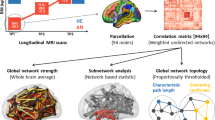
Abstract
Background
Anorexia nervosa (AN) is a disorder characterized by an incapacitating fear of weight gain and by a disturbance in the way the body is experienced, facets that motivate dangerous weight loss behaviors. Multimodal neuroimaging studies highlight atypical neural activity in brain networks involved in interoceptive awareness and reward processing.
Methods
The current study used resting-state neuroimaging to model the architecture of large-scale functional brain networks and characterize network properties of individual brain regions to clinical measures. Resting-state neuroimaging was conducted in 62 adolescents, 22 (21 female) with a history of AN and 40 (39 female) healthy controls (HCs). Sensorimotor and basal ganglia regions, as part of a 165-region whole-brain network, were investigated. Subject-specific functional brain networks were computed to index centrality. A contrast analysis within the general linear model covarying for age was performed. Correlations between network properties and behavioral measures were conducted (significance q < .05).
Results
Compared to HCs, AN had lower connectivity from sensorimotor regions, and greater connectivity from the left caudate nucleus to the right postcentral gyrus. AN demonstrated lower sensorimotor centrality, but higher basal ganglia centrality. Sensorimotor connectivity dyads and centrality exhibited negative correlations with body dissatisfaction and drive for thinness, two essential features of AN.
Conclusions
These findings suggest that AN is associated with greater communication from the basal ganglia, and lower information propagation in sensorimotor cortices. This is consistent with the clinical presentation of AN, where individuals exhibit patterns of rigid habitual behavior that is not responsive to bodily needs, and seem “disconnected” from their bodies.
Plain English summary
Individuals with anorexia nervosa (AN) usually report a fear of gaining weight. They often develop a dislike and distrust of their bodies, feeling that their bodies had somehow let them down. These fears can in turn lead to dangerous weight loss behaviors. Magnetic resonance imaging of the brain is a tool that helps highlight the underlying biological processes associated with AN. In the current study we aim to investigate how the connections in key regions of the brain are related to clinical and behavioral factors associated with AN. We found regions of two main networks were associated with body dissatisfaction and drive for thinness, which are key features of AN. The brain regions involved help explain why patients with AN have characteristics of feeling disconnected from their bodies, having difficulty labeling and regulating emotions, responding to biological needs such as hunger and fatigue, and differentiating experiences that will be rewarding. These results can help guide interventions that will be directed towards helping individuals with AN to better sense, decipher, and act on the various signals being communicated by their body.
Similar content being viewed by others
Introduction
Central to the disorder of anorexia nervosa (AN) is severe weight loss and persistent low-body weight that radically alters typical trajectories of adolescent social and emotional development [1]. Adolescent girls and young adult women are at the greatest risk for AN [2], with evidence indicating that the incidence of AN is emerging at younger ages than prior generations [3]. Fear of weight gain is the diagnostic feature intended to encapsulate the motivation for the intractable drive to lose weight in AN [4]. Compounding this fear is a diagnostic feature described as disturbance in the experience of the body, a feature proposed to be constituted of difficulties with the adaptive integration of interoceptive [5, 6] or proprioceptive [7] cues or with problems of multi-sensory integration more broadly [8]. Critically, body image disturbance in AN is a particularly intractable feature, one that have been found to persist following weight restoration. Advances in our understanding of neural circuit function, particularly in those circuits implicated in the experience of the body, such as the sensorimotor cortex and basal ganglia, may help us to understand the constituents of body image experience and the relentless drive for thinness in AN across the spectrum of weight loss and restoration.
Structural and functional alterations in the sensorimotor network have been consistently reported in patients with AN [9] and have been associated with visuomotor disturbances [10] and deficits in spatial organization [11]. Compared to healthy controls (HCs), individuals with AN have lower gray matter volume in the sensorimotor network [12]. Using a region-of-interest-based approach, it has been shown that individuals with AN have lower functional connectivity between the sensorimotor and visual networks, suggesting that altered visuospatial processing may be related to body image disturbances [10]. Using a network-based statistic approach, it has been shown that a sub-network of regions including the thalamus and posterior insula had lower connectivity compared to HCs, indicating that sensory information may be propagated unreliably or inefficiently [13]. Finally, a seed-based approach found abnormal thalamocortical connectivity that was associated with deficits in cognitive-control tasks in AN [14], revealing potential neurobiological mechanisms underlying cognitive function. When viewed together, such findings may help to explain the discrepancy between individuals’ actual and perceived body state.
Dysregulation in serotonin and dopamine circuits in the basal ganglia may play a role in the hyperactivity of motivational systems - which (may) contribute to the desire to suppress appetitive systems associated with symptoms of AN [15,16,17,18,19]. Increased resting state functional connectivity between the nucleus accumbens and orbital frontal gyrus, and increased structural connectivity, measured via diffusion tensor imaging, has been associated with elevated scores on a self-report measure of eating disorder symptoms in individuals with AN aged 16 to 25 years old [20]. Greater ventral striatal activity in individuals with AN has been observed in response to visual stimuli of under-weight vs normal weight individuals compared to HCs and vise-versa [21], supporting the rewarding value of visual stimuli related to starvation. Similar findings have also been reported in adolescents [22], a response hypothesized to reflect maladaptive conditioning: the reinforcing value of cues associated with food restriction.
Despite the growing understanding of the specific involvement of brain networks in AN, most studies have focused on individual brain regions, and a deeper understanding of the underlying properties and architecture of key brain networks is not fully understood. Graph theory has been used to characterize brain regions, their connections, and the integrity and information flow of brain networks. Measures of centrality are common measures of global connectivity that participate in integrative processing and associated behavioral responses [23,24,25]. Regions with high centrality are influential in communicating and facilitating flow of information with other regions in the brain network and have greater resilience to insult [23]. As centrality accounts for the relationship of the brain region’s properties with the entire functional connectome, brain regions with greater functional centrality indicate a greater amount of influence on the functional performance of the network [25]. Some recent work has been done investigating the structural [26] and functional [27,28,29,30] connectomes using graph theory approaches, and a recent review has shown nodal topological differences in brain regions including the insula, thalamus, basal ganglia, posterior occipital cortex, prefrontal cortex, inferior frontal gyrus, and precentral gyrus have been altered [31].
