Abstract
Background
To determine if tibial tunnel reaming during anatomic single-bundle anterior cruciate ligament (ACL) reconstruction using hamstring autograft can result in anterolateral meniscal root injury, as diagnosed by magnetic resonance imaging (MRI).
Methods
A case series of 104 primary anatomic single-bundle ACL reconstructions using hamstring autograft was retrospectively reviewed. Pre- and post-operative (>1 year) MRIs were radiologically evaluated for each patient, with a lateral meniscus extrusion > 3 mm at the level of the medial collateral ligament midportion on a coronal MRI, to establish anterolateral meniscal root injury.
Results
No patients presented radiological findings of anterolateral meniscal root injury in this case series.
Conclusions
Examining a single-bundle ACL reconstruction technique using hamstring autograft that considered tibial tunnel positioning in the center of the tibial footprint, this case series found no evidence of anterolateral meniscal root injury in patient MRIs, even more than 1-year post-operation.
Similar content being viewed by others
Background
The menisci are essential anatomical structures for tibiofemoral congruity, stabilization, shock absorption, and, possibly, proprioception (Fithian et al. 1990; Koenig et al. 2009). Axial load dissipation is mechanically dependent on meniscal structural integrity (Kim et al. 2012; Kopf et al. 2011), and injuries involving meniscal root detachment affect root biomechanics and can lead to degenerative changes within the knee joint (Kopf et al. 2011; Allaire et al. 2008; Sung et al. 2013; Shelbourne et al. 2011; Shybut et al. 2015; Ziegler et al. 2011). Some reports have demonstrated increased levels of meniscal extrusion after anteromedial horn meniscal tears, which correlate with significant cartilage degeneration (Costa et al. 2004; Lerer et al. 2004; Mariani et al. 2015). Others have even reported that a posterior meniscal root tear has biomechanical consequences similar to total meniscectomy (Allaire et al. 2008; Shelbourne et al. 2011; Ellman et al. 2014).
Anatomic anterior cruciate ligament (ACL) reconstruction advocates for the restoration of native ligament insertion sites by using the center of the ACL footprint as a reference for tunnel positioning (Middleton et al. 2014; Fu et al. 2015). Anatomic ACL reconstruction can yield better clinical and biomechanical results than non-anatomic reconstruction (Hussein et al. 2012). However some studies have suggested that placing the tibial tunnel at the center of the tibial footprint may damage the anterolateral meniscal root (ALMR) (Laprade et al. 2014a; Laprade et al. 2014b). A cadaveric study showed that a tunnel reamed in the center of the tibial footprint can cause a significant decrease in the attachment area and, ultimately, in the strength of the ALMR (Bhatia et al. 2014). A recent study has also shown that a posterolateral location of the tibial tunnel would provide an ALMR injury (Ting & Della Valle 2017).
The purpose of this study was to determine if tibial tunnel reaming during anatomic ACL reconstruction could result in an ALMR tear, as diagnosed by magnetic resonance imaging (MRI). We hypothesized that tibial tunnel reaming during ACL reconstruction would not result in ALMR disruption.
Methods
A series of primary anatomic ACL reconstructions performed between 2008 and 2015 by one senior surgeon were retrospectively reviewed. The inclusion criteria were 1) anatomic single-bundle ACL reconstruction using hamstring autograft, without concomitant anterolateral or posterolateral meniscal root tears, as diagnosed before or during surgery; 2) at least 1 year of follow-up control MRI; and 3) a preoperative MRI. Patients with lateral meniscal tears, multiligamentous injuries or a history of ipsilateral knee surgeries were excluded.
This study was approved by the Ethics Committee at the Department of Orthopaedic Surgery and Traumatology, Hospital del Mar, Universitat Autònoma de Barcelona (2016/7004).
Surgical technique
Classic portals for anatomic ACL reconstruction using hamstring autograft were used (i.e. anterolateral, anteromedial, and accessory anteromedial). To assist with tunnel positioning, 1 to 2 mm of the ACL stump was conserved. Femoral tunnel placement was determined at an equidistant point between the anteromedial and posterolateral femoral footprints using the BullsEye femoral guide (ConMed Linvatec, Largo, FL, USA). Tibial tunnel placement was determined using an ACL aiming guide, which was positioned at the calculated center of the ACL footprint through examination of the tibial insertion site and surrounding structures (Ziegler et al. 2011). This point was regularly 2 to 3 mm anteromedial to the posterior margin of the ALMR, which was always evaluated before reaming (Ziegler et al. 2011) (Fig. 1). A 2.4 mm guide pin was positioned using a 55° drill-guide angle. Tunnel size was established as a diameter 1 mm less than the graft diameter. After reaming, a dilator with a diameter equal to the graft diameter was inserted to smooth and compact the tunnel. The final tunnel diameter was 8 mm at minimum and 11 mm at maximum. For graft fixation, absorbable interference screws were used on the tibial side, and cortical suspension device were used on the femoral side.
