Abstract
Cerebrovascular (CV) dysfunction is emerging as a critical component of Alzheimer’s disease (AD), including altered CV coverage. Angiogenic growth factors (AGFs) are key for controlling CV coverage, especially during disease pathology. Therefore, evaluating the effects of AGFs in vivo can provide important information on the role of CV coverage in AD. We recently demonstrated that epidermal growth factor (EGF) prevents amyloid-beta (Aβ)-induced damage to brain endothelial cells in vitro. Here, our goal was to assess the protective effects of EGF on cognition, CV coverage and Aβ levels using an AD-Tg model that incorporates CV relevant AD risk factors. APOE4 is the greatest genetic risk factor for sporadic AD especially in women and is associated with CV dysfunction. EFAD mice express human APOE3 (E3FAD) or APOE4 (E4FAD), overproduce human Aβ42 and are a well characterized model of APOE pathology. Thus, initially the role of APOE and sex in cognitive and CV dysfunction was assessed in EFAD mice in order to identify a group for EGF treatment. At 8 months E4FAD female mice were cognitively impaired, had low CV coverage, high microbleeds and low plasma EGF levels. Therefore, E4FAD female mice were selected for an EGF prevention paradigm (300 μg/kg/wk, 6 to 8.5 months). EGF prevented cognitive decline and was associated with lower microbleeds and higher CV coverage, but not changes in Aβ levels. Collectively, these data suggest that EGF can prevent Aβ-induced damage to the CV. Developing therapeutic strategies based on AGFs may be particularly efficacious for APOE4-induced AD risk.
Similar content being viewed by others
Introduction
Cerebrovascular (CV) dysfunction is re-emerging as a critical component of Alzheimer’s disease (AD) (reviewed in [36]). An unresolved issue is whether AD pathways induce detrimental effects on CV length and the significance for cognitive dysfunction. There is evidence of both higher [7, 9, 13] and lower [18, 25, 29, 39] vessel length and/or density (vessel coverage) in AD-patients and mice (AD-Tg) that overproduce an important contributor to AD progression; human amyloid-beta (Aβ). Angiogenic growth factors (AGFs) are key for controlling total vessel coverage. Therefore one approach for addressing issues in the field is evaluating the effects of AGFs on AD-like pathology in vitro and in vivo. Positive in vivo effects would also support future therapeutic research focused on developing AGF-like drugs for AD. Despite the promise of AGFs in models of stroke, published data in AD models are limited and for some AGFs equally as conflicting as the data on CV coverage in AD [14, 40, 44, 47], with the possible exception of the vascular endothelial growth factor (VEGF). Peripheral VEGF administration [58] or neuronal overexpression [44] improves cognition and lowers Aβ levels in AD-Tg mice. Beyond these reports review or opinion articles form the basis of our knowledge of AGFs in AD [3, 26, 54]. We recently compared the activity of the main AGFs at preventing Aβ-induced vessel degeneration in vitro [31]. Epidermal growth factor (EGF) prevented oligomeric Aβ42 (oAβ)-induced angiogenesis deficits and disruption of preformed tubes. EGF was also more protective than other AGFs, including VEGF. The role of EGF in AD is unclear as both higher and lower plasma EGF levels have been reported in AD patients [8, 17, 24, 30]. More recently low EGF levels were reported to predict the conversion from amnestic mild cognitive impairment to AD [33], supporting the hypothesis that exogenous EGF will be protective in AD-Tg mice. Contrastingly, there is improved cognition in AD-Tg mice treated with the EGF receptor inhibitor gefitinib [57]. Therefore, the primary goal of this study was to assess the protective effects of EGF on cognition, CV coverage and Aβ-levels using an AD-Tg model that incorporates CV relevant AD risk factors.
APOE4 is the greatest genetic risk factor for sporadic AD, increasing risk up to 12-fold compared to APOE3 (reviewed in [34]). APOE4-induced AD risk is also greater in females [2, 10, 41]. As APOE4 carriers also often respond differentially in clinical trials it is important to identify novel mechanistic processes underlying APOE4-induced AD risk. Increasing evidence indicates that APOE modulates CV morphology and function (reviewed in [50]). In AD and AD-Tg models APOE4 is associated with CV dysfunction including basement membrane degradation and increased leakiness [50]. Thus, incorporating human APOE4 in studies focused on the CV in AD is valuable.
EFAD mice express human APOE3 (E3FAD) or APOE4 (E4FAD), overproduce human Aβ42 and are a well characterized model of APOE modulated Aβ42 pathology [61]. We initially assessed the role of APOE and sex in cognitive and CV dysfunction in EFAD mice in order to identify a group for EGF treatment. E4FAD female mice were cognitively impaired, had high microbleeds, low CV coverage and low plasma EGF levels at 8 months of age. Therefore, E4FAD female mice were selected for an EGF prevention paradigm (300 μg/kg/wk, 6 to 8.5 months). EGF prevented cognitive decline and was associated with lower microbleeds and higher CV coverage, but not with changes in Aβ levels. Collectively these data suggest that EGF can prevent Aβ-induced damage to the CV. Developing therapeutic strategies based on AGFs may be particularly efficacious for APOE4-induced AD risk.
Materials and methods
Experimental design
All protocols follow the UIC Institutional Animal Care and Use Committee protocols. Breeding and colony maintenance was conducted at the UIC as described in [61]. EFAD mice express five Familial Alzheimer’s disease (FAD) mutations (APP K670N/M671L + I716V + V717I and PS1 M146L + L286V) and human APOE3 (E3FAD mice) or APOE4 (E4FAD mice) i.e. 5xFAD+/− APOE +/+. EFAD mice utilized to start our breeding colonies were kindly provided by Dr. M.J.Ladu.
Evaluation of cognitive and CV changes in EFAD mice at 8 months
Male (EFADM) and female (EFADF) E3FAD and E4FAD mice at 8 months of age were sequentially assessed (24 hrs. between tests) using the open field, novel object recognition and spontaneous alternation (Y-maze) tests. EFAD mice were then injected with sodium fluorescein (NaFl, Sigma), anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine), blood drawn via cardiac prick and transcardially perfused with PBS containing protease inhibitors (Millipore). Right hemi-brains were dissected into the cortex, hippocampus, and cerebellum and processed for NaFl extravasation. Left hemi-brains were frozen over dry ice in cryomolds containing O.C.T compound (Tissue-Tek), and stored at −80 °C until processing for immunohistochemical (IHC) analysis. All investigators were blinded for APOE genotype. n = 7 (E3FADM), 7 (E3FADF), 9 (E4FADM), 8 (E4FADF).
Treatment of female E4FAD mice with EGF
Six month old E4FADF mice were administered EGF (Shenandoah, 300 μg/kg/wk) or vehicle control (water) by intraperitoneal injection (i.p.) until 8.5 months. Body weights were monitored prior to every injection. Mice were sequentially assessed (24 hrs. between tests) using the open field, novel object recognition, spontaneous alternation (Y-maze) and novel arm entry (Y-maze) tests. At the end-point behavior was further assessed using the light-dark box and Morris water maze tests and food consumption monitored over 24 h. 30 min prior to sacrifice mice were injected with EGF, dissected right hemi-brains stored at −80 °C until homogenization and left hemi-brains were stored for histological analysis. NaFl extravasation was not conducted on the EGF or VC treated E4FADF mice to enable biochemical analysis of Aβ, apoE and EGF levels in brain homogenates. For all analysis investigators were blinded for treatment. n = 8 per group.