The aim of the current study was to quantify differences in resting-state functional connectivity and centrality in the sensorimotor and basal ganglia networks between individuals with anorexia nervosa (AN) and HCs. We aimed to test the hypothesis that individuals with AN demonstrate lower connectivity and centrality in core regions of sensorimotor networks, and greater connectivity in the basal ganglia networks compared to HCs, and that these differences would be associated with differences in eating disorder symptoms. Specifically, we hypothesized that lower connectivity and centrality in sensorimotor networks would be associated with increased body dissatisfaction, an evaluative measure of body image disturbance. We further hypothesized that greater connectivity and centrality in the basal ganglia networks would be associated with greater drive for thinness, an index of the valence and intensity of threats to weight loss.
Methods and materials
Overview
Adolescents between the ages of 10–20 years old were recruited for a study of “gut feelings”. Recruited individuals participated in a laboratory session and a resting-state fMRI scan as a part of a larger study (reported elsewhere, see [32] and for details about these MRI tasks) [33]. Here we focus on the functional resting-state connectivity and functional network architecture from these scans.
Recruitment
We aimed to recruit all individuals with AN who presented to a specialized outpatient clinic for the treatment of eating disorders at a Southeastern academic medical center. The control sample was recruited from this same medical clinic. Additional recruitment of both the clinical and HC samples was conducted throughout the university associated with this medical center. All participants under the age of 18 had informed consent from a parent and/or legal guardian.
Healthy control recruitment
For our control sample, we recruited from a pediatric primary care practice that was part of a Southeastern academic medical center (see Franz et al. [34] for general screening strategy adapted for this study). The demographic composition of this practice paralleled that of the surrounding county, and thus helped to facilitate the recruitment of a representative control group. Screening occurred on random weekdays.
Clinical recruitment
Clinical participants were also recruited within that medical practice. Recruiters attended every clinic session of pediatricians who were part of a specialized outpatient eating disorder program from the period of 9/1/2009–8/31/2011 to identify and screen all eligible AN participants, whether they currently met criteria for AN or had a history of AN and were attending a medical follow-up appointment.
Inclusion criteria
Study participants were required to have a current or prior diagnosis of AN consistent with symptoms delineated in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (13). Medications were permitted provided the individual was on a stable dose for a period \(\ge\) 3 months. See Additional file 1: Table S1 for medication list.
Exclusion criteria
Adolescents were excluded if either they or their mother did not have fluency in English, had an IQ < 70, failed to meet MRI safety requirements [35,36,37], were suicidal, exhibited symptoms of psychosis, or actively abused substances. In addition, healthy control participants could not have a history of an eating disorder or currently meet criteria for a psychiatric diagnosis as determined by screening for current symptoms (see below) and parent and participant report.
Procedures
Overview
Adolescents and their parents attended an initial laboratory session during which diagnostic information was obtained. The adolescent participated in a mock scanning session to familiarize themselves with the scanning environment and to obtain training in procedures that would maximize the amount of usable scanning data (e.g., teaching to minimize movement). Height and weight were obtained at the time of scanning. Individuals on medications with short half-lives (e.g., stimulant medication) were asked to refrain from taking medication on the day of scanning.
Consent
Written informed consent was obtained from parents and participants above the age of 18, assent was obtained from participants from age 10 up to 18 years. The study was approved by the Institutional Review Board at Duke University Medical Center, and all methods were carried out in accordance with relevant guidelines and regulations from the Declaration of Helsinki.
Assessments
Screening
HC participants were screened for the absence of mental health symptoms using questions used to predict diagnostic status from a prior population cohort study of child and adolescent’s psychopathology [34]. Children who scored above the screen cut-off were excluded from further participation but were given a small prize.
Determination of diagnosis and diagnostic history
We attempted to recruit all individuals with a history of AN who presented at an outpatient medical clinic. These individuals were at various stages of the disorder in terms of degree of weight severity or restoration, and thus the sample had significant heterogeneity. While this had the disadvantage of preventing comparisons between categorical stages of the disorder, it had the advantage of high external validity in that this group reflected individuals presenting for care. We describe individuals dimensionally and categorically. For both, diagnosis and parameters of illness history were determined by systematically combining several sources of data: 1) maternal report of her child’s illness history; 2) adolescent completion of self-report measures of current symptoms; 3) adolescent report of illness history; and 4) medical chart abstraction. This included both BMI and zBMI (i.e., age-adjusted BMI, which accounts for height, weight, and age) and age-adjusted weight percentile. To be classified as a HC, 1) parent report indicated no history of an eating disorder; 2) adolescent self-report of Drive for Thinness values were within 1 standard deviation of normative values; and 3) the medical chart contained no reference to an eating disorder diagnosis.
For the clinical group, we employed the ENIGMA Eating Disorders consortium definitions of weight status to define individuals with AN that were currently ill or partially weight-restored. These definitions were complemented with parent report of disorder history and self-reports of Drive for Thinness as described below. The ENIGMA consortium (http://enigma.ini.usc.edu/about-2/) is an international effort combining data across research sites to accelerate the study of health and disease across development. ENIGMA definitions of weight status are employed in this manuscript for ease of comparison across studies. To these definitions of weight status, we added benchmarks for scores on eating disorder measures as defined below. Acute AN (AN) is a BMI of ≤ 17.5 kg/m2, < 10th for weight according to age-adjusted weight-percentile, and not in a period of rapid weight gain (< 2 kg. over the past 4 weeks). This weight definition was complemented with the following definitions for AN in this study: 1) parent records indicated the child had AN within past 3–6 months; 2) medical chart had a diagnosis of AN; and 3) the adolescent had a Drive for Thinness score > 1 std above normative values. For partially weight-restored AN (ANp), according to ENIGMA: participant does not meet criteria for acute AN and either: A) BMI is < 18.5 kg/m2 or < 10th adjusted percentile; or B) BMI is > 18.5 kg/m2 but < 19.5 kg/m2, age-adjusted percentile is > 10th but < 25th, participant must not have regular menses, and still show significant eating disorder symptoms as defined in this study as > 1 standard deviation of Drive for Thinness Normative Values. In this study, weight-restored AN (ANwr), was defined as: 1) BMI ≥ 18.5 or the parent report indicated that the child was without an eating disorder for 3–6 months; 2) the medical chart review did not contain a current diagnosis of AN and 3) there was no evidence of a medical sign that weight was low (e.g., bradycardia, orthostatic hypotension). To determine length of illness, mothers were asked the age at which their child first developed an eating disorder, the type of eating disorder, and whether this diagnosis was verified by a health care professional. This information was compared and combined with the medical record and referenced against the child’s weight history, current weight, and current symptom endorsement. In only one case was there a discrepancy. In this case, the parent indicated that the child no longer had an eating disorder and had been at a healthy weight for 3–6 months. However, the child’s weight and endorsement of clinical symptoms were both above clinical cut-offs. Of interest, this child had a long duration of illness (> 7 years) and a lowest BMI of 11. Her current BMI of 18, may have seemed to present as significant progress (as it was), yet an anchor of normality had been lost.
