Abstract
Background
Soil carbon-rich organic amendments (biochar, humic substances) may improve the quality and fertility of arable soil. Their co-application can additively enhance the beneficial effect on soil. Hypothetically, the pre-treatment of biochar, by aging via soaking in a solution of commercially available humic substances, could result in synergism, which may exceed the benefit from simple co-application of both amendments to the soil. Therefore, the aim of this study was to investigate the impact of biochar, humic substances, the combination of both, and the impact of biochar aged by humic substances solution on soil microbial activities and plant growth in a short-term pot experiment with lettuce.
Results
The aging of biochar decreased the C:N ratio as compared to non-activated biochar. The co-application of biochar and humic substances into the soil resulted in the highest microbial biomass carbon and respiration activity. The majority of enzyme activities (β-glucosidase, arylsulfatase, N-acetyl-β-d-glucosaminidase, phosphatase) were the highest in humic substances-amended soil. The application of humic substances and biochar with humic substances seemed to stimulate microbial growth and activity followed by the competition of microflora for nutrients with plants, whereas the aged biochar behaved differently. The plants treated by aged biochar achieved the highest values of dry aboveground and root biomass of all variants. However, the assumed rapid uptake of nutrients by plants resulted in lower nutrient availability for microflora, and a decline in microbial viability.
Conclusions
Based on this study, the positive effect of co-applied humic substances and biochar on soil fertility, quality, and health can be concluded. The usability of biochar aging by humic solution requires further study.
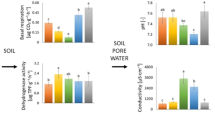
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Soil microorganisms represent an active part of soil. They are important for the transformation of soil organic matter (SOM), nutrient conversion, and circulation in the soil [1]. The quantity, vitality, and diversity of the soil microbial consortium can be assessed by soil microbial respiration (basal and induced by various substrates) [2]. Moreover, microbial metabolism is sensitively indicated by soil enzyme activities, which are closely related to soil fertility, quality, and health [1, 3]. The most studied soil enzymatic activities include β-glucosidase, arylsulfatase, phosphatase, and urease, due to their important roles in the mineralisation of C, S, P and N, respectively [4]. When using soil amendments in sustainable agriculture, it is therefore necessary to assess their effect on soil microbial activity and abundance.
Humic substances-rich peat and lignite [5, 6] and C-rich biochar, belong to the routinely used natural soil amendments [7]. Humic substances, naturally occurring in soil, are produced by complex biogeochemical processes of decomposition and transformation by soil microorganisms [1]. Amendment of humic substances into the soil enhances biological, physical, and chemical properties of soil, and plant growth [1, 8]. Biochar, as a stable C product of biomass pyrolysis, can enhance abundance and activity of soil microbiota and increase crop yields [9, 10]. Therefore, biochar and humic substances represent sustainable soil amendments providing high economic viability, crop productivity, and ecological stability [11, 12].
The positive effect of biochar is usually associated with changes in physical and chemical soil conditions [13]. On the other hand, fresh biochar is not the preferred environment for colonisation by microorganisms [14, 15]. Some studies have even found negative priming effects of biochar [16, 17]. Therefore, the effect of biochar addition on soil microbiome strongly depends on the residence time of biochar in soil [18]. Aged biochar, modified by biotic and abiotic processes, is a more hospitable environment for colonisation by microorganisms than the non-activated biochar. Moreover, aged biochar shows increased nutrient retention capacity resulting from the increased density of surface functional groups and the adsorbed nutrient-rich organic molecules [19].
The individual or combined effect of the soil amendments on soil microbiota and plant growth need to be better understood, because the majority of studies have focused on the environmental protective applications (e.g. [20]). The utilisation of biochar aged by humic substances was investigated only after the introduction of both to the soil [21]. Few studies dealt with liquid aging of biochar before the application to the soil (e.g. [22]). Therefore, the aim of this study was to investigate the effect of the biochar and humic substances on soil microbial properties and plant growth via a novel approach based on the biochar liquid aging. We chose this approach presuming that the biochar composition should be significantly changed after humic substances–biochar interaction in a soilless environment.
The hypotheses of the study were as follows:
-
1
Aging of biochar by humic substances changes the composition of biochar (determined as C, N, H, and O content) via enhanced soilless sorption of humic substances.
-
2
Application of the aged biochar enhances soil microbial activities and plant growth more efficiently than co-application of humic substances and biochar.
-
3
Co-application of humic substances and biochar improves soil microbial activities and plant growth more than their application solely.
-
4
Humic substances mitigate negative priming effect of biochar.
Methods
Soil amendments and their modification
Commercially available biochar (Sonnenerde GmbH, Austria) and humic substances solution, Humac (Envi Produkt, Czech Republic), were used for the experiment.
The biochar was pyrolyzed at 600 °C from agriculture waste consisting of cellulosic fibres and cereal husks. Basic characteristics of the biochar are as follows: specific surface (BET method) 288 m2 g−1, dry matter (DM) 41%, ash content (550 °C) 12% in DM, pH (CaCl2) 8.5, and conductivity 327 µS cm−1.
