathways using corncob: (a) GASIF (methanol production via gasification), (b) PYROL (bio-oil production via fast pyrolysis), and (c) COMB (electricity production via combustion). Results showed that 79% of energy recovery in its products could be achieved with PYROL, 38.4% for production of bio-oil, and 40.6% in the case of biochar, which is higher than methanol production via GASIF (53%). Reduction in carbon dioxide emission for PYROL and GASIF is 73.7% and 35.4%, respectively. They found that all three alternatives are economically feasible for low biomass prices (less than 75.5 USD/t) (Brigagão et al. 2019). Again, the techno-economic prospects to produce methanol using a Mitsubishi methanol converter (super converter) from syngas obtained from the absorption enhanced reforming process were studied and discussed by Amigun et al. Capital investments were termed as the major factor in determining the methanol production cost. From the results, the overall production cost of methanol was found to decrease to about 2.89 R/l in the case of a 2000 MWth methanol plant, from about 10.66 R/l (Rand/litre) in the case of a 10 MWth plant, with intermediate values of 6.44 R/l (for 60 MWth Plant) and 3.95 R/l (for 400 MWth plant) (Amigun et al. 2010).
McFarlan studied the techno-economic feasibility of replacing diesel with clean biofuels (methanol and dimethyl ether) for electricity generation in Canada (McFarlan 2018). The overall cost of electricity production with diesel for the three different sites was found to vary with the annual demand for electricity of 660 USD/MWh (for 3300 MWh plant), 890 USD/MWh (for 1500 MWh plant), and 1460 USD/MWh (for 1400 MWh plant), respectively. The full cost of electricity was found to rise by 46 USD/MWh, 85 USD/MWh, and 66 USD/MWh when methanol was used, and a rise of 40 USD/MWh, 110 USD/MWh, and 44 USD/MWh was found for dimethyl ether. Diesel power generation also had an additional carbon dioxide penalty of 80 USD/MWh (30 USD/ton carbon dioxide), which is not present for biofuel-based electricity generation (McFarlan 2018).
The technical aspects and economic analysis of co-gasification of black liquor and pyrolysis oil to produce methanol were discussed by Andersson et al. by changing the blending ratio and mill capacity. Pure black liquor and up to 50% pyrolysis oil-black liquor blends gasification were found to lower the production costs significantly compared to unblended pyrolysis oil gasification. With an internal rate of return of 20%, the cost of methanol produced was lowered below 110.35 USD/MW for plant capacities between 200 and 800 MW (Andersson et al. 2016).
Techno-economic performance and feasibility of methanol synthesis with a bio-oil integrated gasification process using rapeseed oil, miscanthus seed, and wood as raw materials were designed and discussed by Ng and Sadhukhan (Ng and Sadhukhan 2011). Kempegowda et al. (2012) discussed the techno-economic performance of a combined heat and power process for biomethanol production using raw biofuel, waste biomass, and waste glycerol. The hydrogen-rich gas produced in the methanol synthesis process is sent to the pyrolysis/gasification reactor for recycling to enhance overall efficiency. The initial investment cost for a small-scale biomethanol production plant of 2 t/h capacity in combination with a 2 MWe combined heat and power plant was mentioned as 170.5 million USD. This process of biomethanol production produced a positive net present value (minimum 600 USD/t) and internal rate of return (Kempegowda et al. 2012). Moellenbruck et al. performed an economic analysis considering the investment costs, carbon dioxide emission costs, and the market price of electricity, methanol, and oxygen. The developed thermodynamic model reduced the emission of 150,000 t carbon dioxide/year, generating additional revenue of 5.74 million USD per year (Moellenbruck et al. 2018).
Zakaria et al. (2016) studied different processes and related technologies associated with the methanol production from methane. They discussed the process influencing factors, their properties, challenges, and recent advancement in different conversion processes, like photocatalytic conversion, biological conversion process, plasma technology, and conventional catalytic conversion process. Zhang et al. performed a comparative study, and investigation of solid oxide electrolyser cell (SOEC) integrated with entrained flow gasification-based methanol production system (SOEC case) is with the traditional methanol production from biomass conversion system, integrated with water–gas shift reaction (base case). With a fixed methanol mass yield (both cases) of around 69.4 t/hr, the energy efficiency for the SOEC case (59.1%) was found to be higher than the Base case (47.95%). The biomass consumption for the SOEC case was 51% of the Base case. Integration of SOEC was also found to store renewable electricity of 3.09 kW/kg of methanol produced (Zhang et al. 2019). Häggström et al. successfully synthesized methanol from syngas obtained from black liquor gasification. The reactor was run for 45 h under a pressure of 2.5 MPa and 0.16–0.19 g methanol/g catalyst/h was generated (Häggström et al. 2012). The power-to-methanol concept for Greece was discussed by considering two cases related to investment in power to methanol: a) owner case and b) investor case. Methanol cost for the owner case was estimated at 464.58 USD/t, 40% less than the estimated methanol cost for other cases (640 USD/t) (Kourkoumpas et al. 2016).
A techno-environmental feasibility study of the IBPGM process using rice straw was performed by Im-orb et al. where an increase in carbon dioxide recycle fraction improved the production rate of methanol, but a decrease in total consumed energy and efficiency were seen. The IBPGM process achieved the best results, with a bio-oil and methanol production rate of 0.09 and 0.23 kmol/h, respectively, and a carbon dioxide recycles fraction of 0.2. Biomass input of 1 kmol/h resulted in a 60.7% energy efficiency of the process (Im-orb and Arpornwichanop 2019). Brynolf et al. did a comparative analysis to study the environmental performance of four different fuel sources: (a) biomethanol, (b) liquefied natural gas, (c) liquefied biogas, and (d) methanol. Using biomethanol and liquefied biogas were found to have more positive environmental impacts (Brynolf et al. 2014). Kumabe et al. studied the economic and environmental feasibility of a biomethanol production process concerning carbon dioxide emission. They found a 31% energy recovery rate in the biomass to the methanol production process, much higher than the conventional wood-based power plant efficiency (Kumabe et al. 2008).
Environmental analysis of using renewable methanol as a fuel instead of conventional fossil fuels in the shipping industry was performed by Svanberg et al. Renewable methanol was found to be technically viable with no major challenges in potential supply chain management to reduce emissions from marine shipping. Marine engines were also found suitable for using methanol at a lower purity level than the presently available level (Svanberg et al. 2018). Hasegawa et al. studied and compared carbon dioxide mitigation for the synthesis of methanol and fermentation of ethanol fermentation. According to their study, bioethanol is more preferred as a substitution for gasoline in the short-term perspective, but in the long run, biomethanol was found to have advantages in terms of carbon capture, energy production, and emission reduction. The carbon dioxide emission reduction in the case of biomethanol-operated fuel cell vehicles was found to be 60% larger than that of the other biofuel-employed internal combustion engine cases (Hasegawa et al. 2010).
Using carbon dioxide captured from flue gases to produce methanol is advantageous for the reduction in environmental impacts as well as gaining much popularity in recent pieces of literature. Luu et al. studied and analysed the carbon dioxide utilization process related to methanol production by integrating the geosequestration process and the enhanced gas recovery system. They analysed the methanol synthesis process based on five process flowsheet configurations: (a) productivity of methanol, (b) intensity of carbon dioxide, (c) intensity of thermal energy, (d) intensity of methane, and (e) flexibility of carbon dioxide consumption. They found that integrating the new processes was favourable for higher methanol production with less carbon dioxide emission (Luu et al. 2016).
Ribeiro et al. used pressure swing adsorption technology to perform stoichiometric adjustments of the biosyngas and carbon dioxide capture. The process resulted in recovering 99.7% carbon dioxide and 99.5% hydrogen with a purity above 95% by consuming a total power of 0.841 MW. Process optimization was beneficial for reducing 30% power consumption but resulted in a little decrease in % recovery (Ribeiro et al. 2010). A novel approach to producing biosyngas with high carbon monoxide amount with the help of catalytic (Ni/Al2O3) conversion and biochar during the bio-oil to biomethanol conversion process was developed by Xu et al. According to the results, 99% selectivity and the highest methanol yield of 1.32 kg/h/kg catalyst were achieved. Carbon monoxide production from carbon dioxide is highly influenced by the thermal electrons and nickel present in the Ni/Al2O3 catalyst. After the biosyngas conditioning process, the carbon dioxide-to-carbon monoxide ratio decreased from 6.33 to 0.01–0.28 (Xu 2011).
This section discusses the techno-economic analysis of methanol utilization in different ways. In both modelling and experimental approaches, methanol was widely used as an alternative source of energy and power. Different modelling studies discussed different biomass-based gasification processes to produce methanol using modelling software like Aspen Plus, MATLAB, and Aspen HYSYS. Similarly, in experimental studies, the production and utilization of methanol were studied in different regions of the world using different processes. The efficiency of the processes and economic feasibility were also discussed. Using methanol as a fuel source positively impacted both environment and production cost.
Hybrid energy generation with methanol
Dual-fuel application
Blending methanol with other liquid fuels like diesel/biodiesel (Qi et al. 2010; Duraisamy et al. 2020; Sayin, 2010), dimethyl ether, and natural gas (Wang et al. 2019) (Chen et al. 2019a) has evolved as a promising way of using methanol for reducing carbon dioxide and nitrogen oxide emissions diesel and spark ignition engines. In this section, experimental studies related to methanol-based dual-fuel engine performance have been discussed. Table 5 summarizes the key findings related to the dual-fuel application studies reported.