A similar strategy was employed to determine months of weight restoration. Parents were asked the length of time the child had been at a healthy weight and this was verified relative to the child’s weight history and medical record. Again, there was one discrepancy, noted below.
Self-report measures
The Eating Disorder Inventory (3rd Edition) is one of the most widely used measures of eating disorder symptomatology and associated features [38]. This measure was used to characterize the sample relative to other studies and provide a continuous index of current symptoms. Three subscales that measure the core pathology of eating disorders were administered in the current sample: Drive for Thinness, Bulimia, and Body Dissatisfaction. All scales have extensive validity and reliability information as well as normative data from clinical and non-clinical samples. The Drive for Thinness subscale is a 7-item scale that assesses “an extreme desire to be thinner, preoccupation with weight, and an intense fear of weight gain”. Extensive reliability, construct, and predictive validity have been established [39,40,41]. The internal consistency of this scale was measured via Cronbach’s alpha, which is a measure of internal consistency (between 0 and 1), or how closely related a set of items are as a group. High values indicate high reliability [42]. Cronbach’s alpha for the Drive for Thinness subscale in our sample was α = 0.95. The Bulimia subscale is an 8-item scale used to index the tendency to think about or engage in uncontrollable overeating or eating in response to emotions. The internal consistency in our sample was α = 0.89. The Body Dissatisfaction subscale is a 7-item scale that assesses discontentment with the size and shape of various body parts that are of particular concern to those with eating disorders (e.g., stomach). The internal consistency in our sample was α = 0.94. We also looked at the Perfectionism subscale of the EDI, a 6-item scale that evaluates the personal value that individuals place on personal achievement and meeting their own high standards. The internal consistency in our sample was α = 0.91.
MRI acquisition and quality control
Whole brain structural and functional (resting state) data was acquired using MRI scans conducted on a 3 Tesla General Electric MR 750 system with 50-mT/m gradients and an 8-channel head coil for parallel imaging (General Electric, Waukesha,WI, USA). Noise reducing headphones were used. To control for the state of acute nourishment on brain activity parameters [43], individuals were asked to fast for 2 h prior to the scan and then were asked to consume a small, standardized snack just prior to the scan [43]. Twelve of the participants were on medication the day of scanning.
Structural gray-matter
For registration purposes, a high-resolution structural image was obtained from each subject using a magnetization-prepared rapid acquisition gradient-echo sequence in the axial plane (Ax FSPGR BRAVO, repetition time = 7.58 ms, structural acquisition time = 3 min 22 s, echo time = 2.936 ms, inversion time = 450 ms, flip angle = 12°, slice thickness = 1 mm, 256 slices, 256 × 256 voxel matrix, 1 mm voxel size).
Resting state functional connectivity
Resting state fMRI data was acquired using the following parameters: (34-slice, 150 whole brain volumes, interleaved slices, slice thickness = 3.8 mm, TE: 30 ms, TR 2000 ms, resting-duration (TA) = flip angle = 70°, acquisition matrix = 64 × 128, field of view = 243 mm × 243 mm). Subjects rested with eyes open and instructed to fixate on a cross while functional blood oxygen-level dependent images were acquired.
MRI processing
Resting state functional connectivity pre-processing
Resting state preprocessing was conducted using SPM12 software (Welcome Department of Cognitive Neurology, London, UK). The first two volumes were discarded to allow for stabilization of the magnetic field. Slice timing correction was performed first, followed by rigid six-degree motion-correction realignment. The motion correction parameters in each degree were examined for excessive motion. If any volume-to-volume motion correction parameter was above 2 mm translation or 2° rotation, it was excluded from the dataset. To robustly take account of the effects of motion, root mean squared (RMS) realignment estimates were calculated as robust measures of motion using publicly available MATLAB code from GitHub [44]. Any subjects with a RMS value greater than 0.25 were not included in the analysis [44]. No participants had a RMS value greater than 0.25. The resting state images were then co-registered to their respective anatomical T1 images. Each T1 image was then segmented and normalized to a smoothed template brain in 2 mm Montreal Neurological Institute (MNI) template space. Each subject's T1 normalization parameters were then applied to that subject's resting state image, resulting in an MNI space normalized resting state image. The resulting images were smoothed with a 5 × 5 × 5 mm3 FWHM Gaussian kernel. For each subject, a sample of the volumes was inspected for any artifacts and anomalies. Levels of signal dropout were also visually inspected for excessive dropout in a priori regions of interest.
Functional network connectivity construction
Preprocessed and normalized functional images were entered into the CONN-fMRI functional connectivity toolbox version 17 [45]. Regions from the Destrieux [46] and Harvard–Oxford Subcortical Atlases were entered as ROIs. These atlases were used to accurately capture the ROIs mentioned in the previous research. CompCor, a component-based noise correction method, was applied to remove physiological noise without regressing out the global signal [47]. White-matter, cerebrospinal fluid, six realignment parameters, and first-order temporal derivatives of motion, and RMS were removed using regression. This ensures only signal from gray matter voxels are being considered. Band pass filtering between 0.01 and 0.08 Hz was applied to the residualized time series to reduce the low- and high-frequency noises after regression. Linear measures of ROI-to-ROI functional connectivity were computed using Fisher transformed correlations representing the association between average temporal BOLD time series signals across all voxels in a brain region. The final outputs for each subject consisted of a 165 × 165 matrix consisting of Fisher transformed Z correlation values between each ROI. Overall functional connectivity was computed by taking the mean of all positive values in each individual’s matrix. This was done to determine if proportional thresholding should be used, as because a minimal difference in overall FC can cause a difference in network metrics, which may be due to inherent disease differences [48]. An independent sample t-test was done to determine if there was a significant difference in overall FC between groups.