The basic component of Humac is oxihumolite (leonardite) and its composition is as follows: DM at least 30%, humic substances at least 45% in DM, Ca 1200 mg L−1, Mg 55 mg L−1, Cu 1.70 mg L−1, and Mn 1.97 mg L−1 (as total elements).
The biochar was aged in a gas-washing bottle with a volume of 1 L, which was filled up with 128 g of biochar, 4 mL of Humac, and 640 mL of demineralised water. The doses were chosen considering the application doses in the following pot experiment, in which 32 g of biochar, 1 mL of Humac and 100 ml of watering solution were applied to each pot of specific variants (the content of bottle equaled 4 doses of aged biochar). The suspension in the bottle was intensively aerated at room temperature for 7 days. At the end of the aging process, the content of the bottle was homogenised and filtered through a 42-µm sieve.
Macro-elemental (C, N, H, O) composition of the biochar was determined using TruSpec analyser (LECO, USA). The biochar was dried to a constant weight at 105 °C and sieved through a 0.15-mm mesh prior to analyses.
Pot experiment
The pot experiment with lettuce (Lactuca sativa L. var. capitata L.) cv. Smaragd was carried out in 1-L experimental plastic pots under controlled conditions in a growth chamber, Climacell (BMT Medical Technology, Czech Republic), with full-spectrum stable white LED lighting. Environmental conditions were maintained at a temperature of 18/22 °C (night/day) with a 12-h photoperiod, a light intensity of 370 µmol m−2 s−1, and relative air humidity of 70%.
The topsoil (0–15 cm) used in this pot experiment was collected from a field near the town Troubsko, Czech Republic (49°10′28"N 16°29′32″E). It was classified as silty clay loam (USDA Textural Triangle) Haplic Luvisol (WRB Soil Classification). Before the experiment, the soil was homogenised, sieved through a 2-mm mesh, and mixed with fine quartz sand (0.1–1.0 mm) (1:1, w/w). The basic soil properties were as follows: pH 7.3, total N 1.6 g kg−1, total C 14.0 g kg−1, C:N 8.75, available K 230 mg kg−1, available Ca 3.26 g kg−1, available Mg 240 mg kg−1, and available P 100 g kg−1.
The control pots and the pots with Humac variant (H) were filled up with 1 kg of soil–sand mixture. For variants containing biochar, 1 kg of soil–sand mixture was mixed with 32 g of non-activated biochar (NB) or a quarter of aged biochar (AB), which was equal to 32 g of NB. The obtained mixture was transferred into pots of specific variant. All the pots were watered with 100 mL of fluid: (I) demineralised water for the control, NB and AB variants; and (II) 1 mL of Humac diluted in 99 mL of demineralised water for H and non-activated biochar + Humac variant (NB + H) (Table 1). There were 15 pots in total with three pots per variant.
Lettuce seeds were sprouted on wet filter paper for 2 days. Three sprouted lettuce seeds were sown at about 2 mm deep into each pot. The pots were randomly placed in the growth chamber. All the pots were manually watered with 50 mL of demineralised water every other day and rotated variably during the experiment to ensure homogeneity of treatment. After 10 days, the most robust seedling was left in each pot.
The plants were harvested 6 weeks after sowing [23]. The leaves were cut at ground level and roots were removed from the soil gently and washed with tap water [24]. The weight of fresh aboveground (AGB) and root biomass was measured. The lettuce biomass was dried in a forced-air oven at 70 °C [24] to a constant weight to determine the dry weight of AGB and roots.
Soil analysis
At the end of the experiment, the mixed soil sample was collected from each pot. The soil samples were stored at 4 °C for 14 days before analysis.
The microbial biomass carbon (MBC) content of the soil samples was determined using the fumigation extraction method [25]. Furthermore, the triphenyl tetrazolium chloride-dehydrogenase activity (DHA) was measured according to the methodology based on Casida et al. [26]. DHA was calculated according to the calibration curve. Enzyme activities, namely β-glucosidase (GLU), N-acetyl-β-d-glucosaminidase (NAG), arylsulfatase (ARS), phosphatase (Phos), and urease (Urea), were measured on lyophilised samples by colorimetric methods [27].
Basal (BR) and substrate-induced (SIR) respiration were measured using a MicroResp device (The James Hutton Institute, UK) according to the method by Campbell et al. [28]. BR was measured without the addition of any energy source. SIR was measured after the addition of specific energy sources, namely d-glucose (Glc-SIR), d-trehalose (Tre-SIR), N-acetyl-β-d-glucosamine (NAG-SIR), l-alanine (Ala-SIR), l-lysine (Lys-SIR), and l-arginine (Arg-SIR).
Statistical analysis
All statistical analyses were carried out in the program R version 4.0.2. [29], together with the additional packages ‘ggplot2’ [30] for creating all the statistical graphs. Principal component analysis (PCA) with dependence of different treatments was used for modelling the relation between the soil properties and selected treatments. The results were graphically presented with Rohlf biplot for standardised PCA. Furthermore, the additional packages ‘factoextra’ [31] and ‘FactoMineR’ [32] were used. Pearson correlation analysis was applied for measuring the linear dependence among soil properties. The five-point scale [33] was used for interpreting the size of the correlation coefficient (r).
