Abstract
Background
Increasing prevalence of morbid obesity accompanied by comorbidities like type 2 diabetes mellitus (T2DM) led to a demand for improving therapeutic strategies and pharmacological intervention options. Apart from genetics, inflammation processes have been hypothesized to be of importance for the development of obesity and related aspects like insulin resistance.
Main text
Within this review, we provide an overview of the intricate interplay between chronic inflammation of the adipose tissue and the hypothalamus and the development of obesity. Further understanding of this relationship might improve the understanding of the underlying mechanism and may be of relevance for the establishment of new treatment strategies.
Similar content being viewed by others
Background
The overwhelming increase in obesity and its associated comorbidities worldwide necessitates an advancement of optimal therapeutic intervention. However, an understanding of the underlying mechanism is relevant in order to development new strategies to optimize patient management which includes the reduction of obesity-related comorbidities. Special attention has been paid to elucidating the relationship between chronic inflammation and obesity. In contrast to the transient, acute inflammation type, which is characterized by edema formation and leukocyte migration, chronic inflammation endures over a prolonged period and is marked by the presence of lymphocytes and macrophages, which are integral components of adipose tissue [1]. Chronic inflammation has been extensively studied as a component of the metabolic syndrome due to the release of pro-inflammatory adipokines, such as leptin, interleukin-6 (IL-6), tumor necrosis factor-α (TNFα), and others, by adipose tissue [2]. This chronic inflammatory state plays a pivotal role in the pathogenesis of various conditions including fatty liver disease, cardiovascular disease, insulin resistance in T2DM, asthma, neurodegeneration, certain cancers, and predisposition to autoimmune diseases [3, 4]. The presence of pro-inflammatory cytokines and the recruitment of myeloid cells have been shown to directly correlate with metabolic dysfunction observed in obese patients [5,6,7,8]. Additionally, obesity-related insulin resistance can impact the adaptive immune response [9, 10]. Impaired T cell function has been observed in mice with diet-induced obesity, leading to poorer outcomes in viral infections such as influenza [11, 12]. The inflammasome, a macromolecular sensor found in innate immune cells, represents a critical initiator of the inflammatory response. This multimeric protein complex is activated by cellular nutrients such as glucose or free fatty acids, exerting control over IL-1β production and Caspase-1 activation among others [13, 14]. The concept of “immune metabolism” encompasses the intricate interplay between immunological processes and metabolic abnormalities. This review aims to provide a detailed summary of the interconnections between inflammation in adipose tissue, the hypothalamus, and the leptin-melanocortin signaling pathway and its pharmacological relevance.
Main text
Inflammation and leptin-melanocortin signaling pathway
The leptin-melanocortin signaling pathway plays a crucial role in central appetite regulation. The hormone leptin (LEP), produced by and according to adipose tissue mass, binds to the Leptin receptor (LEPR) in the hypothalamus. This binding stimulates the production of pro-opiomelanocortin (POMC), which is subsequently processed into α-melanocyte-stimulating hormone (MSH) and β-MSH, among other peptides. α- and β-MSH bind to the Melanocortin-4 receptor (MC4R), thereby activating the feeling of satiety, which leads to a reduction in food intake and a modulated energy expenditure [15]. Genetic alterations within this signaling pathway, such as in the LEP and LEPR gene, lead to severe early-onset adiposity due to hyperphagia [16].
In addition to its role in appetite regulation, leptin also triggers proliferative signals in hematopoiesis and lymphopoiesis. It can activate neutrophils, natural killer cells, monocytes, dendritic cells, and macrophages [17,18,19,20,21]. Additionally, there is an enhanced expression of leptin mRNA and cytokines such as TNFα, IL-6, and IL-1β in response to lipopolysaccharide (LPS) stimulation, indicating its role as a mediator in inflammatory activity [22, 23]. In the absence of leptin, dendritic cells exhibit a T helper cell type 2 (Th2)-biased cytokine profile whereas exogenous administration of leptin drives the balance towards a Th1 profile [17, 18]. Th1 responses are present in autoimmune processes, thus reduced levels of Leptin have a protective effect in autoimmune diseases [24,25,26,27]. During acute inflammatory reactions and sepsis, a marked increase in leptin levels is observed in the blood of so far healthy individuals. Leptin acts via binding to LEPR, a class 1 cytokine receptor of the superfamily [28,29,30,31], which is mainly expressed in the hypothalamus, but also in the kidney, lung, and choroid plexus [32].
Both Leptin-deficient ob/ob mice and Leptin receptor-deficient db/db mice display impaired cell-mediated immunity and lymphoid atrophy, making them more susceptible to infections and injuries [33,34,35,36,37]. These animals also exhibit thymic atrophy, which affects the maturation process of thymocytes that require leptin as a survival factor [33]. Consequently, specific alterations in peripheral T cell populations can be observed in these animals. Short-term administration of leptin can restore thymic cellularity, reverse LPS-induced thymic atrophy, and support thymopoiesis [38, 39]. On the other hand, ob/ob mice appear to be partially protected against inflammation and tissue damage, such as in fulminant hepatitis [40]. They are also resistant to dextran sulfate sodium (DSS)-induced colitis [40] and autoimmune glomerulonephritis [41]. Lepr-deficient mice also display impaired lymphopoiesis with reduced numbers of B cells in the bone marrow and permanently reduced levels of B cells and CD4 + T cells in the blood [42]. Hence, a direct role in the proliferation and expansion of hematopoietic stem cells and lymphoid progenitor cells is postulated. Additionally, the development of natural killer (NK) cells is affected, with significantly reduced NK pool size [21].
Patients deficient in LEP and LEPR show reduced lymphocyte proliferation and cytokine production, making them more prone to infections. Particularly in individuals with Leptin deficiency, an increased incidence of infection-related deaths during childhood has been observed [27, 43]. However, the administration of Leptin can restore these immunological abnormalities [26]. Additionally, low levels of Leptin can also play a crucial role in immunosuppression during periods of starvation and malnutrition [44]. On the other hand, in patients with active rheumatoid arthritis, an inverse correlation between disease activity/inflammation and blood leptin concentration has been observed [45].
Several decades ago, it was demonstrated that α-MSH can downregulate pro-inflammatory cytokines, including IL-1, IL-6, TNFα, as well as immunomodulatory cytokines such as IL-2, IL-4, IL-13, and interferon-γ (INFγ) in vitro [46]. Moreover, cell experiments have revealed that α-MSH influences the production of immunoglobulin E (IgE) and nitric oxide (NO) and inhibits IL-1β-induced production of IL-8, growth-regulated protein α (Groα), and nuclear factor “kappa-light-chain-enhancer” of activated B cells (NFκB) [47, 48]. In mouse models, administration of α-MSH suppressed allergic airway inflammation and reduced levels of Il-4 and Il-13 in the bronchoalveolar lavage of allergic mice [49]. Similarly, in mice with DSS-induced colitis, α-MSH administration mitigated disease-induced weight loss and improved the overall outcome of the animals [50]. These effects are believed to be mediated through the melanocortin-1 receptor (MC1R). MC1R, primarily known for its role in melanocyte pigmentation [51, 52], is also expressed in immune cells [53,54,55]. Concordantly, Mc1r-deficient mice exhibited significantly worse outcomes in DSS-induced colitis, characterized by increased weight loss and more pronounced histological changes compared to wild-type mice [56]. Thus, it can be postulated that MC1R serves as an important regulator of mucosal defense. Mutations in MC1R lead to an augmented inflammatory response and are associated with burn-induced systemic inflammatory response syndrome (SIRS) and infectious complications in patients [57, 58]. Furthermore, MC1R is implicated in the development of hypertrophic scarring [59]. Studies have shown that administration of an MC1R agonist (PL-8177) significantly reduced the inflammatory response in mice with experimentally-induced autoimmune uveitis [60] and experimentally-induced inflammatory bowel disease in rats [61]. The MC4R agonist setmelanotide (RM493), which is approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of monogenic obesity in LEPR- and POMC-deficient patients, also binds to MC1R, resulting in skin hyperpigmentation and hair darkening in patients [62,63,64]. In vitro experiments demonstrated that activation of MC4R by setmelanotide in astrocytes exhibits anti-inflammatory and neuroprotective effects. Astrocytoma cells incubated with TNFα and IFNγ and subsequently treated with setmelanotide exhibited reduced expression of chemokine C–C motif ligand 2 (CCL2) and C-X-C motif chemokine 10 (CXCL10), while IL-6 and IL-11 mRNA levels were increased. These chemokines play an important role in the activation of leukocytes in the central nervous system (CNS) [65].
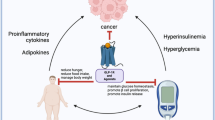
In addition to its role in regulating hunger and satiety, the individual components of the leptin-melanocortin signaling pathway also contribute to immunomodulatory responses. Leptin conveys pro-inflammatory signals via activation of the immune system while, antagonistically, α-MSH displays anti-inflammatory effects for example in inflammatory bowel diseases (Fig. 1).