Computing network metrics
The Graph Theory GLM toolbox (GTG) (http://www.nitrc.org/projects/metalab_gtg) and in-house MATLAB scripts were applied to the subject-specific functional brain networks to compute two local weighted network metrics indexing centrality. We decided to focus on centrality at the microscale level as this allows one to determine characteristic hub roles for specific nodes, or brain regions, which can be easily interpretable by scientists and clinicians alike [49]. Additionally measures of centrality can capture a node’s role in network organization beyond local connections [49]. Measures of centrality quantify the importance of a region’s influence on communication and information flow in large-scale brain networks [25]. These measures include strength and betweenness centrality [23]. Strength represents the weighted sum of the number of connections a given brain region has and reflects a brain region’s total impact in the network [23]. Betweenness centrality describes the degree to which a brain region lies on the shortest path between two other regions [23]. Acting as way stations, regions with high betweenness centrality are topologically primed to control communication between other regions. The magnitude of the Z values represents the weights in the functional network. The Z values in each individual connectivity matrix was thresholded at Z > 0.3, and all other values were set to zero. A threshold of 0.3 was chosen since a correlation of 0.3 represents a medium effect size, and the inclusion of lower correlations could result in the inclusion of less accurate estimates [50]. We did not use a proportional-based thresholding approach because minimal differences in overall functional connectivity may introduce group differences in network metrics in patient vs. control studies [48]. All visualizations were created using in-house visualization schematics along with the BrainNet Viewer [51].
MRI data analysis
Regions of interest
Many of our analyses were based on regions of interest (ROI). For comparison between the combined AN group, consisting of both ANC and ANR individuals, and the HC group, core ROIs of the sensorimotor and basal ganglia networks were examined in relationship to the entire brain parcellated by the Destreiux [46] cortical and Harvard–Oxford subcortical [52,53,54,55] atlases, as well as seed-to-voxel whole brain analyses (Additional file 1: Table S2, Fig. S1). Core regions of the sensorimotor network included the thalamus [Tha], brain stem [Bstem], hippocampus [Hip], paracentral lobule and sulcus [PaCL/S], primary somatosensory cortex [S1], central sulcus [CS], primary motor cortex [M1], precuneus [PrCun], secondary somatosensory cortex [S2], supplementary motor area [M2], middle insula [part of aINS], and posterior insula [pINS] [10, 11]. Core regions of the basal ganglia network included the basal ganglia [BG] and globus pallidus [Pal] [56]. These core seed ROIs were selected from past research in AN and used to look at differences throughout ROIs across the entire brain parcellated by the Destreiux [46] cortical and Harvard–Oxford subcortical [52,53,54,55] atlases, as well as seed-to-voxel whole brain analyses.
A Differences in functional connectivity between anorexia and healthy controls using whole-brain seed-to-voxel analysis: SMN sensorimotor network, BG Basal Ganglia, DMN default mode network, SAL salience network, ERN emotion regulation network, CAN central autonomic network, CEN central executive network, Occ occipital network; BST Brainstem Blue: Anorexia < Healthy Controls, Red: Anorexia > Healthy Controls t: t-score. B Differences in functional centrality between anorexia and healthy controls in the sensorimotor and basal ganglia networks: CaN caudate nucleus, SupFG superior frontal gyrus, SupFS superior frontal sulcus, SubCG subcentral gyrus and sulcus, PreCG precentral gyrus, PosCG postcentral gyrus, PosCS postcentral sulcus, CS central sulcus, PaCLS paracentral lobule and sulcus. Node size represents the degree of centrality of that node based on resting-state functional connectivity. Edge thickness represents the correlation of connectivity between two nodes. Edges between two brain regions in the sensorimotor network are colored in green.
Functional seed-to-voxel whole-brain analysis
In order to determine differences in whole brain connectivity from selected ROIs, we performed a whole brain, seed-to-voxel analysis in CONN utilizing the GLM and controlling for age. This represents the level of functional connectivity between each ROI and every voxel in the brain. The parametric map of t-values were thresholded using an initial height threshold (voxel-level) of p < 0.001 and corrected (using the false discovery rate method); cluster thresholds were set at p(FDR) < 0.05 [57]. In order to perform partial correlations controlling for age with behavioral variables, eigenvalues for each connectivity unit (i.e., the degree of connectivity between the seed and significant cluster of voxels) were extracted from within the CONN toolbox. Significance was set at p < 0.05. Visualizations were done using circus [58] in Linux.
Computing group differences in network metrics
In order to test for disease-related differences, a GLM was applied and the effect of age was included as a covariate in the model. Significance was determined via Freedman & Lane’s non-parametric permutation testing strategy and specifying 10,000 permutations [59]. This method provides good control over type I error rates and is robust to the presence of outliers [60]. Probability values from the permutation testing strategy were corrected using a false discovery rate (FDR) adjusted p-value, where q < 0.05 was considered significant [61, 62]. FDR correction was applied at the whole-brain level. Partial correlations controlling for age were then computed to determine the association between significant network metrics and behavioral measures. Significance was set at p < 0.05.
Behavioral/clinical data
Group differences in clinical and behavioral measures were evaluated by applying linear contrast analyses in a GLM model using Statistical Package for the Social Sciences (SPSS) software (version 22). To quantify the differences between the various contrasts, we calculated Cohen’s effect size d, reflecting differences on the scale of standard deviation units, where values are interpreted as low (d = 0.20), moderate (d = 0.50), and high (d = 0.80) [63]. Correlations between significant findings for group differences in connectivity and measures of centrality were conducted with behavioral variables, while controlling for age.