Behavioral analysis
Behavioral analysis was conducted in the mouse dark cycle, tracked in real time by an overhead camera and videos analyzed using the ANY-Maze software.
Open field. Mice were placed in the center of a white box (l38.5xw30xh30 cm) and allowed to freely move for 10 min. The distance traveled and average speed were measured.
Novel object recognition was conducted as described in [62] with slight modifications. On day 1, mice were habituated in a white testing chamber (38.5x30x30 cm) for 20 min. On day 2, mice were placed in the same testing chamber that contained two identical objects for 7 min, returned to their home cage for 1 hr and placed in the testing chamber with a familiar and a novel object for 7 min. Time spent with each object and the preference index (ratio of time spent with the novel object divided by total investigation time for both objects) were calculated. Mice that did not investigate both objects for longer than 5 s were not included. Data for one E3FADF and one E4FADF were removed (Fig. 1) based on the exclusion criteria.
Cognitive and cerebrovascular dysfunction in E4FADF mice. a E4FADF mice are cognitive impaired when assessed by novel object recognition and spontaneous alternation. b There are higher NaFl levels in the cortex (Cx) and hippocampus (Hp) of E4FADF mice after i.p injection. n = 7(E3FADM), 6 (E3FADF), 9 (E4FADM), 7 (E4FADF). c Prussian blue staining (microbleeds) is higher in the Cx and Hp of E4FADF mice when assessed by IHC analysis. n = 7 (E3FADM), 7 (E3FADF), 9 (E4FADM), 8 (E4FADF). Data expressed as mean +/− S.E.M. *p < 0.05 by two-way ANOVA and Tukey’s post hoc comparisons. #p < 0.05 by two-way AVOVA followed Fisher’s LSD test
Spontaneous alternation (Y-maze)
Mice were placed in one arm of a Y-maze apparatus (38.5x8x13 cm, spaced 120° apart) allowed to explore for 7 min and the sequence of arm entries were recorded. Spontaneous alternation was calculated as the number of alternations (entries into three different arms consecutively) divided by the total possible alternations (the number of arms entered minus 2) and multiplied by 100.
Novel arm entry (Y-maze)
Mice were placed into the maze (plus extra-maze cues) with one of the arms blocked for 10 min, returned to the home cage for 60 min and placed back in the maze with access to all three arms for 5 min. The time spent in the novel arm was calculated.
Light-dark box
The light-dark box (21x42x25 cm) consists of two chambers with an opening in-between (7.3 cm): the light chamber is brightly illuminated with white walls (66.6 % of the box) the dark chamber comprises of dark walls (33 %). Mice were placed into the light side and allowed to move freely for 5 min. The time spent in each chamber was recorded.
Morris water maze (MWM) was conducted as described in [35] with slight modifications in three phases. The circular pool was 120 cm in diameter and 50 cm tall, and the circular escape platform was 10 cm in diameter. The pool was filled with water containing non-toxic tempera paint (maintained at 25 °C) to 10 cm below the top rim and divided into equal-sized imaginary quadrants. Extramaze cues were placed in the four corners for spatial orientation. A single mouse was in the pool for each testing phase/session. MWM testing was comprised of three phases. (a) Visual Cue phase. Mice were trained over the course of 2 days to locate a flagged hidden platform (60s trial time, four trials each day with a 20 min inter-trial interval (ITI)). (b) Acquisition phase. After 2 days, mice were trained for 5 days (60s trial time, four trials each day with a 20 min ITI) to locate the position of the hidden platform (remains on the hidden platform for >2 s). During the visual cue and acquisition phases the mouse’s entry quadrant varied but the platform location remained constant. Latency to find the platform (s) was measured. (c) Probe trial. 1 h following the final acquisition trial, a single 60s probe trial was conducted with the platform removed. The latency to the target area (i.e., where platform was located during acquisition phase) and the time spent in the target quadrant were calculated.
Sodium fluorescein extravasation
Mice were injected i.p. (200 μl) with 2 % NaFl, sacrificed after 30 min and tissue harvest conducted as described above. Dissected brain regions were weighed, homogenized in PBS, suspended in equal volumes of 60 % tricholoroacetic acid, then vortexed and centrifuged (18,000 × g,10 min, 4 °C). Fluorescence levels were measured using a microplate reader (SpectraMax i3x, Molecular Devices) and cleared volumes calculated as: [1/plasma levels (fluorescence units/μl) x total brain fluorescence]/brain weight.
Biochemical analysis
Dissected brains were weighed and sequentially extracted using the 3-step extraction protocol as described in [61].
Protein quantification
Total protein in Tris-buffered saline (TBS) and neutralized formic acid (FA) extracts was quantified using the Ready to Use Bradford Reagent (Bio-Rad) and for TBS-Triton X100 (TBSX) extracts the BCA Protein Assay Kit (Pierce).
For western blot analysis of TBS-X fractions protein (20 μg) was separated on 4–12 % Bis-Tris gels (Invitrogen) and transferred onto low-fluorescence PVDF membranes. Membranes were blocked (1 hr. room temperature) with 5 % non-fat milk in TBS containing 0.1 % Tween 20 (TBST), then incubated (4 °C, overnight) with primary antibodies against post synaptic density protein (PSD-95, 1:1000, Cell Signaling), synaptophysin (1:1000, Cell Signaling), the EGF receptor (1:100, Santa Cruz Biotechnology) or actin (1 hr. room temperature, 1: 20,000, Cell Signaling) in TBST with 5 % bovine serum albumin (BSA). Membranes were incubated in secondary antibodies (Jackson Immunoresearch) with TBST wash (3X5min) in between steps and imaged using an Odyssey ® Fc Imaging System. Image J was utilized to quantify actin normalized proteins.
ELISA
ApoE and Aβ42 (Life Technologies) levels were measured in the TBS, TBSX and FA extracts, and oAβ (Biosensis) in the TBS extracts. The apoE ELISA utilizes α-apoE (1:2000, Millipore) capture and α-apoE biotin-gt (1:5000, Meridian) detection antibodies as described in [48]. EGF levels were measured in the plasma and TBS extracts (R&D).
Histological analysis
Frozen brains were sectioned at 12 μm and fixed using 10 % Neutral Buffered Formalin (Sigma). Nine nonadjacent sections (108 μm apart) were utilized for quantification per animal.