Tibial footprint center. Prior to tunnel drilling, the tibial insertion site and its peripheral structures, including the anterior root of the lateral meniscus (ALMR), were carefully examined to identify the center of the tibial footprint (TF). This point was regularly 2 to 3 mm anteromedial to the posterior margin of the ALMR (a: arthroscopic view; b: schematic view)
Clinical assessments
Clinical patient assessments were performed prior to surgery and periodically thereafter, starting from 1 week to at least 1 year after surgery. Meniscal symptoms were evaluated as part of the standard knee exam, including load-dependent pain, lateral joint line tenderness, posterior knee pain at full flexion, and a positive McMurray test (Ahn et al. 2010; Habata et al. 2004; Vyas & Harner 2012).
Radiological assessments
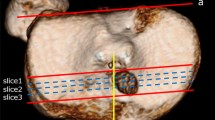
Radiological assessments included an MRI before and at least 1 year after surgery (control exam). All postoperative MRIs were reviewed by two trained orthopedic surgeons to determine the presence of ALMR detachment. The radiological criteria was a meniscal extrusion > 3 mm at the level of the midportion of the medial collateral ligament on a coronal MRI, using at least 3 concomitant coronal slices (Bhatia et al. 2014) (Fig. 2).
Statistical analysis
Categorical variables were presented as frequencies and percentages. The mean and range were calculated for each continuous variable. The Chi-square test was used to compare the results of groups with and without meniscal root tears. Statistical analyses were performed using SPSS 19 (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0.05.
Results
The case series included 31 females (29.8%) and 73 males (70.2%), with a mean age of 32.6 ± 14.2 years-old. The mean follow-up time was 14.6 months (range of 12–18.5 months). Isolated ACL reconstruction was performed in 85 cases (81.7%), while an associated arthroscopic procedure was performed in 19 cases (18.3%, partial medial meniscectomy). Tibial tunnel reamed diameter was 9.32 ± 0.82 mm (8 mm: 14.4%; 9 mm: 46,2%; 10 mm: 31,7%; 11 mm: 7,7%).
Clinical findings
No patients in the evaluated series presented clinical findings of meniscal tearing 1 year after reconstruction surgery.
Radiological findings
The average diameter of MRI-measured tibial tunnels was 9.23 ± 1.1 mm. Considering the radiological MRI criteria needed to establish ALMR detachment, no patients in the present case series presented ALMR tearing, even more than 1 year post-surgery. The medial meniscus roots were also reviewed, and no cases of anterior meniscal root tearing were found.
Discussion
The most important finding of this case series study was that anatomic single-bundle ACL reconstruction using hamstring autograft was not related to MRI criteria of ALMR tearing, even 1 year post-operation. The assessed series was comprised of cases from a single surgeon that performed ACL reconstruction using hamstring autograft by reaming a tibial tunnel in the center of the tibial footprint.
It was recently shown that the anteromedial meniscal root can suffer iatrogenic injury during tibial tunnel creation for ACL reconstruction (Laprade et al. 2014a). This anteromedial injury might obfuscate the recognition of ALMR injury. Nevertheless, a number of cadaveric studies have indicated that anatomic single-bundle ACL reconstruction can significantly decrease ALMR attachment area and strength, as the native ACL tibial insertion is overlapped with the ALMR (Laprade et al. 2014b; Watson et al. 2015). Regarding the attachment area, some studies report that the ALMR is smaller than the anteromedial meniscal root (44.5 mm2 vs 93 mm2) (Johnson et al. 1995; Kohn & Moreno 1995). In contrast, recent anatomical and biomechanical studies provide evidence that the ALMR attachment area is 140.7 mm2 and overlaps up to 40.7% with the tibial ACL footprint (Ellman et al. 2014; Laprade et al. 2014b; Watson et al. 2015; Laprade et al. 2014c; Zantop et al. 2008). A recent study using scanning electron microscopy has shown that the mean percentage of ACL fibers overlapping the ALMR insertion, in the coronal and sagittal planes, was 41.0, 6 8.9 and 53.9, 6 4.3%, respectively (Steineman et al. 2017). Another study has shown that a posterolateral location of the tibial tunnel aperture within the footprint of the native ACL increases extrusion of the lateral meniscus post-reconstruction, where extrusion provides a proxy measure of injury to the anterior root (Ting & Della Valle 2017).