Results
Demographics and clinical variables
AN participants had lower BMI compared to HCs (t(60) = − 2.54, p = 0.01, d = − 0.74), and were older (t(60) = 3.05, p = 0.004, d = 0.84). AN participants had greater scores on the EDI Body Dissatisfaction subscale (t(60) = 6.71, p < 0.0001, d = 1.68), EDI Drive for Thinness subscale (t(60) = 6.47, p < 0.0001, d = 1.62), EDI Bulimia subscale (t(60) = 2.54, p = 0.01, d = 0.58), and EDI Total scores (t(30.74) = 4.34, p = 1.41 × 10−4, d = 1.41), but not the EDI Perfectionism subscale (t(60) = − 0.73, p = 0.47, d = 0.19). See Table 1 for demographic details.
Overall functional connectivity
Overall functional connectivity comparing anorexia (mean = 0.16, SD = 0.040) and healthy controls (mean = 0.23, SD = 0.095) was significantly different (t(57.06) = − 3.92, p = 0.0002, d = − 0.84).
Disease related differences in seed-to-voxel whole brain resting-state functional connectivity
Sensorimotor network
Significant differences were observed looking at whole brain resting-state functional connectivity differences from seeds within the sensorimotor network (46 connectivity dyads, β ranging from − 0.19 to − 0.25, q-values ranging from 0.04 to 0.000007). All connectivity results resulted in AN participants having lower resting-state functional connectivity from sensorimotor regions to other brain areas compared to HCs. See Table 2 and Fig. 1A for details.
Basal ganglia network
AN participants had greater resting-state functional connectivity from the left caudate nucleus to the right postcentral gyrus (k (cluster size) = 46, β = 0.16, t = 5.07, p(FDR) = 0.039), and lower resting-state functional connectivity from the brain stem to the right angular gyrus (k (cluster size) = 85, β = − 0.19, t = − 5.68, p(FDR) = 0.002). See Table 2 and Fig. 1A for details.
Disease related differences in measures of centrality
Significant disease-related differences in measures of centrality between AN and HC participants can be visualized in Table 3 and Fig. 1B. Subsequent analyses were restricted to regions significantly different between the AN and HC groups.
Sensorimotor network
Subjects with AN had lower resting-state functional strength in the left (t(58) = − 3.65, q = 0.02, d = 1.06) and right (t(58) = − 3.59, q = 0.02, d = 1.12) superior frontal gyrus (SMA).
Basal ganglia network
Subjects with AN had greater resting-state functional betweenness centrality in the right caudate nucleus (t(58) = 3.24, q = 0.02, d = 0.63).
Correlations between brain analyses and behavioral variables
Whole brain seed-to-voxel analyses
Lower connectivity between the right postcentral gyrus and right supramarginal gyrus was associated with greater EDI Subscale Total scores (r(16) = − 0.759, p = 0.001, q = 0.03). Connectivity between the hippocampus and supramarginal gyrus was positively associated with time to treatment, and connectivity within the sensorimotor network was associated negatively with age of onset, however these correlations did not survive correction for multiple comparisons. See Table 4.
Measures of centrality
Regions within the sensorimotor network showing significant differences between AN and HC in strength were negatively correlated with the EDI Body Dissatisfaction scale, and the Subscale Total Scores. See Table 4.
Discussion
Anorexia nervosa is a disorder characterized by a seeming lack of synchrony between physical needs and engagement in adaptive behaviors to address those needs (e.g., sensing and responding to hunger, seeming imperviousness to the discomfort of excessive exercise). Our findings showed that AN participants had lower connectivity and centrality within the sensorimotor network, which was associated with more negative evaluations of one’s body image as indexed by the Body Dissatisfaction subscale. We also found that AN participants had greater connectivity and centrality of the caudate nucleus. These findings suggest that individuals suffering from AN have reduced sensory propagation, input that may help guide adaptive behavior, and greater activation of the caudate nucleus, a region which has been shown to be involved in strategic planning, reward, and motor activity among other functions.
Prior work has examined the integrity of the somatosensory/sensorimotor networks and the basal ganglia network as neural substrates of these sensory experiences and rigid driven behaviors respectively. In this study, we aimed to further characterize the integrity of these networks by examining metric dynamics such as centrality within the somatosensory and basal ganglia networks. King et al. [43] described AN as a model of neuroplasticity in which the impact of potentially dangerous weight loss behaviors and the timing and duration of those behaviors could inform how the brain adapts to insult. As such, in this paper we examined not only group-level differences between those with a history of an AN diagnosis relative to typically developing controls, but also more continuous aspects of the disorder such as age of onset, time to treatment, and duration of the disorder. Our findings centered on two networks: the sensorimotor network and the basal ganglia network. Each will be discussed in turn.
First, we found pervasive weakened associations within areas of the sensorimotor network in adolescents with AN relative to HCs and weakened associations of the sensorimotor network with several other networks (i.e., occipital, default mode, central executive, emotion regulation), with the exception of the basal ganglia network in which there was increased connectivity. AN has been described as a disorder in which individuals are seemingly disconnected from somatic experiences: having difficulty labeling emotions, regulating emotions, responding adaptively to biological needs such as hunger and fatigue, and having an incoherent sense of identity often entangling their disorder with their experience of individuality and agency [64]. Thus, broadly, findings with the sensorimotor network are consistent with the phenomenology of AN: weakened associations between networks (sensorimotor) associated with the perception of afferent signals from the viscera and related changes to networks required to integrate such sensations to achieve adaptive actions and self-awareness.
Further, weakened connectivity and centrality within the sensorimotor cortex was associated with several clinical parameters. Greater subjective ratings on the EDI Body Dissatisfaction Subscale, and a combined total EDI subscale score were associated with weakened connectivity within the upper sensorimotor network. As these scales reflect beliefs and behaviors that are incongruent with somatic needs and drives (e.g., the Body Dissatisfaction Subscale assessing discontentment with the overall shape and size of body regions), the negative correlation of this scale with weakened connectivity of this network reflects worsening sensorimotor integration with elevated body dissatisfaction. While duration of illness was not significantly associated with connectivity within the sensorimotor network, a later age of onset was associated with weakened connectivity between regions of the sensorimotor networks and default mode network before correction for multiple comparisons. Past research has also shown similar results where greater sensorimotor network activity was associated with lower body dissatisfaction in control women [65]. At the network level, weakened centrality was documented in AN. Thus, our findings are consistent with prior work documenting weakened connectivity of thalamic and posterior insular regions suggesting weaker propagation of somatic signals to guide adaptive behaviors [29].
