Abstract
Objective
To investigate epidemiological, clinical and oncological outcomes of patients with laryngeal verrucous carcinomas (LVC).
Methods
Two independent authors investigated PubMed, Scopus and Cochrane Library for studies dedicated to epidemiological, clinical and oncological outcomes of patients with LVC. The following outcomes were investigated with PRISMA criteria: age; gender; tobacco/alcohol consumption; HPV infection; anatomical, pathological, therapeutic and survival outcomes. Studies were analyzed for bias through a validated clinical tool.
Results
Of the 212 identified articles, 15 retrospective studies and one prospective uncontrolled study met our inclusion criteria. Three studies reported findings from national databases. The males/females ratio is 9/1. Mean age was 60.3 years, which was younger compared to other laryngeal malignancies. The alcohol, cigarette overuse and the HPV status of patients were lacking in most studies. Glottis and supraglottis were the most common anatomical locations, corresponding to 78.7% and 12.4% of cases, respectively. The main therapeutic approaches consisted of surgery, radiotherapy, surgery followed by radiotherapy. Treatments reported 5-year overall survival and disease-specific survival of 86.3 and 90.8, respectively. The 5- and 10-year local control rate were 83.6 and 72.6, respectively. The 10-year disease-specific survival was 80.2. Heterogeneity between studies was found for inclusion criteria, comorbidity data, and treatments.
Conclusion
LVC is a rare laryngeal cancer associated with better survival and recurrence outcomes than laryngeal squamous cell carcinoma. The role of radiotherapy in the treatment regimen needs to be investigated in future prospective controlled studies.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinomas are the 6th most common adult malignancy worldwide, corresponding to 5.3% of all cancers [1]. Laryngeal cancer (LC) is the second most common head and neck cancer, accounting for 211,000 new cases and 126,000 deaths yearly worldwide, respectively [1]. Laryngeal verrucous carcinoma (LVC) is a well-differentiated variant of laryngeal squamous cell carcinoma (LSCC) and accounts for 1–3.4% of laryngeal malignancies [2, 3]. Since the first case reported by Ackerman in 1948 [4], the management strategies of LVC remain controversial. Surgery has long time been considered as the treatment of choice with overall survival and disease-free survival rates ranging from 86.8 to 100% [2, 3, 5]. The overall survival (OS) and disease-free survival (DFS) of radiotherapy were both lower than surgery with up to 70% of patients with disease free at follow-up [2, 3, 6, 7]. The radiotherapy was moreover controversial regarding the potential risk of anaplastic transformation after treatment [8]. The discussions about the most effective treatment continue regarding recent studies supporting the usefulness of radiation approaches for the treatment of LVC [6, 7].
The aim of this systematic review was to investigate epidemiological, clinical and oncological outcomes of patients with laryngeal verrucous carcinomas (LVC).
Material and methods
The criteria for study inclusion were based on the population, intervention, comparison, outcome, timing and setting (PICOTS) framework [9]. Two authors (JRL and SH) independently reviewed and extracted data according to the PRISMA checklist for systematic reviews [10].
Eligibility criteria
Prospective and retrospective, controlled, uncontrolled, or randomized studies published between January 1960 and Mai 2023 were included if they investigated epidemiological, pathological, therapeutic or oncological outcomes of LVC patients. Authors had to describe the method of diagnostic of LVC, including pathological features or use of international classifications. To date, LVC is considered as a low-grade variant of LSCC with specific morphologic, cytokinetic and clinical features. Microscopically, verrucous carcinoma consists of filiform projections lined by thick, well-differentiated keratinized squamous epithelium, composed of one to a few layers of basal cells, and multiplied, voluminous spinous cells lacking cytological atypia. This carcinoma may invade the stroma with a well-defined, pushing margin [11]. The studies had to be published in English, Spanish, or French peer-reviewed journals. Authors only considered studies reporting data for more than 10 individuals.
Populations, inclusion/exclusion criteria
Authors had to describe inclusion criteria of patients, and, particularly, the pathological features considered for the LVC diagnostic. Controlled studies comparing patients with LVC versus individuals with LSCC were considered. The authors used the levels of evidence (I-V) to characterize the studies [12].
