Abstract
Long noncoding RNAs (lncRNAs) refer to a type of non-protein-coding transcript of more than 200 nucleotides. LncRNAs play fundamental roles in disease development and progression, and lncRNAs are dysregulated in many pathophysiological processes. Thus, lncRNAs may have potential value in clinical applications. The lncRNA, MAF BZIP Transcription Factor G (MAFG)-AS1, is dysregulated in several cancer, including breast cancer, lung cancer, liver cancer, bladder cancer, colorectal cancer, gastric cancer, esophagus cancer, prostate cancer, pancreatic cancer, ovarian cancer, and glioma. Altered MAFG-AS1 levels are also associated with diverse clinical characteristics and patient outcomes. Mechanistically, MAFG-AS1 mediates a variety of cellular processes via the regulation of target gene expression. Therefore, the diagnostic, prognostic, and therapeutic aspects of MAFG-AS1 have been widely explored. In this review, we discuss the expression, major roles, and molecular mechanisms of MAFG-AS1, the relationship between MAFG-AS1 and clinical features of diseases, and the clinical applications of MAFG-AS1.
Similar content being viewed by others
Introduction
Cancer represents a group of heterogeneous diseases, which involve uncontrolled growth of mutated cells, invasion of adjacent organs, and distant metastasis [1,2,3,4]. The application of discoveries and innovations in molecular cancer therapies has significantly improved patient prognoses [5,6,7,8,9]. However, the high incidence and mortality of cancer are still a public health concern [10,11,12,13]. New molecular mechanisms and strategies are still needed to improve therapeutic responses and clinical outcomes [14,15,16,17,18].
Along with advances in high-throughput sequencing technology, an increasing population of noncoding RNAs (ncRNAs) has been discovered [19,20,21,22], including long noncoding RNAs (lncRNAs) [23,24,25,26,27,28]. LncRNAs are transcripts of at least 200 nucleotides that do not have protein-coding capability [23, 29,30,31]. LncRNA dysregulation is involved in diverse human diseases, including neurological diseases, cardiovascular diseases, and cancers [32,33,34], and diverse cellular processes [35,36,37,38], including cell proliferation, differentiation, apoptosis, and migration. In addition, lncRNAs regulate the expression of protein-coding genes and, thus, foster the progression of diseases or tumors. Given these properties, a large proportion of lncRNAs are important in disease diagnosis, prognosis, and therapeutic targets [39,40,41,42].
The lncRNA MAF BZIP Transcription Factor G (MAFG)-AS1, located on human chromosome 17q25.3, was recently identified as an oncogenic lncRNA with a transcript size of 1895 bp. MAFG-AS1 expression is aberrant in diverse diseases, including breast cancer [43,44,45,46,47,48,49], lung cancer [50,51,52], liver cancer [53,54,55,56,57,58], bladder cancer [59,60,61,62,63], colorectal cancer [64,65,66], gastric cancer [67, 68], esophagus cancer [69], prostate cancer [70], pancreatic cancer [71], ovarian cancer [72], glioma [73], periodontitis [74], and coronary artery disease [75]. MAFG-AS1 levels are also strongly associated with clinicopathological characteristics and patient outcomes, such as tumor size, clinical stage, distant metastasis, overall survival (OS), and disease‐free survival (DFS). Experimental studies demonstrate the involvement of MAFG-AS1 in disease development via a series of biological processes, such as cell proliferation, invasion, glycolysis, metastasis, and drug sensitivity. MAFG-AS1 affects cancer progression by regulating target gene expression.
In the present review, we discuss the expression, related clinical features, and biological functions of MAFG-AS1 in diverse cancers. In addition, we discuss the underlying mechanisms and clinical applications of MAFG-AS1.
Characteristics of MAFG-AS1 in human cancers
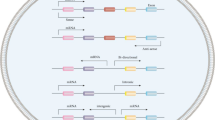
MAFG-AS1 is dysregulated in diverse diseases, including breast cancer, lung cancer, liver cancer, bladder cancer, colorectal cancer, gastric cancer, esophagus cancer, prostate cancer, pancreatic cancer, ovarian cancer, and glioma (Fig. 1). High MAFG-AS1 expression correlates with unfavorable clinical features and prognosis, including lymph node metastasis, histological grade, clinical stage, distant metastasis, OS, and DFS (Table 1). Importantly, MAFG-AS1 often functions as a sponge to interfere with microRNA regulation of gene expression, which affects many biological processes, including cell proliferation, invasion, glycolysis, metastasis, and drug sensitivity (Table 2). In this section, we include a comprehensive description of the relationship between MAFG-AS1 expression and clinical features of diverse cancers.