The feasibility of methanol in spark ignition engine and other fuels in dual-fuel mode has been studied in various ways by researchers. The performance of a novel dual-fuel methanol-natural gas spark ignition (M/N DFSI) engine with methanol port-injection modification was studied by Chen et al. under three load conditions: light, medium, and heavy (brake mean effective pressures of 0.387, 0.775, and 1.163 MPa, respectively), with a relative air–fuel ratio of 1.3 and keeping the engine speed constant at 1600 rpm. Flame development and propagation period were found lower with higher methanol substitution. An increase in peak cylinder pressure and temperature, brake thermal efficiency, and heat release rate was also found with higher methanol addition; however, brake-specific fuel consumption decreased (Chen et al. 2019a). The influence of the injection timings and injection amount/cycle for methanol–liquefied petroleum gas mixture on emissions, combustion, and cold start firing for a dual-fuel methanol–liquefied petroleum gas spark ignition engine was studied by Gong et al. experimentally. The engine's maximum transient speed and maximum cylinder pressure were found to increase with the injection amount/cycle increase for methanol and liquefied petroleum gas. With the minimum injected amount, more reliable engine firing was found in the case of liquefied petroleum gas compared to methanol under low ambient temperature conditions (Gong et al. 2019).
Liu et al. tried to improve the fuel economy and reduce particle number emission through an experimental investigation on a methanol gasoline dual-fuel spark ignition engine. The intake port was used to inject methanol into the engine to reduce particle number emissions and enhance fuel economy. The stoichiometric air–fuel ratio was maintained throughout the experimentation. According to the results, an increase in methanol addition improved fuel economy by reducing brake-specific fuel consumption. Moreover, the total particle number of the engine was found to reduce to a minimum of 5 × 104 N/ml, with a 99.6% reduction compared to that of baseline (Liu et al. 2015a).
The effects of the compression ratio, ignition system, and injector configuration on the combustion characteristics and brake thermal efficiency of a methanol spark ignition engine (high-compression direct-injection) under light load operation were investigated experimentally by Gong et al. The methanol engine was found to have 25% more brake thermal efficiency than that of one using single spark ignition system at a 0.11–0.29 MPa brake mean effective pressure with an engine speed of 1600 rpm. Reducing the compression ratio from 16:1 to 14:1, the brake thermal efficiency increased and decreased under low and high loads, respectively (Gong et al. 2011). Another study of analysis of combustion performance of a natural gas engine under the addition of ethanol, n-butanol and methanol was performed by Chen et al. The experiments were performed using a dual-fuel engine under a light load with stoichiometric equivalent air–fuel ratio, maintaining a constant speed at 1600 rpm, with 0.387 MPa brake mean effective pressure. Four different alcohol fuel energy substitution ratios, viz. 0% (pure natural gas), 19%, 44%, and 60%, were used to analyse the engine performance and found that pure natural gas combustion has the slowest burning rate. Flame development and propagation time, and nitrogen oxide emissions, were found to get reduced with increasing alcohol fuel energy substitution ratios (Chen et al. 2019b).
Studies related to the use of methanol with diesel have recently gained high popularity for the reduction of diesel in the industrial and transport sectors. Duraisamy et al. discussed the effect of methanol-diesel and methanol-polyoxymethylene dimethyl ether (PODE) fuel mix in reactivity-controlled compression ignition (RCCI) combustion using a 3-cylinder turbocharged diesel engine at 1500 rpm under a brake mean effective pressure of 0.34 MPa. Results showed that RCCI combustion with low-methanol-high-PODE fuel mix improved the brake thermal efficiency, ignition lag, and the combustion time compared to diesel-methanol dual-fuel (DMDF) RCCI combustion. The soot, hydrocarbon, and carbon monoxide emissions were found under control using highly reactive PODE as fuel. However, the nitric oxide emissions were higher for methanol-PODE RCCI combustion than DMDF RCCI combustion as PODE has intermolecular oxygen (Duraisamy et al. 2020).
In a similar study, using a turbo-charged, heavy-duty, 6-cylinder modified M/N DFSI engine under excess air–fuel ratio and addition of methanol, the combustion performance, characteristics, and emissions results were analysed by Wang et al. Experiments were performed with low engine load, at a 0.387 MPa brake mean effective pressure, maintaining a fixed 1600 rpm engine speed. In this case, methanol addition also helped decrease the flame development and propagation period, thereby improving brake thermal efficiency and lowering equivalent BSFC along with total hydrocarbon emission. With increasing air–fuel ratio, the brake thermal efficiency and total hydrocarbon emissions increased, while natural gas burning rate and nitric oxide emissions decreased. The burning rate of natural gas increased by adding methanol at air–fuel ratios = 1.5 and 1.6 (Wang et al. 2019). Wei et al. performed an experimental analysis to study the emission and combustion behaviour of a 6-cylinder DMDF engine with a high methanol premixed ratio. Experiments were performed with a maximum methanol premixed ratio of over 70%. Through the intake port, methanol was injected, and ignition was achieved by injecting diesel directly into the cylinder. They found that with high methanol premixed ratio, shorter combustion duration was achieved with increased ignition delay and increased carbon monoxide, hydrocarbon, and formaldehyde emissions. With increasing methanol premixed ratio, the nitrogen oxide emission and dry soot emission decreased, but nitrogen dioxide emission increased (Wei et al. 2015).
Yusaf et al. also observed higher torque and power output in the case of the diesel–methanol fuel blend compared to the pure diesel fuel. There is higher temperature of the exhaust gas and BSFC for pure diesel mode. Adding 10% methanol to the pure diesel was found to have a high influence on the engine's performance (Yusaf et al. 2013). Ma et al. reported improved brake thermal efficiency and reduced replacement ratio (SR) of diesel–methanol dual-fuel combustion by increasing intake air temperature (Ti) up to 65 °C from 35 °C and rising cooling water temperature. Again at 65–100 °C range, SR decreased, but thermal brake efficiency was seen as constant. More cooling loss was seen in the case of diesel–methanol dual-fuel combustion in every case than pure diesel combustion (Ma et al. 2019).
Liu et al. found lower indicated mean effective pressure (IMEP) at low injection pressure for diesel–methanol dual-fuel mode compared to pure diesel (D) mode. With increasing injection pressure, the BSFC in the diesel–methanol dual-fuel mode decreases; however, BSFC mostly is larger than in the diesel combustion mode. Diesel–methanol dual-fuel mode was found to have an ignition delay compared to D combustion mode. Smoke and nitrogen oxide emissions for diesel–methanol dual-fuel mode were lower than the D mode over all test conditions, but nitrogen dioxide emissions increased. Again, compared to the D mode, diesel–methanol dual-fuel mode was found to have higher hydrocarbon and carbon monoxide emissions but lower carbon dioxide emissions (Liu et al. 2015b).
Although diesel–methanol dual-fuel mode of operation was found to have several advantages compared to the pure diesel mode of operation, some mixed results can also be seen in different works of the literature. Pan et al. also conducted testing operations using an intercooled heavy-duty turbocharged diesel engine with 6-cylinder at a fixed load and maintaining a 1500 rpm engine speed. In diesel–methanol dual-fuel mode of operation, reduction in intake air temperature made the exhaust gas's thermal efficiency (indicated) and temperature fall. With a large amount of methanol addition in the fuel blend, a decrease in nitrogen oxides, nitric oxide, and smoke emissions is seen, but formaldehyde, nitrogen dioxide, total hydrocarbon, carbon monoxide, and methanol emissions were found to get higher (Pan et al. 2015).
Intake charge temperature was found to be the most influencing parameter for HCCI methanol combustion on a 4-stroke, 2-cylinder diesel engine, CT2100Q by Zhang et al. Increasing intake temperature was found to increase the cylinder pressure, air–fuel ratio, heat release rate and engine speed. With an increase in the ignition timings, the equivalence ratio was found to increase with the short duration of combustion. The experimental setup was obtained maximum thermal efficiency when CA50 (Crank angle at 50% accumulated heat release rate) locates near 7.5o crank angle with a combustion duration not more than 110o crank angle (Zhang and Wu, 2016). C. Sayin (Sayin, 2010) performed an experimental study on a 4-stroke diesel engine with a single cylinder to analyse the effects of various fuel blends (M5, M10 as methanol-diesel and E5, E10 as ethanol–diesel) on the exhaust and combustion performance. The operating torque of the engine was 30 Nm, with engine speed changing between 1000 and 1800 rpm. The BSFC and nitrogen oxide emissions were found to get increased while smoke opacity, brake thermal efficiency, total hydrocarbon, and carbon monoxide emissions were found to decrease with all fuel blends.
In a similar study, Pranshant et al. (2016) experimentally investigated the relationship between different operating parameters like a) ignition delay, b) pressure change rate, c) heat rate, and d) temperature profile with 20% and 40% methanol blend fuel under 10%, 20%, and 40% load conditions using a 62.5kW, 4-cylinder turbocharged diesel engine. A rise of 3.1%, 14.6%, and 19.5% in the rate of pressure rise was seen under the three different load conditions, respectively. The lowest and highest overall heat release value of 35.93 kJ and 78.07 kJ (at 40% and 10% load conditions) was observed with a 40% methanol blend.
Utilization of methanol with renewable energy sources
The use of renewable sources such as solar, wind, or hydro-energy has seen much popularity recently. To produce biomethanol, the use of renewable sources of energy can increase the overall efficiency and decrease the environmental impact of the whole process. Studies of biomethanol production using renewable sources have been found in different regions mentioned in this section.
Use of concentrating solar power (CSP) for the conversion of methanol-dimethyl ether was reported. A multi-complex model got developed to study the effectiveness of the gasification process with low-temperature solar-powered steam (400–410 °C) produced from the concentrating solar power plant (Ravaghi-Ardebili and Manenti, 2015). A similar study of cotton stalk biomass gasification for syngas production using solar energy with a beam-down optical configuration was performed by Bai et al. Their system had a maximum biomass consumption rate of 100 ktons/year and power capacity of 32.7 MWe with 51.89% and 51.23% of maximum (on-design) energy efficiency and the system exergy efficiency, respectively. The annual electricity and methanol production (off-design) was 50.85 GWh and 54.80 ktons, respectively. The cost of methanol was 361.88 USD/ton, with a 27.33% reduction in biomass consumption rate (Bai et al. 2019).