Outcomes
Two authors (JRL and SH) reviewed the following outcomes: number of patients; criteria of LVC diagnostic; gender ratio; tobacco/alcohol overuse; history of human papilloma virus (HPV) infection; mean or median age; LVC location (glottis, supra- or subglottis, transglottis); tumor stages; therapeutic approaches; and oncological outcomes.
The Tool to Assess Risk of Bias in Cohort Studies developed by the Clarity Group and Evidence Partners was used by two authors (JRL and SH) for the bias/heterogeneity analyses of the included studies [13]. The bias analysis included the evaluation of cofactors that may influence the studies findings, i.e. epidemiological (comorbidities, tobacco/alcohol use, etc.), clinical, histopathological and therapeutic characteristics (radiotherapy protocol).
Authors extracted the following oncological outcomes from studies: OS, DFS, disease-specific survival (DSS), recurrence and second malignancy rates.
Intervention and comparison
The following therapeutic approaches were reviewed for each study: surgery; radiotherapy; chemotherapy; combined treatments; or lack of treatment. The period of the inclusion of patients was considered in the therapeutic analysis regarding the evolution of some therapeutic procedures over the past decades (i.e. chemotherapy, radiotherapy).
Timing and setting
There was no criteria for specific stage or timing in the ‘disease process’ of the study population. Data from population-based registries or clinical hospital studies were considered.
Search strategy
The paper search was carried out by three independent authors (JRL and SH) with PubMED, Scopus, and Cochrane Library databases. The databases were screened for abstracts and titles referring to the description of features of LVC patients. Authors analyzed full texts of the selected papers. Results of the search strategy were reviewed for relevance and the reference lists were examined for additional pertinent studies. Any discrepancies in synthesized data were discussed and resolved by the remaining co-authors. The following MeSH/keywords were considered: ‘larynx’; ‘laryngeal’; ‘cancer’; ‘verrucous’; ‘carcinoma’; ‘treatment’; ‘survival’; ‘outcomes’.
Results
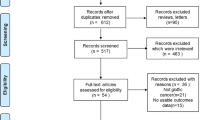
A total of 212 articles were identified. Among them, 16 studies published between 1976 and 2020 met our inclusion criteria (Fig. 1) [3, 8, 12, 14,15,16,17,18,19,20,21,22,23,24,25,26]. Two studies were excluded because overlapping with other case-series [27, 28]. Fifteen studies were retrospective (EL: IV) and there was a prospective uncontrolled study (EL: III) (Table 1). Three studies reported findings from national databases, i.e. the Slovenian national database [22], national cancer database [25], and surveillance, epidemiology, and end results (SEER) [3]. There would be potential overlaps between the two U.S. database studies [3, 25] but authors did not investigate similar findings. The present systematic review reported the features of 948 patients with LVC (Table 2). The characteristics of Table 2 do not include the data of one study [25] because risk of overlapping and, moreover, authors focused on specific population of LVC patients that did not match with the aims of the review. Thus, the patient data of the study of Dubal et al. [3] were only considered, while the data of Jayakrishnan et al. [25] were not included in the data of Table 2.
Epidemiological features
LVC occur in males and females in 88.8% and 10.0% of cases, respectively. The gender proportion was not reported in one study, accounting for 1.2% of cases [14]. Mean or median age were available in 911 cases and were 60.3 and 60.8 years, respectively. Few studies reported tobacco or alcohol overuse [16, 19, 20, 23, 24]. HPV infection was investigated in the study of Fliss et al. who detected HPV-16 and -18 DNA in 13 cases (45%) [17]. Ethnicity data were considered in one SEER study [5]. Among 2039 head and neck verrucous carcinomas, white and black patients accounted for 1765 (86.6%) and 120 (5.9%), respectively. The proportion of black patients was significantly higher in group with head and neck SCC patients compared to head and neck verrucous carcinoma patient group [5]. However, authors did not investigate specific data for LVC. In the study of Dubal et al. the ethnicity analysis reported a white preponderance in LVC (88.4%) and other laryngeal malignancies (82.6%), with blacks constituting 8.1% of LVC and 13.8% of other laryngeal cancers [3].