Breast cancer
MAFG-AS1 overexpression in breast cancer tissue and cells (MCF7, MCF10, SUM149, HCC1937, BT474, Hs578T, SK-BR-3, MDA-MB-468, MDA-MB-231, and T47D) [43,44,45,46,47,48,49] revealed that MAFG-AS1 levels positively correlate with tumor size and ki-67 index [48]. MAFG-AS1 participates in cancer progression via enhanced cell proliferation, invasion, and metastasis and suppression of cell apoptosis and autophagy. Studies using xenograft models confirm the pro‐oncogenic roles of MAFG-AS1 in tumor growth and lung metastasis [44, 46,47,48].
Lung cancer
MAFG-AS1 is also upregulated in lung cancer tissues and H1373, H1975, H1650, HCC827, A549, PC-9, and Calu-3 cells [50,51,52]. High MAFG-AS1 levels are associated with poor prognosis in patients with lung cancer. MAFG-AS1 increases cell proliferation, migration, invasion, and tumor‐forming and metastasis abilities to advance lung cancer [50,51,52].
Liver cancer
MAFG-AS1 upregulation in liver cancer tissues and Huh7, HepG2, LM3, HCCLM3, Hep3B, and MHCC97-H cells is associated with shorter OS [53,54,55,56,57,58]. MAFG-AS1 exerts its pro-cancer roles via increased cell proliferation, migration, invasion, drug resistance, and tumor angiogenesis [54,55,56, 58].
Bladder cancer
MAFG-AS1 is upregulated in bladder cancer cells (HT01197, 5637, BIU87, EJ, RT4, J82, T24, HT-1376, UMUC3, and SVHUC1) and tissues [59,60,61,62,63]. MAFG-AS1 upregulation correlates with aggressive prognosis, shorter DFS and OS, and advanced clinical and TMN stages. Both in vivo and in vitro experimental studies demonstrate that the upregulation of MAFG-AS1 increases proliferation, migration, and invasion, which contribute to the development of bladder cancer.
Colorectal cancer
MAFG-AS1 levels are significantly increased in colorectal cancer tissues and HCT116, HT29, SW1116, SW480, and LoVo cells [64,65,66]. High MAFG-AS1 expression is closely related to invasion depth, advanced TNM stage, and shorter OS and DFS [65, 66]. MAFG-AS1 also facilitates cell migration, proliferation, invasion, glycolysis, and tumor growth to promote colorectal cancer [64, 66].
Gastric cancer
MAFG-AS1 is overexpressed in gastric cancer MKN-45, AGS, and SGC7901 cells and tissues. MAFG-AS1 upregulation is associated with deteriorative clinical stage, depth of invasion, lymph node metastasis, distant metastasis, and unfavorable OS [67, 68]. MAFG-AS1 also plays a pro-cancer role in gastric cancer through the promotion of cell proliferation, migration, and invasion.
Other cancers
MAFG-AS1 is upregulated in esophageal cancer tissues and cells (EC9706, EC109, KYSE30, and KYSE150) and is associated with shorter OS. MAFG-AS1 enhances cell proliferation, migration, invasion, and aerobic glycolysis and, thus, exerts cancer-promoting effects in esophageal cancer [69]. In prostate cancer, MAFG-AS1 is overexpressed in tissues and DU145 and PC-3 cells and participates in cell proliferation and invasion [70]. MAFG-AS1 levels are elevated in pancreatic cancer tissues and Capan 1, CFPAC-1, SW1990, and PANC-1 cells and strengthen cancer development via enhanced cell proliferation and migration [71]. Similarly, increased MAFG-AS1 in ovarian cancer A2780, Caov-3, RMG-I, Caov-4, and CoC1 cells tightly correlates with aggressive tumor stage, size, lymph node metastasis, and poor outcomes. MAFG-AS1 contributes to ovarian tumor progression via enhanced invasion and migration [72]. MAFG-AS1 is upregulated in glioma tissues, and U87 and U-118 cells and is pro-proliferative [73].