Prussian blue stain
Slides were hydrated in distilled water, submerged in equal parts of potassium ferrocyanide and hydrochloric acid (30 min), rinsed in distilled water (1 min) and counter-stained with Nuclear Fast Red (5 min, Rowley Biochemical Institute J-606-3). After another wash in distilled water (1 min) slides were dehydrated (50, 75, 95, & 100 % alcohol series), cleared in xylene and mounted. Slides were scanned at 20x magnification on a Leica Aperio AT2 scanner. The resulting image files were loaded into Aperio eSlide Manager (version 12.3.1.6002) and regions of interest were drawn using Aperio ImageScope (version 12.3.2.5030) around the cortex and hippocampus in separate layers and scored using Definiens Tissue Studio (version 4.2) using the ‘Marker Area’ solution. The solution was modified to identify Nuclear Fast Red and quantify the percent of tissue positive for Prussian blue (indicating microbleeds) in each of the brain regions. Data for multiple sections per brain were combined.
CD31 immunostain
Sections were incubated in 0.3 % hydrogen peroxide (30 min), blocked in 10 % rat-serum (2 hr) and incubated with a rat anti-mouse CD31 antibody (MEC13.3, BD Bioscience 1:10, 4 °C overnight). Sections were stained with the Vectastain ABC Kit according to the manufacturer’s protocol (Vector), dehydrated through alcohol series and cleared in xylene with PBS (3X5min) washes in between steps. Slides were digitally scanned and traced (deep layer cortex and subiculum) as for Prussian blue. The Aperio microvessel algorithm (version 11.2.0.780) was optimized to quantify the percent area of tissue and density occupied by blood vessels per brain region on the slide.
Laminin and Aβ stain
Slides were incubated in 60 % FA (8 min), sections permeabilized with TBS containing 0.25 % TBSX (3X5min) and blocked with 5 % BSA in TBSX (2 hr). Slides were incubated with anti-Aβ (MOAB-2, mouse IgG2b, 1:400 dilution, Biosensis) and anti-laminin (Rabbit, 1:400 dilution, Abcam) antibodies in TBS containing 2 % BSA and 0.10 % Triton X-100 (4 °C, overnight) in a humidified chamber. Slides were washed (3x5min in TBSX), incubated with Alexa fluorophore-conjugated secondary antibodies (AlexaFlour 750 anti-rabbit, Alexafluor 350 anti-mouse IgG2b) diluted 1:200 in TBSX containing 2 % BSA (2 hr) and washed in TBSX (3X5min) followed by TBS (1x5min). Mosaic and single images were captured on a Zeiss Axio Imager M1 under identical capture settings at 20x magnification. Converted images were thresholded equally to diminish background signal (NIH ImageJ software). Identified objects after thresholding were scanned to validate either vessel or Aβ staining. Mosaic images were outlined for the subiculum, deep layer or full cortex and quantified (Analyze Particles function).
Thioflavin-S stain
Slides were incubated in filtered 1 % aqueous Thioflavin-S (5 min, Sigma) protected from light, dehydrated in 70 % ethanol (2X5min) and washed in PBS (2X2min). Mosaic images were captured as for laminin and MOAB-2 staining at 10X magnification. Plaques were evaluated for total number and percent area covered (total Thioflavin-S immunoreactivity/ROI) using Image J as described in [61].
Claudin 5 stain
A representative slide from EGF or VC treated E4FADF mice was stained according to the protocol described for Laminin staining, using an anti-claudin 5 antibody (1:20, Santa Cruz Biotechnology) and AlexaFlour 647 anti-rabbit secondary antibody (1:200).
Statistical analysis
All data are presented as mean +/−S.E.M, and were analyzed using two-way ANOVA followed by either Tukey’s, Fisher’s LSD or Sidak’s post hoc comparisons or using Student’s t-test, as specified in the figure legends using GraphPad Prism version 6.
Results
Female E4FAD mice are cognitively impaired at 8 months of age
EFAD mice overproduce Aβ42 (via 5XFAD mutations)
APP K670N/M671L + I716V + V717I and PS1 M146L + L286V) and express human APOE3 (E3FAD mice) or APOE4 (E4FAD mice). In EFAD mice Aβ pathology is prevalent in the hippocampal formation, primarily the subiculum, and in the deep layers of the frontal cortex [61]. Published data demonstrate that by 7 months soluble Aβ and extracellular plaque levels follow the order: E4FAD female (E4FADF) > E3FAD female (E3FADF) = E4FAD male (E4FADM) > E3FAD male (E4FADM) [12]. Specific for the CV, cortical microbleeds are also reported as highest in E4FADF mice [12]. In order to select a sex and genotype of EFAD mice for treatment with EGF, it was important to identify the association among cognitive decline and CV dysfunction. Initially, cognitive impairment was assessed in E3FADM, E3FADF, E4FADM and E4FADF mice at 8 months of age. E4FADF mice were cognitively impaired compared to all other groups when assessed by both novel object recognition and spontaneous alternation (Y-maze) tests (Fig. 1a, two-way AVOVA followed by Tukey’s post-hoc analysis). Indeed, E4FADF mice performance was ~35 % lower in novel object recognition and ~30 % lower in the spontaneous alternation test.
Higher CV leakiness and lower CV coverage in E4FADF mice
To determine the role of APOE and sex in CV leakiness, brain levels of NaFl were assessed after i.p. injection (Fig. 1b). NaFl levels were significantly higher in the cortex of E4FADF mice compared to E3FADM and E4FADM mice and were higher in hippocampus compared to all other groups (two-way AVOVA followed by Tukey’s post-hoc analysis). NaFl levels were also higher in E3FADF mice compared to EFADM mice (two-way AVOVA followed by Tukey’s post-hoc analysis) in the hippocampus, whereas in the cortex these effects were only significant by two-way AVOVA followed by Fisher’s LSD test. Prussian blue staining was conducted to assess microbleed coverage as a complimentary measure of CV leakiness (Fig. 1c). In the cortex the area of microbleed coverage followed the order E4FADF > E3FADF > E4FADM = E3FADM (Fig. 1c, two-way AVOVA followed by Tukey’s post-hoc analysis). In general cortical microbleed coverage was 20-fold higher in E3FADF mice and 55-fold higher in E4FADF mice compared to EFADM mice. In the hippocampus microbleed coverage was only higher in E4FADF mice when assessed by two-way AVOVA followed by Fisher’s LSD test.
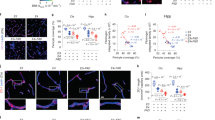
Next, laminin (basement membrane protein)-Aβ co-staining was conducted as an indication of total vessel coverage. Visually laminin staining appeared lower in deeper layers of the frontal cortex and the subiculum in EFADF mice compared to males, an effect particularly pronounced in E4FADF mice (Fig. 2a and b). When quantified for % area covered (Fig. 2c), no differences were observed for laminin staining among the groups for the whole cortex. In the deep layer cortex laminin staining followed the order: E4FADM/E3FADM > E3FADF > E4FADF (two-way AVOVA followed by Tukey’s post-hoc analysis). Data for the subiculum followed the same pattern. Overall, laminin staining was 10 % lower in E3FADF mice and 20 % lower in E4FADF mice in the deep layer cortex and subiculum compared to EFADM mice. Extracellular Aβ levels followed the order E4FADF > E4FADM = E3FADF > E3FADM (Fig. 2d).