Anatomical and pathological features
The tumor location was available in 15 studies [3, 8, 12, 14,15,16,17,18,19,20,21,22,23,24, 26]. Glottis and supraglottis were the most common anatomical locations of LVC, corresponding to 78.7% and 12.4% of cases, respectively (Table 2). In 4.0% of cases, the tumor was transglottic at the time of diagnosis. Compared to other laryngeal malignancies, LVC developed more frequently in glottis and less frequently in supraglottis [3]. The cTNM staging was reported in 14 studies [3, 8, 12, 14,15,16,17, 19,20,21,22,23,24,25]. Some authors only investigated outcomes of cT1 and/or cT2 LVC [12, 14, 23], excluding data from cT3 and cT4 tumors. Considering cohorts without cT stage restriction, LVC was detected in cT1, cT2, and cT3 stages in 41.9%, 29.1%, and 26.3%, respectively (Table 2). cT4 LVC concerned 2.7% of patients at the diagnostic time. Limited data were available for cN and metastasis staging in the studies of the literature. Patients presented with positive nodes at the pathological examination in 1.8% of cases. In the present systematic review, there was no patient with distant metastasis. Most patients were diagnosed with stage 1 LVC (Table 2).
Therapeutic strategies
From retrospective studies published between 1976 and 2020, the main therapeutic approaches consisted of surgery, radiotherapy, surgery followed by radiotherapy or chemotherapy (Table 3). Various surgical approaches were reported, including type I–IV cordectomy, partial or total laryngectomy. At the exception of the study of Huang et al. [24] most authors favored surgery for the treatment. Anaplastic transformation of LVC was investigated in 5 studies and reported in 3 cases [8, 12, 16, 21, 24], accounting for 2.1% of cases (3/140). The three reported cases occurred in the seventies and eighties. The radiotherapy effectiveness was reported in two studies [16, 24]. Hagen et al. included data from 12 LVC patients. Among them, 2 were treated with radiotherapy, which failed in one case (50%) [16]. In the study of Huang et al., 62 patients were treated with radiotherapy and 18 individuals (29%) needed salvage surgery because non-response to radiation [24]. Note that most patients of the study of Huang et al. were treated in the seventies, eighties, and nineties. Radiotherapy was most frequently performed in patients with private insurance compared to those without insurance [25].
Oncological outcomes
Oncological outcomes were reported in 15 studies (Table 3) [3, 8, 12, 14,15,16,17,18,19,20,21,22,23,24,25]. Considering all treatments, the 5-year OS and DSS were 86.3% and 90.8%, respectively. The 5- and 10-year local control rate were 83.6% and 72.6%, respectively. The 10-year DSS was 80.2. Oncological data were analyzed in 13 studies according to the type of treatment [3, 8, 12, 14,15,16,17,18,19,20, 23,24,25]. As found in Table 3, the 5-year OS for surgery and radiotherapy ranged from 62.5 to 100% and 0 to 87.0%, respectively. Because authors assessed OS, DSS and RFS rates at several follow-up time, it is complicated to provide additional rates for treatment-specific oncological outcomes. Moreover, the data of Jayakrishnan et al. were not considered in the assessment of OS, DSS and RFS rates because authors only focused on cTis-T2 LVC [25]. For this specific group of LVC, authors reported 5-years OS for surgery and radiotherapy of 79.0% and 67.0%, respectively. Echanique et al. combined LVC case reports and case-series (N = 282) and reported local recurrence in 33 cases (11.7%) and regional metastasis in 2 cases (0.8%) [2]. This systematic review of case reports/case-series showed that among patients treated with surgery and radiotherapy, 86.8% and 67.8% were disease free at follow-up. Surgery alone was associated with and OS of 80.3% at follow-up, while among the 80 patients treated with combined surgery and radiotherapy, the DFS was 66.7% [2].