The pro-oncogenic mechanisms of MAFG-AS1 in human cancers
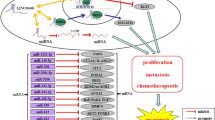
MAFG-AS1 is involved in the development of various cancers and governs numerous biological processes through diverse mechanisms, including cell proliferation, migration, invasion, apoptosis, autophagy, drug resistance, and glycolysis (Table 2). This section briefly introduces the mechanisms for MAFG-AS1 effects in human cancers.
The impaired regulation of cell proliferation, via defective regulatory pathways, mutations in critical genes, and environmental factors [76,77,78,79,80], contributes to tumor formation. Excessively increased migratory and invasive capacities of cancer cells promote cancer progression and higher mortality rates [81,82,83,84,85]. Moreover, energy metabolism also affects the pathogenesis of cancer [3, 86, 87]. Therefore, understanding the molecular mechanisms that govern cell processes is crucial for subsequent cancer management [88,89,90,91]. MAFG-AS1 affects cell proliferation and migration through diverse mechanisms. For example, MAFG-AS1 enhances cell proliferation, invasion, metastasis, and glycolysis to facilitate breast cancer development through multiple mechanisms. MAFG-AS1 plays pro-oncogenic roles through the miR-3612/FKBP4, miR-574-5p/SOD2, miR‑150‑5p/MYB, miR-339-5p/CDK2, miR-339-5p/MMP15, and miR-3196/TFAP2A/JAK2/STAT3 signaling pathways [43, 45,46,47,48,49] (Fig. 2). MAFG-AS1 also stabilizes STC2 expression to promote breast cancer [44]. MAFG-AS1 increases cell proliferation, migration, and invasion of lung cancer cells through the miR-339-5p/MMP15, miR-744-5p/MAFG, and miR-3196/SOX12 axes [50,51,52]. In liver cancer, MAFG-AS1 sponges miR-3196 to increase STRN4 expression, interacts with E2F1 to enhance MAFG levels, combines with miR-3196 to elevate OTX1 transcription, or decreases miR-6852 to increase cell proliferation, migration, and invasion [54,55,56, 58]. MAFG-AS1 promotes the proliferation, migration, and invasion of bladder cancer through miR-125b-5p/SphK1, HuR/PTBP1, miR-143-3p/COX-2, and miR-143-3p/SERPINE1 pathways [59, 60, 62, 63]. MAFG-AS1 may also contribute to colorectal cancer cell migration, proliferation, invasion, and glycolysis [64, 66] by binding to miR-147b to activate NDUFA4 or absorbing miRNA-149-3p to increase HOXB8 expression. In addition, MAFG-AS1 upregulates PLK1 by sponging miR-505 to increase gastric cancer cell proliferation migration, and invasion [67]. In esophageal cancer, MAFG-AS1 increases cell proliferation, migration, invasion, and aerobic glycolysis through interactions with miR-765 and the subsequent upregulation of PDX1 [69]. MAFG-AS1 also sponges miR-3196 to increase NFIX expression and enhance pancreatic cancer cell proliferation and migration [71]. In ovarian cancer, MAFG-AS1 upregulates IGF1 expression by interacting with NFKB1 to facilitate cell invasion and migration [72]. MAFG-AS1 elevates the proliferation of gliomas by decreasing the expression of mature miR-34a [73].
Clinical applications of MAFG-AS1 in human cancers
Despite the continuous strides in disease prevention and treatment, the global burden of cancer remains heavy [92,93,94,95]. In this context, new potent and safe molecules are needed to develop combination therapy strategies.