Kim et al. studied the techno-economic performance of a novel solar-based methanol production process where solar energy is utilized to convert carbon dioxide into carbon monoxide, and then with WGSR, methanol synthesis is performed. According to the results, a system efficiency of 7.1% was found for methanol production with the sole primary energy source of solar energy. The economic analysis shows that for the profitable outcome of this methanol production method, the methanol selling price should not be less than 1.22 USD/kg (Kim et al. 2011). Another solar energy-based and methanol-powered hybrid combined cycle (CC) power system integrated with carbon dioxide capture mechanism- solar-driven methanol-reforming (RFM) and solar-driven methanol decomposition (DCP) process was analysed and proposed by Li et al. The modelling results showed 55% possible exergy efficiency and fuel reduction ratio of 30% with a 20% share of the solar thermal energy. The specific carbon dioxide emissions were reduced to 25 g/kWh, which was 36.8% less compared to a conventional CC (with a carbon dioxide capture process). The primary thermal efficiency for DCP and RFM systems was found to be 28.8% and 28%, respectively. The systems were advantageous for achieving high methanol conversion rates by utilizing the solar heat at only 200–300 °C (Li et al. 2015).
Chen et al. studied the environmental and techno-economic performance of three different process cases: (a) methanol production from coal (Baseline Case), (b) coal-to-methanol conversion with solar energy integration (Case-1), and (c) hybrid solar-biomass route of methanol production from carbon dioxide hydrogenation (Case-2). 45.7% and 57.5% lower environmental effects were found for Case-1 and Case-2, respectively, compared to the baseline case. The production cost of methanol for Case-2 and Case-1 was about 5 × and 3 × times the Baseline Case cost (229.71 USD/ton) (Chen et al. 2019c). With carbon dioxide capture integrated bio-IGCC system for Case-2, negative greenhouse gas emission of (− 1092.1 kgCO2eq) was found in comparison to Case-1 emission (927.8 kgCO2eq) and Baseline case (3607 kgCO2eq). The Case-1 route was found as economically feasible, considering the average carbon tax level (72.08 USD/ton CO2eq) (Chen et al. 2019c). Murray and Furlonge (2020) developed a methanol to power chain integrated model to assess the overall economic viability in the Caribbean region. They estimated that a reduction of up to about 0.10 USD per KWh is possible to achieve, with 6000 MW annual power production through methanol.
Wind energy-based methanol synthesis process using Aspen Plus simulation was reported by Matzen et al. (Matzen et al. 2015), where 97.01 mt of daily methanol production was found to be possible with 138.37 mt CO2/day and 18.56 mt hydrogen/day. Overall energy efficiency for the whole process was around 58%. The plant reduced emissions by − 1.05 CO2e/kg methanol (Matzen et al. 2015). Techno-economic analysis of a solar wind integrated power and methanol production process was studied by Firmansyah et al. (2018). Because of the high initial cost of solar and wind systems, the proposed system could not compete economically with conventional power plants. The initial investment, solar radiation, or wind potential and interest rate were the key factors for the variation in the techno-economic performance of the systems for different locations. Among the three different test locations considered in the study, Beijing (China) was found to perform better economically compared to Denver (US) and Gotland (Sweden) (Firmansyah et al. 2018).
Rivarolo et al. (2016) analysed the use of renewable energy (wind, hydro, and solar) for the methanol synthesis and studied the effects of different electricity costs (0.033-0.044-0.055 USD/kWh) as well as methanol market prices (441.40-551.75-662.10 USD/ton). Hydroelectric source utilization for methanol production was found to be economically more beneficial with higher numbers of equivalent hours (3000) than comparison with the wind (1300) and solar (1100). They found the process profitable with carbon dioxide purchasing and low-cost electricity (0.033 USD/kWh) only with hydroelectric source utilization. They also found that the system's economic performance was enhanced by reducing carbon dioxide emissions (about 33,105.15 USD/year). A study on potential greenhouse gas emissions reduction by producing replacements (hydrogen, methane and methanol) for conventional fossil fuels by using renewable surplus electricity for electrolysis was reported by Uusitalo et al. (2017) studied the. According to the results, reductions in greenhouse gas emissions by using 1 MJ of renewable electricity were 20 g of CO2eq for methane, 60 g of CO2eq for hydrogen, and 40 g of CO2eq for methanol.
The preceding discussion demonstrates that using renewable energy sources to power methanol synthesis is beneficial for reducing global environmental emissions. However, due to the substantial initial investment required for this type of plant, the cost of producing methanol is significantly higher than the current market price.
Production and utilization of dimethyl ether as a fuel with methanol
Dimethyl ether, also known as methoxymethane with the chemical formula CH3OCH3, is chemical fuel which has the potential to be used as a future generation replacement for diesel fuel. Dimethyl ether can be produced from the gasification of different raw materials (like coal, natural gas, biomass, and waste materials). Dimethyl ether has several benefits to be the potential replacement for diesel, such as (a) high cetane number (> 55), (b) low emission, (c) efficient combustion, and d) less smoke formation, along with others (Akhoondi et al. 2021). The production and utilization of dimethyl ether are shown in Fig. 4.
Production and utilization of dimethyl ether, CH3OCH3. Syngas can be produced through gasification from coal, natural gas, biomass, or waste materials. Dimethyl ether can be produced from syngas in two different ways: (a) through methanol (CH3OH) synthesis reaction, 2 CH3OH → CH3OCH3 + H2O, and (b) direct syngas-to-dimethyl ether synthesis, 3 CO+ 3 H2 → CH3OCH3 + CO2. Dimethyl ether can be used as fuel in transport, chemical processes, industrial applications, and power generation
The combustion and engine performance characteristics of methanol- dimethyl ether fuel blend with diesel fuel can be found in different studies. Use of different fuel blends of D80M20, D60M10DME30, D50M30DME20, and D70M20DME10 with different percentages of exhaust gas recirculation was studied by Taghavifar et al. Blending diesel with 30% of methanol and 20% of dimethyl ether (D50M30DME20) at 1400 rpm was found to produce high pressure and accumulated heat with the best mechanical efficiency of 35%, whereas D80M20 blend at 2000 rpm with a 20% exhaust gas recirculation results in poor engine efficiency with defective combustive performance(Taghavifar et al. 2019).
Wattanavichien et al. mentioned a modified dimethyl ether fuelled diesel engine, which achieved 50% of the diesel performance with a reduction in BSFC (Wattanavichien 2009). A similar study is related to dimethyl ether combustion, exhaust gas emissions, and dimethyl ether feasibility in a common rail diesel engine with a single-cylinder was reported by Theinnoi et al. where dimethyl ether was blended to diesel (0–90%) by adding through the engine intake manifold (Theinnoi et al. 2017). Exhaust gas emissions and thermal efficiency were found to get improved in the case of dimethyl ether diesel combustion in comparison to pure diesel combustion (Theinnoi et al. 2017). With 30% dimethyl ether blending, nitric oxide and smoke reductions were also observed. Brake thermal efficiency improvement was found by reducing diesel fuel consumption by 22.36% (Theinnoi et al. 2017). Evaluation of emission control methods for dimethyl ether combustion in CI engines for meeting the emission norms in the near future was also found (Thomas et al. 2014).
Thomas et al. mainly studied the future concerns about the emission characteristics of dimethyl ether combustion and particle matter emission (Thomas et al. 2014). Park et al. also analysed the emission characteristics and combustion performance of biogas–dimethyl ether mix using a high-speed diesel engine. With an increase in biogas/dimethyl ether mixing ratio, ignition timing, nitrogen oxide emission, peak pressure during combustion, and heat release rate were seen to decrease, whereas hydrocarbon and carbon monoxide emission was found to increase (Park et al. 2014).
Kim and Park studied the optimization of operating parameters, start of injection (SOI), fuel injection angle, and pressure for a diesel engine with diesel–dimethyl ether mode. Reduction in nitrogen oxide emissions and fuel consumption was found in diesel-dimethyl ether mode compared to the pure diesel combustion mode. The optimum values for the dimethyl ether combustion process parameters are: start of injection = BTDC 10°, equivalence ratio = 0.7, injection pressure = 110 MPa, and injection angle = 90° (Kim and Park 2016). An optimum configuration with a 0.6% net indicated efficiency improvement and 1% reduction in nitrogen oxide values compared to that of the original case is also found by Benajes et al. for heavy-duty diesel engine combustion using dimethyl ether. An increased net indicated efficiency was seen with exhaust gas recirculation with nitrogen oxide and soot emission control (Benajes et al. 2018). Problems related to the combustion of dimethyl ether in spark ignition engines like leakage in the fuel system, engine wear and cavity were discussed by KRUCZYŃKI et al. Nitrogen oxides, total hydrocarbon, and particle matter emission with the dimethyl ether-fuelled spark ignition engine were found to be 1.19, 0.22, and 0.015 g/kWh, respectively. The requirement of fuel system improvement and the use of additives were suggested for using dimethyl ether in engines (Kruczyński 2017).
This section describes the production and utilization of dimethyl ether alongside methanol, which has a huge potential to be a future generation fuel source. Although dimethyl ether has numerous advantages, issues such as its reduced lower calorific value, low density, and low viscosity may prevent its use in diesel engines. Future research in fuel injection modification can be advantageous for utilizing this potential fuel in existing engines as a replacement for diesel.
Bibliometric mapping
Research in the area of methanol and biomethanol is increasing. A co-occurrence analysis using the keywords “methanol production”, “biomethanol”, and ‘bio-methanol’ on “Web of Science” for the period between 2018 and 2022 highlights that the keywords most associated with methanol over the last 5 years include its production and use as a biofuel for energy production including its use in biodiesel.
Researchers, whether they are specialists in the subject or fresh to the study topic, can use the bibliometric mapping as a visual help. The clusters/family trees of research words depict how the entire research domain is split or subdivided into separate areas/research subjects on the visualization map. The more closely the clusters appear to be connected or are truly connected, the more direct the link and overlap between the subtopics and study areas relevant to methanol production. The larger and thicker circles signify the phrases' perceived effect or the frequency with which they appear in the literature. Furthermore, the fact that they are coloured differently suggests that they are linked to distinctive clusters and trees with off branching words in the relevant literature.