Components of the leptin-melanocortin signaling pathway contribute to immunological functions. Leptin is produced by the adipose tissue and binds centrally to the leptin-receptor. This activates the production and processing of POMC into α-MSH among others. Α-MSH binds to the MC4R, which initiates a feeling of satiety and leading to a reduction and food intake. The components of this pathway also contribute to inflammatory functions. Leptin acts via binding to the Leptin-receptor and increases hematopoiesis and lymphopoiesis. It activates neutrophils, NK, monocytes, dendritic cells, and macrophages and promotes a Th1-type production of pro-inflammatory cytokines. Α-MSH has been shown to downregulate pro-inflammatory cytokines such as IL-1, IL-6, and TNFα and immunomodulatory cytokines like IL-2, IL-4, IL-13, and INFγ. It also reduces the production of IgE and NO
Inflammation and adipose tissue
Multiple hypotheses have been proposed to elucidate the mechanisms underlying chronic adipose tissue inflammation in obesity (Fig. 2). The first hypothesis suggests that excessive nutrient intake leads to the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum, triggering the activation of the unfolded protein response pathway (UPR), which leads to an enhanced expression of pro-inflammatory cytokines [66,67,68,69,70,71]. The second hypothesis postulates that an overloading of adipocytes leads to a substantial infiltration of macrophages. This is accompanied by the differentiation and activation of cytotoxic T cells, which subsequently initiate inflammatory cascades [72,73,74]. Another theory focuses on the expansion of adipose tissue, which results in reduced perfusion, leading to hypoxia and subsequent activation of pro-inflammatory signaling pathways. This hypoxia-induced inflammation contributes to necrosis and infiltration of macrophages within the adipose tissue [75,76,77,78]. Moreover, overloaded adipocytes and mechanical stress directly activate immune pathogen sensors, further promoting chronic inflammation [79]. Additionally, free fatty acids in the adipose tissue can promote inflammation by indirectly binding to Toll-like receptors (TLR4 and TLR2), leading to the activation of NFκB and Janus kinase 1 (JNK1) [80, 81]. This activation, in turn, stimulates the synthesis and secretion of chemokines, such as monocyte chemoattractant protein-1 (MCP1), by adipocytes and macrophages. These chemokines contribute to the infiltration of pro-inflammatory macrophages [82, 83]. As a result, this local adipose tissue inflammation triggers systemic inflammation, which is closely associated with the development of obesity-related comorbidities. This also includes inflammatory vascular changes, which can finally lead to atherosclerotic cardiovascular diseases (CVD). In patients with CVD, plasma adiponectin levels are decreased [84]. Adiponectin is proposed to be protective against CVD by repressing inflammatory mediators such as vascular cell adhesion molecule 1 (VCAM1), TNFα, and IL-6 and by stimulating endothelial NO synthase [85,86,87,88]. Therefore, adipokines are suggested to play an important role in CVD. Furthermore, it has been postulated that advanced glycation end products (AGEs) may play a contributory role in adipose tissue inflammation. AGEs, comprising proteins and lipids subjected to glycation by various sugars, most notably glucose, exhibit their function by binding to cell surfaces or receptors and by catalyzing ROS formation and accumulation [89]. Notably, AGE levels are increased in patients with hyperglycemia, which can activate different signaling pathways, including NF-kB, which regulates the transcription of proteins, such as chemokines, growth factors, or cytokines [90].
Activation of chronic inflammation in the adipose tissue. Several hypotheses suggest possible mechanisms for the activation of adipose tissue inflammation. Hypothesis (a) proposes that an increased nutrient intake leads to an accumulation of misfolded/unfolded proteins, which activates the UPR, activating inflammation. Hypothesis (b) postulates that overloading of adipocytes triggers infiltration of macrophages due to hypoxia, followed by activation of cytotoxic T cells, which subsequently initiate inflammatory cascades. Hypoxia results in necrosis, which further promotes macrophage infiltration (see c). Overloaded adipocytes and consequent mechanical stress can also directly activate immune pathogen sensors (d). Lastly, free fatty acids can promote inflammation via indirectly binding to TLRs, which activates JKN1. This in turn stimulates the secretion of chemokines, such as MCP1 (e). AGEs are also postulated to contribute to inflammation in the adipose tissue (f)
Our adipose tissue depot harbors approximately 2–5 million cells per gram, of which around 65% are leukocytes. Consequently, adipose tissue functions as an autonomous immunological organ [5,6,7,8]. Within our visceral adipose tissue, a diverse array of immune cells exists, including macrophages, dendritic cells, granulocytes, lymphocytes, T cells, and B cells [91]. Remarkably, up to 15 distinct subpopulations of leukocytes can be discerned [92]. In contrast, subcutaneous fat is prominent in lean subjects, and rather serves as a barrier against dermal infection and physical external stress as well as an important regulator of body temperature and is therefore much less immunologically active [93]. Neutrophils are the initial immune cells to infiltrate the visceral adipose tissue in obesity, initiating inflammation within the adipose depot [94]. Macrophages constitute approximately 4% of the healthy visceral adipose tissue, which can escalate to 12% in the context of obesity [83]. Two distinguishable macrophage populations are present: M1 (type 1 macrophage), prevalent in obesity, and M2, predominantly found in lean adipose tissue [95] (Fig. 3). M1 macrophages exhibit an increased production of pro-inflammatory cytokines, such as IL-6, TNFα, IL-12, and IL-23, alongside a reduced synthesis of the anti-inflammatory cytokine IL-10 [95]. M2 macrophages primarily engage in tissue repair processes and generate IL-10, concomitant with decreased IL-12 and IL-23 synthesis [96]. Studies with high-fat-diet (HFD)-induced obesity mouse models have demonstrated a significant increase in NK cells, responsible for the M1 polarization of macrophages through IFNγ production [97, 98]. B cells also exhibit heightened abundance within the adipose tissue of obese individuals [99]. Preventing the accumulation of adipose tissue macrophages (ATMs) or pro-inflammatory macrophages holds the potential to shield obese mice from glucose intolerance and insulin resistance [100,101,102]. Consistently, the reduction of B cells in obesity culminates in enhanced insulin sensitivit [6]. Mice incapable of producing inflammasome molecules exhibit improved glucose tolerance and insulin sensitivity when subjected to HFD compared to their wild-type counterparts [103,104,105,106,107]. Administering obese mice with a caspase-1 inhibitor therapy can restore metabolic functions. Consequently, these inhibitors present a therapeutic potential for inflammasome-targeted interventions [104].
M1-type macrophages are prevalent in obese adipose tissue. In obese adipose tissue, pro-inflammatory M1-type macrophages are predominantly activated. They mainly produce cytokines and chemokines with inflammatory activity such as TNFα, IL-1α, IL-1β, and IL-6. Additionally, Th1 cells are mainly infiltrating the obese adipose tissue. In lean adipose tissue, the anti-inflammatory M2-type macrophage is dominantly represented. These macrophages mainly participate in tissue repair and regeneration and produce cytokines like IL-10 and transforming growth factor β (TGFβ). Apart from M2 macrophages, also Th2 cells are localized in the lean adipose tissue
Pharmaceuticals targeting immunological modulators have been shown to improve insulin sensitivity in humans as well. For example, in patients afflicted with both rheumatoid arthritis and diabetes, IL-1R antagonist has demonstrated the capacity to enhance insulin sensitivity [108]. Clinical trials have unveiled the potential of TNFα antagonists in inhibiting the development of type 2 diabetes [109, 110]. These antagonists have exhibited improvements in glycemic control among obese patients with psoriasis, rheumatoid arthritis, and Crohn's disease who do not have diabetes [111,112,113,114,115,116,117,118]. Additionally, Anakinra, a recombinant human IL-1R antagonist, has been found to ameliorate the secretory function of B cells and reduce glycemic levels [119]. Ongoing clinical trials are presently investigating the effects of neutralizing anti-IL-1β antibodies, particularly in patients with type 2 diabetes [108, 120,121,122].
On the other hand, there is also therapeutic potential focusing on anti-inflammatory adipokines produced by the adipose tissue, such as adiponectin, to prevent the incidence of co-morbidities like insulin resistance [123,124,125,126,127]. Adiponectin exerts beneficial effects on inflammation, atherosclerosis [128], T2DM, and insulin resistance [129, 130]. It enhances local NO production [88], protects against endothelial dysfunction, and inhibits plaque formation and thrombosis. Consequently, it serves as a vasoprotective factor while mitigating oxidative stress [131, 132]. It also improves insulin sensitivity, impedes the uptake of non-esterified fatty acids, reduces gluconeogenesis, and augments oxidative processes. Consistently, in cases of severe weight gain and obesity, adiponectin levels are notably diminished [133, 134].