The comparison of oncological outcomes between LVC and other laryngeal malignancies was available in a large cohort study [3]. Dubal et al. reported that the 1-, 5-, and 10-year DSS rates were 97.5%, 88.0%, and 77.4% for LVC, compared to 87.9%, 64.4%, and 50.4% for other laryngeal malignancies, respectively [3]. Authors showed that patients treated by surgery demonstrated significant higher 5-year DSS (90.2%) than patients who did not benefit from surgery (80.6%), regardless of radiotherapy use. The 5-year DSS of patients receiving primary radiotherapy and/or surgery were 92.3% for surgery alone, 85.6% for radiotherapy in combination with surgery, and 75.8% for radiotherapy alone [3].
Bias analysis
Heterogeneity among studies in inclusion criteria, tobacco/alcohol use evaluations, comorbidities, LVC features and treatments are reported in Table 4. Most studies are retrospective studies (EL: IV). Only one study was prospective but uncontrolled [14]. Inclusion criteria were homogenous and reported in all studies. Authors included all cTNM stages [3, 8, 15,16,17,18,19, 21, 22, 24, 26] or only early stages in their studies [12, 14, 20, 23, 25]. The data about alcohol consumption and tobacco overuse were partly reported in 1 [23] and 5 studies [16, 19, 20, 23, 24], respectively (Table 4). Only Fliss et al. reported data about HPV infection through DNA analyses [17]. There was no full description of comorbidity features in studies. Few authors reported partial data of comorbidities, mostly for the oncological outcome interpretation [8, 12, 14, 20, 25, 26]. Clinical stages of LVC were fully reported in 13 studies according to anatomical location [3, 8, 15,16,17, 19, 21, 22, 24]. The details related to the surgical treatment, e.g. types of surgery and the realization of neck dissection, were available in 9 studies [8, 16, 17, 19], while the others reported partial information [3, 15, 21,22,23, 25]. Of the 12 studies investigating radiotherapy outcomes, the radiation protocols were fully or partly reported in 5 [8, 12, 17, 22, 24] and 3 studies [16, 21, 26], respectively. There was no information in 3 publications [3, 23, 25]. The oncological outcomes were reported considering the type of treatment in 11 studies [3, 8, 12, 14, 17, 18, 20, 23,24,25,26].
Discussion
The number of publications dedicated to therapeutic and oncological outcomes of LVC did not increase over the past few decades with most of studies dating from the twentieth century. Yet, regarding the improvement of radiation protocols, it remains important for the future decades to better understand the differences between LVC and LSCC about surgery and radiotherapy-related oncological outcomes. In this systematic review, we investigated the epidemiological, pathological and oncological outcomes of LVC.
From an etiological standpoint, alcohol and tobacco overuses have long-time been recognized as important contributing factor of the LVC development. However, few studies reported full data information about tobacco and alcohol consumptions. Similar observation may be made for HPV detection in tumors of patients with LVC. Moreover, there was no large-cohort database study comparing the prevalence of alcohol, tobacco, and HPV outcomes between LVC and LSCC, which limits the improvement of etiological knowledge.
In this review, we reported that glottis is the main anatomical region of the LVC development, which may be an element supporting the better oncological outcomes of LVC compared to LSCC [3]. Indeed, glottic carcinomas are commonly diagnosed faster than supraglottic carcinomas according to the rapid impairments of voice quality [29]. From an oncological point of view, the glottic location may support the higher prevalence of early-stage tumors (cT1 and cT2) in LVC compared to LSCC patients [3] and the differences in survival outcomes. The investigations dedicated to the biology of LVC are rare [30, 31]. However, the biological differences between LVC and LSCC should be an additional element supporting the better prognosis of LVC compared to LSCC, as well as the low rate of distant metastasis. Precisely, we did not find report of distant metastasis in the systematic review of case-series, whereas only one study reported distant metastasis in LVC [32]. The occurrence of distant metastasis in this study should be treated with caution because the pathological diagnosis of LVC remains difficult with some LVC confounded with LSCC at the first pathological examination [32]. The lack of investigation of pathological biomarkers of LVC is an important issue for the diagnosis. Indeed, several studies supported that the pathological diagnosis remains difficult and may require several biopsies [8, 15, 22]. In the study of Stojan et al. the LVC diagnosis was carried out with only one biopsy for 36.6% of patients, whereas other patients needed repeated biopsies up to five times to have a definitive pathological diagnosis [22]. Similar findings were reported in the study of Lundgren et al. who made the diagnosis after one biopsy procedure in 21 patients (47.7%), while the 23 remaining individuals required a total of 68 endoscopic procedures to reach the definitive diagnosis [8]. The LVC may be particularly confounded with “hyperkeratosis”, which leads to delay in the diagnosis and the therapeutic management [15]. The challenging issue of histopathological diagnosis of LVC supports the risk of inclusion bias in many studies where some hyperkeratosis glottic (benign) lesions may have been considered as malignancies [15].
