Abstract
CircRNAs, covalently closed noncoding RNAs, are widely expressed in a wide range of species ranging from viruses to plants to mammals. CircRNAs were enriched in the Wnt pathway. Aberrant Wnt pathway activation is involved in the development of various types of cancers. Accumulating evidence indicates that the circRNA/Wnt axis modulates the expression of cancer-associated genes and then regulates cancer progression. Wnt pathway-related circRNA expression is obviously associated with many clinical characteristics. CircRNAs could regulate cell biological functions by interacting with the Wnt pathway. Moreover, Wnt pathway-related circRNAs are promising potential biomarkers for cancer diagnosis, prognosis evaluation, and treatment. In our review, we summarized the recent research progress on the role and clinical application of Wnt pathway-related circRNAs in tumorigenesis and progression.
Similar content being viewed by others
Background
Cancer is one of the main causes of death today and has become a serious public health problem worldwide [1–5]. It is a complex disease that involves changes in a variety of processes, including genetic and epigenetic characteristic changes [6–8]. The molecular changes in cancer genes and related signaling pathways could provide information for cancer diagnosis and targeted therapy [9–11]. This information could contribute to improvements in cancer diagnosis and treatment.
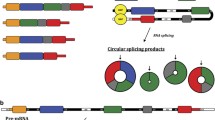
Human genome sequence data indicate that more than 98% of the genome is noncoding genes [12–14]. The transcripts of these genes lack protein-coding ability and are recognized as noncoding RNAs (ncRNAs) [15–18]. ncRNAs were once considered byproducts of transcription [19–21]. With the development of high-throughput sequencing technology, ncRNA features have gradually been revealed. ncRNAs comprise various types of RNA species, including microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs) [22–24]. CircRNA is a single-stranded, covalently closed ncRNA without 5’ end caps or 3’ end poly (A) tails [25–28]. It is generated from its precursor mRNA by noncanonical splicing [29–31] and is widely expressed in a wide range of species ranging from viruses to plants to mammals [32, 33]. circRNAs may act as transcription modulators, miRNA sponges, or protein decoys to exert their function in cancer progression [34–36]. In addition, circRNAs are obviously associated with many clinical characteristics [37–41], which could provide important guidance for the accurate diagnosis and treatment of cancer. Accumulating evidence indicates that circRNAs play a pivotal role in the process of cancer and have the potential to be biomarkers in cancer diagnosis, prognosis, and treatment [42–46].
The Wnt pathway is an evolutionarily conserved pathway [47–49]. It plays a critical role in embryonic development, tissue renewal and regeneration [50–52]. The Wnt pathway can be divided into three classes: Wnt/β-catenin signaling, Wnt/planar cell polarity (PCP) signaling, and Wnt/Ca signaling [47, 53, 54]. Aberrant activation of the Wnt pathway is significantly correlated with a series of cancers, such as lung cancer [55–57], colorectal cancer [58, 59], bladder cancer [60, 61], osteosarcoma [62, 63], glioma [64, 65], and chronic lymphocytic leukemia [66, 67]. Accumulating evidence indicates that circRNAs regulate a series of cellular biological functions by interacting with the Wnt pathway in the cancer process [68–70]. These studies provided novel perspectives into cancer diagnosis and treatment. circRNAs related to the Wnt pathway have been the focus of many cancer research studies [63, 69, 71–73]. In this review, we summarized the recent research progress regarding the molecular mechanisms and functional roles of circRNAs related to the Wnt pathway in tumorigenesis and tumor progression.
The wnt pathway in tumorigenesis
The Wnt gene was first identified in mouse mammary tumors in 1982 [74–76]. At that time, it was designated as int1 [75, 77]. Because of the high homology between the mouse int1 gene and the Drosophila Wingless gene, the researchers merged Wingless with Int1 and assigned the name Wnt gene [78, 79]. The Wnt gene, localized at 12q13, mediates physiological effects in a paracrine and autocrine manner [78, 80]. The signaling pathways regulated by the Wnt gene are collectively termed the Wnt pathway. The Wnt signaling pathway is highly conserved from Drosophila to humans. The pathway [81–83] is critical for a wide variety of cellular functions, such as cell polarity, movement, proliferation, asymmetric division, and muscle tissue development. Wnts are a family of secreted, lipid-modified proteins that bind to Frizzled receptors to activate signaling cascades [84, 85]. The Wnt pathway can be divided into three classes: Wnt/β-catenin signaling, Wnt/planar cell polarity signaling, and Wnt/Ca signaling [86–89] (Fig. 1). Wnt/β-catenin signaling, a canonical Wnt signaling pathway, is involved in the regulation of gene expression [90–92]. Wnt/planar cell polarity signaling regulates cell polarity and directional cell movements [83, 93, 94]. Wnt/Ca signaling is obviously associated with the release of intracellular calcium [95, 96]. Dysregulation of the Wnt pathway has a strong relevance to cancer.
Wnt/β-catenin signaling
The Wnt/β-catenin signaling pathway is characterized by the cellular redistribution and nuclear accumulation of the β-catenin gene [97, 98]. Wnt protein combines with Frz and LRP5/6 on the cell surface to form a trimer, which transmits the signal and activates the protein Disheveled [Dsh/DVL] [99, 100]. This leads to the disassociation of the β-catenin degradation complex adenomatous polyposis coli (APC)/Axin/GSK-3β (glycogen synthase kinase 3β) and increases the cytoplasmic levels of β-catenin [101, 102]. Then, upregulated β-catenin is transferred into the nucleus. Nuclear β-catenin interacts with T cell transcription factor (TCF])/lymphoid enhancer factor (LEF) and finally activates the expression of downstream target genes [98, 103–105]. The Wnt/β-catenin signaling pathway participates in the cancer process by acting as an important modulator [106–108] of cell proliferation, metastasis, and differentiation. Overexpression of the Wnt gene or mutation in one of the components that causes β-catenin degradation leads to activation of the Wnt/β-catenin pathway.
Wnt/PCP signaling
In the Wnt/PCP signaling pathway, Wnt binds to frizzled transmembrane receptors and then activates the protein Disheveled (Dsh/DVL), leading to a series of cell signaling cascades [109–112]. DSH is connected to the downstream effectors Rho and ROCK (Rho-associated kinase) through Daam1. RAC is directly activated by Dsh, and Dsh further activates JNK by activating mitogen-activated protein kinases (MAP3Ks) and MAP2Ks [113, 114]. The PCP pathway is associated with cell polarity, cell alignment and cell migration.
Wnt/Ca 2 + signaling
In the Wnt/Ca 2 + pathway, the Wnt protein is mainly composed of Wnt1, Wnt5A and Wnt11 and binds to the Frizzled transmembrane receptor on the cell surface [115, 116]. The combination of the Wnt protein and Frizzled activates Disheveled, which activates PLC through the G protein [117, 118]. These cellular processes could finally promote the release of intracellular Ca2+. The activation of Disheveled could also activate the cGMP-specific phosphodiesterase PDE6 and reduce intracellular cGMP, which leads to an increase in the intracellular Ca2 + concentration [119–123]. Elevated cytoplasmic Ca2 + concentrations can stimulate the nuclear factor NFAT and other transcription factors [124, 125]. These processes trigger the activation of downstream pathways and a series of altered cell functions. The Wnt/Ca2 + pathway is essential for early embryonic development, interneural communication and the inflammatory response [126, 127].
CircRNA in the wnt pathway
CircRNAs, first found in the 1890s [128], remain enigmatic owing to technological limitations and limited existing knowledge. In 2013, Hansen TB et al. first proposed and confirmed that circRNAs function as miRNA sponges [129]. This finding started a new era in circRNA research [32, 42, 130–133]. Unlike linear RNAs, circRNAs are single-stranded, covalently closed noncoding RNAs without 5’ end caps or 3’ end poly (A) tails [25–28]. CircRNAs are not affected by RNA exonuclease, and their expression is more stable [134, 135]. CircRNAs are formed by reverse splicing events [29–31]. A mechanistic model argued that the RNA is partially folded during the transcription of pre-RNA. Initially, nonadjacent exons are pulled closer by RNA folding, and exon skipping occurs. The spanned region forms a circular RNA intermediate, and then circRNAs are formed by further splicing. Another model suggests that the reverse complement sequence located in the intron region causes the intron region to pair and mediate reverse splicing to form circRNA [136–140].
CircRNAs act mainly through four molecular mechanisms. In regulating gene expression, circRNAs affect the expression of parental gene mRNA by interacting with RNA binding proteins [141–143]. Competitive complementary pairing between introns can strike a balance with linear RNAs during the formation of circRNAs. CircRNAs can also exert their functions by acting as competing endogenous RNAs (ceRNAs) of miRNAs [144–147]. In addition, circRNAs are involved in the immune response [29, 148, 149]. Endogenous circRNAs play a role in the antiviral response, while exogenous circRNAs can stimulate immune signaling in mammalian cells by activating the pattern recognition receptor RIG-L [150–153]. Moreover, although circRNAs are noncoding RNAs, a few circRNAs can also perform regulatory functions by encoding peptides [154–156]. Several previous studies have shown that circRNAs play an important role in tumorigenesis and tumor progression. CircRNA_403658 facilitates aerobic glycolysis and cell growth by upregulating LDHA expression in bladder cancer [157]. CircRNA_103809 functions as an oncogene in the progression of hepatocellular carcinoma [158].
Both circRNAs and the Wnt pathway play a critical role in cancer development and progression. CircRNAs negatively or positively regulate cancer initiation, promotion, and progression by directly or indirectly interacting with the Wnt pathway. The interaction of circRNAs and the Wnt pathway has a noticeable impact on cell growth, metastasis, and other malignant cell behaviors in cancer. The majority of circRNAs act as sponges of miRNAs to activate or inactivate the Wnt pathway. With the deepening of research, more action modes between circRNAs and the Wnt pathway will be found. Related studies are expected to provide new insights for the diagnosis and treatment of cancer.
The role of the circRNA/Wnt axis in cancer
CircRNAs related to the Wnt pathway are aberrantly expressed in many cancers. Emerging evidence suggests that a range of clinical characteristics have been associated with circRNAs related to the Wnt pathway (Table 1). Moreover, the circRNA/Wnt axis contributes to cancer progression by modulating many cell biological functions. In this section, we will introduce the expression, corresponding clinical features, functions and mechanisms of the circRNAs/Wnt axis (Table 2).
Digestive tumors
Esophageal cancer
Elevated levels of circRNA_100367 were observed in radioresistant esophageal cancer cell lines [192], while the expression of cir-ITCH was downregulated in esophageal squamous cell carcinoma (ESCC) tissues [193] (Fig. 2). The expression of cir-ITCH is positively associated with linear ITCH in ESCC. Functionally, colony formation and Cell Counting Kit-8 (CCK-8) assays showed that cir-ITCH could inhibit ESCC tumor growth through the regulation of cell proliferation. Knockdown of circRNA_100367 attenuates cell proliferation, migration, and radioresistance in esophageal cancer [192]. circRNA_100367 decreases radiation sensitivity by regulating the miR-217/Wnt3 pathway. CircRNA_100367 could also affect esophageal cancer cell growth under irradiation in vivo. Using bioinformatics tools, Su et al. [234] found that a large number of circRNAs were closely related to cancer progression. Further studies on these molecules are still required.
Gastric cancer
Some Wnt pathway-related circRNAs (circ0005654, circ-SFMBT2, circ_SMAD4, circRNA_0044516, and circHIPK3) are markedly upregulated in gastric cancer [73, 159, 161, 195, 197]. The expression of circ0005654, circ_SMAD4, circHIPK3, and circheckd1 are positively associated with a poor prognosis in patients with gastric cancer [73, 159–161]. Functionally, these circRNAs all contribute to promoting tumor cell proliferation in gastric cancer [73, 159, 161, 194, 195, 197]. Additionally, circ0005654, circRNA_ASAP2, circ-SFMBT2, and circHIPK3 obviously promote gastric cancer cell migration and invasion. Circ-SFMBT2 upregulation indicates higher levels of oxidative stress in gastric cancer [195]. Mechanistically, in vitro and in vivo studies demonstrated that circ0005654 functions as a ceRNA of miR-363 to upregulate sp1 in the process of gastric cancer [159]. The level of CTNNB1 is regulated by circ-SFMBT2, a sponge of miR-1276 [73]. Circ-SFMBT2 activates the Wnt/β-catenin pathway by upregulating CTNNB1 expression. CircRNA_0044516 affects cancer progression by regulating the miR-149/Wnt1/β-catenin axis [197].
Interestingly, some researchers found that the expression of circCNIH4, cir-ITCH, and circ_0001649 was significantly downregulated in gastric cancer tissues and cells [160, 196, 198]. cir-ITCH is closely related to lymph node metastasis and patient prognosis [160]. CircCNIH4, cir-ITCH, and circ_0001649 markedly reduced cell proliferation, migration, and invasion in gastric cancer cell lines. CircCNIH4 and circ_0001649 also contribute to gastric cancer progression through the regulation of cell apoptosis [196, 198]. CircCNIH4 inhibits the Wnt/β-catenin pathway by upregulating DKK2 and FRZB levels (Fig. 3). Similarly, cir-ITCH reduce miR-17 levels to inactivate the Wnt/β-catenin pathway. Circ_0001649 inhibits the ERK and Wnt/β-catenin signaling pathways by sponging miR‐20a.