The relation between the ALMR and other anatomical structures has also been qualitatively and quantitatively described. Zantop et al. (Zantop et al. 2008) reported that the ALMR center was anteromedial to the apex of the lateral tibial eminence, anteromedial to the closest edge of the articular cartilage of the lateral tibial plateau, anterolateral to the center of the ACL tibial attachment, and anterior to the nearest edge of the posterior lateral meniscal root. Quantitatively, Zantop et al. (Zantop et al. 2008) further reported that the anteromedial bundle of the ACL is 5.2 mm medial and 2.7 mm posterior to the ALMR center, whereas the posterolateral bundle is 11.2 mm posterior and 4.1 mm medial to the ALMR center. Similarly, Ziegler et al. (Ziegler et al. 2011) found that the ACL center was 7.5 mm medial to the ALMR center. Ziegler et al. (Ziegler et al. 2011) also individually described the ACL bundle attachments, with the anteromedial center 8.3 mm medial to the anterior-most fibers of the ALMR, and the posterolateral center 6.6 mm medial to the posterior-most fibers of the ALMR. Finally, Luites et al. (Luites et al. 2007) observed the footprint center and ACL bundles in relation to the tibial eminences (i.e. medial and lateral intercondylar eminences), noting that the average center of the tibial footprint as a whole is approximately two-fifths the interspinous distance medially to laterally.
Nevertheless, these anatomical studies should be interpreted with caution, particularly as a systematic review by Hussein et al. (Hwang et al. 2012) found heterogeneity among the anatomic landmarks used as references for the ACL tibial footprint and in whether the footprint center was observed as a whole or according to the individual anteromedial and posterolateral bundles. Indeed, this review observed that the most consistent arthroscopic landmark for the tibial footprint was the anterior border of the posterior cruciate ligament, with the tibial footprint center 15 mm anterior to the border (Cuomo et al. 2006; Edwards et al. 2007; Heming et al. 2007). However, it is worth noting that Hussein et al. (Hwang et al. 2012) performed a systematic review of subjective interpretations and failed to provide a definitive, quantitative synthesis through meta-analyses. Considering the heterogeneity in anatomic landmarks, placement of the tibial tunnel using an existing footprint remnant might be a better approach than using bony or meniscal landmarks to restore the anatomic position of the ACL. Supporting this, a recent study showed that ACL reconstructions using an existing footprint remnant for tunnel placement provide better objective and subjective clinical results than reconstructions using bony landmarks (Lu et al. 2015).
Currently, MRIs are the best diagnostic tool for detecting ALMR tears, particularly in the absence of highly specific patient history and/or findings through physical examination (Bhatia et al. 2014). While accurate diagnosis with an MRI is dependent on image quality and the skill of the evaluator, the detection of meniscal root tears is significantly improved by using a variety of magnetic resonance sequences and interpretation signs suggestive of root tears (Choi et al. 2012; De Smet et al. 2009; Muhle et al. 2013). For these parameters, T2-weighted sequences provide the highest specificity and sensitivity values (Lee et al. 2008).
The relatively small size of meniscus roots complicates visualizing a clear tear. Therefore, examinations frequently search for a meniscal extrusion, a pathology highly correlated with root tears (Choi et al. 2010; Magee 2008). A meniscal extrusion is defined as partial or total displacement of the meniscus from the tibial articular cartilage (Lerer et al. 2004). A 3 mm extrusion on midcoronal imaging is significantly associated with articular cartilage degeneration, meniscal degeneration, complex tear patterns, and tears involving the meniscus root (Costa et al. 2004; Lerer et al. 2004). In this study, meniscal extrusion was used to assess ALMR disruption.
Worth noting, this study is limited due to its retrospective nature and since MRIs were obtained with patients in a supine position, where meniscal extrusion is theoretically worse under weight-bearing conditions. Also, anterior and posterior extrusions were not evaluated, but, to our knowledge, no method exists for evaluating these extrusions via MRI.
Future studies should aim to establish a methodology for diagnosing potential ALMR injuries associated with ACL reconstruction.