The bibliometric mapping was performed using the following methodology, which is shown in Fig. 5 Search keywords: 'methanol production' (title) or biomethanol (title) or bio-methanol (title).
The database used: Web of science.
Time period: 2018–2022 (publication years).
Figure 5a, b shows the bibliometric mapping analysis utilizing network and density visualizations. Using the search methods mentioned above, the bibliometric mapping study yielded 760 results from the Web of Science. The analysis was carried out by binary counting of co-occurrences or terms that featured at least four times in papers published between 2018 and 2022. Figure 5a shows that the terms methanol, production, methanol production, hydrogen, catalyst, performance, biomass, application are particularly prominent. Figure 5b simultaneously shows these keywords with a high recurrence frequency in vivid yellow in the density visualization map. It can be seen that topics like biomethanol production and dimethyl ether have relatively low occurrence, implying that these need more focus in research.
These keywords are not surprising given the improved environmental performance by mitigating the harmful effects of current rapid carbon dioxide emission (Olah 2005; Ekman et al. 2013). Compared to conventional fossil fuels, renewable methanol combustion reduces nitrogen oxide emission (up to 80%), reduces carbon dioxide emissions (up to 95%), and eliminates sulphur oxide and other particulate matter (Hobson 2018; Pozzo et al. 2015). A 9–38% reduction in total carbon emission per year was found possible by the generation of electricity using biomethanol-fuel cells (Suntana et al. 2009). However, methanol utilization also has several disadvantages. The production cost for biomethanol is reported to be 1.5–4 times more than traditional natural gas-sourced methanol (Broeren 2013). Methanol being electrically conductive accelerates corrosion (Verhelst et al. 2019). Moreover, methanol possesses a low flash point (9–12 °C), necessitating explosion-proof storage (Kumabe et al. 2008; Murray and Furlonge, 2020), and also has a very low cetane number (≤ 5) which makes autoignition difficult (Popa et al. 2001).
This section mainly shows the ongoing research in methanol production and how it relates to other industries. Figure 2a and b illustrates that methanol research has expanded over the past five years. In this review, we examine methanol's efficiency as a fuel for the next generation and as a potential replacement for the use of fossil fuels and energy production.
Conclusion
This review critically assesses the literature concerning methanol's production, utilization, and techno-economic viability as a fuel for the next generation. Various production processes and feedstocks for methanol production were analysed and discussed. Gasification of lignocellulosic biomass was found to be the most effective technology for biomethanol production in the current scenario.
Herein we examine methanol as one of the potential fuels to mitigate the problems associated with greenhouse gas emissions and fuel scarcity by using the modelling and analytical studies to determine the fuel's techno-economic viability. The biomethanol yield and production cost are primarily influenced by feedstock characteristics, initial investment, and plant location.
The use of biomethanol as a fuel in various sectors, including in dual-fuel mode with other fuels such as diesel, natural gas, and dimethyl ether, was found to be beneficial in terms of fuel economy, brake thermal efficiency, and greenhouse gas emissions reduction. The use of renewable sources in the production process increased the overall positive environmental impact and process efficiency, although at a higher cost.
The hydrogen production facility from the electrolysis of water requires a very large investment, and for this reason, the use of renewable energy was also mentioned in numerous studies to reduce both the biomethanol production cost and the overall environmental impact. In contrast with fossil fuels, biomethanol has the potential to meet the energy needs of future generations and has much lower environmental impacts, reducing nitrogen oxide, sulphur oxide, and soot emissions.
Countries with excess forest biomass, such as India, China, and Brazil, should focus on producing and utilizing biomethanol to reduce fossil fuel use for energy generation and transportation. Additionally, biomethanol will positively impact global greenhouse gas emissions and climate change. However, there is still a need for research regarding the entire methanol production and utilization process for large-scale production, considering different biomass resources available in various regions. Conversion of methanol to dimethyl ether and subsequent use of dimethyl ether as fuel was also considered a possibility; however, further research and development of the fuel injection modification will be more effective in using dimethyl ether alongside methanol as dual-fuel blends.
Abbreviations
- WGSR:
-
Water–gas shift reaction
- SOEC:
-
Solid oxide electrolysis cell
- WWTP:
-
Wastewater treatment plant
- IGCC:
-
Integrated gasification combined cycle
- BMEP:
-
Brake mean effective pressure
- BSFC:
-
Brake-specific fuel consumption
- DMDF:
-
Diesel–methanol dual fuel
- M-G DFSI:
-
Methanol–gasoline dual-fuel spark ignition
- IBPGM:
-
Integrated biomass pyrolysis, gasification, and methanol synthesis
- HCCI:
-
Homogenous charge compression ignition
- DMDF:
-
Diesel–methanol dual fuel
References
Abdi H et al (2017) Chapter 5 - Fuel Cells. In: Gharehpetian GB, Mousavi Agah SM (Eds), Distributed generation systems. Butterworth-Heinemann, pp 221–300. https://doi.org/10.1016/B978-0-12-804208-3.00005-4
Abdoulmoumine N et al (2014) Effects of temperature and equivalence ratio on pine syngas primary gases and contaminants in a bench-scale fluidized bed gasifier. Ind Eng Chem Res 53:5767–5777. https://doi.org/10.1021/ie404401n
Adekoya D et al (2019) Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renew Sustain Energy Rev 116:109389. https://doi.org/10.1016/j.rser.2019.109389
Akhoondi A et al (2021) Direct catalytic production of dimethyl ether from CO and CO2: a review. Synth Sinter 1:105–120
Alipour Moghadam R et al (2014) Investigation on syngas production via biomass conversion through the integration of pyrolysis and air–steam gasification processes. Energy Convers Manage. 87:670–675 https://doi.org/10.1016/j.enconman.2014.07.065. https://www.netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/catalytic
AlNouss A et al (2019) A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers Manage 196:664–676. https://doi.org/10.1016/j.enconman.2019.06.019
AlNouss A et al (2020) Production of syngas via gasification using optimum blends of biomass. J Clean Prod 242:118499. https://doi.org/10.1016/j.jclepro.2019.118499
Amigun B et al (2010) Biomethanol production from gasification of non-woody plant in South Africa: optimum scale and economic performance. Energy Policy 38:312–322. https://doi.org/10.1016/j.enpol.2009.09.020
Andersson J et al (2015) Co-gasification of pyrolysis oil and black liquor for methanol production. Fuel 158:451–459. https://doi.org/10.1016/j.fuel.2015.05.044
Andersson J et al (2016) Co-gasification of black liquor and pyrolysis oil: evaluation of blend ratios and methanol production capacities. Energy Convers Manage 110:240–248. https://doi.org/10.1016/j.enconman.2015.12.027
Anicic B et al (2014) Comparison between two methods of methanol production from carbon dioxide. Energy 77:279–289. https://doi.org/10.1016/j.energy.2014.09.069
Arteaga-Pérez LE et al (2016) A modelling approach to the techno-economics of Biomass-to-SNG/Methanol systems: standalone vs Integrated topologies. Chem Eng J 286:663–678. https://doi.org/10.1016/j.cej.2015.11.005
Arutyunov VS et al (1996) Direct high-pressure gas-phase oxidation of natural gas to methanol and other oxygenates. Russ Chem Rev 65:197–224. https://doi.org/10.1070/rc1996v065n03abeh000207
Asadieraghi M, Wan Daud WMA (2015) In-situ catalytic upgrading of biomass pyrolysis vapor: using a cascade system of various catalysts in a multi-zone fixed bed reactor. Energy Convers Manage 101:151–163. https://doi.org/10.1016/j.enconman.2015.05.008
Asadieraghi M et al (2014) Model compound approach to design process and select catalysts for in-situ bio-oil upgrading. Renew Sustain Energy Rev 36:286–303. https://doi.org/10.1016/j.rser.2014.04.050
Atsonios K et al (2016) Investigation of technical and economic aspects for methanol production through CO2 hydrogenation. Int J Hydrogen Energy 41:2202–2214. https://doi.org/10.1016/j.ijhydene.2015.12.074
Babu BV (2008) Biomass pyrolysis: a state-of-the-art review. Biofuels, Bioprod Biorefin 2:393–414. https://doi.org/10.1002/bbb.92
Bai Z et al (2015) A polygeneration system for the methanol production and the power generation with the solar–biomass thermal gasification. Energy Convers Manage 102:190–201. https://doi.org/10.1016/j.enconman.2015.02.031
Bai Z et al (2019) Investigation of a solar-biomass gasification system with the production of methanol and electricity: thermodynamic, economic and off-design operation. Appl Energy 243:91–101. https://doi.org/10.1016/j.apenergy.2019.03.132
Balegedde Ramachandran RP et al (2013) Techno-economic analysis of biomethanol production via hybrid steam reforming of glycerol with natural gas. Energy Fuels 27:5962–5974. https://doi.org/10.1021/ef401323w