One-way analysis of variance (ANOVA) type I (sequential) sum of squares was applied at the significance level of 0.05 separately for each soil property. To detect the difference among the treatments after ANOVA, Tukey's honestly significant difference test from package ‘agricolae’ at 0.05 confidence level [34] was used. The assumption checking of all statistical models was also repeated with the help of the different diagnostic plots.
Results
Impact of biochar aging by humic substances on its macro-elemental composition
The aging process led to a significant increase in both C and N content of biochar (Table 2), which was probably the consequence of humic substances sorption on the biochar surface. On the other hand, the content of O decreased significantly during the aging process. These changes also caused a significant decrease in C:N and O:C ratios.
Total soil biological activity
DHA results showed no significant influence of the amendments as compared to the control (Fig. 1A). However, the increased values of this parameter were obtained for humic substances-influenced variants H and AB, whose values were significantly higher than the values of the NB variant.
Soil biochemical properties and plant biomass. A Dehydrogenase activity, B basal respiration, C microbial biomass carbon, D glucosidase, E respiration induced by d-glucose, F respiration induced by d-trehalose, G N-acetyl-β-d-glucosaminidase, H urease, I respiration induced by N-acetyl-β-d-glucosamine, J respiration induced by l-alanine, K respiration induced by l-lysine, L respiration induced by l-arginine, M arylsulfatase, N phosphatase, O fresh aboveground biomass, P dry aboveground biomass, Q fresh root biomass, R dry root biomass. Mean values ± SD. Statistical difference at the level: *p < 0.05
Soil BR was positively affected by the addition of NB + H (Fig. 1B). Nevertheless, the difference was not statistically significant compared to the control. However, this variant reached significantly higher values of BR compared to other amended variants. Similar results were reached for all SIRs (except Arg-SIR; Fig. 1E–F, I–L), which correlated highly positively with each other (Fig. 2).
Soil carbon pathways
The lowest MBC values were found in the NB variant (Fig. 1C); however, this decrease was not significant as compared to the control. On the other hand, the surplus of nutrients from Humac led to higher microbial biomass gain in the NB + H variant, which exerted significantly higher MBC as compared to the NB and H variants. The effect of the active part of MBC on the microbial activity could be expected due to the low positive correlation with all soil respirations (Fig. 2).
In comparison to the control, the GLU was significantly increased only in variant H (Fig. 1D). In other variants, no significant changes were recorded due to the rather negative priming effect of biochar.
The negative priming effect of biochar is observable, as the Glc-SIR value of the NB variant was significantly lower compared to the control (Fig. 1E). However, Humac application to biochar and their mutual interaction resulted in mitigation of the negative effect of biochar on Glc-SIR in the NB + H variant, in which the Glc-SIR value was significantly higher than that of the NB variant. Their interaction even significantly increased Tre-SIR values in the NB + H variant compared to the control (Fig. 1F).
Soil nitrogen pathways
No significant difference in NAG values were found in any of the tested variants compared to the control (Fig. 1G). However, the effect of the biochar on NAG was neutral to negative as compared to humic substances’ effect in the H variant. This difference was alleviated by the aging of biochar via humic substances in the AB variant.
NB + H addition significantly increased soil Urea activity (Fig. 1H); the remaining amendments had no effect. The NB and NB + H variants showed significantly higher Urea compared to the AB variant.
Statistically significant difference was also found between NB and NB + H for NAG-SIR, Ala-SIR, Lys-SIR (Fig. 1I, J, K), which correlated highly positively with each other (Fig. 2). There was no significant effect of the amendments on the soil Arg-SIR (Fig. 1L), in spite of displaying a similar trend to other amino acid-induced SIRs.
Soil phosphorus and sulphur pathways
ARS and Phos activity moderately positively correlated with each other (Fig. 2). ARS activity was significantly decreased by NB treatment (Fig. 1M). The H variant exerted significantly higher ARS activity in comparison with NB and AB, indicating a positive effect of humic substances on general enzyme activity. Soil Phos activity significantly increased after H addition, whereas significantly decreased in biochar-amended soils (Fig. 1N). The humic substances did not mitigate the negative effects of biochar on Phos.
Lettuce biomass
The addition of Humac had no significant effect on the fresh or dry lettuce biomass (Fig. 1O–R). However, all parameters of plant biomass were increased in the variants with addition of either AB or NB. The low negative correlation between lettuce biomass and all soil respirations (Figs. 2, 3) suggested that soil microorganisms and plants were competitors for nutrient sources. The values of dry AGB and root biomass in the AB were approximately twice as high as the control, H and NB + H, which evidenced a positive impact of biochar aging by Humac tea on plant growth.