In contrast, stronger connectivity between the sensorimotor and basal ganglia networks may reflect the salience or rewarding value of body-related information, given the preoccupation with the body in AN. While somatic information and actions are not adaptively integrated in those with AN (e.g., hunger may not motivate eating), yet body-related information is rewarding in that it may reinforce maladaptive behavior. Thus, the connectivity of the sensorimotor and basal ganglia networks may reflect the salience of somatic signaling with action: albeit maladaptive. It is also important to note that in AN, activity of the caudate nucleus has been shown to be involved in strategic planning and consideration of consequences, as opposed to proximal hedonic responses [66]. Previous imaging studies on AN indicate greater caudate volumes [67] compared to controls, and greater glucose metabolism in the caudate compared to individuals with bulimia [68]. We also know that individuals with AN show greater functional activation in the caudate in response to monetary reward tasks [69]. Thus, findings of greater connectivity between the caudate and somatosensory cortex may indicate strategic planning; that is, alteration of behaviors towards long-term goals (i.e. avoiding food and getting thin) instead of short-term goal (i.e. eating food). Conversely, the basal ganglia network demonstrated weaker connectivity with the default mode network, perhaps reflecting that while focus on the somatic body may be negatively or positively reinforcing, focus on the self, as encapsulated by the self-awareness of the default mode, is not. Connectivity between these networks has been shown to play a key role in reward-based associative learning [70]. This may reflect that individuals with AN have difficulty differentiating experiences that will be rewarding [70].
Limitations
Future studies should also investigate changes in cortico-striatal circuits longitudinally to understand the development of AN, as this cross-sectional study does not allow to make conclusions about causality. While the sample reflected individuals presenting to a specialized outpatient program for eating disorders, it was a mixed sample in the sense that individuals were at various phases of disorder and weight restoration. While such categorical distinctions of disorder, partial recovery, etc. are contested, nonetheless, defining categorical groups may have strengthened our interpretations. These limitations were addressed by examining more continuous measures of AN such as duration and time to treatment. Furthermore, while study participants were on stable doses of psychotrophic medications, a significant proportion of the clinical sample was on various medications and thus these medications may have influenced study results. Medication naïve samples offer a much stronger test and verification of study findings. Moreover, it is important to note that the associations between connectivity/graph theory measures and clinical measures are correlational and does not allow for casual inference. Finally, it should be noted that the atlas for parcellation of nodes was chosen based on prior research in anorexia nervosa. As there is no standard atlas to use for parcellating the brain, future research should test the effect of different brain atlases, as the selection of different atlases may impact final results [71, 72]. We used a structural atlas due to previous research conducted in patients with anorexia. The disadvantage of using a structural atlas is that it may average dissimilar functional signals based on an a-priori definition of a region.
Conclusions and clinical implications
In the presence of a chronic medical condition, adolescents have been reported to develop a dislike and distrust of their bodies, feeling that their bodies had somehow let them down [73]. In AN, there may be a similar adversarial relationship with one’s body and the reported need to control one’s body to feel in control of oneself. Findings from the current study revealed weakened connectivity and centrality in the sensorimotor network, evidence suggesting that the strength, integrity, or efficiency in the processing of sensorimotor signals may be impaired. In adolescents with AN, the altered network metrics were associated with increased body dissatisfaction suggesting that perhaps unreliability of somatic signals can lead to negative evaluations of the body. The question whether such compromised network metrics are a consequence of repeatedly ignoring or being nonresponsive to bodily signals such as hunger or fatigue or whether such network metrics reflect a vulnerability to develop AN cannot be addressed in this cross-sectional study. To answer the question if the unreliability of the body necessitates the need for rigid rules and behaviors because one can’t “trust one’s body” is a topic needing further research. Regardless, our findings speak to the importance of intervention strategies that help individuals with AN to better sense, decipher, and act on the various interoceptive messages communicated by the body.
Availability of data and materials
All data and methods used in the current manuscript are freely available from the authors upon reasonable request.
Abbreviations
- AN:
-
Anorexia nervosa
- HC:
-
Healthy control
- EDI:
-
Eating disorders inventory
- MRI:
-
Magnetic resonance imaging
- fMRI:
-
Functional magnetic resonance imaging
- ROI:
-
Region of interest
- BOLD:
-
Blood oxygen level dependent
- GLM:
-
General linear model
- FDR:
-
False discovery rate
References
Crisp AH. Anorexia nervosa as flight from growth: assessment and treatment based on the model. Handbook of Treatment for Eating Disorders, 2nd ed. Guilford Press; 1997. p. 248–77.
Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–31.
Favaro A, Caregaro L, Tenconi E, Bosello R, Santonastaso P. Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry. 2009;70:1715–21.
Calugi S, El Ghoch M, Conti M, Dalle GR. Preoccupation with shape or weight, fear of weight gain, feeling fat and treatment outcomes in patients with anorexia nervosa: a longitudinal study. Behav Res Ther. 2018;105:63–8.
Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. Altered interoceptive awareness in anorexia nervosa: effects of meal anticipation, consumption and bodily arousal. Int J Eat Disord. 2015;48:889–97.
Khalsa SS, Hassanpour MS, Strober M, Craske MG, Arevian AC, Feusner JD. Interoceptive anxiety and body representation in anorexia nervosa. Front Psychiatry. 2018;9.
Eshkevari E, Rieger E, Longo MR, Haggard P, Treasure J. Persistent body image disturbance following recovery from eating disorders. Int J Eat Disord. 2014;47:400–9.
Gaudio S, Brooks SJ, Riva G. Nonvisual multisensory impairment of body perception in anorexia nervosa: a systematic review of neuropsychological studies. PLoS ONE. 2014;9.
Gaudio S, Carducci F, Piervincenzi C, Olivo G, Schiöth HB. Altered thalamo–cortical and occipital–parietal–temporal–frontal white matter connections in patients with anorexia and bulimia nervosa: a systematic review of diffusion tensor imaging studies. J Psychiatry Neurosci. 2019;44:324–39.