Barrault J, Probst L (1991) Synthesis of higher alcohols from syngas over Ni—Mo Catalysts. Effect of methanol or ethylene. In: Holmen A et al (Eds.), Studies in surface science and catalysis. Elsevier, pp 349–355. https://doi.org/10.1016/S0167-2991(08)60100-7
Bassani A et al (2017) Low impact methanol production from sulfur rich coal gasification. Energy Procedia 105:4519–4524. https://doi.org/10.1016/j.egypro.2017.03.970
Benajes J et al (2018) Computational optimization of a combustion system for a stoichiometric DME fueled compression ignition engine. Fuel 223:20–31. https://doi.org/10.1016/j.fuel.2018.03.022
Ben-Iwo J et al (2016) Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew Sustain Energy Rev 63:172–192. https://doi.org/10.1016/j.rser.2016.05.050
Bergins C et al (2016) Commercialization of low carbon methanol. Atzextra Worldwide 21:22–25. https://doi.org/10.1007/s40111-015-0517-0
Bone WA (1935) Initial formation of methyl alcohol in the oxidation of methane. Nature 136:910–910. https://doi.org/10.1038/136910a0
Bonfim-Rocha L et al (2018) Multi-objective design of a new sustainable scenario for bio-methanol production in Brazil. J Clean Prod 187:1043–1056. https://doi.org/10.1016/j.jclepro.2018.03.267
Boretti A (2013) Renewable hydrogen to recycle CO2 to methanol. Int J Hydrogen Energy 38:1806–1812. https://doi.org/10.1016/j.ijhydene.2012.11.097
Borole AP, Greig AL (2019) Chapter 20 - life-cycle assessment and systems analysis of hydrogen production. In: Pandey A et al (Eds), Biohydrogen (Second Edition). Elsevier, pp 485–512. https://doi.org/10.1016/B978-0-444-64203-5.00020-4
Brigagão GV et al (2019) A techno-economic analysis of thermochemical pathways for corncob-to-energy: fast pyrolysis to bio-oil, gasification to methanol and combustion to electricity. Fuel Process Technol 193:102–113. https://doi.org/10.1016/j.fuproc.2019.05.011
Broeren M, IEA-ETSAP, IRENA (2013). Technology-Policy Brief I08: Production of Bio-methanol. https://iea-etsap.org/E-TechDS/PDF/I09IR_Bio-methanol_MB_Jan2013_final_GSOK.pdf
Brynolf S et al (2014) Environmental assessment of marine fuels: liquefied natural gas, liquefied biogas, methanol and bio-methanol. J Clean Prod 74:86–95. https://doi.org/10.1016/j.jclepro.2014.03.052
Cai Y et al (1997) Synthesis of methanol and isobutanol from syngas over ZrO2-based catalysts. Fuel Process Technol 50:163–170. https://doi.org/10.1016/S0378-3820(96)01060-0
Carvalho L et al (2017) Techno-economic assessment of catalytic gasification of biomass powders for methanol production. Biores Technol 237:167–177. https://doi.org/10.1016/j.biortech.2017.02.019
Carvalho L et al (2018a) Methanol production via black liquor co-gasification with expanded raw material base – Techno-economic assessment. Appl Energy 225:570–584. https://doi.org/10.1016/j.apenergy.2018.04.052
Carvalho L et al (2018b) Alkali enhanced biomass gasification with in situ S capture and a novel syngas cleaning. Part 2: techno-economic assessment. Energy 165:471–482. https://doi.org/10.1016/j.energy.2018.09.159
Chen Z et al (2019a) Experimental investigation on performance and combustion characteristics of spark-ignition dual-fuel engine fueled with methanol/natural gas. Appl Therm Eng 150:164–174. https://doi.org/10.1016/j.applthermaleng.2018.12.168
Chen Z et al (2019b) A comparative study on the combustion and emissions of dual-fuel engine fueled with natural gas/methanol, natural gas/ethanol, and natural gas/n-butanol. Energy Convers Manage 192:11–19. https://doi.org/10.1016/j.enconman.2019.04.011
Chen Q et al (2019c) Comparative environmental and economic performance of solar energy integrated methanol production systems in China. Energy Convers Manage 187:63–75. https://doi.org/10.1016/j.enconman.2019.03.013
Chen L et al (2022) Strategies to achieve a carbon neutral society: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01435-8
Ciuta S et al (2018) Chapter four - field scale developments. In: Ciuta S, et al (Eds), Gasification of waste materials. Academic Press, pp 65–91. https://doi.org/10.1016/B978-0-12-812716-2.00004-2
Clausen LR et al (2010) Technoeconomic analysis of a methanol plant based on gasification of biomass and electrolysis of water. Energy 35:2338–2347. https://doi.org/10.1016/j.energy.2010.02.034
Demirbas A (2008) Biomethanol production from organic waste materials. Energy Sour, Part a: Recov, Utili, Environ Effects 30:565–572. https://doi.org/10.1080/15567030600817167
Dincer I (2000) Renewable energy and sustainable development: a crucial review. Renew Sustain Energy Rev 4:157–175. https://doi.org/10.1016/S1364-0321(99)00011-8
Duraisamy G et al (2020) A comparative study on methanol/diesel and methanol/PODE dual fuel RCCI combustion in an automotive diesel engine. Renew Energy 145:542–556. https://doi.org/10.1016/j.renene.2019.06.044
Dutta PP et al (2014) Down draft gasification modelling and experimentation of some indigenous biomass for thermal applications. Energy Procedia 54:21–34. https://doi.org/10.1016/j.egypro.2014.07.246
Ekman A et al (2013) Bioresource utilisation by sustainable technologies in new value-added biorefinery concepts – two case studies from food and forest industry. J Clean Prod 57:46–58. https://doi.org/10.1016/j.jclepro.2013.06.003
Ellabban O et al (2014) Renewable energy resources: current status, future prospects and their enabling technology. Renew Sustain Energy Rev 39:748–764. https://doi.org/10.1016/j.rser.2014.07.113
Farzad S et al (2016) A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res J 3:483–495
Fawzy S et al (2021) Industrial biochar systems for atmospheric carbon removal: a review. Environ Chem Lett 19:3023–3055. https://doi.org/10.1007/s10311-021-01210-1
Firmansyah H et al (2018) Power and methanol production from biomass combined with solar and wind energy: analysis and comparison. Energy Procedia 145:576–581. https://doi.org/10.1016/j.egypro.2018.04.084
Gahleitner G (2013) Hydrogen from renewable electricity: an international review of power-to-gas pilot plants for stationary applications. Int J Hydrogen Energy 38:2039–2061. https://doi.org/10.1016/j.ijhydene.2012.12.010
Galindo Cifre P, Badr O (2007) Renewable hydrogen utilisation for the production of methanol. Energy Convers Manage 48:519–527. https://doi.org/10.1016/j.enconman.2006.06.011
Ganesh A, Banerjee R (2001) Biomass pyrolysis for power generation — a potential technology. Renew Energy 22:9–14. https://doi.org/10.1016/S0960-1481(00)00026-4
Ge X et al (2014) Biological conversion of methane to liquid fuels: status and opportunities. Biotechnol Adv 32:1460–1475. https://doi.org/10.1016/j.biotechadv.2014.09.004
Geng P et al (2014) Reduction of PM emissions from a heavy-duty diesel engine with diesel/methanol dual fuel. Fuel 123:1–11. https://doi.org/10.1016/j.fuel.2014.01.056
Gesser HD et al (1985) The direct conversion of methane to methanol by controlled oxidation. Chem Rev 85:235–244. https://doi.org/10.1021/cr00068a001
Ghosh S et al (2019) Biogas to methanol: a comparison of conversion processes involving direct carbon dioxide hydrogenation and via reverse water gas shift reaction. J Clean Prod 217:615–626. https://doi.org/10.1016/j.jclepro.2019.01.171
Gondal MA et al (2004) Photocatalytic transformation of methane into methanol under UV laser irradiation over WO3, TiO2 and NiO catalysts. Chem Phys Lett 392:372–377. https://doi.org/10.1016/j.cplett.2004.05.092
Gong C-M et al (2011) Improvement of fuel economy of a direct-injection spark-ignition methanol engine under light loads. Fuel 90:1826–1832. https://doi.org/10.1016/j.fuel.2010.10.032
Gong C et al (2019) Effect of injection strategy on cold start firing, combustion and emissions of a LPG/methanol dual-fuel spark-ignition engine. Energy 178:126–133. https://doi.org/10.1016/j.energy.2019.04.145
Grové J et al (2018) Is MSW derived DME a viable clean cooking fuel in Kolkata, India? Renewable Energy 124:50–60. https://doi.org/10.1016/j.renene.2017.08.039
Gupta KK et al (2010) Bio-fuels for the gas turbine: a review. Renew Sustain Energy Rev 14:2946–2955. https://doi.org/10.1016/j.rser.2010.07.025
Häggström C et al (2012) Catalytic methanol synthesis via black liquor gasification. Fuel Process Technol 94:10–15. https://doi.org/10.1016/j.fuproc.2011.09.019
Hameed A et al (2014) Photocatalytic conversion of methane into methanol: Performance of silver impregnated WO3. Appl Catal A 470:327–335. https://doi.org/10.1016/j.apcata.2013.10.045
Hamelinck CN, Faaij APC (2002) Future prospects for production of methanol and hydrogen from biomass. J Power Sour 111:1–22. https://doi.org/10.1016/S0378-7753(02)00220-3
Han J-S et al (2013) Partial oxidative conversion of methane to methanol through selective inhibition of methanol dehydrogenase in methanotrophic consortium from landfill cover soil. Appl Biochem Biotechnol 171:1487–1499. https://doi.org/10.1007/s12010-013-0410-0
Hasegawa F et al (2010) Methanol or ethanol produced from woody biomass: Which is more advantageous? Biores Technol 101:S109–S111. https://doi.org/10.1016/j.biortech.2009.05.008
Higman C, van der Burgt M (2008) Chapter 5 - gasification processes. In: Higman C, van der Burgt M (Eds), Gasification (Second Edition). Gulf Professional Publishing, Burlington, pp 91–191. https://doi.org/10.1016/B978-0-7506-8528-3.00005-5
Hill J et al (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci 103:11206. https://doi.org/10.1073/pnas.0604600103
Hirano A et al (1998) Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance. Catal Today 45:399–404. https://doi.org/10.1016/S0920-5861(98)00275-2