As the roles of MAFG-AS1 in diverse cancers are revealed, its clinical value has received increased attention. Multiple studies show the overexpression of MAFG-AS1 in tissues and cells and its pro-oncogenic roles in many cancers. MAFG-AS1 overexpression helps distinguish between cancerous and normal tissues and improves early-stage cancer diagnosis. Given the close association between MAFG-AS1 and diverse clinical features, MAFG-AS1 is a powerful prognostic tool for cancers. Kaplan–Meier survival curves demonstrate that high MAFG-AS1 levels correlate with patient’s poor prognoses (such as overall survival and progression free survival) in diverse cancers, including breast, lung, liver, bladder, colorectal, gastric, and esophageal cancers [52, 53, 57, 59, 60, 63, 65, 67,68,69]. Univariate Cox regression analyses in liver and gastric cancer patients also confirm the significant association of MAFG-AS1 with unfavorable OS [53, 57, 68]. Multivariate analyses revealed that MAFG‐AS1 is an independent prognostic biomarker in bladder, colorectal, and gastric cancers [60, 65, 68]. The detection of MAFG‑AS1 levels in cancer tissues and cells may improve the diagnosis and prognosis of several cancers and guide therapeutic approaches. In addition, recent studies suggest that MAFG‑AS1 is involved in important biological processes through diverse molecular mechanisms, especially the regulation of downstream molecules. MAFG‑AS1 knockdown slows cancer progression and is a potential novel therapy. MAFG‑AS1 enhances cancer cell resistance to tamoxifen, which is a target for the treatment of breast cancer [48]. Accordingly, MAFG‑AS1 has great potential in clinical application in terms of cancer diagnosis, prognosis, and therapy. Molecular therapy holds great potential in the field of oncology, albeit with certain challenges [96].
Conclusions
As a novel tumor-related lncRNA, dysregulation of MAFG‑AS1 contributes to multiple human cancers, including breast cancer, lung cancer, liver cancer, bladder cancer, colorectal cancer, gastric cancer, esophagus cancer, prostate cancer, pancreatic cancer, ovarian cancer, and glioma. Elevated MAFG‑AS1 expression is closely associated with diverse undesirable clinical characteristics and poor outcomes. Multiple experimental studies also revealed that MAFG‑AS1 acts on a variety of targets to mediate crucial biological processes, including cell migration, invasion, proliferation, energy metabolism, and drug resistance. Considering its attractive features in diverse cancers, MAFG‑AS1 possesses wide prospects for clinical applications, including diagnosis, prognosis, and treatment.
Data availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Jogalekar MP, et al. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13: 925985.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Pang W, et al. Noninvasive and real-time monitoring of Au nanoparticle promoted cancer metastasis using in vivo flow cytometry. Biomed Opt Express. 2021;12(4):1846–57.
Bernacchioni C, et al. NMR metabolomics highlights sphingosine kinase-1 as a new molecular switch in the orchestration of aberrant metabolic phenotype in cancer cells. Mol Oncol. 2017;11(5):517–33.
Paananen J, Fortino V. An omics perspective on drug target discovery platforms. Brief Bioinform. 2020;21(6):1937–53.
Abramczyk H, et al. Aberrant protein phosphorylation in cancer by using raman biomarkers. Cancers (Basel). 2019;11(12):2017.
Yang S, et al. Recent developments in nanomedicine for pediatric cancer. J Clin Med. 2021;10(7):1437.
Soini EJO, Martikainen JA, Nousiainen T. Treatment of follicular non-Hodgkin’s lymphoma with or without rituximab: cost-effectiveness and value of information based on a 5-year follow-up. Ann Oncol. 2011;22(5):1189–97.
Wu M, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):24.
Papi A, et al. Nuclear receptors agonists exert opposing effects on the inflammation dependent survival of breast cancer stem cells. Cell Death Differ. 2012;19(7):1208–19.
Zugazagoitia J, et al. Current challenges in cancer treatment. Clin Ther. 2016;38(7):1551–66.
Mahajna S, et al. In vitro evaluation of chemically analyzed hypericum triquetrifolium extract efficacy in apoptosis induction and cell cycle arrest of the HCT-116 colon cancer cell line. Molecules. 2019;24(22):4139.
Wassef M, Michaud A, Margueron R. Association between EZH2 expression, silencing of tumor suppressors and disease outcome in solid tumors. Cell Cycle. 2016;15(17):2256–62.
Kong R, et al. Fenofibrate exerts antitumor effects in colon cancer via regulation of DNMT1 and CDKN2A. PPAR Res. 2021;2021:6663782.
Dawood M, Ooko E, Efferth T. Collateral sensitivity of parthenolide via NF-κB and HIF-α inhibition and epigenetic changes in drug-resistant cancer cell lines. Front Pharmacol. 2019;10:542.