The mechanisms of Wnt-associated circRNAs in gastric cancer. A Cir-ITCH downregulates miR-17 expression to inactivate the Wnt/β-catenin pathway. B CircCNIH4 inhibits the Wnt/β-catenin pathway by upregulating DKK2 and FRZB levels in gastric cancer. C Circ_0001649 inhibits the ERK and Wnt/β-catenin signaling pathways by acting as a sponge of miR-20a in gastric cancer
Colorectal cancer [CRC]
CircRNA dysregulation has been discovered to be closely related to the occurrence and progression of CRC. Wnt pathway-associated circRNAs of CRC are shown in Table 1 [70, 162–168, 199–207]. Circ_0082182, circ-PRKDC, circ5615, and circ_0005075 are significantly correlated with advanced tumor-node-metastasis (TNM) stage in CRC [70, 163, 164, 167]. The overexpression of circRASSF2, circ_0082182, circ5615, circcct3, circ_0005075, and circRNA_100290 indicates a poor prognosis in CRC patients [70, 162, 163, 165, 167, 168]. Circ-PRKDC is also associated with lymph node metastasis and tumor size [164]. Circ_0005075 expression is correlated with differentiation and the depth of tumor invasion [162, 167]. Functionally, the expression of cis-HOX facilitates the self-renewal of colorectal tumor-initiating cells [201]. Circ-ABCC1 could regulate malignant phenotypes, such as cell sphere formation ability, cell migration, and cell stemness, in CRC [204]. The role of circ-PRKDC in 5-fluorouracil resistance has been reported [164]. Additionally, the other Wnt pathway-associated upregulated circRNAs (Table 1) inhibit CRC cell growth and metastasis [70, 202, 205,206,207].
Liver cancer
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer [235–238]. The expression of circRNA-SORE, circβ-catenin, circZFR, and circ_0067934 is relatively elevated in HCC [71, 172, 173, 210]. In particular, increased circRNA-SORE levels were found in sorafenib-resistant HCC. CircZFR and circ_0067934 levels are significantly associated with the prognosis of patients with HCC [172, 173]. The expression of circ_0067934 is also markedly correlated with tumor TNM stage in HCC [173]. CircRNA-SORE, cZNF292, circβ-catenin, circZFR, and circ_0067934 markedly facilitates cell proliferation [172, 173, 209, 210, 239]. Additionally, cZNF292, circRNA-SORE, and circ_0067934 reduce cell apoptosis [71, 173, 210, 239], while cZNF292 has no apparent effect on apoptosis [209]. The overexpression of circβ-catenin, circZFR and circ_0067934 increased the migration or invasion of HCC cancer cells [172, 173, 210]. High circRNA-SORE levels are important for maintaining HCC sorafenib resistance [71]. Mechanistically, some circRNAs interact with Wnt/β-catenin via other molecules in HCC. Circ_0067934 regulates HCC cell behaviors by activating the miR-1324/FZD5/wnt/β-catenin axis [173]. cZNF292 increases Wnt/β-catenin pathway activity through the upregulation of sex-determining region Y (SRY)-box 9 (SOX9) nuclear translocation [209].
On the other hand, the expression of circ_0004018, circ_0003418, and circ-ITCH is significantly downregulated in HCC [169, 170, 174, 208]. CircZKSCAN1 and circ-ITCH are potential prognostic biomarkers [171, 174]. Circ_0004018 and circ_0003418 are negatively correlated with tumor size [169, 170]. In addition, the expression of circ_0003418 has been reported to be related to TNM stage and HBsAg levels in HCC [170]. Circ_0004018 and circ_0003418 contribute to cancer development and progression by regulating many cell biological functions, including cell proliferation, migration, and invasion. Knockdown of circZKSCAN1 could inhibit the malignant behaviors of HCC cancer stem cells, such as sphere formation, colony formation, cell proliferation, and metastasis. Circ_0004018 modulates the Wnt/β-catenin pathway to accelerate HCC progression by targeting the miR-626/DKK3 axis. CircZKSCAN1 binds with FMRP to increase Wnt signaling activity in HCC.
Pancreatic cancer
Pancreatic cancer is a digestive tract malignancy with limited treatment options and poor life expectancy [240–243]. Pancreatic ductal adenocarcinoma is the most common primary malignancy of the pancreas [244–246]. The expression of circ_0030167 is significantly elevated in bone marrow mesenchymal stem cells (BM-MSCs) [211]. Yao et al. isolated BM-MSCs from human bone marrow. circ_0030167, obtained from BM-MSC-derived exosomes, attenuates pancreatic cancer cell growth, metastasis, and stemness. Exosomal circ_0030167 activates the WIF1/Wnt8/β-catenin axis by sponging miR-338-5p in pancreatic cancer. An increasing number of Wnt pathway-associated circRNAs have also been found in pancreatic ductal adenocarcinoma [247]. However, the underlying functions and mechanisms still need to be further explored.
The respiratory system tumor
Lung cancer
Lung cancer is the main cause of cancer-associated mortality worldwide [248–252]. It can be classified into non-small-cell lung cancer (NSCLC) and small-cell lung cancer, and NSCLC accounts for the overwhelming majority of lung cancer cases [253–255]. Wnt pathway-associated circRNAs of NSCLC are shown in Table 1 [69, 175–181, 212–216] (Fig. 4). The overexpression of circ_000984 and circ_001569 is significantly correlated with TNM stage and lymph node metastasis in NSCLC [175, 176]. The circ_0001946 expression profile is obviously associated with TNM stage and tumor size in NSCLC [177]. Additionally, circ_000984, circ_001569, and circ_0001946 upregulation predicts a poor prognosis in patients with NSCLC [175–177]. These upregulated circRNAs in NSCLC could promote cell growth by enhancing cell proliferation [69, 175–177, 212–216]. In vitro astray assays showed that silencing circ_0067934 and circ_000984 could inhibit the epithelial-mesenchymal transition (EMT) process to reduce cell metastasis in NSCLC [175, 215]. Circ-PGC could also hinder cancer progression by suppressing glycolysis metabolism [212]. Mechanistically, the majority of circRNAs interact with miRNAs to activate the Wnt/β-catenin pathway in NSCLC [69, 177, 212–216].
Interestingly, circ_0018414, circ_0006427, circ_0007059, and cir-ITCH are remarkably downregulated in NSCLC [178–181]. Circ_0018414 and circ_0006427 are markedly associated with the overall survival rate [178, 179]. Circ_0006427 and circ_0007059 facilitate cell growth and motility in NSCLC [179, 180]. Circ_0018414 enhances stemness features by promoting DKK1 expression in NSCLC [178] (Fig. 5). CircRNAs can inhibit NSCLC tumorigenesis and progression by regulating the circ_0018414/miR-6807-3p/dkk1/Wnt/β-catenin, circ_0006427/ miR-6783-3p/dkk1/Wnt/β-catenin, and circ_0007059/miR-378/Wnt/β-catenin pathways and the cir-ITCH/miR-7/miR-214/ITCH/Wnt/β-catenin axis.
The mechanisms of Wnt-associated circRNA in lung cancer. A In lung cancer, circ_0018414 inhibits cancer progression by regulating miR-6807-3p/DKK1 and the Wnt/β-catenin pathway. DKK1 is upregulated by circ_0018414, a ceRNA of miR-6783-3p. B Circ 0007059 inhibits the Wnt/β-catenin by acting as a sponge for miR-378. C Cir-ITCH upregulates ITCH expression to inactivate Wnt/β-catenin signaling by sponging miR-7 and miR-214
Nervous system neoplasms
Glioma
Malignant gliomas are the most common primary tumors of the central nervous system [256–259]. Wnt pathway-associated circRNAs have drawn much attention in glioma research in recent years [260–263]. The levels of circ_0001730, circKIF4A, circ_0000177, and cZNF292 are upregulated in glioma [182, 183, 217, 218, 264] tissues versus normal brain tissues. Circ_0000177 is related to clinical stage, and patients with increased circ_0000177 expression have a poor prognosis [183]. Circ_0001730, circKIF4A, and circ_0000177 are all involved in tumor cell growth and metastasis in glioma [182, 183, 217]. cZNF292 promotes cancer development by regulating cell proliferation, the cell cycle, and angiogenesis. Mechanistically, circ_0001730 functions as a sponge of miR-326 to positively regulate Wnt/β-catenin pathways in the pathophysiologic processes of glioma. Circ_0001730 could also be upregulated by SP1 [218]. Overexpression of circ_0000177 increases FZD7 levels to activate Wnt signaling mediated by miR-638 in glioma.
Genitourinary tumors
Prostate cancer (PCa)
PCa refers to an epithelial malignancy that occurs in the prostate [265–269]. The expression of cir-ITCH was significantly downregulated in PCa tissues and cell lines [219]. Further experiments showed that cir-ITCH could attenuate PCa cell viability and invasion. Cir-ITCH hinders PCa development by inactivating the Wnt/β-Catenin and PI3K/AKT/mTOR pathways. Not much is known about Wnt pathway-associated circRNAs in PCa. There is a crucial need for Wnt pathway-associated circRNA research in PCa [219].
Female reproductive system cancers
Cancers that originate in the female reproductive system are called female reproductive cancers [270]. Ovarian cancer (OC), endometrial cancer (EC), and cervical cancer are the three most common gynecological malignancies [271–274]. The expression of circ‑ABCB10 is significantly upregulated, while circPLEKHM3 expression is downregulated in OC [184, 220]. Moreover, the level of circPLEKHM3 is positively associated with the overall survival rate in patients with OC [184]. Circ‑ABCB10 remarkably facilitates cell proliferation and invasion and reduces cell apoptosis by miR-1271 in OC [220]. Circ‑ABCB10 plays a critical role in OC progression via the regulation of Capn4/Wnt/β‑catenin. CircPLEKHM3 inhibits cell proliferation and migration by sponging miR-9 and regulating the BRCA1/DNAJB6/KLF4/AKT1/Wnt/β-catenin axis in OC [184]. Circ_0109046 and circ_0002577 are elevated in EC tissues and cell lines [185, 186]. The overexpression of circ_0002577 is positively correlated with advanced FIGO stage and lymph node metastasis in EC. High expression of circ_0109046 and circ_0002577 predicts a poor prognosis in patients with EC. Circ_0109046 activates the Wnt/β-catenin pathway by sponging miR-105 to increase SOX9 levels. Circ_0002577 functions as a sponge of miR-197 to regulate the CTNND1/Wnt/β-catenin axis in EC. CircSAMD11 expression is markedly upregulated in cervical cancer [221]. Silencing of circSAMD11 expression suppressed cell proliferation and metastasis and promoted cell apoptosis in cervical cancer. The circSAMD11/miR-503/sox4/Wnt/β-catenin axis plays an essential role in the progression of cervical cancer [221].
Tumors of the blood system
Hematological malignancies, also known as neoplasms of the blood, lymph nodes and bone marrow, include leukemia, lymphoma, and multiple myeloma [275–278]. The common types of leukemia are acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), and chronic lymphocytic leukemia (CLL) [279–282]. Circ_0121582 expression is significantly decreased in AML [222]. Functional experiments demonstrated that the overexpression of circ_0121582 significantly attenuated cell survival and promoted the cell cycle arrest in AML. Circ_0121582 activates Wnt/β-catenin by sponging miR-224 to increase GSK3β expression in AML. The expression of circ-CBFB was upregulated in CLL, and it has been reported as an independent predictive factor for the prognosis of CLL [223]. Circ-CBFB facilitates CLL cell proliferation and inhibits cell apoptosis by sponging miR-607 and upregulating the FZD3/Wnt/β-catenin axis. Diffuse large B-cell lymphoma (DLBCL) is the most common malignant lymphoma subtype [283–285]. The level of circ-APC is significantly decreased in the tissues, cell lines, and plasma of DLBCL patients versus normal controls [224]. circ-APC inactivates the Wnt/β-catenin pathway to suppress cell proliferation in DLBCL through the regulation of the miR-888/APC and TET1/APC axes.
Tumors of the musculoskeletal systems
Osteosarcoma (OS)
OS is the most common primary malignant neoplasm of the bone and mainly affects children, adolescents, and young adults [286–289]. The level of circMYO10 is significantly elevated [63], while circ_0002052 expression is downregulated in OS tissues and cell lines [187]. The expression of circ_0002052 is positively associated with overall and progression-free survival in patients with OS. circ_0002052 inhibits cell growth and cell motility and enhances cell apoptosis in OS by sponging miR-1205 and modulating the APC2/Wnt/β-catenin axis. CircMYO10 functions as an oncogene in OS progression. The overexpression of circMYO10 facilitates OS cell proliferation and EMT in vitro. CircMYO10 facilitates histone H4K16 acetylation by regulating the miR-370-3p/RUVBL1 axis and activating Wnt/β-catenin signaling in OS. Cisplatin (DDP) is a conventional chemotherapy drug in the treatment of OS [290–293]. Cisplatin resistance is a major challenge for OS chemotherapy application [294, 295]. CircUBAP2 expression is increased in cisplatin-resistant OS tissues and cells [225]. Silencing circUBAP2 inhibits cell proliferation, migration, and invasion and induced apoptosis in OS. CircUBAP2 knockdown also suppresses cisplatin resistance by regulating miR-506-3p/SEMA6D and the Wnt/β-catenin pathway [225].
Tumors of the endocrine system
Thyroid cancer
The incidence rate of thyroid cancer has been increasing throughout the world [296–300]. CircRNA_102171 and circRNA_NEK6 are relatively upregulated [226, 227], while circ-ITCH is downregulated in thyroid cancer tissues and cell lines [188]. The level of circ-ITCH is closely associated with clinical stage, lymph node metastasis, and patient prognosis in thyroid cancer. CircRNA_102171 and circRNA_NEK6 play a promoting role in cell growth and metastasis. CircRNA_102171 activates the Wnt/β-catenin pathway in a CTNNBIP1-dependent way [226]. CircRNA_NEK6 facilitates thyroid cancer progression by sponging miR-370-3p and upregulating the FZD8/Wnt axis [227]. Circ-ITCH exerts its tumor suppressor action by modulating miR-22-3p/CBL/β-catenin in thyroid cancer [188].