Conclusion
Given the close anatomical relation between the ALMR and the ACL tibial footprint, it is possible that the ALMR could incur injury as a result of tibial tunnel reaming during ACL reconstruction. To assess this possibility, the current study examined a case series of single-bundle ACL reconstructions, using hamstring autograft with an average of 9.32 mm tibial tunnel reamed diameter, that considered tunnel positioning in the center of the tibial footprint. No MRI evidence for ALMR injury was found, even more than 1 year post-operation.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ALMR:
-
Anterolateral meniscal root
- MRI:
-
Magnetic resonance imaging
References
Ahn JH, Lee YS, Yoo JC, Chang MJ, Park SJ, Pae YR (2010) Results of arthroscopic all-inside repair for lateral meniscus root tear in patients undergoing concomitant anterior cruciate ligament reconstruction. Arthroscopy 26(1):67–75
Allaire R, Muriuki M, Gilbertson L, Harner CD (2008) Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am 90(9):1922–1931
Bhatia S, Laprade CM, Ellman MB, Laprade RF (2014) Meniscal Root Tears: Significance, Diagnosis, and Treatment. Am J Sports Med 42(12):3016–3030
Choi C-J, Choi Y-J, Lee J-J, Choi C-H (2010) Magnetic resonance imaging evidence of meniscal extrusion in medial meniscus posterior root tear. Arthroscopy 26(12):1602–1606
Choi S-H, Bae S, Ji SK, Chang MJ (2012) The MRI findings of meniscal root tear of the medial meniscus: emphasis on coronal, sagittal and axial images. Knee Surg Sports Traumatol Arthrosc 20(10):2098–2103
Costa CR, Morrison WB, Carrino JA (2004) Medial meniscus extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? Am J Roentgenol 183(1):17–23
Cuomo P, Edwards A, Giron F, Bull AMJ, Amis AA, Aglietti P (2006) Validation of the 65° Howell guide for anterior cruciate ligament reconstruction. Arthroscopy 22(1):70–75
De Smet AA, Blankenbaker DG, Kijowski R, Graf BK, Shinki K (2009) MR diagnosis of posterior root tears of the lateral meniscus using arthroscopy as the reference standard. AJR Am J Roentgenol 192(2):480–486
Edwards A, Bull AMJ, Amis AA (2007) The attachments of the anteromedial and posterolateral fibre bundles of the anterior cruciate ligament: Part 1: tibial attachment. Knee Surg Sports Traumatol Arthrosc 15(12):1414–1421
Ellman MB, Laprade CM, Smith SD, Rasmussen MT, Engebretsen L, Wijdicks CA, Laprade RF (2014) Structural Properties of the Meniscal Roots. Am J Sports Med 42(8):1881–1887
Fithian DC, Kelly MA, Mow VC (1990) Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res 252:19–31
Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS (2015) Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc 23(3):640–648
Habata T, Uematsu K, Hattori K, Takakura Y, Fujisawa Y (2004) Clinical features of the posterior horn tear in the medial meniscus. Arch Orthop Trauma Surg 124(9):642–645
Heming JF, Rand J, Steiner ME (2007) Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med 35(10):1708–1715
Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH (2012) Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction: 281 cases with 3- to 5-year follow-up. Am J Sports Med 40(3):512–520
Hwang MD, Piefer JW, Lubowitz JH (2012) Anterior Cruciate Ligament Tibial Footprint Anatomy: Systematic Review of the 21st Century Literature. Arthroscopy 28(5):728–734
Johnson DL, Swenson TM, Livesay GA, Aizawa H, Fu FH, Harner CD (1995) Insertion-site anatomy of the human menisci: gross, arthroscopic, and topographical anatomy as a basis for meniscal transplantation. Arthroscopy 11(4):386–394
Kim JG, Lee YS, Bae TS, Ha JK, Lee DH, Kim YJ, Ra HJ (2012) Tibiofemoral contact mechanics following posterior root of medial meniscus tear, repair, meniscectomy, and allograft transplantation. Knee Surg Sports Traumatol Arthrosc 21(9):2121–2125
Koenig JH, Ranawat AS, Umans HR, Difelice GS (2009) Meniscal root tears: diagnosis and treatment. Arthroscopy 25(9):1025–1032
Kohn D, Moreno B (1995) Meniscus insertion anatomy as a basis for meniscus replacement: a morphological cadaveric study. Arthroscopy 11(1):96–103
Kopf S, Colvin AC, Muriuki M, Zhang X, Harner CD (2011) Meniscal root suturing techniques: implications for root fixation. Am J Sports Med 39(10):2141–2146
Laprade CM, James EW, Engebretsen L, Laprade RF (2014a) Anterior medial meniscal root avulsions due to malposition of the tibial tunnel during anterior cruciate ligament reconstruction: two case reports. Knee Surg Sports Traumatol Arthrosc 22(5):1119–1123