Deng R, et al. BAP1 suppresses prostate cancer progression by deubiquitinating and stabilizing PTEN. Mol Oncol. 2021;15(1):279–98.
Xue C, et al. The functional roles of the circRNA/Wnt axis in cancer. Mol Cancer. 2022;21(1):108.
Xue C, et al. The mechanism underlying the ncRNA dysregulation pattern in hepatocellular carcinoma and its tumor microenvironment. Front Immunol. 2022;13: 847728.
Xue C, et al. Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct Target Ther. 2021;6(1):400.
Xue C, et al. Progress and assessment of lncRNA DGCR5 in malignant phenotype and immune infiltration of human cancers. Am J Cancer Res. 2021;11(1):1–13.
Okazaki Y, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420(6915):563–73.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–9.
Li Y, et al. Non-coding RNA in bladder cancer. Cancer Lett. 2020;485:38–44.
Aravin AA, et al. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5(2):337–50.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–41.
Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–504.
Jia H, et al. Genome-wide computational identification and manual annotation of human long noncoding RNA genes. RNA. 2010;16(8):1478–87.
Liu C, et al. NONCODE: an integrated knowledge database of non-coding RNAs. Nucleic Acids Res. 2005;33(1):D112–5.
Pauli A, et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res. 2012;22(3):577–91.
Li D, et al. LncRNA, important player in bone development and disease. Endocr Metab Immune Disord Drug Targets. 2020;20(1):50–66.
Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018;22(12):5768–75.
Yang Z, et al. LncRNA: shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res Rev. 2019;52:17–31.
Zhu J, et al. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. 2013;56(10):876–85.
Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–307.
Park JY, et al. Roles of long non-coding RNAs on tumorigenesis and glioma development. Brain Tumor Res Treat. 2014;2(1):1–6.
Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21.
Li Y, et al. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3β signal pathway. Cell Death Dis. 2018;9(9):888.
Wu B, et al. Novel three-lncRNA signature predicts survival in patients with pancreatic cancer. Oncol Rep. 2018;40(6):3427–37.
Guo Y, et al. Role of LncRNAs in regulating cancer amino acid metabolism. Cancer Cell Int. 2021;21(1):209.
Sun CC, et al. FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway. Genome Med. 2020;12(1):77.
Gao Z, et al. LncRNA MAFG-AS1 deregulated in breast cancer affects autophagy and progression of breast cancer by interacting with miR-3612 and FKBP4 invitro. Biochem Biophys Res Commun. 2022;616:95–103.
Di S, et al. Long non-coding RNA MAFG-AS1 promotes proliferation and metastasis of breast cancer by modulating STC2 pathway. Cell Death Discov. 2022;8(1):249.
Dai J, et al. LncRNA MAFG-AS1 affects the tumorigenesis of breast cancer cells via the miR-574-5p/SOD2 axis. Biochem Biophys Res Commun. 2021;560:119–25.
Jia H, et al. Regulatory effect of the MAFG-AS1/miR-150-5p/MYB axis on the proliferation and migration of breast cancer cells. Int J Oncol. 2021;58(1):33–44.
Ding M, et al. Long non-coding RNA MAFG-AS1 knockdown blocks malignant progression in breast cancer cells by inactivating JAK2/STAT3 signaling pathway via MAFG-AS1/miR-3196/TFAP2A axis. Int J Clin Exp Pathol. 2020;13(10):2455–73.
Feng J, et al. Cross-talk between the ER pathway and the lncRNA MAFG-AS1/miR-339-5p/ CDK2 axis promotes progression of ER+ breast cancer and confers tamoxifen resistance. Aging (Albany NY). 2020;12(20):20658–83.
Li H, et al. LncRNA MAFG-AS1 promotes the aggressiveness of breast carcinoma through regulating miR-339-5p/MMP15. Eur Rev Med Pharmacol Sci. 2019;23(7):2838–46.
Wu Q, Jiang J. LncRNA MAFG-AS1 promotes lung adenocarcinoma cell migration and invasion by targeting miR-3196 and regulating SOX12 expression. Mol Biotechnol. 2022;64(9):970–83.
Sui Y, et al. LncRNA MAFG-AS1 boosts the proliferation of lung adenocarcinoma cells via regulating miR-744-5p/MAFG axis. Eur J Pharmacol. 2019;859: 172465.