Discussion
Impact of biochar aging by humic substances on its macro-elemental composition
The decrease in the C:N and O:C ratio in the aged biochar (Table 2) may indicate changes in N availability and biochar stability, respectively. This behaviour is partially related to the increased pore- and surface-blocking effect of humic substances [35], which may have blocked the N leaching and decelerated further oxidation of biochar. We also consider a role of organic matter–microbe–biochar interaction during aging, leading to increased C and N content of the biochar [36]. Lower values of the C:N parameter indicated proportionally higher enrichment of aged biochar with N, implying better availability of N for soil microorganisms and plants [37, 38] in the AB-amended soil. Higher N availability of aged biochar is supported by slightly higher values of plant yield as compared to non-activated biochar. The low content of O-based functional groups indicated more stable biochar [39]. However, it must be noted that AB was presumably enriched with labile available C originating from Humac, which is also characterised by the low O:C ratio [40]. We did not imply that aging removed nitrogenous groups from biochar surface. On the contrary, we assumed N enrichment of the biochar was due to the intensive adsorption of humic acids onto the surface with partial oxidation-blocking effect. Concurrent consumption of O due to the putative respiratory activity (and C mineralisation) of superficial microflora in biochar might have resulted in a lowered O:C ratio. As a consequence, partially mineralised N might become more available. Biochar weathering, in general, leads to enhanced CO2 production and N oxidation [41]. These results suggest that although the difference between NB and AB in O:C is statistically significant, it might have not affected the stability of the aged biochar.
Total soil biological activity
Unlike the findings of Lizarazo et al. [6], the significant increase in soil DHA values after humic substance treatment was not confirmed (Fig. 1A). Significantly decreased DHA of the NB + H variant in comparison to the sole Humac amendment corresponded to a reduced DHA related to the fresh biochar amendment to the soil [42]. This has been attributed to the adverse sorption of substrates or enzymes on the biochar surface [43]. Moreover, this phenomenon was observed in this experiment to a greater degree than the DHA-enhancing effect of Humac, which Bastista et al. [44] reported. Nevertheless, the interaction of biochar with humic substances during the aging process putatively changed the adsorption potential of biochar. This presumption is based on the modified hydrophobicity and polarity of biochar surface structures [45, 46], which might lead to higher DHA values in the AB variant as compared to NB.
The observed effect of biochar addition on BR is variable among literature. There are studies that reported positive [47], neutral [48] or negative effects [49, 50]. Spokas et al. [51] observed a negative effect of NB on the BR, similar to this study. The difference between NB and NB + H was presumably caused by the lower content of amendment-derived available C in the variant NB in comparison with the variant of combined biochar and humic substances (Fig. 1B). The humic substances could decrease sorption and the stabilising effect of biochar [52] and result in putative alleviation of SOM and soil C recalcitrance. The amendment of fresh concentrated Humac and biochar (NB + H) was evidenced to enhance this effect. This feature was also assumed by Al-Maliki et al. [8], who found that the addition of humic substances enhanced the soil respiration as an energy source for the microbial community as well. Based on this, it could be claimed that co-application of biochar and humic substances led to the reduction of the potentially negative effect of biochar on BR. The described feature was general for all determined types of respiration with the most various inducing substrates, as shown by the agonistic relationship between BR and SIRs in PCA (Fig. 3).
Soil carbon pathways
MBC formed by bacteria and fungi and residues of their dead bodies is one of the key soil properties that determine soil C transformation pathways [53]. There was found no significant decrease in MBC values after biochar addition (Fig. 1C), unlike the results of the study by Li et al. [17]. On the other hand, the surplus of nutrients from Humac to the biochar led to higher microbial biomass gain, as the results of MBC in the variants AB and NB + H showed. However, the limited binding capacity of the biochar towards the Humac-derived carbonaceous compounds during the aging phase putatively caused insignificantly but apparently lower final values of the MCB in comparison to the NB + H (Fig. 1C).
The GLU hydrolyses soil carbohydrates, specifically soluble di- and oligo-saccharides [19]. Products of this hydrolysis belong to important sources of energy for soil microbiota, and the enzyme indicates the SOM quality, quantity, and its changes [48]. The decrease of GLU in the NB variant, as compared to the control, might be expected [54,55,56]; however, it was insignificant in this case (Fig. 1D). The significantly higher GLU values in the variants H and NB + H, compared to the NB and AB variants, agreed with observations of other authors [57, 58]. However, they did not support our hypothesis that aged biochar soaked in Humac tea enhances soil biochemical activities most efficiently. Here, again, the authors presumed that the rapidly utilised limited labile C in the Humac tea led to overgrowth of microflora and accelerated exhaustion of easily available C sources in AB, which hindered the enhancement of GLU activity.
The Glc-SIR results showed significant difference among NB and other variants, except AB (Fig. 1E), which proved the previously implied negative priming effect of non-activated biochar on the microbial activity in soil. This effect was not completely mitigated even by aging in Humac tea, and is contradictory to the results of other authors [59]. However, the results again evidenced the importance of the humic substances amendment to the biochar. Similarly, Tre-SIR values (Fig. 1F) supported the assumption of humic substances-related alleviation of the possible adverse effect of biochar on the C stabilisation and enzyme inhibition.
