Abstract
Background
A number of studies have found associations between heavy metals and uterine fibroids, but the results are inconsistent. Here, we conducted this research to demonstrate the relationships between blood heavy metal concentrations and uterine fibroid volume as well as the rate of uterine fibroid presence.
Methods
In a cross-sectional study, we collected data from 308 premenopausal women aged 30–49 years in Seoul; uterine fibroids are ascertained by past history of myomectomy and pelvic ultrasonography. In the analytic phase, we first analyzed the presence of the fibroids and the concentrations of heavy metals via logistic regression. In subgroup analysis, we used simple and multiple linear regression analyses to examine the associations between heavy metals and uterine fibroid volume.
Results
There was no connection between the heavy metal concentrations and the presence of uterine fibroids, but the odds of women having fibroids were higher with three particular metals. In subgroup analysis, the association between blood cadmium concentrations and uterine fibroid volume was statistically significant (adjusted beta coefficient = 2.22, 95% confidential interval: 0.06–4.37). In contrast, blood mercury and lead concentrations were not significantly associated with uterine fibroid volume.
Conclusions
Our findings are the first that we know to report the association of blood cadmium concentrations with the volume of uterine fibroids. We expect that our findings will be used as evidence for supporting policies to improve premenopausal Korean women’s health.
Similar content being viewed by others
Background
Uterine fibroids cause serious symptoms such as uterine bleeding and pelvic pain [1], resulting in major surgery (e.g., hysterectomy). Nearly 70% of Caucasian women show the cumulative incidence of uterine fibroids by the age of 50 [2], and the rate also applies to Asian women [3].
Although the precise causes of fibroids are still unknown, they have been assumed to be associated with reproductive history, body mass index (BMI), and smoking [4]. Fibroids are known for estrogen-dependent diseases, where diseased tissues show more estrogen receptor-α (ERα) than non-diseased tissues [5,6,7]. Because fibroids are hormonally related, it has been hypothesized that exposure to exogenous estrogens is related to their development and size increase [8, 9].
Metalloestrogens are metals that are known to activate the estrogen receptor in the absence of estradiol [10]. In both in vitro and in vivo studies, metalloestrogens have been identified as the cause of ERα activation [11,12,13]. Among metalloestrogens, the following three heavy metals have been reported as toxic to people: cadmium, lead, and mercury. The main sources of exposure to each metal are as follows: cadmium exposure is primarily through cigarette smoke, air pollution, and contaminated food [14]; lead exposure is through lead-based paint, contaminated soil, and dust [15]; and mercury exposure is through fish consumption, air pollution, and dental amalgams [16].
Two epidemiologic studies have found the associations between blood heavy metal concentrations and the existence of uterine fibroids, but the results are not consistent [17, 18]. In addition, Korean women are vulnerable to conventional heavy metal exposure because they consume so much rice and seafood [19, 20]. Hence, here we epidemiologically conduct research to demonstrate the relationships between heavy metal concentration and uterine fibroid volume, mainly based on cell proliferation, as well as the rate of uterine fibroids.
Methods
Study participants
Using a cross-sectional study, we conducted research on the effects of exposure to environmental risk factors in premenopausal women in Seoul. The data were collected for three months (September to November 2014) to clarify how the environment exposure affects their health.
Recruitment and informed consent
The women volunteered following a notice on the bulletin board at the Ewha Womans University Medical Center, a support center for healthy families, a community health center, a community service center, and a community blog for mothers. The study protocol was approved by the institutional review board at Ewha Womans University Medical Center, and written informed consent was obtained from all of the women.
Data and bio-specimen collection
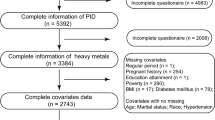
We collected data from 308 premenopausal women in the Republic of Korea, the age range of 30 to 49. For the data analysis, we did not include pregnant or breastfeeding women, whose heavy metal levels at the time might have been influenced by these circumstances and might have been less representative of heavy metal levels at the time of diagnosis [21, 22]; women who had received hysterectomies were also excluded from the analysis. Ultimately, we conducted the statistical analysis with 288 women, 46 with fibroids and 242 without.