Lower laminin coverage in E4FADF mice. a–c Laminin (basement membrane protein) staining is lower in E4FADF mice in brain regions of extracellular Aβ pathology: the subiculum and deep layers of the cortex (Dl-Cx) but not significantly different in the full-length cortex (Fl-Cx) between groups when assessed by IHC analysis. Further, E3FADF mice exhibit lower staining than E3FADM mice. d Extracellular Aβ levels are highest in E4FADF mice (IHC analysis using the antibody MOAB2). e Plasma EGF levels are low in EFADF mice. n = 7(E3FADM), 7 (E3FADF), 9 (E4FADM), 8 (E4FADF) when assessed by ELISA. Data expressed as mean +/− S.E.M. *p < 0.05 by two-way ANOVA and Tukey’s post hoc comparisons. # p < 0.05 by Student’s t-test
Plasma EGF levels are lowest in EFADF mice
EGF plays a key role in controlling total vessel coverage and a recent report indicates that low plasma EGF predict the conversion from MCI to AD [33]. We therefore measured plasma EGF levels in EFAD mice (Fig. 2e). E3FADM mice had higher plasma EGF levels than all other groups (2-way ANOVA, followed by Tukey’s post-hoc comparison). There was also a trend of higher EGF plasma levels in E4FADM mice compared to EFADF mice, which was significant by Student’s t-test. One caveat is that NaFl injection may have modulated plasma EGF levels in the EFADF mice, in an APOE genotype specific manner.
Collectively, these data support that 8 month old EFADF mice are cognitively impaired, have low CV coverage, high CV permeability and low plasma EGF levels. Therefore E4FADF mice were selected for treatment with EGF in a prevention paradigm.
EGF prevents cognitive decline in E4FADF mice
Our overarching goal was to test EGF in a prevention paradigm based on our recent data that EGF prevents Aβ-induced disruption of angiogenesis and degeneration of preformed tubes in vitro [31]. E4FADF mice were treated with EGF (300 μg/kg/wk) by i.p. from 6 to 8.5 months and tested by a battery of tests for cognitive ability (Fig. 3a). 300 μg/kg/wk was selected as a low dose that does not promote cancer or overt signs of toxicity in mice [6]. EGF induced a profound protective effect on cognitive decline in E4FADF mice compared to the vehicle control (VC). When assessed by novel object recognition (Fig. 3b), spontaneous alternation (Fig. 3c, Y-maze) and novel arm entry (Fig. 3d, Y maze) the VC control groups progressively declined in cognitive ability from 6 to 8 months (two-way ANOVA followed by Sidak’s post hoc comparisons which takes into account same animal re-testing). In contrast, no age-dependent decline was observed for EGF treated mice. At the mid-point EGF treated mice performed 46 % higher in the novel object recognition and 37 % higher in the number of spontaneous alternations performed (Y-maze) compared to the VC. At the end-point, EGF treated mice performed ~58 % higher in novel object recognition and spontaneous alternation and spent 477 % longer in the novel arm test (Y-maze).
EGF prevents cognitive deficits in E4FADF mice. a Experimental design for EGF prevention paradigm in E4FADF mice. EP, end-point; MP, mid-point; EP, end-point; OF, open field; SA, spontaneous alternation; NOR, novel object recognition; NAE, novel arm entry; LD, light-dark box; MWM-VC, Morris water maze visual cue phase; MWM-AP, Morris water maze acquisition phase; MWM-PT; Morris water maze probe trial. b–d EGF prevents cognitive decline when assessed by NOR, SA and NAE. Dashed line represents no preference (novel object) or chance alternation (spontaneous alternation). e EGF treatment improves performance in the Morris water maze test (EP). Representative track plots of probe trial is based on latency to previous platform area. f Synaptophysin levels are the same in EGF and VC treated mice when assessed by western blot analysis. g PSD-95 levels are higher in EGF treated E4FADF mice (western blot analysis). h Body weight decreased at 4 weeks but remained constant until 10 weeks with EGF treatment. i–k EGF treatment did not modulate performance in open field, had no effects on food intake (over 24 hrs at EP) and did not change performance in the light-dark box test. l, m EGF treated mice had higher plasma but not brain levels of EGF (ELISA). n There were no changes in EGF receptor levels in the cortex or hippocampus after EGF treatment (western blot). n = 8 per group for a–m and n = 6 for n. Data expressed as mean +/− S.E.M. *p < 0.05 by 2-way ANOVA and Sidak’s post-hoc analysis (B-E, G and H). *p < 0.05 by Students t test (E probe trial, F, I-K)
Cognitive ability was further dissected at the end-point in the Morris water maze test (Fig. 3e). In the acquisition phase EGF (day 1 vs 5 and 2 vs 5 were significant) and VC treated mice (day 1, 3 and 4 vs day 5 were significant) learned the location of the platform by day 5. On day 3 the EGF treated mice located the platform earlier than the VC, indicative of improved learning. In the probe trial (Student’s t-test) EGF treated mice located the previous platform area with a lower latency time (VC = 38.6 s, EGF = 5.15 s) and spent a longer time in the target quadrant (VC = 11.6 s, EGF = 30.9 s). EGF did not modulate levels of the pre-synaptic protein synaptophysin in the cortex or hippocampus (Fig. 3f). However, consistent with the behavior data levels of the postsynaptic protein PSD95 were higher in the cortex (35 %) and hippocampus (50 %) in EGF treated mice (Fig. 3g, Student’s t-test). Thus, in a prevention paradigm EGF-induces a beneficial effect on cognition and post-synaptic neuronal markers.
EGF treatment had no effects on locomotion, food intake, anxiety-like behavior and brain EGF levels
A potential confounding factor for the beneficial effects of EGF on cognition is whether EGF modulates anxiety-like behavior. VC treated mice remained steady in weight until week 4 (7 months old) where they gained ~5 % and then remained at the same weight for the rest of the study. EGF treated mice lost ~ 7 % in weight at week 4 and then remained steady (Fig. 3h). The changes in bodyweight were not related to general locomotion, as in the open field test there were no differences between EGF and VC treated mice (Fig. 3i, two-way ANOVA followed by Sidak’s post hoc). At the end-point (8 months) there were no differences in food intake (Fig. 3j over 24 hrs, Student’s t-test) or anxiety like behavior as assessed in the light-dark box test (Fig. 3k, Student’s t-test). The improved cognition with EGF compared to the VC was not related to general locomotion or anxiety like effects.
EGF can exert pleiotropic actions on many cells within the brain and it was important to determine whether brain EGF levels were increased after treatment. 30 min prior to sacrifice mice were treated with EGF and levels assessed by ELISA in the plasma (Fig. 3l, Student’s t-test), cortex, and hippocampus (Fig. 3m). Plasma EGF levels were ~4 pg/ml in VC treated mice and ~ 250 ng/ml after EGF treatment. There were no differences in hippocampal or cortical EGF levels between VC and EGF treated mice. In addition, when assessed by western blot analysis EGF receptor levels were not altered by EGF treatment in the hippocampus or cortex of E4FADF mice (Fig. 3n and Additional file 1: Figure S1d). However, we cannot exclude that there were cell-specific changes in EGF receptor levels after treatment. Therefore, the beneficial effects of EGF on cognition were likely mediated by peripheral rather than CNS levels of EGF.