Soil nitrogen pathway
NAG is one of three key enzymes participating in degradation of chitin; therefore, it could be considered an indicator of N mineralisation [60]. The NAG assessment showed no significant effect of amendments (Fig. 1G). The NAG is closely related to fungal biomass, hence NAG semi-quantitatively indicates soil fungal biomass [61]. Therefore, our observation was in accordance with Yao et al. [18], who observed no fungal biomass change when biochar was applied under the field conditions. NAG activity was significantly limited by non-activated biochar in the NB and NB + H variants as compared to the H variant, thus there was no significant alleviation of biochar adverse effect by Humac unlike the case of GLU, ARS.
Contrary to the NAG, Urea in soil is produced mainly by bacteria [62]. The significant increase of Urea in the NB + H variant (Fig. 1H) seemed novel due to the referred counteraction of both amendments. The previous study [1] referred to the significant increase in Urea activity after treatment with humic substances. On the other hand, Yao et al. [18] found a significant decrease in Urea activity when biochar was applied under field conditions. The NB and NB + H variants showed significantly higher Urea compared to the AB variant, indicating that ammonium formation rate was negatively affected by biochar aging in Humac tea.
N-Acetyl-β-d-glucosamine utilisation capacity was shown to increase via biochar-stimulated growth of actinomycetes and bacteria [63]. However, this study revealed neutral to opposite results (Fig. 1I). It was previously referred that amino-compounds in the soil, for example l-arginine [64] or l-alanine [65], are negatively affected in their fluxes and availability by sorption on the biochar. We assume also that N-acetyl-β-d-glucosamine might be slightly less accessible for degradation in the NB variant. In addition, a significant increase in the Ala-SIR in the NB + H variant in comparison to the NB may indicate strong sorption of l-alanine to biochar [65]. Thus, it can be deduced that similarly severe unavailability of other amino substances (l-lysine, l-arginine) might occur in the NB variant, causing low respiration potential for the amino substances.
Humac in the NB + H variant not only contributed to the mitigation of this potentially negative effect on NAG-SIR (Fig. 1I), but also on Ala-SIR, Lys-SIR, and potentially Arg-SIR (Fig. 1J, K, L). Therefore, the addition of humic substances to biochar seemed to mitigate the mentioned adverse features; however, the aging process in Humac tea abolished this feature incompletely, or weakly in the case of Lys-SIR and Arg-SIR. This assumption is supported by the high correlation between Ala-SIR and Lys-SIR, and moderate correlation between Arg-SIR and Lys-SIR (Fig. 2), as well as by PCA results (Fig. 3). The authors deduced from this feature and from PCA, which showed a positive relationship between Urea and Arg-SIR, that aging by Humac tea was crucial for the changes in the surface properties of biochar towards the altered binding of organic matter and mainly N sources.
Soil phosphorus and sulphur pathways
Extracellular phosphatases catalyse mineralisation of organic P to the inorganic form, which is available for uptake by plants and soil microorganisms [66]. Thus, Phos activity is closely related to the conversion of organophosphates in the labile mineral P level in soil [67]. Amendment of P-rich organic material, Humac, caused a significant increase of Phos (Fig. 1N). This finding is consistent with results reported by Li et al. [1]. Labile available P is known for its affinity to the biochar [68]. Therefore, all variants amended with biochar showed significantly lower Phos as compared to the control. Similar results under high biochar dosage were already reported [69]. This feature was even stronger than the P-enrichment by co-applied Humac in the variants NB + H and AB. Conversely, the positive effect of humic substances failed to mitigate the negative effects of biochar on Phos activity.
ARS is an enzyme that catalyses the desulfurisation of organic compounds in soil. ARS activity was inhibited by the solo biochar amendment (Fig. 1M) putatively by the same mechanism as Phos, i.e. by sorption and superficial immobilisation. On the other hand, the Humac-derived mitigation of the adverse effect of biochar on ARS was observed.
Lettuce biomass
According to Al-Maliki et al. [8], humic substances amendment increases the fresh and dry weight of crops, and has a positive effect on the length of the roots. However, no significant change in any of the determined biomass properties was detected in this study (Fig. 1O–R), a result similar to other previous observations, for example Holatko et al. [54]. NB and AB caused significantly increased lettuce biomass, a result that Carter et al. [70] also observed after biochar addition in their pot experiment. Moreover, many studies (e.g. [71, 72]) detected significantly increased crop yield after biochar application.
In this experiment, we presumed that NB-derived inhibition of microbial respiration and enzyme activities (Phos, ARS) mitigated the reported [73] plant-microbes’ competition for nutrients, especially N. For example, NB + H stimulated microbial abundance, respirations, and Urea activity; however, the surplus of nutrients provided by both amendments was probably not available for the plants to support their growth. On the other hand, the highest biomass values were obtained for the AB variant, and the biochar aging by Humac tea seemed to result in readily utilised available nutrients in the amended soil, leading to an insignificant change in indicators of soil microflora quality. However, the nutrients preserved in the soil were not competed for by microbes, and thus remained available for plants, thereby enhancing the increase of lettuce biomass, as was anticipated and hypothesised.
Conclusion
The first hypothesis predicting that the aging of biochar by humic substances would change the composition of biochar was confirmed. The aging process in a soilless environment led to a significant increase in both C and N content in biochar, which is probably a consequence of humic substances’ sorption on biochar surface.