Laprade CM, Smith SD, Rasmussen MT, Hamming MG, Wijdicks CA, Engebretsen L, Feagin JA, Laprade RF (2014b) Consequences of Tibial Tunnel Reaming on the Meniscal Roots During Cruciate Ligament Reconstruction in a Cadaveric Model, Part 1: The Anterior Cruciate Ligament. Am J Sports Med 43(1):200–206
Laprade CM, Ellman MB, Rasmussen MT, James EW, Wijdicks CA, Engebretsen L, Laprade RF (2014c) Anatomy of the Anterior Root Attachments of the Medial and Lateral Menisci: A Quantitative Analysis. Am J Sports Med 42(10):2386–2392
Lee SY, Jee W-H, Kim J-M (2008) Radial tear of the medial meniscal root: reliability and accuracy of MRI for diagnosis. AJR Am J Roentgenol 191(1):81–85
Lerer DB, Umans HR, Hu MX, Jones MH (2004) The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol 33(10):569–574
Lu W, Wang D, Zhu W, Li D, Ouyang K, Peng L, Feng W, Li H (2015) Placement of Double Tunnels in ACL Reconstruction Using Bony Landmarks Versus Existing Footprint Remnant: A Prospective Clinical Study With 2-Year Follow-up. Am J Sports Med 43(5):1206–1214
Luites JWH, Wymenga AB, Blankevoort L, Kooloos JGM (2007) Description of the attachment geometry of the anteromedial and posterolateral bundles of the ACL from arthroscopic perspective for anatomical tunnel placement. Knee Surg Sports Traumatol Arthrosc 15(12):1422–1431
Magee T (2008) MR findings of meniscal extrusion correlated with arthroscopy. J Magn Reson Imaging 28(2):466–470
Mariani PP, Iannella G, Cerullo G, Giacobbe M (2015) Avulsion of both posterior meniscal roots associated with acute rupture of the anterior cruciate ligament. J Orthop Traumatol 16(3):259–262, Springer International Publishing
Middleton KK, Hamilton T, Irrgang JJ, Karlsson J, Harner CD, Fu FH (2014) Anatomic anterior cruciate ligament (ACL) reconstruction: a global perspective. Knee Surg Sports Traumatol Arthrosc 22(7):1467–1482
Muhle C, Ahn JM, Dieke C (2013) Diagnosis of ACL and meniscal injuries: MR imaging of knee flexion versus extension compared to arthroscopy. Springerplus 2(1):213, Springer International Publishing
Shelbourne KD, Roberson TA, Gray T (2011) Long-term Evaluation of Posterior Lateral Meniscus Root Tears Left In Situ at the Time of Anterior Cruciate Ligament Reconstruction. Am J Sports Med 39(7):1439–1443
Shybut TB, Vega CE, Haddad J, Alexander JW, Gold JE, Noble PC, Lowe WR (2015) Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med 43(4):905–911
Steineman BD, Moulton SG, Haut Donahue TL, Fontboté CA, Laprade CM, Cram TR, Dean CS, Laprade RF (2017) Overlap Between Anterior Cruciate Ligament and Anterolateral Meniscal Root Insertions. Am J Sports Med 45(2):362–368
Sung JH, Ha JK, Lee DW, Seo WY, Kim JG (2013) Meniscal Extrusion and Spontaneous Osteonecrosis With Root Tear of Medial Meniscus: Comparison With Horizontal Tear. Arthroscopy 29(4):726–732
Ting NT, Valle Della CJ (2017) Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J Arthroplasty. Elsevier (in press)
Vyas D, Harner CD (2012) Meniscus root repair. Sports Med Arthrosc 20(2):86–94
Watson JN, Wilson KJ, Laprade CM, Kennedy NI, Campbell KJ, Hutchinson MR, Wijdicks CA, Laprade RF (2015) Iatrogenic injury of the anterior meniscal root attachments following anterior cruciate ligament reconstruction tunnel reaming. Knee Surg Sports Traumatol Arthrosc 23(8):2360–2366
Zantop T, Wellmann M, Fu FH, Petersen W (2008) Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction: anatomic and radiographic findings. Am J Sports Med 36(1):65–72
Ziegler CG, Pietrini SD, Westerhaus BD, Anderson CJ, Wijdicks CA, Johansen S, Engebretsen L, Laprade RF (2011) Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med 39(4):743–752
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
All authors contributed to the idea, initiation, execution and revision of the present study. All authors read and approved the final manuscript.
Competing interests
The authors declare no potential conflicts of interest regarding the research, authorship, and/or publication of this article.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Irarrázaval, S., Masferrer-Pino, A., Ibañez, M. et al. Does anatomic single-bundle ACL reconstruction using hamstring autograft produce anterolateral meniscal root tearing?. J EXP ORTOP 4, 17 (2017). https://doi.org/10.1186/s40634-017-0093-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-017-0093-5