From a therapeutic standpoint, surgery has long time been considered as the main therapeutic option. The OS, DSS and RFS rates of studies included in the present review were better in surgery groups compared to radiation groups, which corroborated findings of previous systematic review [2] or large database studies [3, 25]. However, most case-series of patients treated with radiotherapy included data from 1970 to 2000. The radiotherapy techniques have considerably improved since the seventies, which led to higher OS, DSS, and RFS rates in patients treated after 2000 for laryngeal malignancies [33, 34]. Thus, the lowest effectiveness of radiotherapy in the management of LVC must be considered with prudence according to the lack of recent controlled study, the low number of patients treated with radiation in the past decade and the potential differences in patient profiles treated with radiotherapy versus surgery. In many studies, radiotherapy was proposed to patients with comorbidities or contraindication to surgery [8, 12, 19], which may bias the assessment of the radiation effectiveness. In the same vein, the post-radiation anaplastic transformation of LVC were reported in the seventies and eighties and concerned old radiotherapy approaches. Nowadays, it appears that the risk of post-radiation anaplastic transformation of LVC may be not considered as an important outcome for the treatment decisions. Because the growth of LVC is slow, it remains complicated to distinguish the evolution of an irradiated tumor versus recurrence [8]. All findings should be considered in future studies investigating the oncological outcomes of radiotherapy versus surgery. Moreover, treatments may differ from one center (country) to another; some physicians favoring surgery, while other prefer radiation when the situation allows several choices.
Conclusion
LVC is a rare laryngeal cancer associated with better survival and recurrence outcomes than laryngeal squamous cell carcinoma. The superiority of surgery over radiotherapy is still not demonstrated regarding the lack of prospective controlled study using current radiation regimens.
Abbreviations
- DFS:
-
Disease-free survival
- DSS:
-
Disease-specific survival
- EL:
-
Evidence-based level
- LSCC:
-
Laryngeal squamous cell carcinoma
- LVC:
-
Laryngeal verrucous carcinoma
- OS:
-
Overall survival
- RFS:
-
Recurrence-free survival
References
Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–68.
Echanique KA, Desai SV, Marchiano E, Spinazzi EF, Strojan P, Baredes S, Eloy JA. Laryngeal verrucous carcinoma. Otolaryngol Head Neck Surg. 2017;156(1):38–45. https://doi.org/10.1177/0194599816662631.
Dubal PM, Svider PF, Kam D, Dutta R, Baredes S, Eloy JA. Laryngeal verrucous carcinoma: a population-based analysis. Otolaryngol Head Neck Surg. 2015;153(5):799–805. https://doi.org/10.1177/0194599815591981.
Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670–8.
Wang N, Huang M, Lv H. Head and neck verrucous carcinoma: a population-based analysis of incidence, treatment, and prognosis. Medicine (Baltimore). 2020;99(2):e18660. https://doi.org/10.1097/MD.0000000000018660.
Kompelli AR, Froehlich MH, Morgan PF, Li H, Sharma AK, Nathan CO, Neskey DM. Definitive radiotherapy versus surgery for the treatment of verrucouscarcinoma of the larynx: a national cancer database study. Int Arch Otorhinolaryngol. 2021;26(3):e348–56. https://doi.org/10.1055/s-0041-1730304.