In order to clarify the statistical significance between uterine fibroid volume and heavy metals, we also performed subgroup analysis among women with fibroids. Because there was bias resulting from the myomectomy histories of women who did not have a medical record of their uterine fibroid volumes, we conducted our subgroup analyses only with women who had not received a myomectomy (n = 40).
We collected the data from interview questionnaires, physical examinations, pelvic ultrasonography, and blood samples; we collected basic health information such as socio-demographic characteristics, prior medical history, reproductive health status, psychosocial status, health behavior, height, weight, and environmental exposure with the assistance of trained researchers using questionnaires.
We collected the data on the presence and volume of the women’s uterine fibroids through pelvic ultrasonography. Specifically, pelvic ultrasounds were performed using the ProSound Alpha 7 ultrasound system (Hitachi Aloka Medical, Tokyo, Japan). We also sampled blood in order to measure the levels of cadmium, mercury and lead; we collected the whole-blood samples in EDTA-treated tubes and stored the mat 4–8 °C throughout the entire analysis period. We conducted these analyses within three days of storage at the Seoul Medical Science Institute.
Analysis of heavy metals in whole blood
We analyzed each heavy metal as follows: We analyzed cadmium levels using graphite furnace atomic absorption spectrophotometry. The limit of quantification (LOQ) was 0.100 μg/L, and no sample had a cadmium level below the LOQ; we analyzed mercury by inductively coupled plasma mass spectrometry. The LOQ was 0.008 μg/L, and no sample had a mercury level below that; we analyzed lead levels by graphite furnace atomic absorption spectrophotometry. The LOQ was 0.030 μg/dL, and again, no sample had a lead level below the LOQ. Standardized quality-control procedures were carried out for the laboratory analyses, including internal and external controls. For each series of analyses, internal quality control was used. Centers for Disease Control and Prevention (CDC)’s Lead and Multi-element Proficiency (LAMP) external quality program were periodically conducted for the precision and accuracy of cadmium, lead, and mercury level measurement. Additional external quality control for lead measurement was executed by College of American Pathologists (CAP) program.
Uterine fibroid ascertainment
Diagnosis of uterine fibroids was based on pelvic ultrasonography and two questions: “Have you ever undergone an operation in the past?” and “If you have ever undergone an operation, what was the operation?”; the pelvic sonograms were taken by a well-trained gynecologist. If a participant had undergone a myomectomy in the past or was diagnosed with uterine fibroids by pelvic ultrasonography during our research, we categorized that woman as having uterine fibroids. In addition, if we could identify fibroids during our research based on the pelvic sonograms, we also gathered data on the fibroids volumes. When study participants had multiple uterine fibroids, we selected the largest fibroids by volume as the dominant or marker fibroids for demonstrating the association between blood heavy metal concentrations and uterine fibroid volumes. We calculated fibroid volume by measuring the longest and shortest diameters to determine an ellipsoid shape [23].
Statistical analysis
All statistical analyses were performed in SAS 9.3 version. Descriptive analyses included evaluating data completeness and comparing women with and without fibroids in relation to socio-demographic, medical, and reproductive history characteristics. We used Chi-square tests to determine statistical significance for the categorical variables. After, the geometric means and standard deviations for the three heavy metal concentrations were calculated by general characteristics and disease status of study participants. Kruskal-Wallis tests were also used to determine statistical significance among non-normally distributed continuous variables. Before moving onto inferential analysis, all concentrations of blood heavy metal and the volume of the largest fibroids were log transformed due to non-normal distribution; this transformation normalized the distributions. In the analytic phase, we first analyzed the presence of uterine fibroids and the heavy metal concentrations via logistic regression. Second, we used simple and multiple linear regressions for the subgroup analyses of the associations between heavy metal concentrations and uterine fibroid volumes. Third, the association between quartiles of heavy metals concentrations and uterine fibroid volumes was assessed using simple and multiple linear regression. The possible confounders were age as continuous, BMI as continuous, the age at menarche as continuous, gravidity categorized as 0, 1, 2, and more than 3, birth control pill administration history dichotomized as never versus used, regularity of menstrual cycle dichotomized as regular versus irregular, hemoglobin level dichotomized as anemia versus non-anemia (<12 g/dL versus ≥12 g/dL), and serum cotinine dichotomized as no versus passive or active exposure (<10 ng/mL versus ≥10 ng/mL).