Tumors of other systems
Breast cancer is one of the most common malignant malignancies among females worldwide [301–304]. Circ-EIF6, circARL8B, circABCC4, circRNA_069718, and circFAT1 expression levels are obviously upregulated in breast cancer [189, 191, 228–230]. CircRNA_069718 overexpression is positively correlated with TNM stage, lymph node metastasis, and overall survival in patients with breast cancer [191]. These upregulated Wnt-associated circRNAs contribute to cancer progression by promoting cell growth and metastasis. In addition, studies also observed that knockdown of circARL8B could induce a suppressive effect on fatty acid metabolism in breast cancer [228]. CircFAT1 enhances oxaliplatin resistance through the miR-525-5p/SKA1 and Wnt pathways in breast cancer [230]. CircARL8B, circABCC4, and CircFAT1 regulate the Wnt pathway by acting as sponges of miRNAs in breast cancer. EIF6-224aa, encoded by circ-EIF6, activates Wnt/β-catenin by regulating the MYH9/Wnt/beta-catenin pathway [189].
Melanoma is a potentially fatal disease with increasing incidence [305–309]. Circ_0027247 was isolated from circ-GLI1 [232]. Circ_0119872, circ_0084043 and circ-GLI1 (circ_0027247) are dramatically upregulated in melanoma tissues and cell lines [231–233]. High levels of circ_0027247 and circ_0084043 can promote cell motility [232], while circ_0119872 has no influence on cell migration and invasion [231]. Circ_0119872 and circ_0027247 are novel negative feedback regulators of angiogenesis in melanoma. Circ_0119872 and circ_0084043 have the same effects on cell proliferation. Circ_0119872 activates the Wnt/β-catenin pathway by interacting with p70S6K2 and upregulates Cyr61 expression in melanoma. The tumorigenesis and progression of melanoma are also regulated by the circ_0119872/ p70S6K2/Wnt/β-catenin and circ_0027247/miR-622/G3BP1/Wnt/β-catenin axes [231].
CircRNA, a potential biomarker in wnt pathway
Despite technological advances, cancer diagnosis and treatment are still a challenge that may require the emergence of new tumor biomarkers [310, 311]. Increasing evidence has revealed that Wnt-associated circRNAs are closely related to cancer progression. Wnt-associated circRNAs may be very promising biomarkers in cancer diagnosis, prognosis, and treatment. In this section, we will further discuss their potential application in clinical practice.
Diagnosis
The early screening and diagnosis of cancer is conducive to the survival of cancer patients [312–316]. Identifying suitable biomarkers has always been a difficult issue in cancer research. Wnt-associated circRNAs may be used to assist early diagnosis in many cancers. They are aberrantly expressed in many kinds of tumors from multiple systems, such as digestive tumors, respiratory system tumors, nervous system neoplasms, genitourinary tumors, musculoskeletal system tumors and endocrine system cancers. Moreover, plasma circ-APC levels are significantly downregulated in DLBCL [224]. This discovery indicates a more convenient clinical application of circ-APC as a diagnostic marker. Studies further evaluated the diagnostic potential for cancer by receiver operating characteristic (ROC) curve analysis. Yang et al. found that the AUC value of circ0005654 was 0.781 in gastric cancer [159]. ROC analysis of circRASSF2 expression levels in colorectal cancer tissues and cells accurately discriminated between CRC patients and healthy controls (AUC: 0.9863) [162]. Further experimental verification and research on circRASSF2 in body fluids is necessary. The corresponding AUC value for circ-CBFB was 0.80 in chronic lymphocytic leukemia [223].
Prognosis prediction
Early prognostic information is important in making treatment decisions [317–321]. A growing amount of evidence shows that Wnt-associated circRNAs can be of important prognostic value. These circRNAs are closely related to overall survival, disease-free survival, recurrence-free survival, 5-year survival rate, and progression-free survival in several cancers. Patients with lower circZKSCAN1 expression have shorter overall and recurrence-free survival in HCC [171]. Li et al. [166] reported that the overexpression of circCCT3 was negatively correlated with the disease-free survival rate in colorectal cancer. Higher circ_0109046 expression predicts a decreased 5-year survival rate in patients with endometrial carcinoma [185]. Such studies have important implications in prognosis evaluation and treatment selection. In addition, Wnt-associated circRNAs are associated with other relevant prognostic factors. For example, downregulated circMTO1 levels predict advanced TNM stage and lymph node metastasis in CRC [70].
Cancer treatment
Despite rapidly progressing treatment modalities, cancer therapy remains one of the most challenging issues in the world. CircRNA-based targeted therapeutic strategies shed new light on the evolution of cancer treatment [42, 43, 262, 322, 323]. CircRNAs regulate many cell biological functions by directly or indirectly interacting with the Wnt pathway. CircRNA_NEK6 activated the FZD8/Wnt axis to facilitate thyroid cancer progression by sponging miR-370-3p [227]. Circ_0121582 promotes GSK3β expression to activate the Wnt/β-catenin pathway by sponging miR-224 in AML [222]. Circ-SFMBT2 contributes to the development and tumorigenesis of gastric cancer via regulation of the miR-1276/CTNNB1/Wnt/β-catenin axis [195]. Controlling Wnt-associated circRNA expression may be an effective approach for cancer treatment. The knockdown of circ_SMAD4 blocked gastric cancer progression by negatively regulating cell growth [73]. Silencing circ-ZNF124 expression inhibited malignant phenotypes in NSCLC cells [73]. In addition, Circ-ITCH is a tumor suppressor in many cancers [160, 174, 181, 188, 190, 193, 207, 208, 219]. Wang et al. found that upregulated circ-ITCH expression suppressed cell proliferation and invasion in papillary thyroid cancer [188]. However, the identification of targeted drugs that can stably control the expression of circRNA and transmit this effect is the current difficulty. This requires a deeper understanding of the structure and function of Wnt-associated circRNAs. The majority of circRNAs act as sponges of miRNAs to activate or inactivate the Wnt pathway. Regulating the target miRNAs of Wnt-associated circRNAs may also be feasible. MiR-582 intervention effectively reversed the cell biological functions regulated by circ_0009361 in CRC [205].
Conclusions and future perspectives
The Wnt signaling pathway is highly involved in cancer development, and essential for a wide variety of cellular functions, such as cell polarity, movement, proliferation, asymmetric division, and muscle tissue development. Both circRNA and the Wnt pathway play a critical role in cancer development and progression. Emerging data suggest that the circRNA/Wnt axis modulates the expression of cancer-associated genes and then regulates tumor progression. CircRNAs are enriched in the Wnt pathway. Wnt-associated circRNAs are abnormally expressed in digestive tumors, respiratory system tumors, nervous system neoplasms, genitourinary tumors, musculoskeletal system tumors, endocrine system cancers and other cancers. Their aberrant expression indicates their potential as diagnostic markers. However, most related experiments are based on tissue and cell research. Ideal and effective molecular markers should be stably expressed in plasma, serum, and other body fluids. Such molecules have greater potential for clinical applications. Wnt-associated circRNAs are also promising potential biomarkers in the treatment of cancer. CircRNAs negatively or positively regulate cancer initiation, promotion, and progression by directly or indirectly interacting with the Wnt pathway. We could enhance the expression of cancer-promoting circRNAs or inhibit the expression of tumor suppressor circRNAs to control cancer progression. The current goal is to find targeted drugs that can stably control the expression of circRNA and induce this effect. We need to further understand the structure and function of Wnt-related circRNAs. Furthermore, the interaction and the related mechanisms between circRNAs involved in the Wnt pathway need more studies to confirm.
Availability of data and materials
Not applicable.
Abbreviations
- ncRNAs:
-
noncoding RNAs
- miRNAs:
-
microRNAs
- lncRNAs:
-
long ncRNAs
- circRNAs:
-
circular RNAs
- PCP:
-
Wnt/planar cell polarity
- GSK-3β:
-
glycogen synthase kinase 3β
- TCF:
-
T cell transcription factor
- LEF:
-
lymphoid enhancer factor
- ceRNAs:
-
competing endogenous RNAs
- ESCC:
-
esophageal squamous cell carcinoma
- CCK-8:
-
Cell Counting Kit-8
- CRC:
-
colorectal cancer
- TNM:
-
tumor-node-metastasis
- HCC:
-
hepatocellular carcinoma
- SRY:
-
sex-determining region Y
- SOX9:
-
sex-determining region Y-box 9
- BM-MSCs:
-
bone marrow mesenchymal stem cells
- NSCLC:
-
non-small-cell lung cancer
- EMT:
-
epithelial-mesenchymal transition
- KPS:
-
Karnofsky Performance Status
- OC:
-
ovarian cancer
- EC:
-
endometrial cancer
- AML:
-
acute myeloid leukemia
- CML:
-
chronic myeloid leukemia
- ALL:
-
acute lymphoblastic leukemia
- CLL:
-
chronic lymphocytic leukemia
- DLBCL:
-
diffuse large B-cell lymphoma
- OS:
-
osteosarcoma
- DDP:
-
cisplatin
- ROC:
-
receiver operating characteristic
References
Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A. Cancer death rates in US congressional districts. CA Cancer J Clin. 2015;65:339–44. https://doi.org/10.3322/caac.21292.
Siegel RL, Miller KD, Jemal A. Cancer statistics. 2020. CA Cancer J Clin. 2020;70:7–30. https://doi.org/10.3322/caac.21590.
Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264–79. https://doi.org/10.3322/caac.21657.
Rinaldi F, Hanieh PN, Del Favero E, Rondelli V, Brocca P, Pereira MC, et al. Decoration of Nanovesicles with pH (Low) Insertion Peptide (pHLIP) for Targeted Delivery. Nanoscale Res Lett. 2018;13:391. https://doi.org/10.1186/s11671-018-2807-8.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493–502. https://doi.org/10.1038/s41575-021-00457-x.
Zhou S, Treloar AE, Lupien M. Emergence of the Noncoding Cancer Genome: A Target of Genetic and Epigenetic Alterations. Cancer Discov. 2016;6:1215–29. https://doi.org/10.1158/2159-8290.Cd-16-0745.
Morel D, Jeffery D, Aspeslagh S, Almouzni G, Postel-Vinay S. Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise. Nat Rev Clin Oncol. 2020;17:91–107. https://doi.org/10.1038/s41571-019-0267-4.
van der Pol Y, Mouliere F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell. 2019;36:350–68. https://doi.org/10.1016/j.ccell.2019.09.003.
Calses PC, Crawford JJ, Lill JR, Dey A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer. 2019;5:297–307. https://doi.org/10.1016/j.trecan.2019.04.001.
Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–918. https://doi.org/10.3390/ijms13021886.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. https://doi.org/10.1038/sj.onc.1210421.
Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, et al. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452–5. https://doi.org/10.1038/nature20149.
Lu S, Zhang J, Lian X, Sun L, Meng K, Chen Y, et al. A hidden human proteome encoded by ‘non-coding’ genes. Nucleic Acids Res. 2019;47:8111–25. https://doi.org/10.1093/nar/gkz646.
Saw PE, Xu X, Chen J, Song EW. Non-coding RNAs: the new central dogma of cancer biology. Sci China Life Sci. 2021;64:22–50. https://doi.org/10.1007/s11427-020-1700-9.
Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16:167–79. https://doi.org/10.1038/nrd.2016.117.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. https://doi.org/10.1038/nrg2521.
Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet 2006;15 Spec No 1:R17–29. https://doi.org/10.1093/hmg/ddl046.
Li G, Zhang T, Huang K, Zhu Y, Xu K, Gu J, et al. Long noncoding RNA GAS8-AS1: A novel biomarker in human diseases. Biomed Pharmacother. 2021;139:111572. https://doi.org/10.1016/j.biopha.2021.111572.
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18. https://doi.org/10.1038/nrc.2017.99.
Wright MW, Bruford EA. Naming ‘junk’: human non-protein coding RNA (ncRNA) gene nomenclature. Hum Genomics. 2011;5:90–8. https://doi.org/10.1186/1479-7364-5-2-90.
Liu J, Liu T, Wang X, He A. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58. https://doi.org/10.1186/s12943-017-0630-y.
Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297–325. https://doi.org/10.1152/physrev.00041.2015.
Chan JJ, Tay Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int J Mol Sci 2018;19. https://doi.org/10.3390/ijms19051310.
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T, Zhang L. Analyzing the Interactions of mRNAs and ncRNAs to Predict Competing Endogenous RNA Networks in Osteosarcoma Chemo-Resistance. Mol Ther. 2019;27:518–30. https://doi.org/10.1016/j.ymthe.2019.01.001.
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94. https://doi.org/10.1186/s12943-017-0663-2.
Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503–17. https://doi.org/10.7150/thno.42174.
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Wu M. Epigenetics in Neurodevelopment: Emerging Role of Circular RNA. Front Cell Neurosci. 2019;13:327. https://doi.org/10.3389/fncel.2019.00327.
Yang X, Ye T, Liu H, Lv P, Duan C, Wu X, et al. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol Cancer. 2021;20:4. https://doi.org/10.1186/s12943-020-01300-8.
Chen X, Yang T, Wang W, Xi W, Zhang T, Li Q, et al. Circular RNAs in immune responses and immune diseases. Theranostics. 2019;9:588–607. https://doi.org/10.7150/thno.29678.
Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G, et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat Plants. 2017;3:17053. https://doi.org/10.1038/nplants.2017.53.
Jiang JY, Ju CJ, Hao J, Chen M, Wang W. JEDI: circular RNA prediction based on junction encoders and deep interaction among splice sites. Bioinformatics. 2021;37:i289-i98. https://doi.org/10.1093/bioinformatics/btab288.