EGF treatment lowers microbleeds and increases CV coverage compared to the VC
Next the effect of EGF on microbleed coverage was assessed. Microbleeds were lower in EGF treated mice compared to the VC. The percent area covered by microbleeds was 55 % lower in the cortex and 66 % lower in the hippocampus in the EGF treated group (Fig. 4a, Student’s t-test). A sub-goal of this study was to determine whether EGF modulates CV coverage in vivo. Thus laminin-Aβ co-staining was conducted (Fig. 4b, Student’s t-test). Visually EGF treated mice had a higher area covered by laminin staining than the VC in the deep layers of the cortex and the subiculum (Fig. 4b–c). Quantification of the laminin stain validates this finding as the percent area covered was 15 % (deep layer cx) and 25 % (subiculum) higher than the VC (Fig. 4d). To further dissect the role of EGF on CV coverage, quantitative IHC was conducted for CD31, a brain endothelial cell marker. In EGF treated mice CD31 coverage was 36 % higher in the deep layer cortex and 90 % higher in the subiculum compared to the VC (Fig. 4f, Student’s t-test). IHC staining for claudin 5 positive vessels revealed a similar staining pattern as for CD31 (Additional file 1: Figure S1a). Vessel coverage (% area covered) is a combination of density and length. There were no changes in laminin (Additional file 1: Figure S1b) or CD31 density (Additional file 1: Figure S1c) between VC and EGF treated mice. These data support that EGF prevents the lowering of CV length in E4FADF mice. It is important to note that distinguishing CV length and density with the IHC techniques utilized here is not straightforward. The optimal technique for assessing length and density entails three dimensional reconstruction of larger brain volumes with read read-outs for multiple parameters including branching points, major nodes, vessel length between nodes and the distribution of vessel lengths. Therefore, we prefer the term CV coverage for the purpose of this manuscript.
EGF prevents cerebrovascular dysfunction in E4FADF mice. a Microbleeds are lower in EGF treated mice (IHC analysis). b–d Laminin staining is higher in the deep layer cortex and subiculum in EGF treated mice (IHC analysis). e, f CD31 staining is higher in the deep layer cortex and subiculum after EGF treatment (IHC analysis). n = 8 per group. Data expressed as mean +/− S.E.M.*p < 0.05 by Students t test
EGF has no effect on Aβ levels
As EFAD mice are a model of APOE modulated Aβ levels and induced dysfunction, we next assessed whether EGF modulates Aβ levels. There were no differences in extracellular amyloid (ThioS, Fig. 5a, Student’s t-test) or extracellular Aβ (Fig. 4b–e and Fig. 5b, Student’s t-test) staining between the EGF and VC treated mice. EGF also had no effect on soluble, detergent soluble or insoluble Aβ42 levels in the cortex or hippocampus when assessed biochemically (Fig. 5c and d). More recent data suggest that soluble oligomeric Aβ is more AD-relevant than extracellular Aβ, however EGF treatment did not modulate soluble oligomeric Aβ levels (Fig. 5e, Student’s t-test). Further, there were no differences in apoE levels between EGF and VC treated mice in any extracted fractions (Fig. 5f–g). Therefore, the protection of EGF on cognitive and CV length was unlikely to be mediated by lowering Aβ42 or changing apoE levels.
EGF has no effect on Aβ levels. a, b ThioS plaque and extracellular Aβ levels were the same in EGF and VC treated E4FADF mice (IHC analysis). c, d When assessed biochemically, EGF also had no effect on soluble, detergent soluble or insoluble Aβ42 levels in the cortex of hippocampus (ELISA). e Soluble oAβ levels were unaffected by EGF treatment (ELISA). f, g ApoE levels were unaffected by EGF treatment (ELISA). n = 8 per group. Data expressed as mean +/− S.E.M. Data non-significant by Students t test
Discussion
AGFs play a key role in controlling total vessel coverage, especially during disease pathology. In AD research the effects that AFGs will induce on AD-like pathology in vivo is a controversial topic. Previously we have demonstrated that EGF can prevent oligomeric Aβ-induced disruption of total vessel length in vitro. Here our goal was to determine whether EGF prevents cognitive and CV dysfunction in an AD-Tg model that incorporates human APOE and high Aβ levels, both of which are important for AD and CV function. We demonstrated that E4FADF mice are cognitively impaired, have higher CV leakiness and lower vessel coverage than other groups at 8 months and also had low plasma EGF levels. Critically, we report for the first time that cognitive performance and vessel coverage was higher in E4FADF mice treated with EGF in a prevention paradigm. These data support further research on the development of AGF-based therapies for AD.
Sex and APOE4-induced cognitive and CV dysfunction in EFAD mice
Our data support that sex and APOE4 impact cognition and CV dysfunction in EFAD mice. In E4FADF the CV dysfunction was accompanied by cognitive dysfunction. In E3FADF mice CV leakiness was higher, CV coverage lower and plasma EGF levels lower compared to EFADM mice. As there were no changes in cognitive function in E3FADF mice compared to EFADM mice, the CV effects may represent early changes that later manifest as cognitive decline. These findings raise important questions on the relationship to human and other in vivo data and the mechanisms underlying sex-induced APOE modulated changes in cognition and CV coverage.
In AD patients APOE4-induced risk is greater in females [2, 19, 20, 41, 45] consistent with our finding that E4FADF are cognitively impaired compared to all other groups tested at 8 months. In partial contrast a few reports have demonstrated impaired cognition in male APOE4-TR mice (which do not overproduce human Aβ) compared to APOE3-TR mice [46, 56]. However, in a separate detailed analysis of APOE-TR mice the deficits in memory were primarily observed in female APOE4-TR mice [21]. Data on CV coverage is lacking in human patients stratified for sex and APOE genotype. In 9 and 12 months old APOE-TR mice lower microvessel coverage has been demonstrated [1, 4], although the effect of sex is unclear. A detailed comparison of EFAD and APOE-TR mice may reveal whether Aβ accelerates the APOE4 and female sex driven cognitive deficits and changes in CV coverage. In AD microbleeds are associated with male sex, higher blood pressure, lower CSF Aβ42 and APOE4 [5]. These data contrast with the female bias observed in EFAD mice [12, 20]. A confounding factor is whether additional AD-risk factors are present in male AD patients assessed for microbleeds. For example, level of exercise, hypertension, hyperglycemia, hyperinsulinemia and insulin resistance observed in type 2 diabetes all modulate AD-risk and the CV. Further, men are at a higher risk of developing cardiovascular events. The controlled laboratory conditions utilized here may be more relevant for studying the interaction among sex, APOE and Aβ in the absence of additional risk factors. Further research could focus on introducing additional AD-risk factors in vivo.