Results
In our study, the prevalence of uterine fibroids was 16.0%. Table 1 shows that the women with uterine fibroids were older than the women without fibroids. Table 2 shows that the research women’s mean blood concentrations of cadmium, lead, and mercury were 0.98 μg/L, 1.36 μg/dL, and 1.61 μg/L, respectively. Women in their 40s had significantly higher cadmium and mercury concentrations than those in their 30s (Table 2). Obese women had a significantly higher mercury concentration than non-obese women and women with anemia had a significantly lower cadmium concentration than women without anemia (Table 2). Although the women with uterine fibroids showed higher blood concentrations of the heavy metals than the women without uterine fibroids, there was no significance between the concentrations and the presence of fibroids, also shown in Table 2. Table 3 shows that there was no significance between the concentrations and the presence of fibroids either before or after adjustment, but the odds of having these three heavy metals in their blood were higher among women who did have uterine fibroids. Tables 4 and 5 shows the results of the subgroup analysis of the women who were found to have uterine fibroids via pelvic ultrasonography. In that subgroup, the mean volume of the largest fibroids was 19.6 ± 56.1 cm3 (range: 0.02–280.55cm3). In Table 4, the association between blood cadmium concentrations and the volume of the largest fibroids was statistically significant after adjusting for confounders (P < 0.05) and the correlation graph between blood cadmium concentrations and the volume of the largest fibroids was represented in Fig. 1. In contrast, blood mercury and lead concentrations were not significantly associated with the volume of the largest fibroids either before or after adjusting for confounders (Table 4). In Table 5, although dose–response association between blood cadmium concentrations and the volume of the largest fibroids did not show a statistically significant, the upper quartile of blood cadmium concentrations had the highest β coefficient.
Discussion
We here demonstrated a significant relationship between blood cadmium concentrations and uterine fibroid volume by statistically adjusting for variables such as age, age at menarche, use of birth control pills prior to diagnosis, gravidity, regularity of menstrual cycle, BMI, hemoglobin level, and cotinine levels. To our knowledge, our findings are the first attempt to report the association of blood cadmium concentrations with uterine fibroid volume; our results show close agreement with the hypothesis that cadmium increases the volume of uterine fibroids.
These findings are similar to the findings of previous in vivo studies. Cadmium exposure is known to increase uterine wet weight and to proliferate human uterine leiomyoma cells based on a number of in vivo studies [24, 25]. Although the mechanisms are not clear, estrogen receptor (ER) stimulation and non-genomic activation of mitogen-activated protein kinase (MAPK) are considered possible biological mechanisms of cadmium’s actions [25, 26]. A number of studies have found more ERs in fibroids than in the homogeneous myometrium [27], and another study suggested that metalloestrogens activate the ER-α [11]. However, more recently there are several efforts to demonstrate that Cd-induced growths in human uterine leiomyoma and smooth muscle cells are not mediated by classical ER mechanism of receptor binding or estrogen response element-mediated gene activation [28], but by non-genomic pathways [27]. Non-genomic pathway involves differential activation of growth factor receptors and subsequent MAPK/extracellular signal-regulated kinase 1/2 phosphorylation [27]. Therefore, more studies are needed to demonstrate the exact mechanisms of estrogenic effects in cadmium exposure.