Zhou WY, Cai ZR, Liu J, Wang DS, Ju HQ, Xu RH. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172. https://doi.org/10.1186/s12943-020-01286-3.
Zhou J, Ge Y, Hu Y, Rong D, Fu K, Wang H, et al. Circular RNAs as novel rising stars with huge potentials in development and disease. Cancer Biomark. 2018;22:597–610. https://doi.org/10.3233/cbm-181296.
Dong W, Dai ZH, Liu FC, Guo XG, Ge CM, Ding J, et al. The RNA-binding protein RBM3 promotes cell proliferation in hepatocellular carcinoma by regulating circular RNA SCD-circRNA 2 production. EBioMedicine. 2019;45:155–67. https://doi.org/10.1016/j.ebiom.2019.06.030.
Wang L, Yi J, Lu LY, Zhang YY, Wang L, Hu GS, et al. Estrogen-induced circRNA, circPGR, functions as a ceRNA to promote estrogen receptor-positive breast cancer cell growth by regulating cell cycle-related genes. Theranostics. 2021;11:1732–52. https://doi.org/10.7150/thno.45302.
He D, Yang X, Kuang W, Huang G, Liu X, Zhang Y. The Novel Circular RNA Circ-PGAP3 Promotes the Proliferation and Invasion of Triple Negative Breast Cancer by Regulating the miR-330-3p/Myc Axis. Onco Targets Ther. 2020;13:10149–59. https://doi.org/10.2147/ott.S274574.
Zeng K, He B, Yang BB, Xu T, Chen X, Xu M, et al. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17:160. https://doi.org/10.1186/s12943-018-0914-x.
Zeng K, Wang S. Circular, RNAs. The crucial regulatory molecules in colorectal cancer. Pathol Res Pract. 2020;216:152861. https://doi.org/10.1016/j.prp.2020.152861.
Liu P, Zou Y, Li X, Yang A, Ye F, Zhang J, et al. circGNB1 Facilitates Triple-Negative Breast Cancer Progression by Regulating miR-141-5p-IGF1R Axis. Front Genet. 2020;11:193. https://doi.org/10.3389/fgene.2020.00193.
Shen C, Wu Z, Wang Y, Gao S, Da L, Xie L, et al. Downregulated hsa_circ_0077837 and hsa_circ_0004826, facilitate bladder cancer progression and predict poor prognosis for bladder cancer patients. Cancer Med. 2020;9:3885–903. https://doi.org/10.1002/cam4.3006.
Li Y, Shi P, Zheng T, Ying Z, Jiang D. Circular RNA hsa_circ_0131242 Promotes Triple-Negative Breast Cancer Progression by Sponging hsa-miR-2682. Onco Targets Ther. 2020;13:4791–8. https://doi.org/10.2147/ott.S246957.
Xu X, Zhang J, Tian Y, Gao Y, Dong X, Chen W, et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol Cancer. 2020;19:128. https://doi.org/10.1186/s12943-020-01246-x.
Yang H, Li X, Meng Q, Sun H, Wu S, Hu W, et al. CircPTK2 (hsa_circ_0005273) as a novel therapeutic target for metastatic colorectal cancer. Mol Cancer. 2020;19:13. https://doi.org/10.1186/s12943-020-1139-3.
Szabo L, Salzman J. Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet. 2016;17:679–92. https://doi.org/10.1038/nrg.2016.114.
Bai H, Lei K, Huang F, Jiang Z, Zhou X. Exo-circRNAs: a new paradigm for anticancer therapy. Mol Cancer. 2019;18:56. https://doi.org/10.1186/s12943-019-0986-2.
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Zhang Y, et al. The Landscape of Circular RNA in Cancer. Cell 2019;176:869 – 81.e13. https://doi.org/10.1016/j.cell.2018.12.021.
Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19:165. https://doi.org/10.1186/s12943-020-01276-5.
Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10:101. https://doi.org/10.1186/s13045-017-0471-6.
Schunk SJ, Floege J, Fliser D, Speer T. WNT-β-catenin signalling - a versatile player in kidney injury and repair. Nat Rev Nephrol. 2021;17:172–84. https://doi.org/10.1038/s41581-020-00343-w.
Bengoa-Vergniory N, Kypta RM. Canonical and noncanonical Wnt signaling in neural stem/progenitor cells. Cell Mol Life Sci. 2015;72:4157–72. https://doi.org/10.1007/s00018-015-2028-6.
Mah AT, Yan KS, Kuo CJ. Wnt pathway regulation of intestinal stem cells. J Physiol. 2016;594:4837–47. https://doi.org/10.1113/jp271754.
Blagodatski A, Klimenko A, Jia L, Katanaev VL. Small Molecule Wnt Pathway Modulators from Natural Sources: History, State of the Art and Perspectives. Cells 2020;9. https://doi.org/10.3390/cells9030589.
Arredondo SB, Valenzuela-Bezanilla D, Mardones MD, Varela-Nallar L. Role of Wnt Signaling in Adult Hippocampal Neurogenesis in Health and Disease. Front Cell Dev Biol. 2020;8:860. https://doi.org/10.3389/fcell.2020.00860.
Camilli TC, Weeraratna AT. Striking the target in Wnt-y conditions: intervening in Wnt signaling during cancer progression. Biochem Pharmacol 2010;80:702 – 11. https://doi.org/10.1016/j.bcp.2010.03.002.
Pan J, Fang S, Tian H, Zhou C, Zhao X, Tian H, et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer. 2020;19:9. https://doi.org/10.1186/s12943-020-1133-9.
Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124. https://doi.org/10.1186/s12943-017-0700-1.
Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222:1–10. https://doi.org/10.1016/j.canlet.2004.08.040.
Tang Q, Chen J, Di Z, Yuan W, Zhou Z, Liu Z, et al. TM4SF1 promotes EMT and cancer stemness via the Wnt/β-catenin/SOX2 pathway in colorectal cancer. J Exp Clin Cancer Res. 2020;39:232. https://doi.org/10.1186/s13046-020-01690-z.
Han P, Li JW, Zhang BM, Lv JC, Li YM, Gu XY, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer. 2017;16:9. https://doi.org/10.1186/s12943-017-0583-1.
Tan TZ, Rouanne M, Tan KT, Huang RY, Thiery JP. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-cohort Analysis of 2411 Tumors. Eur Urol. 2019;75:423–32. https://doi.org/10.1016/j.eururo.2018.08.027.
Zhan Y, Zhang L, Yu S, Wen J, Liu Y, Zhang X. Long non-coding RNA CASC9 promotes tumor growth and metastasis via modulating FZD6/Wnt/β-catenin signaling pathway in bladder cancer. J Exp Clin Cancer Res. 2020;39:136. https://doi.org/10.1186/s13046-020-01624-9.
Matsuoka K, Bakiri L, Wolff LI, Linder M, Mikels-Vigdal A, Patiño-García A, et al. Wnt signaling and Loxl2 promote aggressive osteosarcoma. Cell Res. 2020;30:885–901. https://doi.org/10.1038/s41422-020-0370-1.
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z, et al. CircMYO10 promotes osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to enhance the transcriptional activity of β-catenin/LEF1 complex via effects on chromatin remodeling. Mol Cancer. 2019;18:150. https://doi.org/10.1186/s12943-019-1076-1.
Yue X, Lan F, Xia T. Hypoxic Glioma Cell-Secreted Exosomal miR-301a Activates Wnt/β-catenin Signaling and Promotes Radiation Resistance by Targeting TCEAL7. Mol Ther. 2019;27:1939–49. https://doi.org/10.1016/j.ymthe.2019.07.011.
Zhang J, Cai H, Sun L, Zhan P, Chen M, Zhang F, et al. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37:225. https://doi.org/10.1186/s13046-018-0864-6.
Mangolini M, Götte F, Moore A, Ammon T, Oelsner M, Lutzny-Geier G, et al. Notch2 controls non-autonomous Wnt-signalling in chronic lymphocytic leukaemia. Nat Commun. 2018;9:3839. https://doi.org/10.1038/s41467-018-06069-5.
Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:3118–23. https://doi.org/10.1073/pnas.0308648100.
Huang G, Liang M, Liu H, Huang J, Li P, Wang C, et al. CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell Death Dis. 2020;11:1065. https://doi.org/10.1038/s41419-020-03276-1.
Gao N, Ye B. Circ-SOX4 drives the tumorigenesis and development of lung adenocarcinoma via sponging miR-1270 and modulating PLAGL2 to activate WNT signaling pathway. Cancer Cell Int. 2020;20:2. https://doi.org/10.1186/s12935-019-1065-x.
Ge Z, Li LF, Wang CY, Wang Y, Ma WL. CircMTO1 inhibits cell proliferation and invasion by regulating Wnt/β-catenin signaling pathway in colorectal cancer. Eur Rev Med Pharmacol Sci. 2018;22:8203–9. https://doi.org/10.26355/eurrev_201812_16513.
Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L, et al. N(6)-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol Cancer. 2020;19:163. https://doi.org/10.1186/s12943-020-01281-8.
He J, Xie Q, Xu H, Li J, Li Y. Circular RNAs and cancer. Cancer Lett 2017;396:138 – 44. https://doi.org/10.1016/j.canlet.2017.03.027.
Wang L, Li B, Yi X, Xiao X, Zheng Q, Ma L. Circ_SMAD4 promotes gastric carcinogenesis by activating wnt/β-catenin pathway. Cell Prolif. 2021;54:e12981. https://doi.org/10.1111/cpr.12981.
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. https://doi.org/10.1016/0092-8674(82)90409-3.
Chen X, Yang J, Evans PM, Liu C. Wnt signaling: the good and the bad. Acta Biochim Biophys Sin (Shanghai). 2008;40:577 – 94. https://doi.org/10.1111/j.1745-7270.2008.00440.x.
Korzh V. Winding roots of Wnts. Zebrafish. 2008;5:159–68. https://doi.org/10.1089/zeb.2008.0532.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469 – 80. https://doi.org/10.1016/j.cell.2006.10.018.
Perochon J, Carroll LR, Cordero JB. Wnt Signalling in Intestinal Stem Cells: Lessons from Mice and Flies. Genes (Basel). 2018;9. https://doi.org/10.3390/genes9030138.
Mukherjee T, Balaji KN. The WNT Framework in Shaping Immune Cell Responses During Bacterial Infections. Front Immunol. 2019;10:1985. https://doi.org/10.3389/fimmu.2019.01985.
Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA 2nd, Rucker EB 3. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80:989–1000. https://doi.org/10.1095/biolreprod.108.075416. rd, et al.
Poindexter KM, Matthew S, Aronchik I, Firestone GL. Cooperative antiproliferative signaling by aspirin and indole-3-carbinol targets microphthalmia-associated transcription factor gene expression and promoter activity in human melanoma cells. Cell Biol Toxicol. 2016;32:103–19. https://doi.org/10.1007/s10565-016-9321-5.
Su X, Zhao Y, Wang Y, Zhang L, Zan L, Wang H. Overexpression of the Rybp Gene Inhibits Differentiation of Bovine Myoblasts into Myotubes. Int J Mol Sci 2018;19. https://doi.org/10.3390/ijms19072082.
Vladar EK, Königshoff M. Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem Soc Trans. 2020;48:231–43. https://doi.org/10.1042/bst20190597.
Tan Y, Yu D, Busto GU, Wilson C, Davis RL. Wnt signaling is required for long-term memory formation. Cell Rep. 2013;4:1082–9. https://doi.org/10.1016/j.celrep.2013.08.007.
Cerpa W, Farías GG, Godoy JA, Fuenzalida M, Bonansco C, Inestrosa NC. Wnt-5a occludes Abeta oligomer-induced depression of glutamatergic transmission in hippocampal neurons. Mol Neurodegener. 2010;5:3. https://doi.org/10.1186/1750-1326-5-3.
Pandit AA, Gandham RK, Mukhopadhyay CS, Verma R, Sethi RS. Transcriptome analysis reveals the role of the PCP pathway in fipronil and endotoxin-induced lung damage. Respir Res. 2019;20:24. https://doi.org/10.1186/s12931-019-0986-1.
Bian J, Dannappel M, Wan C, Firestein R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells 2020;9. https://doi.org/10.3390/cells9092125.
Van Steenwinckel J, Schang AL, Krishnan ML, Degos V, Delahaye-Duriez A, Bokobza C, et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain. 2019;142:3806–33. https://doi.org/10.1093/brain/awz319.
Murillo-Garzón V, Gorroño-Etxebarria I, Åkerfelt M, Puustinen MC, Sistonen L, Nees M, et al. Frizzled-8 integrates Wnt-11 and transforming growth factor-β signaling in prostate cancer. Nat Commun. 2018;9:1747. https://doi.org/10.1038/s41467-018-04042-w.
Ueno A, Masugi Y, Yamazaki K, Komuta M, Effendi K, Tanami Y, et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014;61:1080–7. https://doi.org/10.1016/j.jhep.2014.06.008.
Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, et al. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31:3729–42. https://doi.org/10.1523/jneurosci.4270-10.2011.
Niell N, Larriba MJ, Ferrer-Mayorga G, Sánchez-Pérez I, Cantero R, Real FX, et al. The human PKP2/plakophilin-2 gene is induced by Wnt/β-catenin in normal and colon cancer-associated fibroblasts. Int J Cancer. 2018;142:792–804. https://doi.org/10.1002/ijc.31104.
Kaucká M, Petersen J, Janovská P, Radaszkiewicz T, Smyčková L, Daulat AM, et al. Asymmetry of VANGL2 in migrating lymphocytes as a tool to monitor activity of the mammalian WNT/planar cell polarity pathway. Cell Commun Signal. 2015;13:2. https://doi.org/10.1186/s12964-014-0079-1.