Takenaka Y, Uno A, Tanaka H, Takemoto N, Inohara H. Early-stage glottic cancer of rare squamous cell carcinoma variants: a population-based study. Acta Otolaryngol. 2023;143(1):70–6. https://doi.org/10.1080/00016489.2022.2161626.
Lundgren JA, van Nostrand AW, Harwood AR, Cullen RJ, Bryce DP. Verrucous carcinoma (Ackerman’s tumor) of the larynx: diagnostic and therapeutic considerations. Head Neck Surg. 1986;9(1):19–26. https://doi.org/10.1002/hed.2890090105.
Thompson M, Tiwari A, Fu R, Moe E, Buckley DI. A framework to facilitate the use of systematic reviews and meta-analyses in the design of primary research studies. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012. http://www.ncbi.nlm.nih.gov/books/NBK83621/. Accessed 22 Feb 2020.
McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96. https://doi.org/10.1001/jama.2017.19163.
Odar K, Boštjančič E, Gale N, Glavač D, Zidar N. Differential expression of microRNAs miR-21, miR-31, miR-203, miR-125a-5p and miR-125b and proteins PTEN and p63 in verrucous carcinoma of the head and neck. Histopathology. 2012;61:257–65. https://doi.org/10.1111/j.1365-2559.2012.04242.x.
Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–10. https://doi.org/10.1097/PRS.0b013e318219c171.
Viswanathan M, Berkman ND, Dryden DM, Hartling L. Assessing risk of bias and confounding in observational studies of interventions or exposures: further development of the RTI item bank. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. http://www.ncbi.nlm.nih.gov/books/NBK154461/. Accessed 20 Oct 2019.
Blakeslee D, Vaughan CW, Shapshay SM, Simpson GT, Strong MS. Excisional biopsy in the selective management of T1 glottic cancer: a three-year follow-up study. Laryngoscope. 1984;94(4):488–94. https://doi.org/10.1288/00005537-198404000-00012.
Sllamniku B, Bauer W, Painter C, Sessions D. Clinical and histopathological considerations for the diagnosis and treatment of verrucous carcinoma of the larynx. Arch Otorhinolaryngol. 1989;246(3):126–32. https://doi.org/10.1007/BF00456652.
Hagen P, Lyons GD, Haindel C. Verrucous carcinoma of the larynx: role of human papillomavirus, radiation, and surgery. Laryngoscope. 1993;103(3):253–7. https://doi.org/10.1288/00005537-199303000-00003.
Fliss DM, Noble-Topham SE, McLachlin M, Freeman JL, Noyek AM, van Nostrand AW, Hartwick RW. Laryngeal verrucous carcinoma: a clinicopathologic study and detection of human papillomavirus using polymerase chain reaction. Laryngoscope. 1994;104(2):146–52. https://doi.org/10.1288/00005537-199402000-00005.
Longarela Herrero Y, Morales Angulo C, Rubio Suárez A, González-Rodilla I, Rama Quintela J. Verrucous carcinoma of the larynx. Acta Otorrinolaringol Esp. 1995;46(1):49–52.
Maurizi M, Cadoni G, Ottaviani F, Rabitti C, Almadori G. Verrucous squamous cell carcinoma of the larynx: diagnostic and therapeutic considerations. Eur Arch Otorhinolaryngol. 1996;253(3):130–5. https://doi.org/10.1007/BF00615109.
Damm M, Eckel HE, Schneider D, Arnold G. CO2 laser surgery for verrucous carcinoma of the larynx. Lasers Surg Med. 1997;21(2):117–23. https://doi.org/10.1002/(sici)1096-9101(1997)21:2%3c117::aid-lsm2%3e3.0.co;2-t.
McCaffrey TV, Witte M, Ferguson MT. Verrucous carcinoma of the larynx. Ann Otol Rhinol Laryngol. 1998;107(5 Pt 1):391–5. https://doi.org/10.1177/000348949810700505.