Females are at a higher risk for AD, which is often attributed to the loss of sex hormones during the menopause [45]. In the mouse model utilized here, with no ovariectomy, there may be inherent sex-differences that modulate CV and AD-like pathology. Extracellular Aβ levels from highest to lowest were: E4FADF > E4FADM = E3FADF > E3FADM consistent with previous reports [12, 51, 61]. Thus, higher levels of Aβ in E4FADF mice could induce or accelerate signaling cascades that cause CV degeneration. As described above CV coverage is lower in APOE4-TR mice which supports an acceleration rather than primary causation of CV deficits caused by Aβ. The low CV coverage and EGF levels in E3FADF mice compared to E4FADM mice are not associated with higher Aβ levels, supporting an Aβ-level independent mechanism. Females prior to menopause are at an increased risk for some autoimmune diseases and there are a number of proposed mechanisms underlying these effects [28]. For example, females may have a greater immune/inflammatory response in the periphery and the brain [28]. Although potentially beneficial for fighting infection an enhanced or prolonged immune/inflammatory response in females may predispose, or potentiate Aβ-induced direct and indirect damage to the CV. It is also noteworthy that lower vessel coverage was associated with low plasma EGF levels in EFADF mice. Low EGF plasma appear to increase the conversion of MCI to AD [33] but a sex bias has not been reported. Early reports in mice have demonstrated lower plasma EGF levels in females compared to males [42]. Further, plasma EGF levels in female mice are altered by the circadian rhythm, pregnancy, [53] and are increased with testosterone treatment [52]. Although 17-β estradiol is protective against brain endothelial damage in vitro [38], perhaps the male sex hormones exert a greater protective effect. A possibility is that low EGF levels in females result in lower protection of the CV to damage in AD, which is potentiated by APOE4 and accelerated by Aβ. Speculating further, the low plasma levels of EGF in female mice may be indicative general growth factor suppression or a form of accelerated aging/senescence.
The effects of APOE in AD are multifactorial, complex at the structural and mechanistic level and an area of intense research (reviewed extensively in [23, 34, 37, 43, 55, 59]). On a simplified level apoE4 can effect CV length through modulating Aβ levels and via signaling to cells at the blood-brain barrier (BBB) to alter Aβ-dependent and independent effects. As discussed, there are higher Aβ levels in APOE4 AD patients and E4FADF mice in this study. Aβ can cause vessel disruption by directly signaling to brain endothelial cells and indirectly via neuronal dysfunction and activation of astrocytes, microglia and pericytes. ApoE also signals to different cells types in the brain to disrupt vessel length. In astrocytes and microglia apoE4 is associated with a detrimental stress-induced (including Aβ) neuroinflammatory response [49]; mediators described as inflammatory can affect brain endothelial function (e.g. cytokines, matrix metalloproteases). In pericytes apoE4 is less effective at suppressing motility [15] and preventing MMP9 production [4, 22], both of which disrupt total vessel length. ApoE4 also directly disrupts brain endothelial cell tight junctions and effects peripheral lipid metabolism to cause brain endothelial dysfunction.
Overall, the female sex-induced, APOE4 modulated, Aβ accelerated changes in homeostatic signaling at the CV may ultimately converge to predispose and/or amplify stress-induced brain endothelial damage. Our ongoing studies are focused on delineating the role of APOE, aging, sex and peripheral risk factors on CV length.
EGF prevents cognitive and CV dysfunction in E4FADF mice
An important finding here is that EGF prevented CV dysfunction in E4FADF mice. These data are in agreement with the lower EGF plasma levels observed in humans and the beneficial effects of EGF in models of stroke and traumatic brain injury. In contrast improved cognition after blocking the tyrosine kinase activity of the EGF receptor (EGFR) with gefitinib has been reported in drosophilia and AD-Tg mice [57]. Aside methodological differences that commonly impact data (e.g. mouse model, time of treatment), the specificity of gefitinib for the EGFR has been drawn into question. Recently, gefitinib was demonstrated to antagonize a number of G-protein coupled receptors including adrenoreceptors, chemokine receptors, histamine receptors and other neuronal receptors [60]. Gefitinib is reported as a brain penetrant and therefore its beneficial effects in AD-Tg mice could be mediated by non-EGFR targets that play a role in AD-like pathology.
As EGF induced a pronounced effect on preventing cognitive decline in E4FADF mice, it is important to consider potential mechanisms of action. Our data support that the primary mode of action is not on cells within the brain, since brain EGF levels were not altered after EGF treatment. One caveat is that the technique used to measured EGF (ELISA of whole cortex or hippocampus) may have failed to detect regional high concentrations of EGF. Further, EGF did not modulate Aβ levels unlike VEGF in AD-Tg mice [58]. Although VEGF lowers Aβ levels in slice cultures [11] a role for EGF in this process has not been assessed. As EGF did not change Aβ levels, one hypothesis is that EGF acts directly on brain endothelial cells to prevent disrupted signaling induced by the interactive effects of female sex, APOE4 and Aβ in E4FADF mice. This is consistent with our in vitro data [31], the beneficial effect of EGF in wound healing, the promotion of angiogenesis in cancers that produce high EGF levels or that contain a mutation in erbb2 (a dimerization receptor partner of the EGFR), the protective effect of EGF-induced angiogenesis in stroke models and the general protective function of EGF in epithelial tissue (reviewed in [16]). Further, during the course of treatment EGF may have prevented a loss of key proteins involved in the homeostatic functions of brain endothelial cells, prior to vessel degeneration. For example key proteins involved in the nutrient transport/signaling molecule supply, waste product removal and efflux/metabolic/structural barrier functions of brain endothelial cells. For microbleeds a key question remains on whether EGF prevented global leakiness deficits in capillaries (e.g. via preventing tight junction changes or basement membrane degradation) and/or dysfunction at the post capillary venule level (the site of cellular trafficking).
An alternative hypothesis is that EGF activated receptors outside of brain endothelial cells in the CNS and/or the periphery to cause functional effects that prevent changes in CV coverage. For example, subtle increases in brain EGF levels may induce a beneficial response in pericytes, astrocytes, microglia or neurons to modulate levels of soluble molecules that maintain CV length. In the periphery EGF can signal in cells of virtually every organ in the body [15] and is associated with changes in metabolism and insulin signaling, both of which may protect the CV. An indicator of the peripheral effects is the lower body weight in EGF treated mice. EGF may also prevent cognitive decline independent of any CV changes through the same CNS and peripheral processes described above, in addition to promoting neurogenesis. Our data provide the basis for further studies dissecting if brain endothelial cells are the primary target of EGF.
CV length and AGFs as potential therapeutic targets in AD
What do the data mean in the context of CV length in AD and therapeutic options for AD patients? There is no clear in vitro or in vivo consensus on whether there is higher or lower CV in AD. One proposal is that lower CV density contributes to AD progression by disrupting cerebral blood flow and the complex transport and metabolic systems of the CV [18, 25, 29, 39]. The counter argument is that AD-pathways induce angiogenesis, causing hypersprouting and tight junction disruption, which increase CV permeability [7, 9, 13]. Traditional angiogenic signaling, typically observed over the short-term is likely not applicable to a chronic neurodegenerative condition such as AD. Pathological angiogenesis, described as the angiogenesis hypothesis [3] may be more applicable for AD: AD-processes not only cause direct vessel degeneration but also induce signaling cascades consistent with increased angiogenesis, however there is a failure to complete maturation and vessel formation. AD-risk factors both in the CNS and periphery could predispose and amplify the disrupted angiogenic signaling in brain endothelial cells.