Unlike the results for blood cadmium, the blood lead and mercury levels were not significantly associated with fibroid volume in our study, which can be explained by a ranking of estrogenicity based on an in vitro study [12] that measured thee estrogenicity of major heavy metals by estrogen-receptor-dependent transcriptional expression assay. The results show the following estrogenicity ranking: cadmium chloride > lead acetate > lead nitrate > mercuric chloride [12]. Prior human studies regarding breast cancer and heavy metals also show this estrogenicity ranking [10]. Breast cancer is known to depend on estrogen, as uterine fibroids do. Studies of breast cancer and metalloestrogens have found major concerns with cadmium [10], and many epidemiological studies demonstrate the association between cadmium and breast cancer [29,30,31].
The increased volume of uterine fibroids is clinically important because most fibroids are asymptomatic [32], but larger fibroids can cause “bulk” symptoms including bowel and bladder dysfunction and abdominal pain [33]; consequently, large uterine fibroids need to be treated by surgery or medication. Because of this importance of uterine fibroid volume, our finding is medically meaningful for identifying strategies to prevent adverse effects of uterine fibroids.
In our study, although there was no clear statistical significance between the existence of uterine fibroids and heavy metals, the women with uterine fibroids showed higher geometric mean heavy metal concentrations and greater odds of having these three heavy metals in their blood than did the women without fibroids. Previously, two epidemiologic studies demonstrated the relationship between heavy metal blood concentrations and uterine fibroids [17, 18]. Research based on the National Health and Nutrition Examination Survey (NHANES) presented that uterine fibroids were not significantly associated with blood levels of heavy metals [17], whereas research based on an operative cohort of the Endometriosis: Natural History, Diagnosis, and Outcomes (ENDO) study showed the effects of blood cadmium and lead on uterine fibroids [18]. In terms of exposure, our study participants had higher blood concentrations of heavy metals than did participants in either of the previously reported two studies; our study participants showed, respectively, mean blood concentrations of 0.98 μg/L, 1.36 μg/dL, and 1.61 μg/L of cadmium, lead, and mercury, whereas in the NHANES, the participants showed means of 0.42 μg/L, 1.13 μg/dL, and 1.00 μg/L of cadmium, lead, and mercury, respectively [17]. In the ENDO study, the research participants who had uterine fibroids showed blood concentration means of 0.37 μg/L, 0.83 μg/dL, and 0.93 μg/L, and those without fibroids showed 0.30 μg/L, 0.61 μg/dL, and 0.55 μg/L of cadmium, lead, and mercury blood concentrations, respectively [18]. Regarding the study outcomes, the NHANES [17] is based on a self-reported questionnaire and thus determining whether the patients had uterine fibroids had limited sensitivity. The prevalence of uterine fibroids was 8% in the NHANES [17], much lower than the prevalence that had been previously reported from utilizing ultrasonography for the detection [2]. In contrast, ENDO study [18] showed uterine fibroid prevalence of 21% with the postoperative diagnosis. Although postoperative diagnosis clearly showed fibroids, small submucosal and intramural fibroids may have escaped detection. To diagnose the presence of fibroids, our study used both the questionnaire and pelvic ultrasonography, and prevalence was lower than expected (16.3%). Additionally, ENDO study [18] had more participants (n = 495) than our study (n = 282).
Our study does have a number of limitations; first, the study design was cross-sectional, and thus there was no temporality. Second, our participants consisted of healthy volunteers, and nearly all of them had given birth; thus, our study results could have been biased by this characteristic of the subjects. Third, our study population was smaller than that in previous studies, which could have reduced the statistical power in our study.
Although this study did have its limitations, it also has strengths. Our advanced diagnostic method with the questionnaire and the pelvic sonograms allowed us to confirm the history of myomectomy and asymptomatic uterine fibroids, respectively. This outstanding measurement method showed clearly reliable diagnosis results that were unattainable with the NHANES [17] and ENDO study [18]. This approach also enabled us to obtain the data on uterine fibroid volume, which allowed us to analyze the relationship between blood heavy metal concentrations and fibroid volumes.