Hobson C (2018) Renewable methanol report. Methanol Institute, https://www.methanol.org/wp-content/uploads/2019/01/MethanolReport.pdf
Holmgren KM et al (2012) System aspects of biomass gasification with methanol synthesis – Process concepts and energy analysis. Energy 45:817–828. https://doi.org/10.1016/j.energy.2012.07.009
Huber GW et al (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106:4044–4098. https://doi.org/10.1021/cr068360d
Iaquaniello G et al (2017) Waste-to-methanol: process and economics assessment. Biores Technol 243:611–619. https://doi.org/10.1016/j.biortech.2017.06.172
Im-orb K, Arpornwichanop A (2019) Process and sustainability analyses of the integrated biomass pyrolysis, gasification, and methanol synthesis process for methanol production. Energy. https://doi.org/10.1016/j.energy.2019.116788
IPCC (2011) Special report on renewable energy sources and climate change mitigation. IPCC. https://www.ipcc.ch/report/renewable-energy-sources-and-climate-change-mitigation/
Jadhav SG et al (2014) Catalytic carbon dioxide hydrogenation to methanol: a review of recent studies. Chem Eng Res Des 92:2557–2567. https://doi.org/10.1016/j.cherd.2014.03.005
Jali S et al (2011) The effect of Mo(CO)6 as a co-catalyst in the carbonylation of methanol to methyl formate catalyzed by potassium methoxide under CO, syngas and H2 atmospheres. HP-IR observation of the methoxycarbonyl intermediate of Mo(CO)6. J Mol Catal a: Chem 348:63–69. https://doi.org/10.1016/j.molcata.2011.08.002
Karagoz P et al (2019) Lignocellulosic ethanol production: evaluation of new approaches, cell immobilization and reactor configurations. Renew Energy 143:741–752. https://doi.org/10.1016/j.renene.2019.05.045
Kasmuri N et al (2016) Potential of biomass for biomethanol production. Int J Appl Eng Res. 11:10016–10019. https://www.researchgate.net/publication/309399650_Potential_of_Biomass_for_Biomethanol_Production
van Kasteren JMN (2016) 16 - Production of bioalcohols via gasification. In: Luque R et al (Eds), Handbook of Biofuels Production (Second Edition). Woodhead Publishing, pp 495–507. https://doi.org/10.1016/B978-0-08-100455-5.00016-3
Kempegowda RS et al (2012) Process synthesis and economics of combined biomethanol and CHP energy production derived from biomass wastes. J Chem Technol Biotechnol 87:897–902. https://doi.org/10.1002/jctb.3696
Kim HJ, Park SH (2016) Optimization study on exhaust emissions and fuel consumption in a dimethyl ether (DME) fueled diesel engine. Fuel 182:541–549. https://doi.org/10.1016/j.fuel.2016.06.001
Kim J et al (2011) Methanol production from CO2 using solar-thermal energy: process development and techno-economic analysis. Energy Environ Sci 4:3122–3132. https://doi.org/10.1039/C1EE01311D
Kim YD et al (2013) Air-blown gasification of woody biomass in a bubbling fluidized bed gasifier. Appl Energy 112:414–420. https://doi.org/10.1016/j.apenergy.2013.03.072
Kirubakaran V et al (2009) A review on gasification of biomass. Renew Sustain Energy Rev 13:179–186. https://doi.org/10.1016/j.rser.2007.07.001
Kourkoumpas DS et al (2016) Implementation of the Power to Methanol concept by using CO2 from lignite power plants: techno-economic investigation. Int J Hydrogen Energy 41:16674–16687. https://doi.org/10.1016/j.ijhydene.2016.07.100
Kruczyński S, Slęzak M, Gis W et al (2017) Problems in fuelling spark ignition engines with dimethyl ether. Combust Eng 170(3):154–158
Kruse A (2009) Hydrothermal biomass gasification. The J Supercrit Fluids 47:391–399. https://doi.org/10.1016/j.supflu.2008.10.009
Kumabe K et al (2008) Environmental and economic analysis of methanol production process via biomass gasification. Fuel 87:1422–1427. https://doi.org/10.1016/j.fuel.2007.06.008
Kumar S, Shukla S (2016) A review on recent gasification methods for biomethane gas production. Int J Energy Eng 2016:32–43. https://doi.org/10.5923/s.ijee.201601.05
Kumar A et al (2015) A review on biomass energy resources, potential, conversion and policy in India. Renew Sustain Energy Rev 45:530–539. https://doi.org/10.1016/j.rser.2015.02.007
Kumar A et al (2020) Utilization of lignin: a sustainable and eco-friendly approach. J Energy Inst 93:235–271. https://doi.org/10.1016/j.joei.2019.03.005
Lange J-P (2001) Methanol synthesis: a short review of technology improvements. Catal Today 64:3–8. https://doi.org/10.1016/S0920-5861(00)00503-4
Leduc S et al (2010) Location of a biomass based methanol production plant: a dynamic problem in northern Sweden. Appl Energy 87:68–75. https://doi.org/10.1016/j.apenergy.2009.02.009
Lee J et al (2018) Experimental investigation on the performance and emissions characteristics of ethanol/diesel dual-fuel combustion. Fuel 220:72–79. https://doi.org/10.1016/j.fuel.2018.02.002
Lee WJ et al (2019) The effect of metal additives in Cu/Zn/Al2O3 as a catalyst for low-pressure methanol synthesis in an oil-cooled annulus reactor. Catal Today. https://doi.org/10.1016/j.cattod.2019.03.041
Li H et al (2010) Analysis of a feasible polygeneration system for power and methanol production taking natural gas and biomass as materials. Appl Energy 87:2846–2853. https://doi.org/10.1016/j.apenergy.2009.07.001
Li Y et al (2015) Performance comparison of two low-CO2 emission solar/methanol hybrid combined cycle power systems. Appl Energy 155:740–752. https://doi.org/10.1016/j.apenergy.2015.06.052
Li P et al (2019) Extraction techniques in sustainable biofuel production: a concise review. Fuel Process Technol 193:295–303. https://doi.org/10.1016/j.fuproc.2019.05.009
Liu H et al (2015a) Methanol-gasoline Dual-fuel Spark Ignition (DFSI) combustion with dual-injection for engine particle number (PN) reduction and fuel economy improvement. Energy 89:1010–1017. https://doi.org/10.1016/j.energy.2015.06.051
Liu J et al (2015b) Effects of diesel injection pressure on the performance and emissions of a HD common-rail diesel engine fueled with diesel/methanol dual fuel. Fuel 140:192–200. https://doi.org/10.1016/j.fuel.2014.09.109
Liu Z et al (2016) Circulating fluidized bed gasification of biomass for flexible end-use of syngas: a micro and nano scale study for production of bio-methanol. J Clean Prod 129:249–255. https://doi.org/10.1016/j.jclepro.2016.04.076
López-Martín Á et al (2017) Photochemical methane partial oxidation to methanol assisted by H2O2. J Photochem Photobiol, A 349:216–223. https://doi.org/10.1016/j.jphotochem.2017.09.039
Luu MT et al (2015) A comparative study of CO2 utilization in methanol synthesis with various syngas production technologies. J CO2 Util 12:62–76. https://doi.org/10.1016/j.jcou.2015.07.001
Luu MT et al (2016) Analysis of CO2 utilization for methanol synthesis integrated with enhanced gas recovery. J Clean Prod 112:3540–3554. https://doi.org/10.1016/j.jclepro.2015.10.119
Ma Y et al (2008) A sulfur-tolerant Pd/CeO2 catalyst for methanol synthesis from syngas. J Nat Gas Chem 17:387–390. https://doi.org/10.1016/S1003-9953(09)60014-1
Ma B et al (2019) Experimental study on energy balance of different parameters in diesel methanol dual fuel engine. Appl Therm Eng 159:113954. https://doi.org/10.1016/j.applthermaleng.2019.113954
Mahinpey N, Gomez A (2016) Review of gasification fundamentals and new findings: reactors, feedstock, and kinetic studies. Chem Eng Sci 148:14–31. https://doi.org/10.1016/j.ces.2016.03.037
Maity SK (2015) Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew Sustain Energy Rev 43:1427–1445. https://doi.org/10.1016/j.rser.2014.11.092
Manenti F et al (2015) First-principles models and sensitivity analysis for the lignocellulosic biomass-to-methanol conversion process. Comput Chem Eng 84:558–567. https://doi.org/10.1016/j.compchemeng.2015.05.012
Mao D et al (2009) Highly effective conversion of syngas to dimethyl ether over the hybrid catalysts containing high-silica HMCM-22 zeolites. Catal Commun 10:620–624. https://doi.org/10.1016/j.catcom.2008.11.003
Matzen M et al (2015) Chemical storage of wind energy by renewable methanol production: feasibility analysis using a multi-criteria decision matrix. Energy 93:343–353. https://doi.org/10.1016/j.energy.2015.09.043
McFarlan A (2018) Techno-economic assessment of pathways for electricity generation in northern remote communities in Canada using methanol and dimethyl ether to replace diesel. Renew Sustain Energy Rev 90:863–876. https://doi.org/10.1016/j.rser.2018.03.076
McKendry P (2002) Energy production from biomass (part 2): conversion technologies. Biores Technol 83:47–54. https://doi.org/10.1016/S0960-8524(01)00119-5
Miao Q et al (2014) Model validation of a CFB biomass gasification model. Renew Energy 63:317–323. https://doi.org/10.1016/j.renene.2013.09.040
Michailos S et al (2016) A multicriteria comparison of utilizing sugar cane bagasse for methanol to gasoline and butanol production. Biomass Bioenerg 95:436–448. https://doi.org/10.1016/j.biombioe.2016.06.019
Mignard D, Pritchard C (2008) On the use of electrolytic hydrogen from variable renewable energies for the enhanced conversion of biomass to fuels. Chem Eng Res Des 86:473–487. https://doi.org/10.1016/j.cherd.2007.12.008
Milani D et al (2015) A model-based analysis of CO2 utilization in methanol synthesis plant. J CO2 Util 10:12–22. https://doi.org/10.1016/j.jcou.2015.02.003
Minteer SD (2011) 11 - Biochemical production of other bioalcohols: biomethanol, biopropanol, bioglycerol, and bioethylene glycol. In: Luque R et al (Eds), Handbook of Biofuels Production. Woodhead Publishing, pp 258–265. https://doi.org/10.1533/9780857090492.2.258