The second hypothesis predicting that the application of aged biochar would enhance soil biochemical activities and plant growth more than only co-application of humic substances and biochar was partly confirmed; however, only in case of plant biomass. The values of dry AGB and root biomass reached by the AB variant were approximately twice as high as the control, H and NB + H.
The last two hypotheses predicting that the co-application of humic substances and biochar would improve soil biochemical activities and plant growth more than the application of each amendment solely, and would mitigate the negative priming effect of biochar were also partly confirmed. The highest values of MBC, basal, and all substrate-induced respirations were observed for the NB + H variant. It clearly contrasts with the lowest values of DHA, MBC, basal, and all substrate-induced respirations and all enzymatic activities (except urease) obtained after NB addition. Therefore, it can be concluded that the co-application of humic substances and biochar could enhance amount of soil microorganism (MBC) and their viability, represented by DHA and respirations activities. However, the negative effect of biochar on enzymatic activities was stronger than the positive effect of humic substances. Thus, the co-application of humic substances and biochar did not show complete mitigation of adverse effect on these parameters (only in the case of arylsulfatase and β-glucosidase).
Based on this study, the positive effect of co-application of humic substances and biochar on soil fertility, quality, and health can be concluded. The possibility of biochar aging by humic solution requires further study.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
27 October 2021
A Correction to this paper has been published: https://doi.org/10.1186/s40538-021-00265-0
Abbreviations
- AB:
-
Aged biochar
- AGB:
-
Aboveground biomass
- Ala-SIR:
-
Respiration induced by l-alanine
- Arg-SIR:
-
Respiration induced by l-arginine
- ARS:
-
Arylsulfatase
- BR:
-
Basal respiration
- DHA:
-
Dehydrogenase activity
- Glc-SIR:
-
Respiration induced by d-glucose
- GLU:
-
β-Glucosidase
- H:
-
Humac
- Lys-SIR:
-
Respiration induced by l-lysine
- MBC:
-
Microbial biomass carbon
- NAG:
-
N-Acetyl-β-d-glucosaminidase
- NAG-SIR:
-
Respiration induced by N-acetyl-β-d-glucosamine
- NB:
-
Non-activated biochar
- PCA:
-
Principal component analysis
- Phos:
-
Phosphatase
- SD:
-
Standard deviation
- SIR:
-
Substrate-induced respiration
- SOM:
-
Soil organic matter
- Tre-SIR:
-
Respiration induced by d-trehalose
- Urea:
-
Urease
References
Li Y, Fang F, Wei J, Wu X, Cui R, Li G, et al. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: a three-year experiment. Sci Rep. 2019;9(1):12014.
Onica BM, Vidican R, Sandor M. A short review about using MicroResp method for the assessment of community level physiological profile in agricultural soils. Bull Univ Agric Sci Vet Med Cluj-Napoca Agric. 2018;75(1):24.
Aon MA, Colaneri AC II. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. Appl Soil Ecol. 2001;18(3):255–70.
Kuzyakov Y, Blagodatskaya E. Microbial hotspots and hot moments in soil: concept and review. Soil Biol Biochem. 2015;83:184–99.
Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR. Chapter two—a meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. In: Sparks DL, editor. Advances in agronomy, vol. 124. San Diego: Academic Press; 2014. p. 37–89.
Lizarazo LM, Jordá JD, Juárez M, Sánchez-Andreu J. Effect of humic amendments on inorganic N, dehydrogenase and alkaline phosphatase activities of a Mediterranean soil. Biol Fertil Soils. 2005;42(2):172–7.
Tisserant A, Cherubini F. Potentials, limitations, co-benefits, and trade-offs of biochar applications to soils for climate change mitigation. Land. 2019;8(12):34.
Al-Maliki S, Al-Mammory H, Scullion J. Interactions between humic substances and organic amendments affecting soil biological properties and growth of Zea mays L. in the arid land region. Arid Land Res Manag. 2018;32(4):455–70.
Domene X, Mattana S, Hanley K, Enders A, Lehmann J. Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol Biochem. 2014;72:152–62.
Arif M, Talha J, Muhammad R, Fahad S, Muhammad A, Amanullah, et al. Biochar; a remedy for climate change. In: Fahad S, Hasanuzzaman M, Alam M, Ullah H, Saeed M, Khan AK, et al. editors. Environment, climate, plant and vegetation growth. Cham: Springer International Publishing; 2020. p. 151–172. https://doi.org/10.1007/978-3-030-49732-3
Palanivell P, Ahmed OH, Latifah O, Majid NMA. Economic viability of including crude humic substances, chicken litter biochar, and clinoptilolite zeolite in rice cultivation on acid soils. Bulg J Agric Sci. 2019;25(1):79–96.
Zhang L, Sun XY, Tian Y, Gong XQ. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci Hortic. 2014;176:70–8.
Pandian K, Subramaniayan P, Gnasekaran P, Chitraputhirapillai S. Effect of biochar amendment on soil physical, chemical and biological properties and groundnut yield in rainfed Alfisol of semi-arid tropics. Arch Agron Soil Sci. 2016;62(9):1293–310.
Luo L, Gu JD. Alteration of extracellular enzyme activity and microbial abundance by biochar addition: Implication for carbon sequestration in subtropical mangrove sediment. J Environ Manag. 2016;182:29–36.