Conclusions
In summary, we attempted to determine the influence of heavy metals on the prevalence and volume of uterine fibroids. As demonstrated in our statistical results, which derived from a questionnaire and pelvic ultrasonography results, the women who had uterine fibroid showed higher geometric mean blood heavy metal concentrations as well as greater odds of having these three metals in their blood than the women who did not have fibroids. In addition, we also examined the relationship between uterine fibroid volume and the heavy metal concentrations. The blood cadmium concentration was significantly associated with fibroid volume, although the blood levels of lead and mercury were not.
Symptomatic uterine fibroids seriously reduce the quality of life in premenopausal women, and their volume has the most important influence on the symptoms. Therefore, well-designed studies considering the limitations of our study are needed for demonstrating the clear associations between heavy metal exposure and uterine fibroids in premenopausal women.
Abbreviations
- BMI:
-
Body mass index
- ENDO:
-
Endometriosis: Natural History, Diagnosis and Outcomes
- ER:
-
Estrogen receptor
- LOQ:
-
Limit of quantification
- MAPK:
-
Mitogen-activated protein kinase
- NHANES:
-
National Health and Nutrition Examination Survey
References
Chabbert-Buffet N, Esber N, Bouchard P. Fibroid growth and medical options for treatment. Fertil Steril. 2014;102(3):630–9.
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–7.
Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967–73.
Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153(1):1–10.
Wang H, Wu X, Englund K, Masironi B, Eriksson H, Sahlin L. Different expression of estrogen receptors alpha and beta in human myometrium and leiomyoma during the proliferative phase of the menstrual cycle and after GnRHa treatment. Gynecol Endocrinol. 2001;15(6):443–52.
Brandon DD, Erickson TE, Keenan EJ, Strawn EY, Novy MJ, Burry KA, et al. Estrogen receptor gene expression in human uterine leiomyomata. J Clin Endocrinol Metab. 1995;80(6):1876–81.
Benassayag C, Leroy MJ, Rigourd V, Robert B, Honoré JC, Mignot TM, et al. Estrogen receptors (ERalpha/ERbeta) in normal and pathological growth of the human myometrium: pregnancy and leiomyoma. Am J Physiol. 1999;276(6 Pt 1):E1112–8.
Hunter DS, Hodges LC, Eagon PK, Vonier PM, Fuchs-Young R, Bergerson JS, et al. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect. 2000;108(Suppl 5):829–34.
Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108(Suppl 5):821–7.
Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Metals and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):63–73.
Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14(4):545–53.
Choe SY, Kim SJ, Kim HG, Lee JH, Choi Y, Lee H, et al. Evaluation of estrogenicity of major heavy metals. Sci Total Environ. 2003;312(1-3):15–21.
Zhang X, Wang Y, Zhao Y, Chen X. Experimental study on the estrogen-like effect of mercuric chloride. Biometals. 2008;21(2):143–50.
Registry(ASTDR) AoTSaD. Toxicological Profile for Cadmium. In: US Department of Health and Human Services. Atlanta: Public Health Service; 2012.
(ASTDR) AfTSaDR. Toxicological Profile for lead. In: US Department of Health and Human Services. Atlanta: Public Health Service; 2007.
(ATSDR) AfTSaDR. Toxicological Profile for mercury. In: US Department of Health and Human Services. Atlanta: Public Health Service; 1999.
Jackson LW, Zullo MD, Goldberg JM. The association between heavy metals, endometriosis and uterine myomas among premenopausal women. National Health and Nutrition Examination Survey 1999-2002. Hum Reprod. 2008;23(3):679–87.
Johnstone EB, Louis GM, Parsons PJ, Steuerwald AJ, Palmer CD, Chen Z, et al. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: findings from the ENDO study. Reprod Toxicol. 2014;49:27–32.
Park S, Lee BK. Strong positive association of traditional Asian-style diets with blood cadmium and lead levels in the Korean adult population. Int J Environ Health Res. 2013;23(6):531–43.