Moellenbruck F et al (2018) Cogeneration of power and methanol based on a conventional power plant in Germany. J Energy Storage 19:393–401. https://doi.org/10.1016/j.est.2018.08.018
Morone P, Cottoni L (2016) 4 - Biofuels: technology, economics, and policy issues. In: Luque R et al (Eds), Handbook of Biofuels Production (Second Edition). Woodhead Publishing, pp 61–83. https://doi.org/10.1016/B978-0-08-100455-5.00004-7
Murray R, Furlonge H (2020) Market and economic assessment of using methanol for power generation in the Caribbean Region. The J Assoc Prof Eng Trinidad Tobago. 38:88–99. https://www.methanol.org/wp-content/uploads/2016/06/Market-Economic-Assessment-of-Using-Methanol-for-Power-Generation-in-the-Caribbean-Region.pdf
Nakagawa H et al (2007) Biomethanol production and CO2 emission reduction from forage grasses, trees, and crop residues. Jpn Agric Res Quart: JARQ 41:173–180. https://doi.org/10.6090/jarq.41.173
Ng KS, Sadhukhan J (2011) Process integration and economic analysis of bio-oil platform for the production of methanol and combined heat and power. Biomass Bioenerg 35:1153–1169. https://doi.org/10.1016/j.biombioe.2010.12.003
Nozaki T et al (2011) A single step methane conversion into synthetic fuels using microplasma reactor. Chem Eng J 166:288–293. https://doi.org/10.1016/j.cej.2010.08.001
Ogunkunle O, Ahmed NA (2019) A review of global current scenario of biodiesel adoption and combustion in vehicular diesel engines. Energy Rep 5:1560–1579. https://doi.org/10.1016/j.egyr.2019.10.028
Olabi AG (2012) Developments in sustainable energy and environmental protection. Energy 39:2–5. https://doi.org/10.1016/j.energy.2011.12.037
Olah GA (2005) Beyond oil and gas: the methanol economy. Angew Chem Int Ed 44:2636–2639. https://doi.org/10.1002/anie.200462121
Olah GA et al (2009) Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J Org Chem 74:487–498. https://doi.org/10.1021/jo801260f
Osman AI, Abu-Dahrieh JK (2018) Kinetic investigation of η-Al2O3 catalyst for dimethyl ether production. Catal Lett 148:1236–1245. https://doi.org/10.1007/s10562-018-2319-2
Osman AI et al (2012) Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl Catal B 127:307–315. https://doi.org/10.1016/j.apcatb.2012.08.033
Osman AI et al (2017) Silver-modified η-Al2O3 catalyst for DME production. The J Phys Chem c 121:25018–25032. https://doi.org/10.1021/acs.jpcc.7b04697
Osman AI et al (2021a) Recent advances in carbon capture storage and utilisation technologies: a review. Environ Chem Lett 19:797–849. https://doi.org/10.1007/s10311-020-01133-3
Osman AI et al (2021b) Bioethanol and biodiesel: Bibliometric mapping, policies and future needs. Renew Sustain Energy Rev 152:111677. https://doi.org/10.1016/j.rser.2021.111677
Osman AI et al (2021c) Conversion of biomass to biofuels and life cycle assessment: a review. Environ Chem Lett 19:4075–4118. https://doi.org/10.1007/s10311-021-01273-0
Osman AI et al (2022a) Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-022-01424-x
Osman AI et al (2022b) Hydrogen production, storage, utilisation and environmental impacts: a review. Environ Chem Lett 20:153–188. https://doi.org/10.1007/s10311-021-01322-8
Pala LPR et al (2017) Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: an Aspen Plus model. Renew Energy 101:484–492. https://doi.org/10.1016/j.renene.2016.08.069
Pan W et al (2015) The impact of intake air temperature on performance and exhaust emissions of a diesel methanol dual fuel engine. Fuel 162:101–110. https://doi.org/10.1016/j.fuel.2015.08.073
Park SH et al (2014) Mixing effects of biogas and dimethyl ether (DME) on combustion and emission characteristics of DME fueled high-speed diesel engine. Energy 66:413–422. https://doi.org/10.1016/j.energy.2014.02.007
Parthasarathy P, Narayanan KS (2014) Hydrogen production from steam gasification of biomass: influence of process parameters on hydrogen yield – a review. Renew Energy 66:570–579. https://doi.org/10.1016/j.renene.2013.12.025
Parvez AM et al (2018) Bio-DME production based on conventional and CO2-enhanced gasification of biomass: a comparative study on exergy and environmental impacts. Biomass Bioenerg 110:105–113. https://doi.org/10.1016/j.biombioe.2018.01.016
Patel SKS et al (2016) Improvement in methanol production by regulating the composition of synthetic gas mixture and raw biogas. Biores Technol 218:202–208. https://doi.org/10.1016/j.biortech.2016.06.065
Pauls JH et al (2016) Simulation of air-steam gasification of woody biomass in a bubbling fluidized bed using Aspen Plus: a comprehensive model including pyrolysis, hydrodynamics and tar production. Biomass Bioenerg 95:157–166. https://doi.org/10.1016/j.biombioe.2016.10.002
Pellegrini LA et al (2011) Economic analysis of a combined energy–methanol production plant. Appl Energy 88:4891–4897. https://doi.org/10.1016/j.apenergy.2011.06.028
Pham TN et al (2014) Evaluating strategies for catalytic upgrading of pyrolysis oil in liquid phase. Appl Catal B 145:10–23. https://doi.org/10.1016/j.apcatb.2013.01.002
Phillips SD et al (2011) Gasoline from Woody biomass via thermochemical gasification, methanol synthesis, and methanol-to-gasoline technologies: a technoeconomic analysis. Ind Eng Chem Res 50:11734–11745. https://doi.org/10.1021/ie2010675
Popa MG et al (2001) Results obtained by methanol fuelling diesel engine. SAE Int. https://doi.org/10.4271/2001-01-3748
Pozzo M et al (2015) Enhanced biomass-to-liquid (BTL) conversion process through high temperature co-electrolysis in a solid oxide electrolysis cell (SOEC). Fuel 145:39–49. https://doi.org/10.1016/j.fuel.2014.12.066
Prashant GK et al (2016) Investigations on the effect of methanol blend on the combustion parameters of dual fuel diesel engine. Appl Therm Eng 103:187–194. https://doi.org/10.1016/j.applthermaleng.2016.04.061
Puig-Gamero M et al (2018) Three integrated process simulation using aspen plus®: pine gasification, syngas cleaning and methanol synthesis. Energy Convers Manage 177:416–427. https://doi.org/10.1016/j.enconman.2018.09.088
Qi DH et al (2010) Performance and combustion characteristics of biodiesel–diesel–methanol blend fuelled engine. Appl Energy 87:1679–1686. https://doi.org/10.1016/j.apenergy.2009.10.016
Raudaskoski R et al (2009) Catalytic activation of CO2: use of secondary CO2 for the production of synthesis gas and for methanol synthesis over copper-based zirconia-containing catalysts. Catal Today 144:318–323. https://doi.org/10.1016/j.cattod.2008.11.026
Ravaghi-Ardebili Z, Manenti F (2015) Unified modeling and feasibility study of novel green pathway of biomass to methanol/dimethylether. Appl Energy 145:278–294. https://doi.org/10.1016/j.apenergy.2015.02.019
Riaz A et al (2013) A review of cleaner production methods for the manufacture of methanol. J Clean Prod 57:19–37. https://doi.org/10.1016/j.jclepro.2013.06.017
Ribeiro AM et al (2010) PSA design for stoichiometric adjustment of bio-syngas for methanol production and co-capture of carbon dioxide. Chem Eng J 163:355–363. https://doi.org/10.1016/j.cej.2010.08.015
Rivarolo M et al (2016) Feasibility study of methanol production from different renewable sources and thermo-economic analysis. Int J Hydrogen Energy 41:2105–2116. https://doi.org/10.1016/j.ijhydene.2015.12.128
Rivera-Tinoco R et al (2016) Investigation of power-to-methanol processes coupling electrolytic hydrogen production and catalytic CO2 reduction. Int J Hydrogen Energy 41:4546–4559. https://doi.org/10.1016/j.ijhydene.2016.01.059
Rodionova MV et al (2017) Biofuel production: challenges and opportunities. Int J Hydrogen Energy 42:8450–8461. https://doi.org/10.1016/j.ijhydene.2016.11.125
Roode-Gutzmer QI et al (2019) Renewable methanol synthesis. Chembioeng Rev 6:209–236. https://doi.org/10.1002/cben.201900012
Saboori B et al (2014) Economic growth, energy consumption and CO2 emissions in OECD (Organization for Economic Co-operation and Development)’s transport sector: a fully modified bi-directional relationship approach. Energy 66:150–161. https://doi.org/10.1016/j.energy.2013.12.048
Saito M et al (1996) Development of copper/zinc oxide-based multicomponent catalysts for methanol synthesis from carbon dioxide and hydrogen. Appl Catal A 138:311–318. https://doi.org/10.1016/0926-860X(95)00305-3
Santos ROD et al (2018) Simulation and optimization of a methanol synthesis process from different biogas sources. J Clean Prod 186:821–830. https://doi.org/10.1016/j.jclepro.2018.03.108
Sayin C (2010) Engine performance and exhaust gas emissions of methanol and ethanol–diesel blends. Fuel 89:3410–3415. https://doi.org/10.1016/j.fuel.2010.02.017
Shamsul NS et al (2014) An overview on the production of bio-methanol as potential renewable energy. Renew Sustain Energy Rev 33:578–588. https://doi.org/10.1016/j.rser.2014.02.024
Shi Z et al (2018) Ignition properties of lean DME/H2 mixtures at low temperatures and elevated pressures. Fuel 226:545–554. https://doi.org/10.1016/j.fuel.2018.04.043
Sikarwar VS et al (2016) An overview of advances in biomass gasification. Energy Environ Sci 9:2939–2977. https://doi.org/10.1039/C6EE00935B
Sikarwar VS et al (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248. https://doi.org/10.1016/j.pecs.2017.04.001
Sindhu R et al (2019) Chapter 5 - biofuel production from biomass: toward sustainable development. In: Kumar S et al (Eds.), Current developments in biotechnology and bioengineering. Elsevier, pp 79–92. https://doi.org/10.1016/B978-0-444-64083-3.00005-1