There is a drive to develop novel strategies for AD as an alternative or complimentary approach for Aβ or tau-based therapeutics. AGF-like drugs may meet this criteria, yet are frequently overlooked in drug development programs. Indeed, an alternative focus is proposed of using therapeutics to block angiogenesis-like processes [54]. Our data with EGF and published data with other factors that increase total vessel coverage support an alternative approach [3, 26, 27, 32], which is to stabilize brain endothelial cells and maintain or increase vessel length. Further research is required to fully dissect the use of AGF-based treatments for AD. Key research areas include the potential for AGFs to reverse AD-like pathology in vivo, distinguishing CV versus peripheral effects and dissecting whether EGF-like molecules act on cells within the brain (i.e., would a brain penetrant AGF-like drug or treatment protocol be more efficacious than non-CNS penetrant). Further research is needed to address the concerns that agonists for AGF receptors could increase cancer risk. Cancer cells themselves are often the origin of high AGF levels; therefore finding a dosing strategy that negates this concern could be important in the elderly AD population.
Conclusions
In summary, our data demonstrate that EGF prevents cognitive decline in female mice that express APOE4 and overproduce Aβ. Further, there are lower microbleeds and increased CV coverage but no changes in Aβ levels in EGF treated mice. Developing therapeutic strategies based on AGFs could provide alternative therapeutic options for AD patients.
Abbreviations
- AD:
-
Alzheimer’s disease
- AD-Tg:
-
Alzheimer’s disease transgenic mice overproducing Aβ
- AGFs:
-
Angiogenic growth factors
- ApoE:
-
Apolipoprotein E
- Aβ:
-
Amyloid beta
- BBB:
-
Blood brain barrier
- CD31:
-
Cluster of differentiation 31
- CV:
-
Cerebrovascular
- EFAD:
-
Mice that express 5xFAD mutations and human APOE
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EP:
-
End-point
- FAD:
-
Familial Alzheimer’s disease
- LD:
-
Light-dark box
- MCI:
-
Mild cognitive impairment
- MP:
-
Mid-point
- MWM:
-
Morris water maze
- MWM-AP:
-
Morris water maze-acquisition phase
- MWM-PT:
-
Morris water maze-probe trial
- MWM-VC:
-
Morris water maze-visual cue
- NAE:
-
Novel arm entry
- NaFl:
-
Sodium fluorescein
- NOR:
-
Novel object recognition
- OF:
-
Open field
- PSD-95:
-
Post-synaptic density protein 95
- SA:
-
Spontaneous alternation
- VC:
-
Vehicle control
- VEGF:
-
Vascular endothelial growth factor
References
Alata W, Ye Y, St-Amour I, Vandal M, Calon F. Human apolipoprotein E varepsilon4 expression impairs cerebral vascularization and blood–brain barrier function in mice. J Cereb Blood Flow Metab. 2015;35:86–94.
Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75:563–73.
Ambrose CT. The role of capillaries in the lesser ailments of old age and in Alzheimer's disease and vascular dementia: the potential of Pro-therapeutic angiogenesis. J Alzheimers Dis. 2016;54:31–43.
Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–6.
Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer's disease. Neurobiol Aging. 2013;34:2488–94.
Berlanga-Acosta J, Gavilondo-Cowley J, Lopez-Saura P, Gonzalez-Lopez T, Castro-Santana MD, Lopez-Mola E, Guillen-Nieto G, Herrera-Martinez L. Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6:331–46.
Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS One. 2011;6:e23789.
Bjorkqvist M, Ohlsson M, Minthon L, Hansson O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer's disease. PLoS One. 2012;7:e29868.
Boscolo E, Folin M, Nico B, Grandi C, Mangieri D, Longo V, Scienza R, Zampieri P, Conconi MT, Parnigotto PP, et al. Beta amyloid angiogenic activity in vitro and in vivo. Int J Mol Med. 2007;19:581–7.
Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Finch CE, Henderson VW. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216–21.
Burger S, Noack M, Kirazov LP, Kirazov EP, Naydenov CL, Kouznetsova E, Yafai Y, Schliebs R. Vascular endothelial growth factor (VEGF) affects processing of amyloid precursor protein and beta-amyloidogenesis in brain slice cultures derived from transgenic Tg2576 mouse brain. Int J Dev Neurosci. 2009;27:517–23.
Cacciottolo M, Christensen A, Moser A, Liu J, Pike CJ, Smith C, LaDu MJ, Sullivan PM, Morgan TE, Dolzhenko E, et al. The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer's disease of humans and mice. Neurobiol Aging. 2016;37:47–57.
Cameron DJ, Galvin C, Alkam T, Sidhu H, Ellison J, Luna S, Ethell DW. Alzheimer's-related peptide amyloid-beta plays a conserved role in angiogenesis. PLoS One. 2012;7:e39598.
Cantara S, Donnini S, Morbidelli L, Giachetti A, Schulz R, Memo M, Ziche M. Physiological levels of amyloid peptides stimulate the angiogenic response through FGF-2. Faseb j. 2004;18:1943–5.
Casey CS, Atagi Y, Yamazaki Y, Shinohara M, Tachibana M, Fu Y, Bu G, Kanekiyo T. Apolipoprotein E inhibits cerebrovascular pericyte mobility through a RhoA Protein-mediated pathway. J Biol Chem. 2015;290:14208–21.
Chen J, Zeng F, Forrester SJ, Eguchi S, Zhang M-Z, Harris RC. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev. 2016;96:1025–69.
Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, Mondal A, Bedo J, Bush AI, Brown B, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–25.
Donnini S, Solito R, Cetti E, Corti F, Giachetti A, Carra S, Beltrame M, Cotelli F, Ziche M. Abeta peptides accelerate the senescence of endothelial cells in vitro and in vivo, impairing angiogenesis. FASEB J. 2010;24:2385–95.
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278:1349–56.
Finch CE, Shams S. Apolipoprotein E and sex bias in cerebrovascular aging of Men and mice. Trends Neurosci. 2016;39:625–37.
Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159:1–14.
Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV. Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer's disease. J Cereb Blood Flow Metab. 2016;36:216–27.
Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis 72. 2014; Pt A: 3–12
Humpel C, Hochstrasser T. Cerebrospinal fluid and blood biomarkers in Alzheimer's disease. World J Psychiatry. 2011;1:8–18.
Jantaratnotai N, Ryu JK, Schwab C, McGeer PL, McLarnon JG. Comparison of vascular perturbations in an abeta-injected animal model and in AD brain. Int J Alzheimers Dis. 2011;2011:918280.
Jefferies WA, Price KA, Biron KE, Fenninger F, Pfeifer CG, Dickstein DL. Adjusting the compass: new insights into the role of angiogenesis in Alzheimer's disease. Alzheimers Res Ther. 2013;5:64.
Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13 Suppl 3:S115–123.
Kipp M, Hochstrasser T, Schmitz C, Beyer C. Female sex steroids and glia cells: Impact on multiple sclerosis lesion formation and fine tuning of the local neurodegenerative cellular network. Neurosci Biobehav Rev. 2016;67:125–36.