Park S, Lee BK. Strong positive associations between seafood, vegetables, and alcohol with blood mercury and urinary arsenic levels in the Korean adult population. Arch Environ Contam Toxicol. 2013;64(1):160–70.
Akesson A, Berglund M, Schütz A, Bjellerup P, Bremme K, Vahter M. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92(2):284–7.
Hertz-Picciotto I, Schramm M, Watt-Morse M, Chantala K, Anderson J, Osterloh J. Patterns and determinants of blood lead during pregnancy. Am J Epidemiol. 2000;152(9):829–37.
Zivković N, Zivković K, Despot A, Paić J, Zelić A. Measuring the volume of uterine fibroids using 2- and 3-dimensional ultrasound and comparison with histopathology. Acta Clin Croat. 2012;51(4):579–89.
Zhang W, Yang J, Wang J, Xia P, Xu Y, Jia H, et al. Comparative studies on the increase of uterine weight and related mechanisms of cadmium and p-nonylphenol. Toxicology. 2007;241(1-2):84–91.
Gao X, Yu L, Moore AB, Kissling GE, Waalkes MP, Dixon D. Cadmium and proliferation in human uterine leiomyoma cells: evidence of a role for EGFR/MAPK pathways but not classical estrogen receptor pathways. Environ Health Perspect. 2015;123(4):331–6.
Nasiadek M, Swiatkowska E, Nowinska A, Krawczyk T, Wilczynski JR, Sapota A. The effect of cadmium on steroid hormones and their receptors in women with uterine myomas. Arch Environ Contam Toxicol. 2011;60(4):734–41.
Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjöblom P, Norgren A, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83(11):4092–6.
Ali I, Penttinen-Damdimopoulou PE, Mäkelä SI, Berglund M, Stenius U, Akesson A, et al. Estrogen-like effects of cadmium in vivo do not appear to be mediated via the classical estrogen receptor transcriptional pathway. Environ Health Perspect. 2010;118(10):1389–94.
McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98(12):869–73.
Gallagher CM, Chen JJ, Kovach JS. Environmental cadmium and breast cancer risk. Aging (Albany NY). 2010;2(11):804–14.
Julin B, Wolk A, Bergkvist L, Bottai M, Akesson A. Dietary cadmium exposure and risk of postmenopausal breast cancer: a population-based prospective cohort study. Cancer Res. 2012;72(6):1459–66.
Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59(1):2–24.
Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372(17):1646–55.
Acknowledgements
Sources of funding for all authors
SH Ye received funding from Ewha Womans University scholarship of 2015 and ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’, a project of the Ministry of Environment, Republic of Korea.
HW Chung, KA Jeong, SY Park, YA Sung, HJ Lee, EH Ha received funding from ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’, a project of the Ministry of Environment, Republic of Korea
HJ Kim did not receive funding.
Funding
This study was supported by ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’, a project of the Ministry of Environment, Republic of Korea and by the Ewha Womans University scholarship of 2015.
The roles of funding from a project of the Ministry of Environment are design of the study and collection, analysis, and interpretation of data.
The roles of funding from Ewha Womans University scholarship are analysis and interpretation of data.
Availability of data and materials
Due to data protection laws, data is only available to the participating scientists, involved in ‘Research on Health Effects of Exposure to Environmental Risk Factors in Reproductive Age Women’.
Authors’ contributions
SHY and EHH conceptualized and designed the study, collected the data, conducted analysis, and drafted and finalized the manuscript. HWC, KAJ, YAS, HJL, and SYP conceptualized and designed the study, collected the data, and reviewed the draft manuscript. HJK reviewed the draft manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the institutional review board at Ewha Womans University Hospital, and written informed consent was obtained from all of the participants.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ye, S., Chung, H.W., Jeong, K. et al. Blood cadmium and volume of uterine fibroids in premenopausal women. Ann of Occup and Environ Med 29, 22 (2017). https://doi.org/10.1186/s40557-017-0178-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40557-017-0178-8