Jia YC, et al. LncRNA MAFG-AS1 facilitates the migration and invasion of NSCLC cell via sponging miR-339-5p from MMP15. Cell Biol Int. 2019;43(4):384–93.
Huang K, et al. Construction of a ceRNA network and a genomic-clinicopathologic nomogram to predict survival for HBV-related HCC. Hum Cell. 2021;34(6):1830–42.
Chen T, Huang B, Pan Y. Long NON-coding RNA MAFG-AS1 promotes cell proliferation, migration, and EMT by miR-3196/STRN4 in drug-resistant cells of liver cancer. Front Cell Dev Biol. 2021;9: 688603.
Zhang F, et al. HBx-upregulated MAFG-AS1 promotes cell proliferation and migration of hepatoma cells by enhancing MAFG expression and stabilizing nonmuscle myosin IIA. Faseb j. 2021;35(5): e21529.
Hu ZQ, et al. Long noncoding RNA MAFG-AS1 facilitates the progression of hepatocellular carcinoma via targeting miR-3196/OTX1 axis. Eur Rev Med Pharmacol Sci. 2020;24(23):12131–43.
Du W, et al. Identification of prognostic biomarkers of hepatocellular carcinoma via long noncoding RNA expression and copy number alterations. Epigenomics. 2020;12(15):1303–15.
Ouyang H, et al. Long noncoding RNA MAFG-AS1 promotes proliferation, migration and invasion of hepatocellular carcinoma cells through downregulation of miR-6852. Exp Ther Med. 2019;18(4):2547–53.
Tang C, et al. LncRNA MAFG-AS1 regulates miR-125b-5p/SphK1 axis to promote the proliferation, migration, and invasion of bladder cancer cells. Hum Cell. 2021;34(2):588–97.
Xiao M, et al. MAFG-AS1 promotes tumor progression via regulation of the HuR/PTBP1 axis in bladder urothelial carcinoma. Clin Transl Med. 2020;10(8): e241.
Qing L, et al. Extracellular matrix-related Six-lncRNA signature as a novel prognostic biomarker for bladder cancer. Onco Targets Ther. 2020;13:12521–38.
Li D, et al. LncRNA MAFG-AS1 promotes the progression of bladder cancer by targeting the miR-143-3p/COX-2 axis. Pathobiology. 2020;87(6):345–55.
Sun X, et al. Long noncoding RNA MAFG-AS1 facilitates bladder cancer tumorigenesis via regulation of miR-143-3p/SERPINE1 axis. Transl Cancer Res. 2020;9(11):7214–26.
Ruan Z, et al. Downregulation of long non-coding RNA MAFG-AS1 represses tumorigenesis of colorectal cancer cells through the microRNA-149-3p-dependent inhibition of HOXB8. Cancer Cell Int. 2020;20:511.
Cui W, et al. High-expression of LncRNA MAFG-AS1 is associated with the prognostic of patients with colorectal cancer. Rev Assoc Med Bras (1992). 2020;66(11):1530–5.
Cui S, et al. LncRNA MAFG-AS1 promotes the progression of colorectal cancer by sponging miR-147b and activation of NDUFA4. Biochem Biophys Res Commun. 2018;506(1):251–8.
Fu Y, et al. LncRNA MAFG-AS1 upregulates polo-like kinase-1 by sponging miR-505 to promote gastric adenocarcinoma cell proliferation. Crit Rev Eukaryot Gene Expr. 2021;31(5):27–32.
Li C, Wu R, Xing Y. MAFG-AS1 is a novel clinical biomarker for clinical progression and unfavorable prognosis in gastric cancer. Cell Cycle. 2020;19(5):601–9.
Qian CJ, et al. LncRNA MAFG-AS1 accelerates cell migration, invasion and aerobic glycolysis of esophageal squamous cell carcinoma cells via miR-765/PDX1 axis. Cancer Manag Res. 2020;12:6895–908.
Wang K, et al. 5-methylcytosine RNA methyltransferases-related long non-coding RNA to develop and validate biochemical recurrence signature in prostate cancer. Front Mol Biosci. 2021;8: 775304.
Ye L, et al. MAFG-AS1 aggravates the progression of pancreatic cancer by sponging miR-3196 to boost NFIX. Cancer Cell Int. 2020;20(1):591.