Our microbiome also exerts a significant influence on the immune response and could serve as a prospective therapeutic target in managing insulin resistance and chronic inflammation associated with obesity [135]. A research group has demonstrated that patients who undergo Roux-en-Y gastric bypass surgery display diminished infiltration of macrophages in adipose tissue, resulting in reduced inflammation [136].
Inflammation within the adipose tissue has been a long-known regulator of the development of metabolic syndrome. In recent years, targeting this chronic state of inflammation has led to the development of new pharmacological strategies to protect against insulin resistance and diet-induced obesity (DIO), indicating that a more extensive understanding of underlying mechanisms can contribute to improved therapy strategies for patients with obesity and metabolic syndrome.
Inflammation and hypothalamus
Gut inflammation is a consequential outcome of an HFD and potentially contributes to the onset of obesity [137, 138]. The intricate interplay between dietary components of HFD, the microbiome, and neuronal inflammation holds substantial importance [139]. HFD induces noteworthy alterations in the diversity of the microbiome and triggers oxidative stress within the hypothalamus [140]. As a consequence, the permeability of the blood–brain barrier is enhanced due to a potential downregulation of tight junction proteins [141,142,143]. This enables the infiltration of peripheral macrophages into the hypothalamus [144, 145]. These infiltrating macrophages originate from adipose tissue and share similar surface markers with ATMs. Displaying a pro-inflammatory M1 phenotype may contribute to neuropathological conditions such as cerebral ischemia and dementia [146].
In male mice, an HFD leads to a rise in macrophage population from 1.3 to 2.9% of all hypothalamic cells. Concurrently, the proportion of macrophages in visceral adipose tissue also increases from 5.3% to as high as 22.8% in these animals. Furthermore, the proportion of microglia cells in the hypothalamus of male mice increases from 31 to 52% following HFD [146]. Hypothalamic microglial cells are believed to have an orchestrating role in the inflammatory response as sensors within the hypothalamus [147]. Simultaneously, an increase in the population of these cells has been associated with neurodegeneration [148]. Microglial cells, known as the brain's macrophages, play a crucial role in hypothalamic inflammation [149, 150]. The proportion of microglial cells also influences the strength of the inflammatory response, impacts neuronal stress, and regulates satiety-signaling neurons [151]. This inflammatory response triggers reactive gliosis, characterized by increased infiltration of microglia and proliferation of astrocytes [139, 152]. Among the regulators of microglial cell activation, uncoupling protein 2 (UCP2) plays a significant role. HFD induces mitochondrial changes in microglial cells through an increase in UCP2, leading to the production of reactive oxygen species (ROS) and activation of inflammation [153, 154]. It is therefore highly expressed in activated microglia cells [155]. Concordantly, genetic ablation of UCP2 in microglia cells of mice led to protection against DIO and made POMC-neurons more sensible towards glucose [156]. UCP2 can inhibit the activation of POMC neurons induced by glucose while activating NPY/AgRP neurons through ROS [157] thereby promoting orexigenic signaling. It is distributed throughout the organism including the spleen, kidney, immune system, and within the CNS [158,159,160,161,162] and genetic variants of UCP2 have been associated with obesity and insulin resistance [163,164,165]. Interestingly, UCP2 has a dual function of protecting against ROS and supporting fatty acid oxidation [153, 154] and also presents anti-inflammatory effects by having a protective role in acute and chronic neurodegeneration and inflammatory brain diseases [166].
Hypothalamic inflammation has been connected to obesity in the past. Following HFD, pro-inflammatory proteins such as Tnfα, Il-6, and Jnk3 are upregulated in the hypothalamus in rats [167]. Prolonged exposure to HFD in rodents also leads to hypothalamic inflammation, resulting in hypothalamic leptin resistance and subsequent development of obesity due to reduced leptin effectiveness [139, 152, 168]. This inflammation in the hypothalamus has also been observed in humans and correlates with elevated levels of serum inflammatory proteins, including IL-6 and C-reactive protein (CRP) [139, 152]. Interestingly, obesity resistance has been associated with increased expression of IL-6 in the hypothalamus. It plays a role in neurogenesis within the hypothalamus. This process involves the expression of transcription factors Sox2 and Sox6, which are crucial for neurogenic transcriptional regulation [169,170,171]. Produced in response to exercise, primarily by muscle tissue, IL-6 has been found to mitigate memory loss in Alzheimer’s disease models [172, 173]. Moreover, exercise-induced IL-6 can reduce diet-induced inflammation and restore abnormal regulation of food intake [174, 175]. In mice fed HFD, administration of IL-6 protected against weight gain regardless of calorie intake [176].
Although discussed controversially as it is not known yet whether leptin resistance actually occurs in humans, its development within the CNS might be partially mediated by the activation of pro-inflammatory suppressor of cytokine signaling 3 (SOCS3) (Fig. 4). The binding of leptin to its receptor triggers the activation of the janus-kinase-signal transducer and activators of transcription-SOCS3 (JAK-STAT-SOCS3) signaling pathway [177,178,179]. Within this pathway, SOCS3 serves as a negative feedback regulator, exerting control over the effects of leptin and dampening the downstream activation of MC4R [179]. Accordingly, in situations where there is heightened production of leptin due to increased adipose tissue, there can also be an upregulation of SOCS3 expression. This elevated SOCS3 expression can contribute to the development of both leptin resistance and insulin resistance within the brain and peripheral tissues [179,180,181].
Pro-inflammatory cytokine SOCS3 has been proposed to play an important role in the development of leptin and insulin resistance in the CNS and the periphery. SOCS3 is activated and produced by DIO, injection of TNFα, or due to lipid infusion in several different organs. It is also activated upon increased levels of insulin and leptin and acts as a negative regulator for both, i.e., by inhibiting phosphorylation and p85 binding to insulin receptor substrate 1 (IRS-1), inhibiting insulin-stimulated glucose uptake or reducing the binding of leptin to the leptin-receptor in the hypothalamus, leading to orexigenic signaling. Chronic inflammation increases levels of IL-6, which in turn activates SOCS3 in the adipose tissue
Inflammation and liver
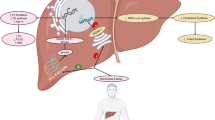
In liver disease, there is a dysregulation of the tolerogenic mechanism, leading to an excessive inflammatory response [182]. In non-alcoholic steatohepatitis (NASH), there is a persistent occurrence of apoptosis in Kupffer cells, which are subsequently replaced by monocyte-derived recruited hepatic macrophages [183] (see Figs. 5 and 6). Additionally, there is an accumulation of collagen within the liver [184, 185]. Hepatocytes become steatotic, primarily attributed to enhanced de novo lipogenesis, and exhibit a ballooned morphology characteristic of NASH [186]. Furthermore, the abnormal accumulation of triglycerides in hepatocytes, along with oxidative stress and lipid peroxidation, collectively contribute to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) [187,188,189]. In obese individuals, adipose tissue inflammation results in the secretion of inflammatory cytokines that further promote hepatic inflammation [190]. Moreover, dysregulated hepatic lipid and cholesterol metabolism contributes to an increased production of ROS [190]. Additionally, alterations in the gut can lead to augmented infiltration of LPS, thereby inducing hepatic inflammation, hepatocyte damage, and activation of hepatic stellate cells (HSCs), which produce extracellular matrix and therefore contribute to fibrotic changes within the liver tissue [191]. Leptin also plays a significant role in the activation of HSCs [192]. Stimulation by leptin results in enhanced expression of TGFβ in Kupffer cells and upregulation of hedgehog signaling pathways that sustain the activated phenotype of HSCs [193]. Additionally, LEPR-deficient rats exhibit a protective effect against the progression of liver fibrosis induced by carbon tetrachloride (CCl4) [194]. On the other hand, adiponectin suppresses HSC activation in NASH [195, 196]. Administration of recombinant adiponectin has been shown to ameliorate hepatic steatosis and inflammation in obese mice [197]. Dual agonist of adiponectin receptors AdipoR1/AdipR2 improved NASH and fibrosis in rodents by reducing HSC activation [198]. Several potential treatment options have emerged to attenuate the progression of liver fibrosis in NASH by targeting inflammatory pathways. Among these approaches is the inhibition of cytokine-mediated processes, such as the utilization of anti-interleukin-17 (anti-IL-17) biological therapy to impede HSC stimulation [199]. Additionally, promising effects have been observed in a phase 2 clinical trial of the CCR2/5 antagonist cenicriviroc [200], which suppresses monocyte recruitment to the liver and has demonstrated a reduction of liver fibrosis in rodents [201,202,203,204,205]. Furthermore, the neutralization of TGFβ using fresolimumab (GC1008), a human anti-TGFβ1 monoclonal antibody, has shown successful suppression of liver fibrosis development in mouse models [206,207,208,209]. Hyperinsulinemia can directly stimulate the proliferation of HSCs and subsequently trigger the secretion of type 1 collagen [210]. In obese rats, HFD and the consequent insulin resistance were observed to elevate the expression of TGFβ1 [211]. Moreover, hyperglycemia itself can also activate HSCs [212]. Interestingly, a meta-analysis revealed that nearly all patients with T2DM also exhibit NASH [213]. Insulin inhibits lipolysis in adipocytes [214]. Upon insulin resistance in adipose tissue, elevated release of free fatty acids (FFAs) can be examined. These FFAs activate NFκB among others, and lead to lipotoxicity, which can result in lipid accumulation in the liver [190, 215]. Lipid overload potentiates oxidative stress and liver damage. Accordingly, patients with NAFLD show significantly increased serum FFA levels [216]. Additionally, overexpression of TNFα and IL-6, which occur in obese adipose tissue, can be involved in the progression of NAFLD [217, 218]. Secretion of IL-6 can elevate the expression of hepatic SOCS3, which can contribute to the development of hepatic insulin resistance [219] (see Fig. 7). Hepatic insulin resistance can also occur due to inhibitory serine phosphorylation of the insulin signaling molecules Irs1 and Irs2. This is caused by overactivation of JNK in hepatocytes in response to pro-inflammatory cytokines, ER stress and ROS [220]. Conclusively, the liver might contribute to the development of metabolic syndrome and morphologic changes in hepatic tissue are a result of increased body mass. Therefore, targeting inflammation within this organ may improve the outcome of metabolic diseases.