Phillipou LA, Castle DJ, Hughes ME, Nibbs RG, Gurvich C, Rossell SLAA. Resting state functional connectivity in anorexia nervosa. Psychiatry Res. 2016;251:45–52.
Favaro P, Manara R, Bosello R, Bommarito G, Tenconi E, Di Salle F. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry. 2012;72:864–70.
Amianto F, Caroppo P, D’agata F, Spalatro A, Lavagnino L, Caglio M, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Res Neuroimaging. 2013;213:210–6.
Ehrlich S, Lord AR, Geisler D, Borchardt V, Boehm I, Seidel M, Ritschel F, Schulze A, King JA, Weidner K, Roessner V. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Hum Brain Map. 2015;36(5):1772–81.
Biezonski D, Cha J, Steinglass J, Posner J. Evidence for thalamocortical circuit abnormalities and associated cognitive dysfunctions in underweight individuals with anorexia nervosa. Neuropsychopharmacology. 2016;41:1560–8.
Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopzzamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11 C]raclopride. Biol Psychiatry. 2005;58:908–12.
Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431.
Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Prog Brain Res. 2000;126:193–215.
Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–83.
Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–16.
Cha J, Ide JS, Bowman FD, Simpson HB, Posner J, Steinglass JE. Abnormal reward circuitry in anorexia nervosa: a longitudinal, multimodal MRI study. Hum Brain Mapp. 2016;37:3835–46.
Fladung AK, Grön G, Grammer K, Herrnberger B, Schilly E, Grasteit S, Wolf RC, Walter H, von Wietersheim J. A neural signature of anorexia nervosa in the ventral striatal reward system. Am J Psychiatry. 2010;167(2):206–12.
Fladung A-K, Schulze U, Schöll F, Bauer K, Grön G. Role of the ventral striatum in developing anorexia nervosa. Transl Psychiatry. 2013;3:e315.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69.
Bullmore E, Sporns O. The economy of brain network organization. Nat Neurosci Rev. 2012;13:336–49.
Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–62.
Collantoni E, Meneguzzo P, Tenconi E, Meregalli V, Manara R, Favaro A. Shift toward randomness in brain networks of patients with anorexia nervosa: the role of malnutrition. Front Neurosci. 2021;15:210.
Geisler D, Borchardt V, Boehm I, King JA, Tam FI, Marxen M, et al. Altered global brain network topology as a trait marker in patients with anorexia nervosa. Psychol Med. 2020;50:107–15.
Collantoni E, Meneguzzo P, Solmi M, Tenconi E, Manara R, Favaro A. Functional connectivity patterns and the role of 5-httlpr polymorphism on network architecture in female patients with anorexia nervosa. Front Neurosci. 2019;13:1056.
Ehrlich S, Lord AR, Geisler D, Borchardt V, Boehm I, Seidel M, et al. Reduced functional connectivity in the thalamo-insular subnetwork in patients with acute anorexia nervosa. Hum Brain Mapp. 2015;36:1772–81.
Lord A, Ehrlich S, Borchardt V, Geisler D, Seidel M, Huber S, Murr J, Walter M. Brain parcellation choice affects disease-related topology differences increasingly from global to local network levels. Psychiatry Res Neuroimaging. 2016;249:12–9.
Collantoni E, Alberti F, Meregalli V, Meneguzzo P, Tenconi E, Favaro A. Brain networks in eating disorders: a systematic review of graph theory studies. Eating and weight disorders. Springer Science and Business Media Deutschland GmbH; 2021.
Kragel PA, Zucker NL, Covington VE, LaBar KS. Developmental trajectories of cortical-subcortical interactions underlying the evaluation of trust in adolescence. Soc Cogn Affect Neurosci. 2015;10:240–324.
Sweitzer MM, Watson KK, Erwin SR, Winecoff AA, Datta N, Huettel S, et al. Neurobiology of social reward valuation in adults with a history of anorexia nervosa. PLoS ONE. 2018;13:e0205085.
Franz L, Angold A, Copeland W, Costello EJ, Towe-Goodman N, Egger H. Preschool anxiety disorders in pediatric primary care: Prevalence and comorbidity. J Am Acad Child Adolesc Psychiatry. 2013;52:1294–303.
Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology. 2016;41:521–8.
Li D, Zucker NL, Kragel PA, Covington VE, LaBar KS. Adolescent development of insula-dependent interoceptive regulation. Dev Sci. 2017;20:e12438.
Zucker NL, Kragel PA, Wagner HR, Keeling L, Mayer E, Wang J, et al. The clinical significance of posterior insular volume in adolescent anorexia nervosa. Psychosom Med. 2017;79:1025–2035.
Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int J Eat Disord. 1983;2:125–41.
Clausen L, Rosenvinge JH, Friborg O, Rokkedal K. Validating the eating disorder inventory-3 (EDI-3): a comparison between 561 female eating disorders patients and 878 females from the general population. J Psychopathol Behav Assess. 2011;33:101–10.
Wildes JE, Ringham RM, Marcus MD. Emotion avoidance in patients with anorexia nervosa: initial test of a functional model. Int J Eat Disord. 2010;43:398–404.
Espelage DL, Mazzeo SE, Aggen SH, Quittner AL, Sherman R, Thompson R. Examining the construct validity of the eating disorder inventory. Psychol Assess. 2003;15:71–80.
Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–5.
King JA, Frank GKW, Thompson PM, Ehrlich S. Structural neuroimaging of anorexia nervosa: future directions in the quest for mechanisms underlying dynamic alterations. Biol Psychiatry. 2018;83:224–34.
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–41.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
van den Heuvel MP, de Lange SC, Zalesky A, Seguin C, Yeo BTT, Schmidt R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. Neuroimage. 2017;152:437–49.
Medaglia JD. Graph theoretic analysis of resting state functional MR imaging. Neuroimaging Clin N Am. 2017;27:593–607.
Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8:467–74.
Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910.
Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–71.
Frazier JA, Chiu S, Breeze JL, Nikos Makris M, Lange N, David Kennedy SN, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–65.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Goldstein JM, Seidman LJ, Makris N, Ahern T, O’brien LM, Caviness VS, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61:935–45.