Bai Y, et al. LncRNA MAFG-AS1 promotes the malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther. 2022;29(3–4):277–91.
Zhao H, et al. LncRNA MAFG-AS1 suppresses the maturation of miR-34a to promote glioblastoma cell proliferation. Cancer Manag Res. 2021;13:3493–501.
Wangzhou K, et al. LncRNA MAFG-AS1 regulates human periodontal ligament stem cell proliferation and Toll-like receptor 4 expression. Oral Dis. 2020. https://doi.org/10.1111/odi.13330.
Wu L, et al. LncRNA TONSL-AS1 participates in coronary artery disease by interacting with miR-197. Microvasc Res. 2021;136: 104152.
Przytycki PF, Singh M. Differential allele-specific expression uncovers breast cancer genes dysregulated by Cis noncoding mutations. Cell Syst. 2020;10(2):193-203.e4.
Totsuka Y, Watanabe M, Lin Y. New horizons of DNA adductome for exploring environmental causes of cancer. Cancer Sci. 2021;112(1):7–15.
Lu J, et al. Clinical features and treatment outcome in newly diagnosed Chinese patients with multiple myeloma: results of a multicenter analysis. Blood Cancer J. 2014;4(8): e239.
Fashoyin-Aje L, et al. Integrating genetic and genomic information into effective cancer care in diverse populations. Ann Oncol. 2013;24(Suppl 7):vii48-54.
Inhoffen J, et al. Deficiency of iPLA2β primes immune cells for proinflammation: potential involvement in age-related mesenteric lymph node lymphoma. Cancers (Basel). 2015;7(4):2427–42.
Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–23.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009.
Iuliano JN, et al. Metastatic bladder cancer cells distinctively sense and respond to physical cues of collagen fibril-mimetic nanotopography. Exp Biol Med (Maywood). 2015;240(5):601–10.
Hoock SC, et al. RITA modulates cell migration and invasion by affecting focal adhesion dynamics. Mol Oncol. 2019;13(10):2121–41.
Esa R, et al. The role of methionine aminopeptidase 2 in lymphangiogenesis. Int J Mol Sci. 2020;21(14):5148.
Zu XL, Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun. 2004;313(3):459–65.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47.
Kashima Y, et al. Combinatory use of distinct single-cell RNA-seq analytical platforms reveals the heterogeneous transcriptome response. Sci Rep. 2018;8(1):3482.
Xu D, et al. LncRNA DLEU1 contributes to the growth and invasion of colorectal cancer via targeting miR-320b/PRPS1. Front Oncol. 2021;11: 640276.
Pal J, et al. Systematic analysis of migration factors by MigExpress identifies essential cell migration control genes in non-small cell lung cancer. Mol Oncol. 2021;15(7):1797–817.
Xu P, et al. Stretch-induced tenomodulin expression promotes tenocyte migration via F-actin and chromatin remodeling. Int J Mol Sci. 2021;22(9):4928.
Miebach L, et al. Tumor cytotoxicity and immunogenicity of a novel V-jet neon plasma source compared to the kINPen. Sci Rep. 2021;11(1):136.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–91.
Talagala IA, Nawarathne M, Arambepola C. Novel risk factors for primary prevention of oesophageal carcinoma: a case-control study from Sri Lanka. BMC Cancer. 2018;18(1):1135.
Liu J, et al. Ginsenoside Rh2 pretreatment and withdrawal reactivated the pentose phosphate pathway to ameliorate intracellular redox disturbance and promoted intratumoral penetration of adriamycin. Redox Biol. 2020;32: 101452.
Ascione CM, et al. Role of FGFR3 in bladder cancer: treatment landscape and future challenges. Cancer Treat Rev. 2023;115: 102530.
Funding
This work was supported by the Henan provincial Medical Science and Technology Research Project (LHGJ20230453), and Science and technology Research program of Henan Province (212102310191).
Author information
Authors and Affiliations
Contributions
PL and XM contributed equally to this work. PL designed the work, PL, XM and XG wrote this manuscript, and made figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, P., Ma, X. & Gu, X. LncRNA MAFG-AS1 is involved in human cancer progression. Eur J Med Res 28, 497 (2023). https://doi.org/10.1186/s40001-023-01486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01486-9