Babayeva S, Rocque B, Aoudjit L, Zilber Y, Li J, Baldwin C, et al. Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem. 2013;288:24035–48. https://doi.org/10.1074/jbc.M113.452904.
Vargas JY, Loria F, Wu YJ, Córdova G, Nonaka T, Bellow S, et al. The Wnt/Ca(2+) pathway is involved in interneuronal communication mediated by tunneling nanotubes. Embo j. 2019;38:e101230. https://doi.org/10.15252/embj.2018101230.
Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: a convergent model of Wnt/Ca2 + and Wnt/β-catenin pathways. J Biol Chem. 2013;288:35651–9. https://doi.org/10.1074/jbc.M112.437913.
Jamieson C, Lui C, Brocardo MG, Martino-Echarri E, Henderson BR. Rac1 augments Wnt signaling by stimulating β-catenin-lymphoid enhancer factor-1 complex assembly independent of β-catenin nuclear import. J Cell Sci. 2015;128:3933–46. https://doi.org/10.1242/jcs.167742.
Nguyen VHL, Hough R, Bernaudo S, Peng C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J Ovarian Res. 2019;12:122. https://doi.org/10.1186/s13048-019-0596-z.
Henson JH, Samasa B, Shuster CB, Wikramanayake AH. The nanoscale organization of the Wnt signaling integrator Dishevelled in the vegetal cortex domain of an egg and early embryo. PLoS One. 2021;16:e0248197. https://doi.org/10.1371/journal.pone.0248197.
Guo J, Cagatay T, Zhou G, Chan CC, Blythe S, Suyama K, et al. Mutations in the human naked cuticle homolog NKD1 found in colorectal cancer alter Wnt/Dvl/beta-catenin signaling. PLoS One. 2009;4:e7982. https://doi.org/10.1371/journal.pone.0007982.
Ji L, Lu B, Wang Z, Yang Z, Reece-Hoyes J, Russ C, et al. Identification of ICAT as an APC Inhibitor, Revealing Wnt-Dependent Inhibition of APC-Axin Interaction. Mol Cell. 2018;72:37–47.e4. https://doi.org/10.1016/j.molcel.2018.07.040.
Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 2012;149:1192 – 205. https://doi.org/10.1016/j.cell.2012.05.012.
Doumpas N, Lampart F, Robinson MD, Lentini A, Nestor CE, Cantù C, et al. TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. Embo j 2019;38. https://doi.org/10.15252/embj.201798873.
Danek P, Kardosova M, Janeckova L, Karkoulia E, Vanickova K, Fabisik M, et al. β-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood. 2020;136:2574–87. https://doi.org/10.1182/blood.2019004664.
Fu X, Zhu X, Qin F, Zhang Y, Lin J, Ding Y, et al. Linc00210 drives Wnt/β-catenin signaling activation and liver tumor progression through CTNNBIP1-dependent manner. Mol Cancer. 2018;17:73. https://doi.org/10.1186/s12943-018-0783-3.
Huang K, Zhang JX, Han L, You YP, Jiang T, Pu PY, et al. MicroRNA roles in beta-catenin pathway. Mol Cancer. 2010;9:252. https://doi.org/10.1186/1476-4598-9-252.
Yu CY, Kuo HC. The emerging roles and functions of circular RNAs and their generation. J Biomed Sci. 2019;26:29. https://doi.org/10.1186/s12929-019-0523-z.
Jo S, Yoon S, Lee SY, Kim SY, Park H, Han J, et al. DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells 2020;9. https://doi.org/10.3390/cells9010236.
Yu JJS, Maugarny-Calès A, Pelletier S, Alexandre C, Bellaiche Y, Vincent JP, et al. Frizzled-Dependent Planar Cell Polarity without Secreted Wnt Ligands. Dev Cell 2020;54:583 – 92.e5. https://doi.org/10.1016/j.devcel.2020.08.004.
Rogers S, Scholpp S. Vertebrate Wnt5a - At the crossroads of cellular signalling. Semin Cell Dev Biol. 2021;125:3–10. https://doi.org/10.1016/j.semcdb.2021.10.002.
Lapébie P, Borchiellini C, Houliston E. Dissecting the PCP pathway: one or more pathways?: Does a separate Wnt-Fz-Rho pathway drive morphogenesis? Bioessays. 2011;33:759–68. https://doi.org/10.1002/bies.201100023.
Muñoz-Descalzo S, Gómez-Cabrero A, Mlodzik M, Paricio N. Analysis of the role of the Rac/Cdc42 GTPases during planar cell polarity generation in Drosophila. Int J Dev Biol. 2007;51:379–87. https://doi.org/10.1387/ijdb.062250sm.
Park E, Kim GH, Choi SC, Han JK. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev Biol. 2006;292:344–57. https://doi.org/10.1016/j.ydbio.2006.01.011.
Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–68. https://doi.org/10.1002/dvdy.20262.
De A. Wnt/Ca2 + signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 2011;43:745–56. https://doi.org/10.1093/abbs/gmr079.
Zhuang X, Zhang H, Li X, Li X, Cong M, Peng F, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. 2017;19:1274–85. https://doi.org/10.1038/ncb3613.
Flores-Hernández E, Velázquez DM, Castañeda-Patlán MC, Fuentes-García G, Fonseca-Camarillo G, Yamamoto-Furusho JK, et al. Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell Signal. 2020;72:109636. https://doi.org/10.1016/j.cellsig.2020.109636.
Gong B, Shen W, Xiao W, Meng Y, Meng A, Jia S. The Sect. 14-like phosphatidylinositol transfer proteins Sec14l3/SEC14L2 act as GTPase proteins to mediate Wnt/Ca(2+) signaling. Elife 2017;6. https://doi.org/10.7554/eLife.26362.
Ma L, Wang HY. Mitogen-activated protein kinase p38 regulates the Wnt/cyclic GMP/Ca2 + non-canonical pathway. J Biol Chem. 2007;282:28980–90. https://doi.org/10.1074/jbc.M702840200.
Wang H, Lee Y, Malbon CC. PDE6 is an effector for the Wnt/Ca2+/cGMP-signalling pathway in development. Biochem Soc Trans. 2004;32:792–6. https://doi.org/10.1042/bst0320792.
Chen Y, Chen Z, Tang Y, Xiao Q. The involvement of noncanonical Wnt signaling in cancers. Biomed Pharmacother. 2021;133:110946. https://doi.org/10.1016/j.biopha.2020.110946.
Burst HV. RCM Annual Meetings. New frontiers. Nurs Times. 1982;78:suppl 3–7.
Malbon CC, Wang HY. Dishevelled: a mobile scaffold catalyzing development. Curr Top Dev Biol. 2006;72:153–66. https://doi.org/10.1016/s0070-2153(05)72002-0.
Fiedler B, Wollert KC. Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes. Cardiovasc Res. 2004;63:450–7. https://doi.org/10.1016/j.cardiores.2004.04.002.
Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2 + pathway. J Biol Chem. 2006;281:30990–1001. https://doi.org/10.1074/jbc.M603603200.
Jiang X, Guan Y, Zhao Z, Meng F, Wang X, Gao X, et al. Potential Roles of the WNT Signaling Pathway in Amyotrophic Lateral Sclerosis. Cells 2021;10. https://doi.org/10.3390/cells10040839.
Weerackoon N, Gunawardhana KL, Mani A. Wnt Signaling Cascades and Their Role in Coronary Artery Health and Disease. J Cell Signal. 2021;2:52–62. https://doi.org/10.33696/Signaling.2.035.
Fang N, Ding GW, Ding H, Li J, Liu C, Lv L, et al. Research Progress of Circular RNA in Gastrointestinal Tumors. Front Oncol. 2021;11:665246. https://doi.org/10.3389/fonc.2021.665246.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. https://doi.org/10.1038/nature11993.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91. https://doi.org/10.1038/s41576-019-0158-7.
Gao Y, Shang S, Guo S, Li X, Zhou H, Liu H, et al. Lnc2Cancer 3.0: an updated resource for experimentally supported lncRNA/circRNA cancer associations and web tools based on RNA-seq and scRNA-seq data. Nucleic Acids Res. 2021;49:D1251-d8. https://doi.org/10.1093/nar/gkaa1006.
Patop IL, Wüst S, Kadener S. Past, present, and future of circRNAs. Embo j. 2019;38:e100836. https://doi.org/10.15252/embj.2018100836.
Chen L, Wang C, Sun H, Wang J, Liang Y, Wang Y, et al. The bioinformatics toolbox for circRNA discovery and analysis. Brief Bioinform. 2021;22:1706–28. https://doi.org/10.1093/bib/bbaa001.
Xiao MS, Wilusz JE. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3’ ends. Nucleic Acids Res. 2019;47:8755–69. https://doi.org/10.1093/nar/gkz576.
Aufiero S, Reckman YJ, Pinto YM, Creemers EE. Circular RNAs open a new chapter in cardiovascular biology. Nat Rev Cardiol. 2019;16:503–14. https://doi.org/10.1038/s41569-019-0185-2.
Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. https://doi.org/10.1016/j.molcel.2014.08.019.
Eger N, Schoppe L, Schuster S, Laufs U, Boeckel JN. Circular RNA Splicing. Adv Exp Med Biol. 2018;1087:41–52. https://doi.org/10.1007/978-981-13-1426-1_4.
Wilusz JE. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip Rev RNA. 2018;9:e1478. https://doi.org/10.1002/wrna.1478.
Pervouchine DD. Circular exonic RNAs: When RNA structure meets topology. Biochim Biophys Acta Gene Regul Mech. 2019;1862:194384. https://doi.org/10.1016/j.bbagrm.2019.05.002.
Liu X, Hu Z, Zhou J, Tian C, Tian G, He M, et al. Interior circular RNA. RNA Biol. 2020;17:87–97. https://doi.org/10.1080/15476286.2019.1669391.
Du WW, Zhang C, Yang W, Yong T, Awan FM, Yang BB. Identifying and Characterizing circRNA-Protein Interaction. Theranostics. 2017;7:4183–91. https://doi.org/10.7150/thno.21299.
Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: An important part of circRNA maintenance and function. J Neurosci Res. 2020;98:87–97. https://doi.org/10.1002/jnr.24356.
Okholm TLH, Sathe S, Park SS, Kamstrup AB, Rasmussen AM, Shankar A, et al. Transcriptome-wide profiles of circular RNA and RNA-binding protein interactions reveal effects on circular RNA biogenesis and cancer pathway expression. Genome Med. 2020;12:112. https://doi.org/10.1186/s13073-020-00812-8.
Li X, Ding J, Wang X, Cheng Z, Zhu Q. NUDT21 regulates circRNA cyclization and ceRNA crosstalk in hepatocellular carcinoma. Oncogene. 2020;39:891–904. https://doi.org/10.1038/s41388-019-1030-0.
Wang J, Zhao X, Wang Y, Ren F, Sun D, Yan Y, et al. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11:32. https://doi.org/10.1038/s41419-020-2230-9.
Li H, Xu JD, Fang XH, Zhu JN, Yang J, Pan R, et al. Circular RNA circRNA_000203 aggravates cardiac hypertrophy via suppressing miR-26b-5p and miR-140-3p binding to Gata4. Cardiovasc Res. 2020;116:1323–34. https://doi.org/10.1093/cvr/cvz215.
Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–12. https://doi.org/10.1158/0008-5472.Can-13-1568.
Yan L, Chen YG. Circular RNAs in Immune Response and Viral Infection. Trends Biochem Sci. 2020;45:1022–34. https://doi.org/10.1016/j.tibs.2020.08.006.
Yang J, Cheng M, Gu B, Wang J, Yan S, Xu D. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κB axis. Cell Death Dis. 2020;11:833. https://doi.org/10.1038/s41419-020-03038-z.
Su H, Zheng W, Pan J, Lv X, Xin S, Xu T. Circular RNA. circSamd4a Regulates Antiviral Immunity in Teleost Fish by Upregulating STING through Sponging miR-29a-3p. J Immunol. 2021;207:2770–84. https://doi.org/10.4049/jimmunol.2100469.
Su H, Chu Q, Zheng W, Chang R, Gao W, Zhang L, et al. Circular RNA circPIKfyve acts as a sponge of miR-21-3p to enhance antiviral immunity through regulating MAVS in teleost fish. J Virol 2021;95. https://doi.org/10.1128/jvi.02296-20.
Zheng W, Chu Q, Yang L, Sun L, Xu T. Circular. RNA circDtx1 regulates IRF3-mediated antiviral immune responses through suppression of miR-15a-5p-dependent TRIF downregulation in teleost fish. PLoS Pathog. 2021;17:e1009438. https://doi.org/10.1371/journal.ppat.1009438.
Wesselhoeft RA, Kowalski PS, Parker-Hale FC, Huang Y, Bisaria N, Anderson DG. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol Cell 2019;74:508 – 20.e4. https://doi.org/10.1016/j.molcel.2019.02.015.
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. https://doi.org/10.1186/s12943-020-1147-3.
Wang Y, Liu B. Circular RNA in Diseased Heart. Cells 2020;9. https://doi.org/10.3390/cells9051240.
Wang J, Zhu S, Meng N, He Y, Lu R, Yan GR. ncRNA-Encoded Peptides or Proteins and Cancer. Mol Ther. 2019;27:1718–25. https://doi.org/10.1016/j.ymthe.2019.09.001.
Wei Y, Zhang Y, Meng Q, Cui L, Xu C. Hypoxia-induced circular RNA has_circRNA_403658 promotes bladder cancer cell growth through activation of LDHA. Am J Transl Res. 2019;11:6838–49.