Zhang A, Leow A, Zhan L, Gadelkarim J, Moody T, Khalsa S, et al. Brain connectome modularity in weight-restored anorexia nervosa and body dysmorphic disorder. Psychol Med. 2016.
Woo C-W, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–9.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45.
Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–8.
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97.
Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507.
Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60–83.
Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2–18.
Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. Anorexia nervosa. Nat Rev Dis Primers. 2015;1.
McFadden KL, Tregellas JR, Shott ME, Frank GK. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neuroscience JPN. 2014;39(3):178–88.
Lopez C, Tchanturia K, Stahl D, Booth R, Holliday J, Treasure J. An examination of the concept of central coherence in women with anorexia nervosa. Int J Eat Disord. 2008;41:143–52.
Yue L, Wang Y, Kaye WH, Kang Q, Huang J, Cheung EFC, et al. Structural alterations in the caudate nucleus and precuneus in un-medicated anorexia nervosa patients. Psychiatry Res Neuroimaging. 2018;281:12–8.
Krieg V, Schreiber W, Pirke KM, Herholz K. Glucose metabolism in the caudate nuclei of patients with eating disorders, measured by PET. Eur Arch Psychiatry Clin Neurosci. 1991;240:331–3.
Wagner H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, Frank GK, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WHAA. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–9.
Vatansever D, Manktelow AE, Sahakian BJ, Menon DK, Stamatakis EA. Cognitive flexibility: a default network and basal ganglia connectivity perspective. Brain Connect. 2016;6:201–7.
Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26:288–303.
Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61.
Cole MAT, Qu’d D, Wild MG, Russell AC, Caillet AR, Stone AL. “My body hates me”: a qualitative analysis of the experience of functional nausea in adolescent girls and their mothers. Children. 2020;7:83.
Acknowledgements
Not applicable.
Funding
Support: Supported by NIH Grants K23 DK106528 (AG), R01 DK048351 (EAM), P30 DK041301 (EAM), RC1-MH-088678 (NZ/KL), Duke Institute for Brain Sciences.
Author information
Authors and Affiliations
Contributions
AG: conception and design of the work, analysis, interpretation of data, revising manuscript critically for important intellectual content, final approval of the version to be published; RRB: design of the work, analysis, interpretation of data, revising manuscript critically for important intellectual content, final approval of the version to be published; ARC: acquisition, analysis; RM: analysis; PAK: revising manuscript critically for important intellectual content, final approval of the version to be published; TR: analysis; JLG: revising manuscript critically for important intellectual content, final approval of the version to be published; AA: analysis; KH: analysis; PPV: analysis; BDN: revising manuscript critically for important intellectual content, final approval of the version to be published; JSL: revising manuscript critically for important intellectual content, final approval of the version to be published; KSL: conception or design of the work, acquisition, revising manuscript critically for important intellectual content, final approval of the version to be published; EAM: conception and design of the work, interpretation of data, drafting the manuscript and revising it critically for important intellectual content, final approval of the version to be published; NLZ: conception and design of the work, acquisition, interpretation of data, drafting the manuscript and revising it critically for important intellectual content, final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board at Duke University Medical Center, and all methods were carried out in accordance with relevant guidelines and regulations from the Declaration of Helsinki. Each participant provided informed written consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
Each of the authors’ report that no conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1
. Psychotropic Medications of Clinical Sample. Notes: 1. n = 12 individuals on medication, 3 on multiple medications. 2. For all short-acting and PRN medications (e.g., Lorazepam, Methylphenidate), participants were instructed not to take the medication the day of the scan. Compliance was assessed prior to scanning. There was no need to reschedule a scanning session based on this instruction. 12 individuals were on medication on the day of scanning; Table 2. Regions of Interest (ROIs). Sensorimotor Network: Thalamus [Includes Anterior, Central-medial Thalamus (Tha)], Hippocampus (Hip), Paracentral lobule and sulcus (PaCL/S), Primary Somatosensory Cortex/S1 [Includes Postcentral gyrus (PosCG), Postcentral sulcus (PosCS)], Central sulcus (Rolando's Fissure, CS), Precentral (Primary Motor Cortex/M1) [Includes Inferior part of the precentral sulcus (InfPrCS), Superior part of the precentral sulcus (SupPrCs), Precentral gyrus (PRCG)], Precuneus (PrCun), Secondary Somatosensory Cortex/S2 [Includes Subcentral gyrus (central operculum) and sulci (SbCG_S)], Supplementary Motor Area/M2 [Includes BA6/Superior Frontal Gyrus (SupFG), BA6/Superior Frontal Sulcus (SupFS), Posterior Insula (pINS) [Includes Long insular gyrus and central sulcus of the insula (LoInG/CInS), Inferior segment of the circular sulcus of the insula (InfCirInS), Posterior ramus (or segment) of the lateral sulcus (or fissure) (PosLS)]. Basal Ganglia Network: Putamen (Pu), Caudate nucleus (CaN), Nucleus Accumbens (Nacc)], Globus Pallidus [Includes Pallidum (Pal)], Brain Stem (Bstem). Figure 1 Regions of Interest (ROIs): Tha: thalamus, Hipp: hippocampus, PaCL: paracentral lobule, PosCG: postcentral gyrus, PosCS: postcentral sulcus, CS: central sulcus, InfPrCS; inferior part of the precentral sulcus, SupPrCS; superior part of the precentral sulcus, PrCG: precentral gyrus, PrCu: precuneus, SubCG/S: subcentral gyrus and sulcus, SupFG: superior frontal gyrus, SupFS: superior frontal sulcus, SupCirIns: superior part of the circular sulcus of the insula, InfCirIns: inferior part of the circular sulcus of the insula, LoInG/CInS: long insular gyrus and central sulcus of the insula, PosLS: posterior ramus of the lateral sulcus, Put: putamen, CaN: caudate nucleus, NAcc: nucleus accumbens, Pal: pallidum, BStem: brainstem.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gupta, A., Bhatt, R.R., Rivera-Cancel, A. et al. Complex functional brain network properties in anorexia nervosa. J Eat Disord 10, 13 (2022). https://doi.org/10.1186/s40337-022-00534-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40337-022-00534-9