Kitaguchi H, Ihara M, Saiki H, Takahashi R, Tomimoto H. Capillary beds are decreased in Alzheimer's disease, but not in Binswanger's disease. Neurosci Lett. 2007;417:128–31.
Komurcu HF, Kilic N, Demirbilek ME, Akin KO. Plasma levels of vitamin B12, epidermal growth factor and tumor necrosis factor alpha in patients with alzheimer dementia. Int J Res Med Sci. 2016;4:734–8.
Koster KP, Thomas R, Morris AW, Tai LM. Epidermal growth factor prevents oligomeric amyloid-beta induced angiogenesis deficits in vitro. J Cereb Blood Flow Metab. 2016. [Epub ahead of print].
Lee ST, Chu K, Park JE, Jung KH, Jeon D, Lim JY, Lee SK, Kim M, Roh JK. Erythropoietin improves memory function with reducing endothelial dysfunction and amyloid-beta burden in Alzheimer's disease models. J Neurochem. 2012;120:115–24.
Lim NS, Swanson CR, Cherng HR, Unger TL, Xie SX, Weintraub D, Marek K, Stern MB, Siderowf A, Investigators P, et al. Plasma EGF and cognitive decline in Parkinson's disease and Alzheimer's disease. Ann Clin Transl Neurol. 2016;3:346–55.
Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18.
Liu DS, Pan XD, Zhang J, Shen H, Collins NC, Cole AM, Koster KP, Ben Aissa M, Dai XM, Zhou M, et al. APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice. Mol Neurodegener. 2015;10:7.
Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131:645–58.
Mahley RW, Rall Jr SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37.
Na W, Lee JY, Kim WS, Yune TY, Ju BG. 17beta-estradiol ameliorates tight junction disruption via repression of MMP transcription. Mol Endocrinol. 2015;29:1347–61.
Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–5.
Patel NS, Mathura VS, Bachmeier C, Beaulieu-Abdelahad D, Laporte V, Weeks O, Mullan M, Paris D. Alzheimer's beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J Neurochem. 2010;112:66–76.
Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–11.
Perheentupa J, Lakshmanan J, Hoath SB, Beri U, Kim H, Macaso T, Fisher DA. Epidermal growth factor measurements in mouse plasma: method, ontogeny, and sex difference. Am J Physiol. 1985;248:E391–396.
Phillips MC. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life. 2014;66:616–23.
Religa P, Cao R, Religa D, Xue Y, Bogdanovic N, Westaway D, Marti HH, Winblad B, Cao Y. VEGF significantly restores impaired memory behavior in Alzheimer's mice by improvement of vascular survival. Sci Rep. 2013;3:2053.
Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134–47.
Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn Mem. 2013;20:256–66.
Solito R, Corti F, Fossati S, Mezhericher E, Donnini S, Ghiso J, Giachetti A, Rostagno A, Ziche M. Dutch and arctic mutant peptides of beta amyloid(1–40) differentially affect the FGF-2 pathway in brain endothelium. Exp Cell Res. 2009;315:385–95.
Tai LM, Bilousova T, Jungbauer L, Roeske SK, Youmans KL, Yu C, Poon WW, Cornwell LB, Miller CA, Vinters HV, et al. Levels of soluble apolipoprotein E/amyloid-beta (Abeta) complex are reduced and oligomeric Abeta increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J Biol Chem. 2013;288:5914–26.
Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, et al. APOE-modulated Abeta-induced neuroinflammation in Alzheimer's disease: current landscape, novel data and future perspective. J Neurochem. 2015;133(4):465–88.
Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, Bu G. The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 2016;131(5):709–23.
Tai LM, Youmans KL, Jungbauer L, Yu C, Ladu MJ. Introducing human APOE into abeta transgenic mouse models. Int J Alzheimers Dis. 2011;2011:810981.
Tuomela T, Miettinen P, Pesonen K, Viinikka L, Perheentupa J. Epidermal growth factor in mice: effects of estradiol, testosterone and dexamethasone. Acta Endocrinol (Copenh). 1990;123:211–7.
Tuomela T, Viinikka L, Perheentupa J. Epidermal growth factor in mice: changes during circadian and female reproductive cycles. Acta Endocrinol (Copenh). 1990;123:643–8.
Vagnucci Jr AH, Li WW. Alzheimer's disease and angiogenesis. Lancet. 2003;361:605–8.
Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–52.
Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166:883–91.
Wang L, Chiang HC, Wu W, Liang B, Xie Z, Yao X, Ma W, Du S, Zhong Y. Epidermal growth factor receptor is a preferred target for treating amyloid-beta-induced memory loss. Proc Natl Acad Sci U S A. 2012;109:16743–8.
Wang P, Xie ZH, Guo YJ, Zhao CP, Jiang H, Song Y, Zhu ZY, Lai C, Xu SL, Bi JZ. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2011;411:620–6.
Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–21.
Yadav M, Singh AK, Kumar H, Rao G, Chakravarti B, Gurjar A, Dogra S, Kushwaha S, Vishwakarma AL, Yadav PN, et al. Epidermal growth factor receptor inhibitor cancer drug gefitinib modulates cell growth and differentiation of acute myeloid leukemia cells via histamine receptors. Biochim Biophys Acta Gen Subj. 2016;1860:2178–90.
Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW, et al. APOE4-specific changes in abeta accumulation in a New transgenic mouse model of Alzheimer disease. J Biol Chem. 2012;287:41774–86.
Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Timson BF, Csernansky JG. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426–32.
Funding
LMT is supported by University of Illinois at Chicago start-up funds. Histology/Imaging services were provided by the Research Resources Center - Research Histology and Tissue Imaging Core at the University of Illinois at Chicago established with the support of the Vice Chancellor of Research.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon request.
Authors’ contributions
RT treated mice, conducted behavioral analysis, sacrificed mice, sliced brains and conducted IHC analysis, aided in manuscript and figure preparation. PZ sliced brains, stained for CD31, coordinated the majority of Prussian blue staining and aided in the development of IHC standard operating procedures, AWJM aided in experimental design and mouse sacrifice, developed the standard operating procedure for microbleed and CD31 analysis. FM conducted the protein assays, western blots, Aβ, apoE and oAβ ELISAs and contributed to the figure design and methods. SS developed the methods and performed Prussian blue staining. RD and PG were responsible for imaging and quantification of microbleeds and CD31 staining. LMT conceived the study, conducted experiments, interpreted data and wrote the manuscript. All authors read and approved the final manuscript.
Author’s information
None.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
All protocols follow the UIC Institutional Animal Care and Use Committee protocols.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
EGF had no effect on a laminin or b CD31 density (IHC analysis) in E4FADF mice. Data expressed as mean +/− S.E.M. Data non-significant by Students t test. n = 8 per group. c. Claudin 5 stained vessels (red) are higher in E4FADF mice after EGF treatment. d Representative full western blot of EGF receptor levels after EGF or VC treatment (the top band was quantified). (PDF 2906 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Thomas, R., Zuchowska, P., Morris, A.W.J. et al. Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. acta neuropathol commun 4, 111 (2016). https://doi.org/10.1186/s40478-016-0387-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-016-0387-3