Zhan W, Liao X, Chen Z, Li L, Tian T, Yu L, et al. Circular RNA hsa_circRNA_103809 promoted hepatocellular carcinoma development by regulating miR-377-3p/FGFR1/ERK axis. J Cell Physiol. 2020;235:1733–45. https://doi.org/10.1002/jcp.29092.
Yang C, Han S. The circular RNA circ0005654 interacts with specificity protein 1 via microRNA-363 sequestration to promote gastric cancer progression. Bioengineered. 2021;12:6305–17. https://doi.org/10.1080/21655979.2021.1971031.
Peng Y, Wang HH. Cir-ITCH inhibits gastric cancer migration, invasion and proliferation by regulating the Wnt/β-catenin pathway. Sci Rep. 2020;10:17443. https://doi.org/10.1038/s41598-020-74452-8.
Liu WG, Xu Q. Upregulation of circHIPK3 promotes the progression of gastric cancer via Wnt/β-catenin pathway and indicates a poor prognosis. Eur Rev Med Pharmacol Sci. 2019;23:7905–12. https://doi.org/10.26355/eurrev_201909_19004.
Yang L, Bi T, Zhou S, Lan Y, Zhang R. CircRASSF2 facilitates the proliferation and metastasis of colorectal cancer by mediating the activity of Wnt/β-catenin signaling pathway by regulating the miR-195-5p/FZD4 axis. Anticancer Drugs. 2021;32:919–29. https://doi.org/10.1097/cad.0000000000001084.
Liu R, Deng P, Zhang Y, Wang Y, Peng C. Circ_0082182 promotes oncogenesis and metastasis of colorectal cancer in vitro and in vivo by sponging miR-411 and miR-1205 to activate the Wnt/β-catenin pathway. World J Surg Oncol. 2021;19:51. https://doi.org/10.1186/s12957-021-02164-y.
Chen H, Pei L, Xie P, Guo G. Circ-PRKDC Contributes to 5-Fluorouracil Resistance of Colorectal Cancer Cells by Regulating miR-375/FOXM1 Axis and Wnt/β-Catenin Pathway. Onco Targets Ther. 2020;13:5939–53. https://doi.org/10.2147/ott.S253468.
Ma Z, Han C, Xia W, Wang S, Li X, Fang P, et al. circ5615 functions as a ceRNA to promote colorectal cancer progression by upregulating TNKS. Cell Death Dis. 2020;11:356. https://doi.org/10.1038/s41419-020-2514-0.
Li W, Xu Y, Wang X, Cao G, Bu W, Wang X, et al. circCCT3 Modulates Vascular Endothelial Growth Factor A and Wnt Signaling to Enhance Colorectal Cancer Metastasis Through Sponging miR-613. DNA Cell Biol. 2020;39:118–25. https://doi.org/10.1089/dna.2019.5139.
Jin YD, Ren YR, Gao YX, Zhang L, Ding Z. Hsa_circ_0005075 predicts a poor prognosis and acts as an oncogene in colorectal cancer via activating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23:3311–9. https://doi.org/10.26355/eurrev_201904_17693.
Fang G, Ye BL, Hu BR, Ruan XJ, Shi YX. CircRNA_100290 promotes colorectal cancer progression through miR-516b-induced downregulation of FZD4 expression and Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2018;504:184–9. https://doi.org/10.1016/j.bbrc.2018.08.152.
Zhu P, Liang H, Huang X, Zeng Q, Liu Y, Lv J, et al. Circular RNA Hsa_circ_0004018 Inhibits Wnt/β-Catenin Signaling Pathway by Targeting microRNA-626/DKK3 in Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:9351–64. https://doi.org/10.2147/ott.S254997.
Chen H, Liu S, Li M, Huang P, Li X. circ_0003418 Inhibits Tumorigenesis And Cisplatin Chemoresistance Through Wnt/β-Catenin Pathway In Hepatocellular Carcinoma. Onco Targets Ther. 2019;12:9539–49. https://doi.org/10.2147/ott.S229507.
Zhu YJ, Zheng B, Luo GJ, Ma XK, Lu XY, Lin XM, et al. Circular RNAs negatively regulate cancer stem cells by physically binding FMRP against CCAR1 complex in hepatocellular carcinoma. Theranostics. 2019;9:3526–40. https://doi.org/10.7150/thno.32796.
Tan A, Li Q, Chen L. CircZFR promotes hepatocellular carcinoma progression through regulating miR-3619-5p/CTNNB1 axis and activating Wnt/β-catenin pathway. Arch Biochem Biophys. 2019;661:196–202. https://doi.org/10.1016/j.abb.2018.11.020.
Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D, et al. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun. 2018;497:626–32. https://doi.org/10.1016/j.bbrc.2018.02.119.
Guo W, Zhang J, Zhang D, Cao S, Li G, Zhang S, et al. Polymorphisms and expression pattern of circular RNA circ-ITCH contributes to the carcinogenesis of hepatocellular carcinoma. Oncotarget. 2017;8:48169–77. https://doi.org/10.18632/oncotarget.18327.
Li XY, Liu YR, Zhou JH, Li W, Guo HH, Ma HP. Enhanced expression of circular RNA hsa_circ_000984 promotes cells proliferation and metastasis in non-small cell lung cancer by modulating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23:3366–74. https://doi.org/10.26355/eurrev_201904_17700.
Ding L, Yao W, Lu J, Gong J, Zhang X. Upregulation of circ_001569 predicts poor prognosis and promotes cell proliferation in non-small cell lung cancer by regulating the Wnt/β-catenin pathway. Oncol Lett. 2018;16:453–8. https://doi.org/10.3892/ol.2018.8673.
Yao Y, Hua Q, Zhou Y, Shen H. CircRNA has_circ_0001946 promotes cell growth in lung adenocarcinoma by regulating miR-135a-5p/SIRT1 axis and activating Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019;111:1367–75. https://doi.org/10.1016/j.biopha.2018.12.120.
Yao Y, Zhou Y, Hua Q. circRNA hsa_circ_0018414 inhibits the progression of LUAD by sponging miR-6807-3p and upregulating DKK1. Mol Ther Nucleic Acids. 2021;23:783–96. https://doi.org/10.1016/j.omtn.2020.12.031.
Yao Y, Hua Q, Zhou Y. CircRNA has_circ_0006427 suppresses the progression of lung adenocarcinoma by regulating miR-6783-3p/DKK1 axis and inactivating Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2019;508:37–45. https://doi.org/10.1016/j.bbrc.2018.11.079.
Gao S, Yu Y, Liu L, Meng J, Li G. Circular RNA hsa_circ_0007059 restrains proliferation and epithelial-mesenchymal transition in lung cancer cells via inhibiting microRNA-378. Life Sci. 2019;233:116692. https://doi.org/10.1016/j.lfs.2019.116692.
Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Circular RNA-ITCH Suppresses Lung Cancer Proliferation via Inhibiting the Wnt/β-Catenin Pathway. Biomed Res Int. 2016;2016:1579490. https://doi.org/10.1155/2016/1579490.
Lu Y, Deng X, Xiao G, Zheng X, Ma L, Huang W. circ_0001730 promotes proliferation and invasion via the miR-326/Wnt7B axis in glioma cells. Epigenomics. 2019;11:1335–52. https://doi.org/10.2217/epi-2019-0121.
Chen Z, Duan X. hsa_circ_0000177-miR-638-FZD7-Wnt Signaling Cascade Contributes to the Malignant Behaviors in Glioma. DNA Cell Biol. 2018;37:791–7. https://doi.org/10.1089/dna.2018.4294.
Zhang L, Zhou Q, Qiu Q, Hou L, Wu M, Li J, et al. CircPLEKHM3 acts as a tumor suppressor through regulation of the miR-9/BRCA1/DNAJB6/KLF4/AKT1 axis in ovarian cancer. Mol Cancer. 2019;18:144. https://doi.org/10.1186/s12943-019-1080-5.
Li Y, Liu J, Piao J, Ou J, Zhu X. Circ_0109046 promotes the malignancy of endometrial carcinoma cells through the microRNA-105/SOX9/Wnt/β-catenin axis. IUBMB Life. 2021;73:159–76. https://doi.org/10.1002/iub.2415.
Shen Q, He T, Yuan H. Hsa_circ_0002577 promotes endometrial carcinoma progression via regulating miR-197/CTNND1 axis and activating Wnt/β-catenin pathway. Cell Cycle. 2019;18:1229–40. https://doi.org/10.1080/15384101.2019.1617004.
Wu Z, Shi W, Jiang C. Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/β-catenin pathway by regulating miR-1205/APC2 axis. Biochem Biophys Res Commun. 2018;502:465–71. https://doi.org/10.1016/j.bbrc.2018.05.184.
Wang M, Chen B, Ru Z, Cong L. CircRNA circ-ITCH suppresses papillary thyroid cancer progression through miR-22-3p/CBL/β-catenin pathway. Biochem Biophys Res Commun. 2018;504:283–8. https://doi.org/10.1016/j.bbrc.2018.08.175.
Li Y, Wang Z, Su P, Liang Y, Li Z, Zhang H, et al. circ-EIF6 encodes EIF6-224aa to promote TNBC progression via stabilizing MYH9 and activating the Wnt/beta-catenin pathway. Mol Ther. 2022;30:415–30. https://doi.org/10.1016/j.ymthe.2021.08.026.
Wang ST, Liu LB, Li XM, Wang YF, Xie PJ, Li Q, et al. Circ-ITCH regulates triple-negative breast cancer progression through the Wnt/β-catenin pathway. Neoplasma. 2019;66:232–9. https://doi.org/10.4149/neo_2018_180710N460.
Zhang J, Xu HD, Xing XJ, Liang ZT, Xia ZH, Zhao Y. CircRNA_069718 promotes cell proliferation and invasion in triple-negative breast cancer by activating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23:5315–22. https://doi.org/10.26355/eurrev_201906_18198.
Liu J, Xue N, Guo Y, Niu K, Gao L, Zhang S, et al. CircRNA_100367 regulated the radiation sensitivity of esophageal squamous cell carcinomas through miR-217/Wnt3 pathway. Aging (Albany NY). 2019;11:12412–27. https://doi.org/10.18632/aging.102580.
Li F, Zhang L, Li W, Deng J, Zheng J, An M, et al. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget. 2015;6:6001–13. https://doi.org/10.18632/oncotarget.3469.
Hu J, Hu B, Deng L, Cheng L, Fan Q, Lu C. Arsenic sulfide inhibits the progression of gastric cancer through regulating the circRNA_ASAP2/Wnt/β-catenin pathway. Anticancer Drugs. 2022;33:e711-e9. https://doi.org/10.1097/cad.0000000000001246.
He Y, Zhang Z, Wang Z, Jiao Y, Kang Q, Li J. Downregulation of circ-SFMBT2 blocks the development of gastric cancer by targeting the miR-885-3p/CHD7 pathway. Anticancer Drugs. 2022;33:e247-e59. https://doi.org/10.1097/cad.0000000000001195.
Shi Q, Zhou C, Xie R, Li M, Shen P, Lu Y, et al. CircCNIH4 inhibits gastric cancer progression via regulating DKK2 and FRZB expression and Wnt/β-catenin pathway. J Biol Res (Thessalon). 2021;28:19. https://doi.org/10.1186/s40709-021-00140-x.
Fang J, Chen W, Meng X. Downregulating circRNA_0044516 Inhibits Cell Proliferation in Gastric Cancer Through miR-149/Wnt1/β-catenin Pathway. J Gastrointest Surg. 2021;25:1696–705. https://doi.org/10.1007/s11605-020-04834-w.
Sun H, Wang Q, Yuan G, Quan J, Dong D, Lun Y, et al. Hsa_circ_0001649 restrains gastric carcinoma growth and metastasis by downregulation of miR-20a. J Clin Lab Anal. 2020;34:e23235. https://doi.org/10.1002/jcla.23235.
Gu H, Xu Z, Zhang J, Wei Y, Cheng L, Wang J. circ_0038718 promotes colon cancer cell malignant progression via the miR-195-5p/Axin2 signaling axis and also effect Wnt/β-catenin signal pathway. BMC Genomics. 2021;22:768. https://doi.org/10.1186/s12864-021-07880-z.
Zhang X, Yao J, Shi H, Gao B, Zhou H, Zhang Y, et al. Hsa_circ_0026628 promotes the development of colorectal cancer by targeting SP1 to activate the Wnt/β-catenin pathway. Cell Death Dis. 2021;12:802. https://doi.org/10.1038/s41419-021-03794-6.
Chen Z, Wu J, Liu B, Zhang G, Wang Z, Zhang L, et al. Identification of cis-HOX-HOXC10 axis as a therapeutic target for colorectal tumor-initiating cells without APC mutations. Cell Rep. 2021;36:109431. https://doi.org/10.1016/j.celrep.2021.109431.
Yang S, Gao S, Liu T, Liu J, Zheng X, Li Z. Circular. RNA SMARCA5 functions as an anti-tumor candidate in colon cancer by sponging microRNA-552. Cell Cycle. 2021;20:689–701. https://doi.org/10.1080/15384101.2021.1899519.
Zhang L, Dong X, Yan B, Yu W, Shan L. CircAGFG1 drives metastasis and stemness in colorectal cancer by modulating YY1/CTNNB1. Cell Death Dis. 2020;11:542. https://doi.org/10.1038/s41419-020-2707-6.
Zhao H, Chen S, Fu Q. Exosomes from CD133(+) cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. J Cell Biochem. 2020;121:3286–97. https://doi.org/10.1002/jcb.29600.
Geng Y, Zheng X, Hu W, Wang Q, Xu Y, He W, et al. Hsa_circ_0009361 acts as the sponge of miR-582 to suppress colorectal cancer progression by regulating APC2 expression. Clin Sci (Lond). 2019;133:1197–213. https://doi.org/10.1042/cs20190286.