Singh NB et al (2014) Potential production of bioenergy from biomass in an Indian perspective. Renew Sustain Energy Rev 39:65–78. https://doi.org/10.1016/j.rser.2014.07.110
Soam S et al (2016) Global warming potential and energy analysis of second generation ethanol production from rice straw in India. Appl Energy 184:353–364. https://doi.org/10.1016/j.apenergy.2016.10.034
Songolzadeh M et al (2014) Carbon dioxide separation from flue gases: a technological review emphasizing reduction in greenhouse gas emissions. The Sci World J 2014:828131–828131. https://doi.org/10.1155/2014/828131
Soni DK, Gupta R (2016) Optimization of methanol powered diesel engine: a CFD approach. Appl Therm Eng 106:390–398. https://doi.org/10.1016/j.applthermaleng.2016.06.026
Specht M et al (1999) Synthesis of methanol from biomass/CO2 resources. Elsevier Science Ltd, United Kingdom. http://inis.iaea.org/search/search.aspx?orig_q=RN:31023189
Speight JG (2011) Chapter 3 - Fuels for fuel cells. In: Shekhawat D et al (Eds.), Fuel cells: technologies for fuel processing. Elsevier, Amsterdam, pp 29–48. https://doi.org/10.1016/B978-0-444-53563-4.10003-3
Speight JG (2014) Composition and properties. The chemistry and technology of petroleum, Fifth Edition. CRC Press, pp 185–186. https://doi.org/10.1201/b16559-10
Ståhl K, Neergaard M (1998) IGCC power plant for biomass utilisation, Värnamo. Sweden Biomass and Bioenergy 15:205–211. https://doi.org/10.1016/S0961-9534(98)00025-7
Su Y-C et al (2019) Bioaugmented methanol production using ammonia oxidizing bacteria in a continuous flow process. Biores Technol 279:101–107. https://doi.org/10.1016/j.biortech.2019.01.092
Suntana AS et al (2009) Bio-methanol potential in Indonesia: forest biomass as a source of bio-energy that reduces carbon emissions. Appl Energy 86:S215–S221. https://doi.org/10.1016/j.apenergy.2009.05.028
Svanberg M et al (2018) Renewable methanol as a fuel for the shipping industry. Renew Sustain Energy Rev 94:1217–1228. https://doi.org/10.1016/j.rser.2018.06.058
Taghavifar H et al (2019) Combustion and exergy analysis of multi-component diesel-DME-methanol blends in HCCI engine. Energy 187:115951. https://doi.org/10.1016/j.energy.2019.115951
Taghizadeh-Alisaraei A et al (2019) Ethanol production from date wastes: adapted technologies, challenges, and global potential. Renew Energy 143:1094–1110. https://doi.org/10.1016/j.renene.2019.05.048
Taher E, Chandran K (2013) High-rate, high-yield production of methanol by ammonia-oxidizing bacteria. Environ Sci Technol 47:3167–3173. https://doi.org/10.1021/es3042912
Taylor CE, Noceti RP (2000) New developments in the photocatalytic conversion of methane to methanol. Catal Today 55:259–267. https://doi.org/10.1016/S0920-5861(99)00244-8
Theinnoi K et al (2017) Engine performance of dual fuel operation with in-cylinder injected diesel fuels and in-port injected DME. Energy Procedia 142:461–467. https://doi.org/10.1016/j.egypro.2017.12.072
Thomas G et al (2014) Emissions from DME combustion in diesel engines and their implications on meeting future emission norms: a review. Fuel Process Technol 119:286–304. https://doi.org/10.1016/j.fuproc.2013.10.018
Tijmensen MJA et al (2002) Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenerg 23:129–152. https://doi.org/10.1016/S0961-9534(02)00037-5
Torres S et al (2017) Direct transesterification of microalgae biomass and biodiesel refining with vacuum distillation. Algal Res 28:30–38. https://doi.org/10.1016/j.algal.2017.10.001
Trudewind CA et al (2014) Photocatalytic methanol and methane production using captured CO2 from coal-fired power plants. Part I – a life cycle assessment. J Clean Prod 70:27–37. https://doi.org/10.1016/j.jclepro.2014.02.014
Tsita KG et al (2019) Next generation biofuels derived from thermal and chemical conversion of the Greek transport sector. Thermal Sci Eng Progress. https://doi.org/10.1016/j.tsep.2019.100387
Uusitalo V et al (2017) Potential for greenhouse gas emission reductions using surplus electricity in hydrogen, methane and methanol production via electrolysis. Energy Convers Manage 134:125–134. https://doi.org/10.1016/j.enconman.2016.12.031
van de Water LGA et al (2018) Understanding methanol synthesis from CO/H2 feeds over Cu/CeO2 catalysts. J Catal 364:57–68. https://doi.org/10.1016/j.jcat.2018.04.026
Van-Dal ÉS, Bouallou C (2013) Design and simulation of a methanol production plant from CO2 hydrogenation. J Clean Prod 57:38–45. https://doi.org/10.1016/j.jclepro.2013.06.008
Verhelst S et al (2019) Methanol as a fuel for internal combustion engines. Prog Energy Combust Sci 70:43–88. https://doi.org/10.1016/j.pecs.2018.10.001
Verma P, Sharma MP (2016) Review of process parameters for biodiesel production from different feedstocks. Renew Sustain Energy Rev 62:1063–1071. https://doi.org/10.1016/j.rser.2016.04.054
Vita A et al (2018) Methanol synthesis from biogas: a thermodynamic analysis. Renew Energy 118:673–684. https://doi.org/10.1016/j.renene.2017.11.029
Wang L et al (2019) Effect of excess air/fuel ratio and methanol addition on the performance, emissions, and combustion characteristics of a natural gas/methanol dual-fuel engine. Fuel 255:115799. https://doi.org/10.1016/j.fuel.2019.115799
Wattanavichien K (2009) Implementation of DME in a small direct injection diesel engine. https://doi.org/10.14456/iire.2009.7
Wei L et al (2015) Combustion and emission characteristics of a turbocharged diesel engine using high premixed ratio of methanol and diesel fuel. Fuel 140:156–163. https://doi.org/10.1016/j.fuel.2014.09.070
Xiao J et al (2009) Integrated analysis of energy, economic, and environmental performance of biomethanol from rice straw in China. Ind Eng Chem Res 48:9999–10007. https://doi.org/10.1021/ie900680d
Xu Y (2011) High efficient conversion of CO2-rich bio-syngas to CO-rich bio-syngas using biomass char: a useful approach for production of bio-methanol from bio-oil. Bioresour Technol. https://doi.org/10.1016/j.biortech.2011.02.069
Yang L et al (2014) Progress and perspectives in converting biogas to transportation fuels. Renew Sustain Energy Rev 40:1133–1152. https://doi.org/10.1016/j.rser.2014.08.008
Yang S et al (2018) Biomass-to-Methanol by dual-stage entrained flow gasification: design and techno-economic analysis based on system modeling. J Clean Prod 205:364–374. https://doi.org/10.1016/j.jclepro.2018.09.043
Yang L, Ge X (2016) Chapter three - biogas and syngas upgrading. Advances in Bioenergy. Elsevier, pp 125–188. https://doi.org/10.1016/bs.aibe.2016.09.003
Yin X et al (2005) Characteristics of the synthesis of methanol using biomass-derived syngas. Energy Fuels 19:305–310. https://doi.org/10.1021/ef0498622
Yusaf TF et al (2013) The effect of methanol-diesel blended ratio on CI engine performance. Int J Autom Mech Eng 8:1385–1395
Zakaria Z, Kamarudin SK (2016) Direct conversion technologies of methane to methanol: an overview. Renew Sustain Energy Rev 65:250–261. https://doi.org/10.1016/j.rser.2016.05.082
Zhang C, Wu H (2016) Combustion characteristics and performance of a methanol fueled homogenous charge compression ignition (HCCI) engine. J Energy Inst 89:346–353. https://doi.org/10.1016/j.joei.2015.03.005
Zhang S et al (2005) Upgrading of liquid fuel from the pyrolysis of biomass. Biores Technol 96:545–550. https://doi.org/10.1016/j.biortech.2004.06.015
Zhang Y et al (2009) Simulation of methanol production from biomass gasification in interconnected fluidized beds. Ind Eng Chem Res 48:5351–5359. https://doi.org/10.1021/ie801983z
Zhang L et al (2010) Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers Manage 51:969–982. https://doi.org/10.1016/j.enconman.2009.11.038
Zhang H et al (2011) Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Biores Technol 102:4258–4264. https://doi.org/10.1016/j.biortech.2010.12.075
Zhang Y et al (2013) Process simulation and optimization of methanol production coupled to tri-reforming process. Int J Hydrogen Energy 38:13617–13630. https://doi.org/10.1016/j.ijhydene.2013.08.009
Zhang H et al (2019) Solid-oxide electrolyzer coupled biomass-to-methanol systems. Energy Procedia 158:4548–4553. https://doi.org/10.1016/j.egypro.2019.01.755
Zheng Y et al (2017) A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem Soc Rev 46:1427–1463. https://doi.org/10.1039/C6CS00403B
Zhou DZ et al (2015) A numerical study on RCCI engine fueled by biodiesel/methanol. Energy Convers Manage 89:798–807. https://doi.org/10.1016/j.enconman.2014.10.054
Acknowledgements
The authors wish to acknowledge the support of The Bryden Centre project (Project ID VA5048), which was awarded by The European Union’s INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB), with match funding provided by the Department for the Economy in Northern Ireland and the Department of Business, Enterprise and Innovation in the Republic of Ireland.
Disclaimer
The views and opinions expressed in this review do not necessarily reflect those of the European Commission or the Special EU Programs Body (SEUPB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer: The views and opinions expressed in this review do not necessarily reflect those of the European Commission or the Special EU Programmes Body (SEUPB).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deka, T.J., Osman, A.I., Baruah, D.C. et al. Methanol fuel production, utilization, and techno-economy: a review. Environ Chem Lett 20, 3525–3554 (2022). https://doi.org/10.1007/s10311-022-01485-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01485-y