Pathophysiology from the healthy liver to NASH. In response to pathogens and FFAs pro-inflammatory cytokines are produced within the hepatic tissue by RHMs, Kupffer cells, and hepatocytes. Dietary fat due to HFD leads to lipid accumulation within the liver. Pro-inflammatory cytokines, especially an overactivation of NFκB lead to the activation of HSC, which in turn produces extracellular matrix contributing to the progression of fibrosis. Kupffer cells undergo apoptosis in a state of increased inflammation and are replaced by RHMs
Inflammation and fibrosis in hepatic tissue. Alcohol consumption and viral infections can lead to morphologic changes in hepatocytes which proceed to undergo apoptosis. These triggers also lead to an infiltration of lymphocytes into the hepatic tissue and an inhibition of NK cells. Pro-inflammatory cytokines which are produced by adipose tissue of obese individuals can also initiate activation of HSC. Additionally, microbial changes in the gut can lead to a disturbed barrier and an increased infiltration of LPS and bacterial molecules. These bind to TLR and promote the production of pro-inflammatory cytokines via Kupffer cells and HSC activation
Gut hormone-derived incretins contribute to hypothalamic inflammation and modulate insulin- and leptin-resistance. Over-nutrition activates GIP production in the gut, which in turn activates glucose-dependent insulin secretion in the pancreas and also exhibits extra-pancreatic functions. Interestingly, in mice, it has been shown that deletion and overexpression of GIP is associated with improved diabetes and resistance to DIO. Centrally administered GIP leads to a reduction of JAK-STAT-activation and therefore diminishes leptin activity in the hypothalamus and upregulates SOCS3 and IL-6 in mice. Concordantly, intracerebral application of monoclonal antibodies against GIPR leads to a suppression of SOCS3 and IL-6 and induces weight loss in mice and non-human primates. This effect is enhanced when combined with GLP-1R agonist. Paradoxically, the GIPR-GLP-1R co-agonist also leads to weight loss, reduced food intake, and a decrease in fat mass. These incretins and their receptors propose immense pharmacological potential in targeting DIO and its co-morbidities
This is especially relevant for pediatric patients as it has been shown, that in obese adolescents insulin sensitivity and glucose tolerance as well as the risk for the development of T2DM are directly linked to liver steatosis [221, 222]. Interestingly, adolescents with NASH present with higher serum TNFα and MCP1 and lower serum adiponectin levels, thereby displaying a pro-inflammatory trend [223].
Inflammation and incretins
Gut-derived hormones such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) also contribute to the development and mitigation of inflammation within the whole body including the hypothalamus (Fig. 7). Studies have demonstrated that GIP is associated with increased expression of pro-inflammatory cytokines and chemokines, while GIP infusion induces elevated levels of adipokines and pro-inflammatory cytokines in adipocytes in vitro [224,225,226,227,228,229]. Centrally administered GIP leads to an increase in pro-inflammatory cytokines and factors such as Il-6 and Socs3 in the hypothalamus in mice [230], diminishes the anorectic effects of insulin in the brain and attenuates the impact of leptin, resulting in leptin resistance [231]. Loss of GIP action is therefore associated with a better outcome in diabetes and resistance towards DIO in mice [232, 233], but contradictory a transgenic overexpression of GIP also promotes resistance to DIO and leads to a reduced fat mass in mice [234]. Genetic elimination of GIP or its receptor in mice has yielded long-term metabolic protection against diet-induced obesity and insulin resistance [224, 235,236,237,238,239]. In the liver, a reduction of GIP ameliorates lipid accumulation and lowers the expression of markers of inflammation [235, 240, 241]. As GIP receptor (GIPR) is also expressed by myeloid cell lines, which include monocytes and macrophages as well as bone marrow-derived T cells, Gipr deletion in rodents impacts hematopoiesis by decreasing the number of myeloid-progenitor cells, as well as circulating monocytes and macrophages [242, 243]. Moreover, GIPR deficiency and the application of an antagonistic GIP receptor antibody significantly diminish the levels of pro-inflammatory cytokines such as Il-6 and Socs3 in the hypothalamus in mice [230]. Acute inhibition of GIPR using neutralizing antibodies has shown the ability to substantially reduce body weight and improve obesity by enhancing the effectiveness of leptin [231, 235, 244, 245]. In humans, elevated plasma GIP levels have also been correlated with increased expression of pro-inflammatory genes in obese individuals [246]. Therefore, GIP is considered a pivotal factor in driving leptin resistance and plays a significant role in hypothalamic inflammation.
GLP-1 has also been identified as an important regulator of inflammation and metabolic diseases and has been targeted as a therapeutic opportunity to improve cardiovascular and metabolic outcomes of patients with obesity and T2DM. It is synthesized in the large and small bowel and colon as well as in the brain [247, 248]. In the pancreas, the binding of GLP-1 to its receptor (GLP-1R) stimulates insulin secretion and also increases glucose metabolism by promoting insulin synthesis [249]. Additionally, it preserves beta-cell mass through stimulation of cell proliferation and inhibition of apoptosis and is therefore improving glycemic control via chronic alterations [250,251,252,253].
The secretion of GLP-1 is induced by various factors such as inflammation, microbial metabolites, and cytokines [254,255,256,257]. Accordingly, in hospitalized patients with critical illness, plasma levels of GLP-1 correlate with the severity and survival [258, 259]. Conclusively, GLP-1 receptor agonists (GLP-1R) can reduce systemic inflammation as well as tissue inflammation in rodents independent of body weight changes [260, 261]. However, the underlying mechanism remains poorly understood [262]. In the liver, GLP-1 also reduces hepatic steatosis and inflammation and can additionally attenuate hepatocyte injury in preclinical studies with models of non-alcoholic steatohepatitis (NASH). This effect has also been shown in humans with NASH, partly independent of weight changes [263,264,265]. There are currently ongoing clinical studies to investigate the potential of liraglutide and semaglutide, GLP-1R agonists, to reduce hepatic inflammation in people with NASH [266, 267]. In obese patients, liraglutide administered daily for 48 weeks improved liver histology and decreased the progression of fibrosis [267]. The exact underlying mechanism remains unclear. Interestingly, GLP-1R has been detected in the endothelium, the coronary arteries, and the smooth muscle cells of the heart [268, 269]. In cardiovascular outcome trials, GLP-1RA reduced the rates of major adverse cardiovascular events (MACEs) and liraglutide administration reduced total mortality, cardiovascular death, and number of myocardial infarctions in patients [270, 271]. It has also been shown that liraglutide improved behavioral profile and induced re-myelination in a mouse model of multiple sclerosis (MS). These effects are proposed to be due to anti-inflammatory, autophagic flux activation, and inflammasome suppression [272]. In an in vivo model for experimental autoimmune encephalitis (EAE), liraglutide could ameliorate the disease score, was able to delay the disease onset and reduce demyelination and inflammation scores in the lumbar spinal cord [273]. These results suggest the anti-inflammatory effects of GLP-1R agonists in the central nervous system and a potential therapy option for patients with MS or autoimmune encephalitis.
As GLP-1R agonists like liraglutide and semaglutide have shown very promising results in clinical studies, the development of dual- and tri-agonists, which also have agonist effects on glucagon-receptors, holds immense promise to improve metabolic outcomes for people with obesity, T2DM or liver diseases [248]. The dual agonist tirzepatide is achieving tremendous weight loss in patients with T2DM, improves blood glucose levels, and reduces hepatic steatosis. Interestingly, tri-agonists also show neuroprotective effects in rodent models of Alzheimer's disease and Parkinson’s disease [241, 274,275,276,277,278,279,280,281].