Jin Y, Yu LL, Zhang B, Liu CF, Chen Y. Circular RNA hsa_circ_0000523 regulates the proliferation and apoptosis of colorectal cancer cells as miRNA sponge. Braz J Med Biol Res. 2018;51:e7811. https://doi.org/10.1590/1414-431x20187811.
Huang G, Zhu H, Shi Y, Wu W, Cai H, Chen X. cir-ITCH plays an inhibitory role in colorectal cancer by regulating the Wnt/β-catenin pathway. PLoS One. 2015;10:e0131225. https://doi.org/10.1371/journal.pone.0131225.
Yang B, Zhao J, Huo T, Zhang M, Wu X. Effects of CircRNA-ITCH on proliferation and apoptosis of hepatocellular carcinoma cells through inhibiting Wnt/β-catenin signaling pathway. J buon. 2020;25:1368–74.
Yang W, Liu Y, Gao R, Xiu Z, Sun T. Knockdown of cZNF292 suppressed hypoxic human hepatoma SMMC7721 cell proliferation, vasculogenic mimicry, and radioresistance. Cell Signal. 2019;60:122–35. https://doi.org/10.1016/j.cellsig.2019.04.011.
Liang WC, Wong CW, Liang PP, Shi M, Cao Y, Rao ST, et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol. 2019;20:84. https://doi.org/10.1186/s13059-019-1685-4.
Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y, et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 2021;512:38–50. https://doi.org/10.1016/j.canlet.2021.04.030.
Xia D, Chen Z, Liu Q. Circ-PGC increases the expression of FOXR2 by targeting miR-532-3p to promote the development of non-small cell lung cancer. Cell Cycle. 2021;20:2195–209. https://doi.org/10.1080/15384101.2021.1974788.
Gao F, Jia L, Han J, Wang Y, Luo W, Zeng Y. Circ-ZNF124 downregulation inhibits non-small cell lung cancer progression partly by inactivating the Wnt/β-catenin signaling pathway via mediating the miR-498/YES1 axis. Anticancer Drugs. 2021;32:257–68. https://doi.org/10.1097/cad.0000000000001014.
Jin Z, Gao B, Gong Y, Guan L. Depletion of circ-BIRC6, a circular RNA, suppresses non-small cell lung cancer progression by targeting miR-4491. Biosci Trends. 2021;14:399–407. https://doi.org/10.5582/bst.2020.03310.
Zhao M, Ma W, Ma C. Circ_0067934 promotes non-small cell lung cancer development by regulating miR-1182/KLF8 axis and activating Wnt/β-catenin pathway. Biomed Pharmacother. 2020;129:110461. https://doi.org/10.1016/j.biopha.2020.110461.
Tian F, Yu CT, Ye WD, Wang Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;493:1260–6. https://doi.org/10.1016/j.bbrc.2017.09.136.
Huo LW, Wang YF, Bai XB, Zheng HL, Wang MD. circKIF4A promotes tumorogenesis of glioma by targeting miR-139-3p to activate Wnt5a signaling. Mol Med. 2020;26:29. https://doi.org/10.1186/s10020-020-00159-1.
Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu C, et al. Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the Wnt/β-catenin signaling pathway. Oncotarget. 2016;7:63449–55. https://doi.org/10.18632/oncotarget.11523.
Li S, Yu C, Zhang Y, Liu J, Jia Y, Sun F, et al. Circular RNA cir-ITCH Is a Potential Therapeutic Target for the Treatment of Castration-Resistant Prostate Cancer. Biomed Res Int. 2020;2020:7586521. https://doi.org/10.1155/2020/7586521.
Lin X, Chen Y, Ye X, Xia X. Circular. RNA ABCB10 promotes cell proliferation and invasion, but inhibits apoptosis via regulating the microRNA–1271–mediated Capn4/Wnt/β–catenin signaling pathway in epithelial ovarian cancer. Mol Med Rep. 2021;23. https://doi.org/10.3892/mmr.2021.12026.
Pan Q, Meng X, Li J, Qin X, Chen H, Li Y. CircSAMD11 facilitates progression of cervical cancer via regulating miR-503/SOX4 axis through Wnt/β-catenin pathway. Clin Exp Pharmacol Physiol. 2022;49:175–87. https://doi.org/10.1111/1440-1681.13593.
Chen JJ, Lei P, Zhou M. hsa_circ_0121582 inhibits leukemia growth by dampening Wnt/β-catenin signaling. Clin Transl Oncol. 2020;22:2293–302. https://doi.org/10.1007/s12094-020-02377-9.
Xia L, Wu L, Bao J, Li Q, Chen X, Xia H, et al. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;503:385–90. https://doi.org/10.1016/j.bbrc.2018.06.045.
Hu Y, Zhao Y, Shi C, Ren P, Wei B, Guo Y, et al. A circular RNA from APC inhibits the proliferation of diffuse large B-cell lymphoma by inactivating Wnt/β-catenin signaling via interacting with TET1 and miR-888. Aging (Albany NY). 2019;11:8068–84. https://doi.org/10.18632/aging.102122.
Gong X, Li W, Dong L, Qu F. CircUBAP2 promotes SEMA6D expression to enhance the cisplatin resistance in osteosarcoma through sponging miR-506-3p by activating Wnt/β-catenin signaling pathway. J Mol Histol. 2020;51:329–40. https://doi.org/10.1007/s10735-020-09883-8.
Bi W, Huang J, Nie C, Liu B, He G, Han J, et al. CircRNA circRNA_102171 promotes papillary thyroid cancer progression through modulating CTNNBIP1-dependent activation of β-catenin pathway. J Exp Clin Cancer Res. 2018;37:275. https://doi.org/10.1186/s13046-018-0936-7.
Chen F, Feng Z, Zhu J, Liu P, Yang C, Huang R, et al. Emerging roles of circRNA_NEK6 targeting miR-370-3p in the proliferation and invasion of thyroid cancer via Wnt signaling pathway. Cancer Biol Ther. 2018;19:1139–52. https://doi.org/10.1080/15384047.2018.1480888.
Wu H, Xu J, Gong G, Zhang Y, Wu S. CircARL8B Contributes to the Development of Breast Cancer Via Regulating miR-653-5p/HMGA2 Axis. Biochem Genet. 2021;59:1648–65. https://doi.org/10.1007/s10528-021-10082-7.
Jiang J, Cheng X. Circular RNA. circABCC4 acts as a ceRNA of miR-154-5p to improve cell viability, migration and invasion of breast cancer cells in vitro. Cell Cycle. 2020;19:2653–61. https://doi.org/10.1080/15384101.2020.1815147.
Yao Y, Li X, Cheng L, Wu X, Wu B. Circular RNA FAT atypical cadherin 1 (circFAT1)/microRNA-525-5p/spindle and kinetochore-associated complex subunit 1 (SKA1) axis regulates oxaliplatin resistance in breast cancer by activating the notch and Wnt signaling pathway. Bioengineered. 2021;12:4032–43. https://doi.org/10.1080/21655979.2021.1951929.
Liu S, Chen L, Chen H, Xu K, Peng X, Zhang M. Circ_0119872 promotes uveal melanoma development by regulating the miR-622/G3BP1 axis and downstream signalling pathways. J Exp Clin Cancer Res. 2021;40:66. https://doi.org/10.1186/s13046-021-01833-w.
Chen J, Zhou X, Yang J, Sun Q, Liu Y, Li N, et al. Circ-GLI1 promotes metastasis in melanoma through interacting with p70S6K2 to activate Hedgehog/GLI1 and Wnt/β-catenin pathways and upregulate Cyr61. Cell Death Dis. 2020;11:596. https://doi.org/10.1038/s41419-020-02799-x.
Chen Z, Chen J, Wa Q, He M, Wang X, Zhou J, et al. Knockdown of circ_0084043 suppresses the development of human melanoma cells through miR-429/tribbles homolog 2 axis and Wnt/β-catenin pathway. Life Sci. 2020;243:117323. https://doi.org/10.1016/j.lfs.2020.117323.
Su H, Lin F, Deng X, Shen L, Fang Y, Fei Z, et al. Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J Transl Med. 2016;14:225. https://doi.org/10.1186/s12967-016-0977-7.
Fan G, Wei X, Xu X. Is the era of sorafenib over? A review of the literature. Ther Adv Med Oncol. 2020;12:1758835920927602. https://doi.org/10.1177/1758835920927602.
Saini A, Wallace A, Alzubaidi S, Knuttinen MG, Naidu S, Sheth R, et al. History and Evolution of Yttrium-90 Radioembolization for Hepatocellular Carcinoma. J Clin Med 2019;8. https://doi.org/10.3390/jcm8010055.
Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;9:171. https://doi.org/10.1186/1479-5876-9-171.
Sasaki R, Kanda T, Fujisawa M, Matsumoto N, Masuzaki R, Ogawa M, et al. Different Mechanisms of Action of Regorafenib and Lenvatinib on Toll-Like Receptor-Signaling Pathways in Human Hepatoma Cell Lines. Int J Mol Sci 2020;21. https://doi.org/10.3390/ijms21093349.
Chen CH, Su YJ, Ding H, Duan J, Wang J. Circular. RNA ZNF292 affects proliferation and apoptosis of hepatocellular carcinoma cells by regulating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2020;24:12124–30. https://doi.org/10.26355/eurrev_202012_24001.
Wang C, Zhang T, Liao Q, Dai M, Guo J, Yang X, et al. Metformin inhibits pancreatic cancer metastasis caused by SMAD4 deficiency and consequent HNF4G upregulation. Protein Cell. 2021;12:128–44. https://doi.org/10.1007/s13238-020-00760-4.
Bibok A, Kim DW, Malafa M, Kis B. Minimally invasive image-guided therapy of primary and metastatic pancreatic cancer. World J Gastroenterol. 2021;27:4322–41. https://doi.org/10.3748/wjg.v27.i27.4322.
Iglesia D, Avci B, Kiriukova M, Panic N, Bozhychko M, Sandru V, et al. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. United Eur Gastroenterol J. 2020;8:1115–25. https://doi.org/10.1177/2050640620938987.
Bushnell GG, Orbach SM, Ma JA, Crawford HC, Wicha MS, Jeruss JS, et al. Disease-induced immunomodulation at biomaterial scaffolds detects early pancreatic cancer in a spontaneous model. Biomaterials. 2021;269:120632. https://doi.org/10.1016/j.biomaterials.2020.120632.
Li L, Bao J, Wang H, Lei JH, Peng C, Zeng J, et al. Upregulation of amplified in breast cancer 1 contributes to pancreatic ductal adenocarcinoma progression and vulnerability to blockage of hedgehog activation. Theranostics. 2021;11:1672–89. https://doi.org/10.7150/thno.47390.
Elaskalani O, Domenichini A, Abdol Razak NB, D ED, Falasca M, Metharom P. Antiplatelet Drug Ticagrelor Enhances Chemotherapeutic Efficacy by Targeting the Novel P2Y12-AKT Pathway in Pancreatic Cancer Cells. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12010250.
O’Reilly EM, Hechtman JF. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Ann Oncol. 2019;30:viii36–40. https://doi.org/10.1093/annonc/mdz385.
Zhang Q, Wang JY, Zhou SY, Yang SJ, Zhong SL. Circular RNA expression in pancreatic ductal adenocarcinoma. Oncol Lett. 2019;18:2923–30. https://doi.org/10.3892/ol.2019.10624.
Khan N, Mukhtar H. Dietary agents for prevention and treatment of lung cancer. Cancer Lett. 2015;359:155–64. https://doi.org/10.1016/j.canlet.2015.01.038.
Zha JH, Xia YC, Ye CL, Hu Z, Zhang Q, Xiao H, et al. The Anti-Non-Small Cell Lung Cancer Cell Activity by a mTOR Kinase Inhibitor PQR620. Front Oncol. 2021;11:669518. https://doi.org/10.3389/fonc.2021.669518.
Teng K, Zhang Y, Hu X, Ding Y, Gong R, Liu L. Nimotuzumab enhances radiation sensitivity of NSCLC H292 cells in vitro by blocking epidermal growth factor receptor nuclear translocation and inhibiting radiation-induced DNA damage repair. Onco Targets Ther. 2015;8:809–18. https://doi.org/10.2147/ott.S77283.
Lin L, Zhao J, Hu J, Huang F, Han J, He Y, et al. Comparison of the efficacy and tolerability of gefitinib with pemetrexed maintenance after first-line platinum-based doublet chemotherapy in advanced lung adenocarcinoma: single-center experience. Onco Targets Ther. 2016;9:6305–14. https://doi.org/10.2147/ott.S113374.
Ardesch FH, Ruiter R, Mulder M, Lahousse L, Stricker BHC, Kiefte-de Jong JC. The Obesity Paradox in Lung Cancer: Associations With Body Size Versus Body Shape. Front Oncol. 2020;10:591110. https://doi.org/10.3389/fonc.2020.591110.
Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165-72. https://doi.org/10.1016/s1470-2045(14)71180-5.
Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol 2017;7. https://doi.org/10.1098/rsob.170070.
Wahab A, Kesari K, Chaudhary S, Khan M, Khan H, Smith S, et al. Sequential occurrence of small cell and non-small lung cancer in a male patient: Is it a transformation? Cancer Biol Ther. 2017;18:940–3. https://doi.org/10.1080/15384047.2017.1394546.
Sun X, Turcan S. From Laboratory Studies to Clinical Trials: Temozolomide Use in IDH-Mutant Gliomas. Cells 2021;10. https://doi.org/10.3390/cells10051225.
Sturm D, Pfister SM, Jones DTW. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J Clin Oncol. 2017;35:2370–7. https://doi.org/10.1200/jco.2017.73.0242.