Quilliam RS, Marsden KA, Gertler C, Rousk J, DeLuca TH, Jones DL. Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agric Ecosyst Environ. 2012;158:192–9.
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil. 2012;354(1–2):311–24.
Li Y, Li Y, Chang SX, Yang Y, Fu S, Jiang P, et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol Biochem. 2018;122:173–85.
Yao Q, Liu JJ, Yu ZH, Li YS, Jin J, Liu XB, et al. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol Biochem. 2017;110:56–67.
Lehmann J, Joseph S. Biochar for environmental management: science, technology and implementation. 2nd ed. London: Routledge; 2015.
Pukalchik M, Mercl F, Panova M, Brendova K, Terekhova VA, Tlustos P. The improvement of multi-contaminated sandy loam soil chemical and biological properties by the biochar, wood ash, and humic substances amendments. Environ Pollut. 2017;229:516–24.
Mukherjee A, Lal R, Zimmerman AR. Impacts of 1.5-year field aging on biochar, humic acid, and water treatment residual amended soil. Soil Sci. 2014;179(7):333–9.
Quan GX, Fan QY, Zimmerman AR, Sun JX, Cui LQ, Wang LH, et al. Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products. J Hazard Mater. 2020;382:9.
Trinchera A, Baratella V, Rinaldi S, Renzaglia M, Marcucci A, Rea E. Greenhouse lettuce: assessing nutrient use efficiency of digested livestock manure as organic n-fertiliser. Acta Hort. 2014;1041:63–9.
Iocoli GA, Zabaloy MC, Pasdevicelli G, Gómez MA. Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci Total Environ. 2019;647:11–9.
Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 1987;19(6):703–7.
Casida LE, Klein DA, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98(6):371–6.
ISO 20130. Soil quality—measurement of enzyme activity patterns in soil samples using colorimetric substrates in micro-well plates. Stockholm: SIS; 2018.
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol. 2003;69(6):3593–9.
R CORE TEAM. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020.
Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2016.
Kassambara A, Mundt F. factoextra: extract and visualize the results of multivariate data analyses. Package version 1.0.5. 2017.
Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25(1):1–18.
Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. 5th ed. Boston: Houghton Mifflin; 2003.
Mendiburu DF. agricolae: Statistical procedures for agricultural research. R package version 1.3–1. 2020.
Pignatello JJ, Kwon S, Lu Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (Char): attenuation of surface activity by humic and fulvic acids. Environ Sci Technol. 2006;40(24):7757–63.
Mukherjee A, Zimmerman AR, Hamdan R, Cooper WT. Physicochemical changes in pyrogenic organic matter (biochar) after 15 months of field aging. Solid Earth. 2014;5(2):693–704.
Lehmann J, Pereira da Silva Jr J, Steiner C, Nehls T, Zech W, Glaser B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil. 2003;249(2):343–57.
Mukome FND, Parikh SJ. Chemical, physical, and surface characterization of biochar. In: Wong MH, Ok YS, editors. Biochar: production, characterization and applications. Boca Raton: CRC Press, Taylor and Francis Group; 2016. p. 68–96.
Özçimen D, Ersoy-Meriçboyu A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew Energy. 2010;35(6):1319–24.
Jindo K, Sánchez-Monedero MA, Matsumoto K, Sonoki T. The efficiency of a low dose of biochar in enhancing the aromaticity of humic-like substance extracted from poultry manure compost. Agronomy. 2019;9(5):248.
Spokas KA. Impact of biochar field aging on laboratory greenhouse gas production potentials. Glob Change Biology Bioenergy. 2013;5(2):165–76.
Brtnicky M, Dokulilova T, Holatko J, Pecina V, Kintl A, Latal O, et al. Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy. 2019;9(11):747.
Lammirato C, Miltner A, Kaestner M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol Biochem. 2011;43(9):1936–42.
Batista EMCC, Shultz J, Matos TTS, Fornari MR, Ferreira TM, Szpoganicz B, et al. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci Rep. 2018;8(1):10677.
Wang F, Sun H, Ren X, Liu Y, Zhu H, Zhang P, et al. Effects of humic acid and heavy metals on the sorption of polar and apolar organic pollutants onto biochars. Environ Pollut. 2017;231:229–36.
Zhang J, Wei Y, Liu J, Yuan J, Liang Y, Ren J, et al. Effects of maize straw and its biochar application on organic and humic carbon in water-stable aggregates of a Mollisol in Northeast China: a five-year field experiment. Soil Tillage Res. 2019;190:1–9.
Watzinger A, Feichtmair S, Kitzler B, Zehetner F, Kloss S, Wimmer B, et al. Soil microbial communities responded to biochar application in temperate soils and slowly metabolized 13C-labelled biochar as revealed by 13C PLFA analyses: results from a short-term incubation and pot experiment. Eur J Soil Sci. 2013;65(1):40–51.
Wu F, Jia Z, Wang S, Chang SX, Startsev A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol Fertil Soils. 2013;49(5):555–65.
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem. 2009;41(2):210–9.