Strojan P, Smid L, Cizmarevic B, Zagar T, Auersperg M. Verrucous carcinoma of the larynx: determining the best treatment option. Eur J Surg Oncol. 2006;32(9):984–8. https://doi.org/10.1016/j.ejso.2006.03.025.
Hod R, Feinmesser R, Shvero J. Carbon dioxide laser cordectomy for verrucous carcinoma of vocal folds. J Laryngol Otol. 2010;124(1):55–8. https://doi.org/10.1017/S002221510999140X.
Huang SH, Lockwood G, Irish J, Ringash J, Cummings B, Waldron J, Kim J, Dawson LA, Bayley A, Hope A, O’Sullivan B. Truths and myths about radiotherapy for verrucous carcinoma of larynx. Int J Radiat Oncol Biol Phys. 2009;73(4):1110–5. https://doi.org/10.1016/j.ijrobp.2008.05.021.
Jayakrishnan TT, Abel S, Interval E, Colonias A, Wegner RE. Patterns of care and outcomes in verrucous carcinoma of the larynx treated in the modern era. Front Oncol. 2020;10:1241. https://doi.org/10.3389/fonc.2020.01241.
Ferlito A, Recher G. Ackerman’s tumor (verrucous carcinoma) of the larynx: a clinicopathologic study of 77 cases. Cancer. 1980;46(7):1617–30. https://doi.org/10.1002/1097-0142(19801001)46:7%3c1617::aid-cncr2820460722%3e3.0.co;2-t.
Orvidas LJ, Olsen KD, Lewis JE, Suman VJ. Verrucous carcinoma of the larynx: a review of 53 patients. Head Neck. 1998;20(3):197–203. https://doi.org/10.1002/(sici)1097-0347(199805)20:3%3c197::aid-hed3%3e3.0.co;2-w.
O’Sullivan B, Warde P, Keane T, Irish J, Cummings B, Payne D. Outcome following radiotherapy in verrucous carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1995;32(3):611–7. https://doi.org/10.1016/0360-3016(95)00021-P.
Raitiola H, Pukander J, Laippala P. Glottic and supraglottic laryngeal carcinoma: differences in epidemiology, clinical characteristics and prognosis. Acta Otolaryngol. 1999;119(7):847–51. https://doi.org/10.1080/00016489950180531.
Marioni G, Pillon M, Bertolin A, Staffieri A, Marino F. The role of survivin expression in the differential diagnosis of laryngeal (glottic) verrucous squamous cell carcinoma. Eur J Surg Oncol. 2007;33(2):229–33. https://doi.org/10.1016/j.ejso.2006.09.027.
Marioni G, Agostini M, Cappellesso R, Bedin C, Ottaviano G, Marchese-Ragona R, Lovato A, Cacco T, Giacomelli L, Nitti D, Blandamura S, Stellini E, de Filippis C. miR-19a and SOCS-1 expression in the differential diagnosis of laryngeal (glottic) verrucous squamous cell carcinoma. J Clin Pathol. 2016;69(5):415–21. https://doi.org/10.1136/jclinpath-2015-203308.
Varshney S, Singh J, Saxena RK, Kaushal A, Pathak VP. Verrucous carcinoma of larynx. Indian J Otolaryngol Head Neck Surg. 2004;56:54–6.
Tomeh C, Holsinger FC. Laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):147–53. https://doi.org/10.1097/MOO.0000000000000032.
Gupta A, Wong KH, Newbold K, Bhide S, Nutting C, Harrington KJ. Early-stage glottic squamous cell carcinoma in the era of image-guided radiotherapy. Front Oncol. 2021;11:753908. https://doi.org/10.3389/fonc.2021.753908.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
JRL: design, acquisition of data, data analysis and interpretation, drafting, final approval, and accountability for the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SH: design, acquisition of data, data analysis and interpretation, drafting, final approval, and accountability for the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. IRB was not required for a systematic review.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lechien, J.R., Hans, S. Epidemiological, clinical and oncological outcomes of laryngeal verrucous carcinomas: a systematic review. J of Otolaryngol - Head & Neck Surg 52, 81 (2023). https://doi.org/10.1186/s40463-023-00666-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40463-023-00666-1