Conclusion
There has been clear evidence in vitro, in rodents and in humans, that obesity and inflammation are significantly interconnected and effect each other on several metabolic levels. Additionally, targeting inflammation in the adipose tissue or the hypothalamus introduces new possibilities to prevent diet-induced obesity as well as insulin and leptin resistance. In this review, we displayed the important interplay between gut hormones, adipose tissue, and the hypothalamus in regard to inflammation as this is an important pathomechanism in advancing therapy options for obesity. This is of strong importance for pediatric patients because the conversion from impaired glucose tolerance to the development of T2DM does not seem to be a linear process. The progression appears much faster in children and adolescents compared to adults [282]. Additionally, it has been shown that the 20-year survival rate free of liver transplant for children with NAFLD was about 80% compared to 99% in the reference population [283]. There is an urgent need for a deeper understanding of the development of comorbidities and the interplay of different organ systems, hormones, and cytokines, especially in early life stages.
Availability of data and materials
Not applicable.
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- IL-6:
-
Interleukin-6
- TNFa:
-
Tumor necrosis factor-α
- LEPR:
-
Leptin-receptor
- POMC:
-
Proopiomelanocortin
- MSH:
-
Melanocyte-stimulating hormone
- MC4R:
-
Melanocortin-4-receptor
- LEP:
-
Leptin
- LPS:
-
Lipopolysaccharide
- Th:
-
T helper cell
- DSS:
-
Dextran sulfate sodium
- NK cells:
-
Natural killer cells
- INFγ:
-
Interferon-γ
- IgE:
-
Immunoglobulin E
- NO:
-
Nitric oxide
- Groα:
-
Growth-regulated protein α
- NFκB:
-
Nuclear factor “kappa-light-chain-enhancer” of activated B cells
- MC1R:
-
Melanocortin-1-receptor
- SIRS:
-
Systemic inflammatory response syndrome
- FDA:
-
Food and Drug Administration
- EMA:
-
European Medicines Agency
- CCL2:
-
Chemokine C–C motif ligand 2
- CXCL10:
-
C-X-C motif chemokine 10
- CNS:
-
Central nervous system
- UPR:
-
Unfolded protein response pathway
- TLR:
-
Toll-like receptors
- JNK1:
-
Janus kinase 1
- MCP1:
-
Monocyte chemoattractant protein-1
- CVD:
-
Cardiovascular diseases
- VCAM1:
-
Vascular cell adhesion molecule 1
- M1:
-
Macrophage type 1
- HFD:
-
High-fat diet
- ATM:
-
Adipose tissue macrophage
- DIO:
-
Diet-induced obesity
- UCP2:
-
Uncoupling protein 2
- ROS:
-
Reactive oxygen species
- CRP:
-
C-reactive protein
- SOCS3:
-
Suppressor of cytokine signaling 3
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- GLP-1:
-
Glucagon-like peptide 1
- GIPR:
-
GIP receptor
- GLP-1R:
-
GLP-1 receptor
- GLP-1RA:
-
GLP-1 receptor agonists
- NASH:
-
Non-alcoholic steatohepatitis
- MACE:
-
Major adverse cardiovascular event
- MS:
-
Multiple sclerosis
- EAE:
-
Experimental autoimmune encephalitis
- TGFβ:
-
Transforming growth factor β
- IRS-1:
-
Insulin receptor substrate 1
- RHM:
-
Recruited hepatic macrophage
- HSC:
-
Hepatic stellate cells
- NAFLD:
-
Non-alcoholic fatty liver disease
- FFA:
-
Free fatty acid
- AGE:
-
Advanced glycation end product
References
Seki H, Tani Y, Arita M (2009) Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat 89(3–4):126–130
Lafontan M (2005) Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol 45:119–146
Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J et al (2009) Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15(8):921–929
Kanneganti TD, Dixit VD (2012) Immunological complications of obesity. Nat Immunol 13(8):707–712
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808
Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G et al (2011) B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 17(5):610–617
Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F et al (2010) Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 185(3):1836–1845
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15(8):914–920
Milner JJ, Beck MA (2012) The impact of obesity on the immune response to infection. Proc Nutr Soc 71(2):298–306
Yang H, Lang S, Zhai Z, Li L, Kahr WH, Chen P et al (2009) Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood 114(2):425–436
Smith AG, Sheridan PA, Harp JB, Beck MA (2007) Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 137(5):1236–1243
Karlsson EA, Sheridan PA, Beck MA (2010) Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 184(6):3127–3133
Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG (2012) The inflammasome puts obesity in the danger zone. Cell Metab 15(1):10–18
Lamkanfi M, Kanneganti TD (2012) The inflammasome: a remote control for metabolic syndrome. Cell Res 22(7):1095–1098
Farooqi IS, O’Rahilly S (2008) Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4(10):569–577
Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ et al (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387(6636):903–908
Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M (2005) Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol 174(11):6820–6828
Lam QL, Liu S, Cao X, Lu L (2006) Involvement of leptin signaling in the survival and maturation of bone marrow-derived dendritic cells. Eur J Immunol 36(12):3118–3130
Caldefie-Chezet F, Poulin A, Vasson MP (2003) Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res 37(8):809–814
Zhao Y, Sun R, You L, Gao C, Tian Z (2003) Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun 300(2):247–252
Tian Z, Sun R, Wei H, Gao B (2002) Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun 298(3):297–302
Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gomez-Reino JJ et al (2005) Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579(2):295–301
Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ 3rd et al (1997) Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 185(1):171–175
Fraser DA, Thoen J, Reseland JE, Forre O, Kjeldsen-Kragh J (1999) Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol 18(5):394–401
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394(6696):897–901
Lam QL, Lu L (2007) Role of leptin in immunity. Cell Mol Immunol 4(1):1–13
Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C et al (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110(8):1093–1103
Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H et al (1996) The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A 93(16):8374–8378
Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R et al (1995) Identification and expression cloning of a leptin receptor. OB-R Cell 83(7):1263–1271
Guan XM, Hess JF, Yu H, Hey PJ, van der Ploeg LH (1997) Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol 133(1):1–7
Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H et al (1996) Molecular cloning of rat leptin receptor isoform complementary DNAs–identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun 225(1):75–83
Bjorbaek C, Uotani S, da Silva B, Flier JS (1997) Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272(51):32686–32695
Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA et al (1999) Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest 104(8):1051–1059
Chandra RK (1980) Cell-mediated immunity in genetically obese C57BL/6J ob/ob) mice. Am J Clin Nutr 33(1):13–16
Mandel MA, Mahmoud AA (1978) Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol 120(4):1375–1377
Webb SR, Loria RM, Madge GE, Kibrick S (1976) Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J Exp Med 143(5):1239–1248
Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR et al (1999) Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol 276(1):R136–R142
Gruver AL, Sempowski GD (2008) Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol 84(4):915–923
Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD (2006) Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol 177(1):169–176
Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G (2002) Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol 32(2):552–560
Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM (2004) Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol 164(2):385–390
Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W (1996) A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6(9):1170–1180
Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM et al (2007) Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356(3):237–247
Schaible UE, Kaufmann SH (2007) Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4(5):e115
Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW (2005) Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis 64(8):1195–1198
Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM (1996) alpha-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol 59(2):248–253
Brzoska T, Kalden DH, Scholzen T, Luger TA (1999) Molecular basis of the alpha-MSH/IL-1 antagonism. Ann N Y Acad Sci 885:230–238
Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM (1995) Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A 92(17):8016–8020
Raap U, Brzoska T, Sohl S, Path G, Emmel J, Herz U et al (2003) Alpha-melanocyte-stimulating hormone inhibits allergic airway inflammation. J Immunol 171(1):353–359
Rajora N, Boccoli G, Catania A, Lipton JM (1997) alpha-MSH modulates experimental inflammatory bowel disease. Peptides 18(3):381–385
Searle AG (1968) An extension series in the mouse. J Hered 59(6):341–342
Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E et al (1993) Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72(6):827–834
Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC (2006) Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol 126(9):1966–1975
Brzoska T, Luger TA, Maaser C, Abels C, Bohm M (2008) Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev 29(5):581–602
Catania A (2008) Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci 31(7):353–360
Maaser C, Kannengiesser K, Specht C, Lugering A, Brzoska T, Luger TA et al (2006) Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut 55(10):1415–1422
Carter D, Warsen A, Mandell K, Cuschieri J, Maier RV, Arbabi S (2014) Delayed topical p38 MAPK inhibition attenuates full-thickness burn wound inflammatory signaling. J Burn Care Res 35(2):e83-92
Carter DW, Sood RF, Seaton ME, Muffley LA, Honari S, Hocking AM et al (2018) MC1R gene polymorphisms are associated with dysfunctional immune responses and wound infection after burn injury. J Surg Res 231:448–452
Sood RF, Hocking AM, Muffley LA, Ga M, Honari S, Reiner AP et al (2015) Race and melanocortin 1 receptor polymorphism R163Q are associated with post-burn hypertrophic scarring: a prospective cohort study. J Invest Dermatol 135(10):2394–2401
Spana C, Taylor AW, Yee DG, Makhlina M, Yang W, Dodd J (2018) Probing the role of melanocortin type 1 receptor agonists in diverse immunological diseases. Front Pharmacol 9:1535