Paz-Ferreiro J, Gasco G, Gutierrez B, Mendez A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils. 2012;48(5):511–7.
Spokas KA, Koskinen WC, Baker JM, Reicosky DC. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere. 2009;77(4):574–81.
Koelmans AA, Meulman B, Meijer T, Jonker MTO. Attenuation of polychlorinated biphenyl sorption to charcoal by humic acids. Environ Sci Technol. 2009;43(3):736–42.
Gil-Sotres F, Trasar-Cepeda C, Leirós MC, Seoane S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol Biochem. 2005;37(5):877–87.
Holatko J, Hammerschmiedt T, Datta R, Baltazar T, Kintl A, Latal O, et al. Humic acid mitigates the negative effects of high rates of biochar application on microbial activity. Sustainability. 2020;12(22):9524.
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol. 2013;71:33–44.
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils. 2012;48(5):511–7.
Bastida F, Jindo K, Moreno JL, Hernández T, García C. Effects of organic amendments on soil carbon fractions, enzyme activity and humus–enzyme complexes under semi-arid conditions. Eur J Soil Biol. 2012;53:94–102.
Fincheira-Robles P, Martínez-Salgado M, Ortega-Blu R, Janssens M. Compost and humic substance effects on soil parameters of Vitis vinifera L cv Thompson seedless. Sci Agropec. 2016;7:291–6.
Hamer U, Marschner B, Brodowski S, Amelung W. Interactive priming of black carbon and glucose mineralisation. Org Geochem. 2004;35(7):823–30.
Ekenler M, Tabatabai MA. Effects of trace elements on β-glucosaminidase activity in soils. Soil Biol Biochem. 2002;34(11):1829–32.
Parham JA, Deng SP. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol Biochem. 2000;32(8–9):1183–90.
Dick RP. Methods of soil enzymology. Madison: Soil Science Society of America; 2011.
Jiang L-L, Han G-M, Lan Y, Liu S-N, Gao J-P, Yang X, et al. Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J Integr Agric. 2017;16(3):713–24.
Hill RA, Hunt J, Sanders E, Tran M, Burk GA, Mlsna TE, et al. Effect of biochar on microbial growth: a metabolomics and bacteriological investigation in E. coli. Environ Sci Technol. 2019;53(5):2635–46.
Dippold M, Biryukov M, Kuzyakov Y. Sorption affects amino acid pathways in soil: Implications from position-specific labeling of alanine. Soil Biol Biochem. 2014;72:180–92.
Gianfreda L, Rao MA, Sannino F, Saccomandi F, Violante A. Enzymes in soil: properties, behavior and potential applications. In: Violante A, Huang PM, Bollag J-M, Gianfreda L, editors. Developments in soil science. Elsevier: Amsterdam; 2002. p. 301–27.
Fujita K, Kunito T, Moro H, Toda H, Otsuka S, Nagaoka K. Microbial resource allocation for phosphatase synthesis reflects the availability of inorganic phosphorus across various soils. Biogeochemistry. 2017;136(3):325–39.
Shepherd JG, Sohi SP, Heal KV. Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development. Water Res. 2016;94:155–65.
Huang D, Liu L, Zeng G, Xu P, Huang C, Deng L, et al. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere. 2017;174:545–53.
Carter S, Shackley S, Sohi S, Suy T, Haefele S. The impact of biochar application on soil properties and plant growth of pot grown lettuce (Lactuca sativa) and cabbage (Brassica chinensis). Agronomy. 2013;3(2):404–18.
Arif M, Ali S, Ilyas M, Riaz M, Akhtar K, Ali K, et al. Enhancing phosphorus availability, soil organic carbon, maize productivity and farm profitability through biochar and organic-inorganic fertilizers in an irrigated maize agroecosystem under semi-arid climate. Soil Use Manag. 2021;37(1):104–19.
Spokas KA, Cantrell KB, Novak JM, Archer DW, Ippolito JA, Collins HP, et al. Biochar: a synthesis of its agronomic impact beyond carbon sequestration. J Environ Qual. 2012;41(4):973–89.
Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85(3):591–602.
Acknowledgements
Not applicable.
Funding
This research has been financially supported by the project of Technology Agency of the Czech Republic TH03030319: “Promoting the functional diversity of soil organisms by applying classical and modified stable organic matter while preserving the soil's production properties” and by the Ministry of Education, Youth and Sports of the Czech Republic under the project FCH-S-21-7398.
Author information
Authors and Affiliations
Contributions
TH was involved in conceptualisation, formal analysis, investigation, writing—original draft; JH, VP, DH, MR and MK were involved in writing—review and editing, resources; OL, AK, MZG and SM were involved in writing—review and editing, MN and NA data curation, resources; TB was involved in data curation and visualisation; MB was involved in project administration, supervision, conceptualisation, writing—review and editing, investigation and validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the affiliation has been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hammerschmiedt, T., Holatko, J., Pecina, V. et al. Assessing the potential of biochar aged by humic substances to enhance plant growth and soil biological activity. Chem. Biol. Technol. Agric. 8, 46 (2021). https://doi.org/10.1186/s40538-021-00242-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-021-00242-7