Dodd J, Jordan R, Makhlina M, Barnett K, Roffel A, Spana C et al (2023) A novel oral formulation of the melanocortin-1 receptor agonist PL8177 resolves inflammation in preclinical studies of inflammatory bowel disease and is gut restricted in rats, dogs, and humans. Front Immunol 14:1083333
Kanti V, Puder L, Jahnke I, Krabusch PM, Kottner J, Vogt A et al (2021) A melanocortin-4 receptor agonist induces skin and hair pigmentation in patients with monogenic mutations in the leptin-melanocortin pathway. Skin Pharmacol Physiol 34(6):307–316
Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C et al (2018) MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med 24(5):551–555
Clement K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H et al (2020) Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol 8(12):960–970
Kamermans A, Verhoeven T, van Het Hof B, Koning JJ, Borghuis L, Witte M et al (2019) Setmelanotide, a novel, selective melanocortin receptor-4 agonist exerts anti-inflammatory actions in astrocytes and promotes an anti-inflammatory macrophage phenotype. Front Immunol 10:2312
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444(7121):860–867
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666
Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N et al (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24(23):10161–10168
Gregor MF, Hotamisligil GS (2007) Thematic review series: adipocyte biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res. 48(9):1905–14
Surmi BK, Hasty AH (2008) Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol 3(5):545–556
Yudkin JS (2003) Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord 27(Suppl 3):S25–S28
Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V (2000) Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148(2):209–214
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K et al (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56(4):901–911
Ye J, Gao Z, Yin J, He Q (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293(4):E1118–E1128
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16(10):1151–1162
Semenza GL (2001) HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol 13(2):167–171
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Invest 121(6):2094–2101
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116(11):3015–3025
Suganami T, Nishida J, Ogawa Y (2005) A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 25(10):2062–2068
Oh DY, Olefsky JM (2012) Omega 3 fatty acids and GPR120. Cell Metab 15(5):564–565
Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E et al (2007) Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92(6):2240–2247
Matsuzawa Y (2006) The metabolic syndrome and adipocytokines. FEBS Lett 580(12):2917–2921
Hopkins TA, Ouchi N, Shibata R, Walsh K (2007) Adiponectin actions in the cardiovascular system. Cardiovasc Res 74(1):11–18
Qiao L, Zou C, van der Westhuyzen DR, Shao J (2008) Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 57(7):1824–1833
Aldhahi W, Hamdy O (2003) Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep 3(4):293–298
Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ (2003) Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278(45):45021–45026
Twarda-Clapa A, Olczak A, Bialkowska AM, Koziolkiewicz M (2022) Advanced Glycation End-Products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 11(8):1312
Kuzan A (2021) Toxicity of advanced glycation end products (Review). Biomed Rep 14(5):46
Psaila AM, Vohralik EJ, Quinlan KGR (2022) Shades of white: new insights into tissue-resident leukocyte heterogeneity. FEBS J 289(2):308–318
Weinstock A, Brown EJ, Garabedian ML, Pena S, Sharma M, Lafaille J et al (2019) Single-cell RNA Sequencing of visceral adipose tissue leukocytes reveals that caloric restriction following obesity promotes the accumulation of a distinct macrophage population with features of phagocytic cells. Immunometabolism 1:e190008
Kwok KH, Lam KS, Xu A (2016) Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med 48(3):e215
Elgazar-Carmon V, Rudich A, Hadad N, Levy R (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 49(9):1894–1903
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32(5):593–604
Sun S, Ji Y, Kersten S, Qi L (2012) Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr 32:261–286
Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S et al (2015) NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol 16(4):376–385
Dungan LS, McGuinness NC, Boon L, Lynch MA, Mills KH (2014) Innate IFN-gamma promotes development of experimental autoimmune encephalomyelitis: a role for NK cells and M1 macrophages. Eur J Immunol 44(10):2903–2917
DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR et al (2013) B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A 110(13):5133–5138
Desai HR, Sivasubramaniyam T, Revelo XS, Schroer SA, Luk CT, Rikkala PR et al (2017) Macrophage JAK2 deficiency protects against high-fat diet-induced inflammation. Sci Rep 7(1):7653
Dror E, Dalmas E, Meier DT, Wueest S, Thevenet J, Thienel C et al (2017) Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol 18(3):283–292
Lee YS, Olefsky J (2021) Chronic tissue inflammation and metabolic disease. Genes Dev 35(5–6):307–328
Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D et al (2011) Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108(37):15324–15329
Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA et al (2010) The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12(6):593–605
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL et al (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17(2):179–188
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG et al (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464(7293):1357–1361
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT et al (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12(5):408–415
Ruscitti P, Masedu F, Alvaro S, Airo P, Battafarano N, Cantarini L et al (2019) Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med 16(9):e1002901
Antohe JL, Bili A, Sartorius JA, Kirchner HL, Morris SJ, Dancea S et al (2012) Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti-tumor necrosis factor alpha therapy. Arthritis Care Res (Hoboken) 64(2):215–221
Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S (2011) Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 305(24):2525–2531
Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J et al (2006) Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol 24(1):83–86
Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ (2007) Improved insulin sensitivity by anti-TNFalpha antibody treatment in patients with rheumatic diseases. Ann Rheum Dis 66(4):558–559
Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA (2005) Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis 64(5):765–766
Marra M, Campanati A, Testa R, Sirolla C, Bonfigli AR, Franceschi C et al (2007) Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol 20(4):731–736
Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H et al (2011) TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 96(1):E146–E150
Timper K, Hruz P, Beglinger C, Donath MY (2013) Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care 36(7):e90–e91
Yazdani-Biuki B, Mueller T, Brezinschek HP, Hermann J, Graninger W, Wascher TC (2006) Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care 29(7):1712–1713
Yazdani-Biuki B, Stelzl H, Brezinschek HP, Hermann J, Mueller T, Krippl P et al (2004) Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur J Clin Invest 34(9):641–642
Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B et al (2007) Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356(15):1517–1526
Cavelti-Weder C, Furrer R, Keller C, Babians-Brunner A, Solinger AM, Gast H et al (2011) Inhibition of IL-1beta improves fatigue in type 2 diabetes. Diabetes Care 34(10):e158
Rissanen A, Howard CP, Botha J, Thuren T, Global I (2012) Effect of anti-IL-1beta antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab 14(12):1088–1096
Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A et al (2013) Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care 36(8):2239–2246
Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N et al (2003) Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 42(1):76–81
Decochez K, Rippley RK, Miller JL, De Smet M, Yan KX, Matthijs Z et al (2006) A dual PPAR alpha/gamma agonist increases adiponectin and improves plasma lipid profiles in healthy subjects. Drugs R D 7(2):99–110
Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF et al (2008) Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 57(6):1526–1535
Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L et al (2003) Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52(3):667–674
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7(8):941–946
Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N et al (2002) Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 277(40):37487–91
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H et al (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17(1):4–12
Goldstein BJ, Scalia R (2004) Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab 89(6):2563–2568
Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M et al (2004) Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart 90(5):528–533
Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K et al (2006) Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes 55(6):1840–1846
Harmancey R, Wilson CR, Taegtmeyer H (2008) Adaptation and maladaptation of the heart in obesity. Hypertension 52(2):181–187
De Rosa A, Monaco ML, Capasso M, Forestieri P, Pilone V, Nardelli C et al (2013) Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur J Endocrinol 169(1):37–43
Papatriantafyllou M (2011) Mucosal immunology: inflammasome shapes the microbiota. Nat Rev Immunol 11(7):439
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al (2005) Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54(8):2277–2286
Kim KA, Gu W, Lee IA, Joh EH, Kim DH (2012) High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7(10):e47713
Tilg H, Zmora N, Adolph TE, Elinav E (2020) The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol 20(1):40–54
Kreutzer C, Peters S, Schulte DM, Fangmann D, Turk K, Wolff S et al (2017) Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 66(9):2407–2415
Fouesnard M, Zoppi J, Petera M, Le Gleau L, Migne C, Devime F et al (2021) Dietary switch to Western diet induces hypothalamic adaptation associated with gut microbiota dysbiosis in rats. Int J Obes (Lond) 45(6):1271–1283
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596
Kim DW, Glendining KA, Grattan DR, Jasoni CL (2016) Maternal obesity in the mouse compromises the blood-brain barrier in the arcuate nucleus of offspring. Endocrinology 157(6):2229–2242
Van Dyken P, Lacoste B (2018) Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci 12:930
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M et al (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 6(263):263ra158
Tang W, Zhu H, Feng Y, Guo R, Wan D (2020) The impact of gut microbiota disorders on the blood-brain barrier. Infect Drug Resist 13:3351–3363
Chen KE, Lainez NM, Nair MG, Coss D (2021) Visceral adipose tissue imparts peripheral macrophage influx into the hypothalamus. J Neuroinflammation 18(1):140
Valdearcos M, Douglass JD, Robblee MM, Dorfman MD, Stifler DR, Bennett ML et al (2017) Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 26(1):185–97.e3
Andre C, Guzman-Quevedo O, Rey C, Remus-Borel J, Clark S, Castellanos-Jankiewicz A et al (2017) Inhibiting microglia expansion prevents diet-induced hypothalamic and peripheral inflammation. Diabetes 66(4):908–919
Mendes NF, Kim YB, Velloso LA, Araujo EP (2018) Hypothalamic microglial activation in obesity: a mini-review. Front Neurosci 12:846
Rahman MH, Kim MS, Lee IK, Yu R, Suk K (2018) Interglial crosstalk in obesity-induced hypothalamic inflammation. Front Neurosci 12:939
Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK (2014) Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9(6):2124–2138
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1):153–162
Kim JD, Yoon NA, Jin S, Diano S (2019) Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metab. 30(5):952–62.e5
De Simone R, Ajmone-Cat MA, Pandolfi M, Bernardo A, De Nuccio C, Minghetti L et al (2015) The mitochondrial uncoupling protein-2 is a master regulator of both M1 and M2 microglial responses. J Neurochem 135(1):147–156
Arsenijevic D, Clavel S, Sanchis D, Plamondon J, Huang Q, Ricquier D et al (2007) Induction of Ucp2 expression in brain phagocytes and neurons following murine toxoplasmosis: an essential role of IFN-gamma and an association with negative energy balance. J Neuroimmunol 186(1–2):121–132
Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY et al (2007) Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449(7159):228–232
Diano S, Horvath TL (2012) Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med 18(1):52–58
Affourtit C, Crichton PG, Parker N, Brand MD (2007) Novel uncoupling proteins. Novartis Found Symp. 287:70–80. Discussion -91
Echtay KS (2007) Mitochondrial uncoupling proteins–what is their physiological role? Free Radic Biol Med 43(10):1351–1371
Krauss S, Zhang CY, Lowell BB (2005) The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol 6(3):248–261
Alan L, Smolkova K, Kronusova E, Santorova J, Jezek P (2009) Absolute levels of transcripts for mitochondrial uncoupling proteins UCP2, UCP3, UCP4, and UCP5 show different patterns in rat and mice tissues. J Bioenerg Biomembr 41(1):71–78
Andrews ZB, Diano S, Horvath TL (2005) Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci 6(11):829–840
Le Fur S, Le Stunff C, Dos Santos C, Bougneres P (2004) The common -866 G/A polymorphism in the promoter of uncoupling protein 2 is associated with increased carbohydrate and decreased lipid oxidation in juvenile obesity. Diabetes 53(1):235–239
Bulotta A, Ludovico O, Coco A, Di Paola R, Quattrone A, Carella M et al (2005) The common -866G/A polymorphism in the promoter region of the UCP-2 gene is associated with reduced risk of type 2 diabetes in Caucasians from Italy. J Clin Endocrinol Metab 90(2):1176–1180
Su M, Chen X, Chen Y, Wang C, Li S, Ying X et al (2018) UCP2 and UCP3 variants and gene-environment interaction associated with prediabetes and T2DM in a rural population: a case control study in China. BMC Med Genet 19(1):43
Mehta SL, Li PA (2009) Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J Cereb Blood Flow Metab 29(6):1069–1078
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC et al (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146(10):4192–4199
Jais A, Bruning JC (2017) Hypothalamic inflammation in obesity and metabolic disease. J Clin Invest 127(1):24–32
Ohta S, Misawa A, Lefebvre V, Okano H, Kawakami Y, Toda M (2013) Sox6 up-regulation by macrophage migration inhibitory factor promotes survival and maintenance of mouse neural stem/progenitor cells. PLoS One 8(9):e74315
Wegner M, Stolt CC (2005) From stem cells to neurons and glia: a Soxist’s view of neural development. Trends Neurosci 28(11):583–588
Zhang J, Jiao J (2015) Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int 2015:727542
Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T (2009) Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res 200(1):192–196
Gomes da Silva S, Simoes PS, Mortara RA, Scorza FA, Cavalheiro EA, da Graca Naffah-Mazzacoratti M et al (2013) Exercise-induced hippocampal anti-inflammatory response in aged rats. J Neuroinflammation. 10:61
Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J et al (2010) IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 8(8):e100046
Silva VRR, Micheletti TO, Katashima CK, Lenhare L, Morari J, Moura-Assis A et al (2018) Exercise activates the hypothalamic S1PR1-STAT3 axis through the central action of interleukin 6 in mice. J Cell Physiol 233(12):9426–9436
Bobbo VC, Engel DF, Jara CP, Mendes NF, Haddad-Tovolli R, Prado TP et al (2021) Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J Neuroinflammation 18(1):192
Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS (1999) The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274(42):30059–30065
Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1(4):619–625
Wunderlich CM, Hovelmeyer N, Wunderlich FT (2013) Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAKSTAT 2(2):e23878
Yang Z, Hulver M, McMillan RP, Cai L, Kershaw EE, Yu L et al (2012) Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS One 7(10):e47493
Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J et al (2014) Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab 3(6):608–618
Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V (2015) Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 63(5):1272–1284
Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H et al (2020) Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity. 52(6):1057–74.e7
Loomba R, Gindin Y, Jiang Z, Lawitz E, Caldwell S, Djedjos CS et al (2018) DNA methylation signatures reflect aging in patients with nonalcoholic steatohepatitis. JCI Insight. 3(2):e96685
Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184(10):2537–2564
Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD et al (2003) Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125(2):437–443
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8):1038–1048
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ et al (2015) Nonalcoholic fatty liver disease. Nat Rev Dis Primers 1:15080
Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B (2013) Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58(4):1497–1507
Cusi K (2012) Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 142(4):711–25.e6
Schnabl B, Brenner DA (2014) Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146(6):1513–1524
Potter JJ, Rennie-Tankesley L, Mezey E (2003) Influence of leptin in the development of hepatic fibrosis produced in mice by Schistosoma mansoni infection and by chronic carbon tetrachloride administration. J Hepatol 38(3):281–288
Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM et al (2009) Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 137(2):713–723
Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ et al (2002) Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology 122(5):1399–1410
De Minicis S, Seki E, Oesterreicher C, Schnabl B, Schwabe RF, Brenner DA (2008) Reduced nicotinamide adenine dinucleotide phosphate oxidase mediates fibrotic and inflammatory effects of leptin on hepatic stellate cells. Hepatology 48(6):2016–2026
Adachi M, Brenner DA (2008) High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47(2):677–685
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112(1):91–100
Xu H, Zhao Q, Song N, Yan Z, Lin R, Wu S et al (2020) AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat Commun 11(1):5807
Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G et al (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2(52):52ra72
Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L et al (2016) Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials 47:356–365
Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA et al (2009) CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest 119(7):1858–1870
Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N et al (2009) CCR2 promotes hepatic fibrosis in mice. Hepatology 50(1):185–197
Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V et al (2009) Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol 174(5):1766–1775
Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E (2012) Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 302(11):G1310–G1321
Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H et al (2010) Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest 120(11):4129–4140
Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S et al (2013) Transforming growth factor beta neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. PLoS One 8(1):e54499
Lacouture ME, Morris JC, Lawrence DP, Tan AR, Olencki TE, Shapiro GI et al (2015) Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor beta by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol Immunother 64(4):437–446
Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M et al (2014) Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 9(3):e90353
Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H et al (2011) A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79(11):1236–1243
Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G et al (1999) Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology 29(6):1743–1751
Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M et al (2007) Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology 132(1):282–293
Liang T, Zhang Q, Sun W, Xin Y, Zhang Z, Tan Y et al (2015) Zinc treatment prevents type 1 diabetes-induced hepatic oxidative damage, endoplasmic reticulum stress, and cell death, and even prevents possible steatohepatitis in the OVE26 mouse model: Important role of metallothionein. Toxicol Lett 233(2):114–124
Masarone M, Rosato V, Aglitti A, Bucci T, Caruso R, Salvatore T et al (2017) Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS One 12(6):e0178473
Londos C, Honnor RC, Dhillon GS (1985) cAMP-dependent protein kinase and lipolysis in rat adipocytes. III. Multiple modes of insulin regulation of lipolysis and regulation of insulin responses by adenylate cyclase regulators. J Biol Chem 260(28):15139–45
Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P et al (2005) Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes 54(12):3458–3465
Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J et al (2014) Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep 4:5832
Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A et al (2001) Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34(6):1158–1163
Coulon S, Francque S, Colle I, Verrijken A, Blomme B, Heindryckx F et al (2012) Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine 59(2):442–449
Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ et al (2008) A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322(5907):1539–1543
Czaja MJ (2010) JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab 21(12):707–713
Nadeau KJ, Klingensmith G, Zeitler P (2005) Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr 41(1):94–98
Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X et al (2006) Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 91(11):4287–4294
Sheldon RD, Kanosky KM, Wells KD, Miles L, Perfield JW 2nd, Xanthakos S et al (2016) Transcriptomic differences in intra-abdominal adipose tissue in extremely obese adolescents with different stages of NAFLD. Physiol Genomics 48(12):897–911
Joo E, Harada N, Yamane S, Fukushima T, Taura D, Iwasaki K et al (2017) Inhibition of gastric inhibitory polypeptide receptor signaling in adipose tissue reduces insulin resistance and hepatic steatosis in high-fat diet-fed mice. Diabetes 66(4):868–879
Nie Y, Ma RC, Chan JC, Xu H, Xu G (2012) Glucose-dependent insulinotropic peptide impairs insulin signaling via inducing adipocyte inflammation in glucose-dependent insulinotropic peptide receptor-overexpressing adipocytes. FASEB J 26(6):2383–2393
Timper K, Grisouard J, Sauter NS, Herzog-Radimerski T, Dembinski K, Peterli R et al (2013) Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am J Physiol Endocrinol Metab 304(1):E1-13
Chen S, Okahara F, Osaki N, Shimotoyodome A (2015) Increased GIP signaling induces adipose inflammation via a HIF-1alpha-dependent pathway and impairs insulin sensitivity in mice. Am J Physiol Endocrinol Metab 308(5):E414–E425
Gogebakan O, Osterhoff MA, Schuler R, Pivovarova O, Kruse M, Seltmann AC et al (2015) GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: a randomised trial. Diabetologia 58(8):1759–1768
Beaudry JL, Kaur KD, Varin EM, Baggio LL, Cao X, Mulvihill EE et al (2019) Physiological roles of the GIP receptor in murine brown adipose tissue. Mol Metab 28:14–25
Fu Y, Kaneko K, Lin HY, Mo Q, Xu Y, Suganami T et al (2020) Gut hormone GIP induces inflammation and insulin resistance in the hypothalamus. Endocrinology. 161(9):bqaa102
Kaneko K, Fu Y, Lin HY, Cordonier EL, Mo Q, Gao Y et al (2019) Gut-derived GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. J Clin Invest 129(9):3786–3791
Campbell JE, Drucker DJ (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17(6):819–837
Finan B, Muller TD, Clemmensen C, Perez-Tilve D, DiMarchi RD, Tschop MH (2016) Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med 22(5):359–376
Kim SJ, Nian C, Karunakaran S, Clee SM, Isales CM, McIntosh CH (2012) GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One 7(7):e40156
Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H et al (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8(7):738–742
Bates HE, Campbell JE, Ussher JR, Baggio LL, Maida A, Seino Y et al (2012) Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr(-/-) mice. Diabetes 61(1):40–48
Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y et al (2007) Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 117(1):143–152
Nasteska D, Harada N, Suzuki K, Yamane S, Hamasaki A, Joo E et al (2014) Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 63(7):2332–2343
Campbell JE, Ussher JR, Mulvihill EE, Kolic J, Baggio LL, Cao X et al (2016) TCF1 links GIPR signaling to the control of beta cell function and survival. Nat Med 22(1):84–90
Althage MC, Ford EL, Wang S, Tso P, Polonsky KS, Wice BM (2008) Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J Biol Chem 283(26):18365–18376
Killion EA, Wang J, Yie J, Shi SD, Bates D, Min X et al (2018) Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci Transl Med. 10(472):eaat3392
Mantelmacher FD, Fishman S, Cohen K, Pasmanik Chor M, Yamada Y, Zvibel I et al (2017) Glucose-dependent insulinotropic polypeptide receptor deficiency leads to impaired bone marrow hematopoiesis. J Immunol 198(8):3089–3098
Pujadas G, Varin EM, Baggio LL, Mulvihill EE, Bang KWA, Koehler JA et al (2020) The gut hormone receptor GIPR links energy availability to the control of hematopoiesis. Mol Metab 39:101008
Ravn P, Madhurantakam C, Kunze S, Matthews E, Priest C, O’Brien S et al (2013) Structural and pharmacological characterization of novel potent and selective monoclonal antibody antagonists of glucose-dependent insulinotropic polypeptide receptor. J Biol Chem 288(27):19760–19772
Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y et al (1999) Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 96(26):14843–14847
Goralska J, Razny U, Polus A, Stancel-Mozwillo J, Chojnacka M, Gruca A et al (2018) Pro-inflammatory gene expression profile in obese adults with high plasma GIP levels. Int J Obes (Lond) 42(4):826–834
Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL et al (2018) Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 61(2):284–294
Hammoud R, Drucker DJ (2023) Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol 19(4):201–216
Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A 84(10):3434–3438
Arakawa M, Ebato C, Mita T, Hirose T, Kawamori R, Fujitani Y et al (2009) Effects of exendin-4 on glucose tolerance, insulin secretion, and beta-cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem Biophys Res Commun 390(3):809–814
Kawamori D, Shirakawa J, Liew CW, Hu J, Morioka T, Duttaroy A et al (2017) GLP-1 signalling compensates for impaired insulin signalling in regulating beta cell proliferation in betaIRKO mice. Diabetologia 60(8):1442–1453
Park S, Dong X, Fisher TL, Dunn S, Omer AK, Weir G et al (2006) Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J Biol Chem 281(2):1159–1168
Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M (2004) Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 47(5):806–815
Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT et al (2011) Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17(11):1481–1489
Lebrun LJ, Lenaerts K, Kiers D, Pais de Barros JP, Le Guern N, Plesnik J et al (2017) Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 21(5):1160–8
Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F (2014) Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep 9(4):1202–1208
Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J et al (2016) Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 23(2):324–34
Brakenridge SC, Moore FA, Mercier NR, Cox M, Wu Q, Moldawer LL et al (2019) Persistently elesvated glucagon-like peptide-1 levels among critically Ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J Am Coll Surg. 229(1):58-67.e1
Lebherz C, Schlieper G, Mollmann J, Kahles F, Schwarz M, Brunsing J et al (2017) GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am J Med. 130(7):833–41.e3
Wong CK, Yusta B, Koehler JA, Baggio LL, McLean BA, Matthews D et al (2022) Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 34(10):1514–31.e7
Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK et al (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127(1):74–85
Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756
Trevaskis JL, Griffin PS, Wittmer C, Neuschwander-Tetri BA, Brunt EM, Dolman CS et al (2012) Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol 302(8):G762–G772
Somm E, Montandon SA, Loizides-Mangold U, Gaia N, Lazarevic V, De Vito C et al (2021) The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl Res 227:75–88
McLean BA, Wong CK, Kaur KD, Seeley RJ, Drucker DJ (2021) Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight 6(22):e153732
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V et al (2021) A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 384(12):1113–1124
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387(10019):679–690
Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B et al (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287(6):E1209–E1215
Wei Y, Mojsov S (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358(3):219–224
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA et al (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375(19):1834–1844
Ammar RA, Mohamed AF, Kamal MM, Safar MM, Abdelkader NF (2022) Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: role of AMPK/SIRT1 signaling and NLRP3 inflammasome. Inflammopharmacology 30(3):919–934
Song S, Guo R, Mehmood A, Zhang L, Yin B, Yuan C et al (2022) Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci Ther 28(3):422–434
Svendsen B, Capozzi ME, Nui J, Hannou SA, Finan B, Naylor J et al (2020) Pharmacological antagonism of the incretin system protects against diet-induced obesity. Mol Metab 32:44–55
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193
Finan B, Ma T, Ottaway N, Muller TD, Habegger KM, Heppner KM et al (2013) Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 5(209):209ra151
Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB et al (2018) LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab 18:3–14
Mroz PA, Finan B, Gelfanov V, Yang B, Tschop MH, DiMarchi RD et al (2019) Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab 20:51–62
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B et al (2022) Tirzepatide once weekly for the treatment of obesity. N Engl J Med 387(3):205–216
Gastaldelli A, Cusi K, Fernandez Lando L, Bray R, Brouwers B, Rodriguez A (2022) Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 10(6):393–406
Li T, Jiao JJ, Su Q, Holscher C, Zhang J, Yan XD et al (2020) A GLP-1/GIP/Gcg receptor triagonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca(2+) homeostasis in 3xTg-AD mice. Neuropharmacology 170:108042
Kleber M, Lass N, Papcke S, Wabitsch M, Reinehr T (2010) One-year follow-up of untreated obese white children and adolescents with impaired glucose tolerance: high conversion rate to normal glucose tolerance. Diabet Med 27(5):516–521
Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P (2009) The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 58(11):1538–1544
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. LR is supported by the BIH Charité Junior Clinician Scientist Program, PK is supported by Germany Research Foundation (DFG) KU 2673/6–1, KU 2673/7–1, CRC1423/B02 (PK), CRC/ TR 296/ P04 and ERC_CoG_101043991 (E-VarEndo).
Author information
Authors and Affiliations
Contributions
LR wrote the first manuscript version. SW and PK performed editing of the manuscript. All authors read and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruck, L., Wiegand, S. & Kühnen, P. Relevance and consequence of chronic inflammation for obesity development. Mol Cell Pediatr 10, 16 (2023). https://doi.org/10.1186/s40348-023-00170-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-023-00170-6