Groblewska M, Litman-Zawadzka A, Mroczko B. The Role of Selected Chemokines and Their Receptors in the Development of Gliomas. Int J Mol Sci 2020;21. https://doi.org/10.3390/ijms21103704.
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16:896–913. https://doi.org/10.1093/neuonc/nou087.
Ding C, Yi X, Wu X, Bu X, Wang D, Wu Z, et al. Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett. 2020;479:1–12. https://doi.org/10.1016/j.canlet.2020.03.002.
Chen J, Chen T, Zhu Y, Li Y, Zhang Y, Wang Y, et al. circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res. 2019;38:398. https://doi.org/10.1186/s13046-019-1376-8.
Sun J, Li B, Shu C, Ma Q, Wang J. Functions and clinical significance of circular RNAs in glioma. Mol Cancer. 2020;19:34. https://doi.org/10.1186/s12943-019-1121-0.
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P, et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475. https://doi.org/10.1038/s41467-018-06862-2.
Du Q, Zhang W, Feng Q, Hao B, Cheng C, Cheng Y, et al. Comprehensive circular RNA profiling reveals that hsa_circ_0001368 is involved in growth hormone-secreting pituitary adenoma development. Brain Res Bull. 2020;161:65–77. https://doi.org/10.1016/j.brainresbull.2020.04.018.
Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer. 2006;5:14. https://doi.org/10.1186/1476-4598-5-14.
Chen K, Xu H, Zhao J. Bloom Syndrome Protein Activates AKT and PRAS40 in Prostate Cancer Cells. Oxid Med Cell Longev. 2019;2019:3685817. https://doi.org/10.1155/2019/3685817.
Mollica V, Di Nunno V, Cimadamore A, Lopez-Beltran A, Cheng L, Santoni M, et al. Molecular Mechanisms Related to Hormone Inhibition Resistance in Prostate Cancer. Cells 2019;8. https://doi.org/10.3390/cells8010043.
Xu L, Zhang G, Zhang X, Bai X, Yan W, Xiao Y, et al. External Validation of the Extraprostatic Extension Grade on MRI and Its Incremental Value to Clinical Models for Assessing Extraprostatic Cancer. Front Oncol. 2021;11:655093. https://doi.org/10.3389/fonc.2021.655093.
Wang L, Liu X, Liu Z, Wang Y, Fan M, Yin J, et al. Network models of prostate cancer immune microenvironments identify ROMO1 as heterogeneity and prognostic marker. Sci Rep. 2022;12:192. https://doi.org/10.1038/s41598-021-03946-w.
Getahun F, Mazengia F, Abuhay M, Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in Northwest Ethiopia. BMC Cancer. 2013;13:2. https://doi.org/10.1186/1471-2407-13-2.
Farrand L, Oh SW, Song YS, Tsang BK. Phytochemicals: a multitargeted approach to gynecologic cancer therapy. Biomed Res Int. 2014;2014:890141. https://doi.org/10.1155/2014/890141.
Wang G, Liu X, Wang D, Sun M, Yang Q. Identification and Development of Subtypes With Poor Prognosis in Pan-Gynecological Cancer Based on Gene Expression in the Glycolysis-Cholesterol Synthesis Axis. Front Oncol. 2021;11:636565. https://doi.org/10.3389/fonc.2021.636565.
Zhang H, Wang S, Cacalano N, Zhu H, Liu Q, Xie M, et al. Oncogenic Y68 frame shift mutation of PTEN represents a mechanism of docetaxel resistance in endometrial cancer cell lines. Sci Rep. 2019;9:2111. https://doi.org/10.1038/s41598-019-38585-9.
Li C, Ao H, Chen G, Wang F, Li F. The Interaction of CDH20 With β-Catenin Inhibits Cervical Cancer Cell Migration and Invasion via TGF-β/Smad/SNAIL Mediated EMT. Front Oncol. 2019;9:1481. https://doi.org/10.3389/fonc.2019.01481.
Li T, Yang Z, Jiang S, Di W, Ma Z, Hu W, et al. Melatonin: does it have utility in the treatment of haematological neoplasms? Br J Pharmacol. 2018;175:3251–62. https://doi.org/10.1111/bph.13966.
Howell DA, Wang HI, Roman E, Smith AG, Patmore R, Johnson MJ, et al. Preferred and actual place of death in haematological malignancy. BMJ Support Palliat Care. 2017;7:150–7. https://doi.org/10.1136/bmjspcare-2014-000793.
Auberger P, Tamburini-Bonnefoy J, Puissant A. Drug Resistance in Hematological Malignancies. Int J Mol Sci 2020;21. https://doi.org/10.3390/ijms21176091.
Deshantri AK, Varela Moreira A, Ecker V, Mandhane SN, Schiffelers RM, Buchner M, et al. Nanomedicines for the treatment of hematological malignancies. J Control Release. 2018;287:194–215. https://doi.org/10.1016/j.jconrel.2018.08.034.
Jin MW, Xu SM, An Q, Wang P. A review of risk factors for childhood leukemia. Eur Rev Med Pharmacol Sci. 2016;20:3760–4.
Li AJ, Dhanraj JP, Lopes G, Parker JL. Clinical trial risk in leukemia: Biomarkers and trial design. Hematol Oncol. 2021;39:105–13. https://doi.org/10.1002/hon.2818.
Zhao H, Wang D, Du W, Gu D, Yang R. MicroRNA and leukemia: tiny molecule, great function. Crit Rev Oncol Hematol. 2010;74:149–55. https://doi.org/10.1016/j.critrevonc.2009.05.001.
Morelli MB, Liberati S, Amantini C, Nabiss M, Santoni M, Farfariello V, et al. Expression and function of the transient receptor potential ion channel family in the hematologic malignancies. Curr Mol Pharmacol. 2013;6:137–48. https://doi.org/10.2174/187446720603140415215431.
Battistello E, Katanayeva N, Dheilly E, Tavernari D, Donaldson MC, Bonsignore L, et al. Pan-SRC kinase inhibition blocks B-cell receptor oncogenic signaling in non-Hodgkin lymphoma. Blood. 2018;131:2345–56. https://doi.org/10.1182/blood-2017-10-809210.
Zhu J, Yang Y, Tao J, Wang SL, Chen B, Dai JR, et al. Association of progression-free or event-free survival with overall survival in diffuse large B-cell lymphoma after immunochemotherapy: a systematic review. Leukemia. 2020;34:2576–91. https://doi.org/10.1038/s41375-020-0963-1.
Caramuta S, Lee L, Ozata DM, Akçakaya P, Georgii-Hemming P, Xie H, et al. Role of microRNAs and microRNA machinery in the pathogenesis of diffuse large B-cell lymphoma. Blood Cancer J. 2013;3:e152. https://doi.org/10.1038/bcj.2013.49.
Mateu-Sanz M, Tornín J, Brulin B, Khlyustova A, Ginebra MP, Layrolle P, et al. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12010227.
Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O 3. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol. 2016;34:3031–8. https://doi.org/10.1200/jco.2015.65.5381. rd, et al.
Lallier M, Marchandet L, Moukengue B, Charrier C, Baud’huin M, Verrecchia F, et al. Molecular Chaperones in Osteosarcoma: Diagnosis and Therapeutic Issues. Cells 2021;10. https://doi.org/10.3390/cells10040754.
Hou CH, Lin FL, Hou SM, Liu JF. Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway. Mol Cancer. 2014;13:236. https://doi.org/10.1186/1476-4598-13-236.
Li C, Cai J, Ge F, Wang G. TGM2 knockdown reverses cisplatin chemoresistance in osteosarcoma. Int J Mol Med. 2018;42:1799–808. https://doi.org/10.3892/ijmm.2018.3753.
Wen JF, Jiang YQ, Li C, Dai XK, Wu T, Yin WZ. LncRNA-SARCC sensitizes osteosarcoma to cisplatin through the miR-143-mediated glycolysis inhibition by targeting Hexokinase 2. Cancer Biomark. 2020;28:231–46. https://doi.org/10.3233/cbm-191181.
Yong L, Ma Y, Liang C, He G, Zhao Z, Yang C, et al. Oleandrin sensitizes human osteosarcoma cells to cisplatin by preventing degradation of the copper transporter 1. Phytother Res. 2019;33:1837–50. https://doi.org/10.1002/ptr.6373.
Guo J, Dou D, Zhang T, Wang B. HOTAIR Promotes Cisplatin Resistance of Osteosarcoma Cells by Regulating Cell Proliferation, Invasion, and Apoptosis via miR-106a-5p/STAT3 Axis. Cell Transpl. 2020;29:963689720948447. https://doi.org/10.1177/0963689720948447.
Yang D, Xu T, Fan L, Liu K, Li G. microRNA-216b enhances cisplatin-induced apoptosis in osteosarcoma MG63 and SaOS-2 cells by binding to JMJD2C and regulating the HIF1α/HES1 signaling axis. J Exp Clin Cancer Res. 2020;39:201. https://doi.org/10.1186/s13046-020-01670-3.
Zhang L, Wang Y, Li X, Xia X, Li N, He R, et al. ZBTB7A Enhances Osteosarcoma Chemoresistance by Transcriptionally Repressing lncRNALINC00473-IL24 Activity. Neoplasia. 2017;19:908–18. https://doi.org/10.1016/j.neo.2017.08.008.
Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid. 2021;31:183–92. https://doi.org/10.1089/thy.2020.0330.
Yang Z, Wei X, Pan Y, Xu J, Si Y, Min Z, et al. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. 2021;12:51. https://doi.org/10.1038/s41419-020-03294-z.
Cao Y, Zhong X, Diao W, Mu J, Cheng Y, Jia Z. Radiomics in Differentiated Thyroid Cancer and Nodules: Explorations, Application, and Limitations. Cancers (Basel). 2021;13. https://doi.org/10.3390/cancers13102436.
Xu B, Ibrahimpasic T, Wang L, Sabra MM, Migliacci JC, Tuttle RM, et al. Clinicopathologic Features of Fatal Non-Anaplastic Follicular Cell-Derived Thyroid Carcinomas. Thyroid. 2016;26:1588–97. https://doi.org/10.1089/thy.2016.0247.
Loehrer AP, Murthy SS, Song Z, Lubitz CC, James BC. Association of Insurance Expansion With Surgical Management of Thyroid Cancer. JAMA Surg. 2017;152:734–40. https://doi.org/10.1001/jamasurg.2017.0461.
Li S, Chen C, Xiong X, Huang Y, Hu J, Fan Z, et al. Type Iγ phosphatidylinositol phosphate kinase dependent cell migration and invasion are dispensable for tumor metastasis. Am J Cancer Res. 2019;9:959–74.
Hu PC, Li K, Tian YH, Pan WT, Wang Y, Xu XL, et al. CREB1/Lin28/miR-638/VASP Interactive Network Drives the Development of Breast Cancer. Int J Biol Sci. 2019;15:2733–49. https://doi.org/10.7150/ijbs.36854.
Chen Y, Chen L, Zhang JY, Chen ZY, Liu TT, Zhang YY, et al. Oxymatrine reverses epithelial-mesenchymal transition in breast cancer cells by depressing α(V)β(3) integrin/FAK/PI3K/Akt signaling activation. Onco Targets Ther. 2019;12:6253–65. https://doi.org/10.2147/ott.S209056.
Chen H, Sun Y, Yang Z, Yin S, Li Y, Tang M, et al. Metabolic heterogeneity and immunocompetence of infiltrating immune cells in the breast cancer microenvironment (Review). Oncol Rep. 2021;45:846–56. https://doi.org/10.3892/or.2021.7946.
Colombino M, Paliogiannis P, Cossu A, De Re V, Miolo G, Botti G, et al. BRAF Mutations and Dysregulation of the MAP Kinase Pathway Associated to Sinonasal Mucosal Melanomas. J Clin Med 2019;8. https://doi.org/10.3390/jcm8101577.
Katsarelias D, Eriksson H, Mikiver R, Krakowski I, Nilsson JA, Ny L, et al. The Effect of Beta-Adrenergic Blocking Agents in Cutaneous Melanoma-A Nation-Wide Swedish Population-Based Retrospective Register Study. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12113228.
Vogelsang M, Wilson M, Kirchhoff T. Germline determinants of clinical outcome of cutaneous melanoma. Pigment Cell Melanoma Res. 2016;29:15–26. https://doi.org/10.1111/pcmr.12418.
Kwiatkowska-Borowczyk E, Czerwińska P, Mackiewicz J, Gryska K, Kazimierczak U, Tomela K, et al. Whole cell melanoma vaccine genetically modified to stem cells like phenotype generates specific immune responses to ALDH1A1 and long-term survival in advanced melanoma patients. Oncoimmunology. 2018;7:e1509821. https://doi.org/10.1080/2162402x.2018.1509821.
Tang L, Long J, Li K, Zhang X, Chen X, Peng C. A novel chalcone derivative suppresses melanoma cell growth through targeting Fyn/Stat3 pathway. Cancer Cell Int. 2020;20:256. https://doi.org/10.1186/s12935-020-01336-2.
Zhang Y, Ma JA, Zhang HX, Jiang YN, Luo WH. Cancer vaccines: Targeting KRAS-driven cancers. Expert Rev Vaccines. 2020;19:163–73. https://doi.org/10.1080/14760584.2020.1733420.
Mun EJ, Babiker HM, Weinberg U, Kirson ED, Von Hoff DD. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin Cancer Res. 2018;24:266–75. https://doi.org/10.1158/1078-0432.Ccr-17-1117.
Maida M, Macaluso FS, Ianiro G, Mangiola F, Sinagra E, Hold G, et al. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther. 2017;17:1131–46. https://doi.org/10.1080/14737140.2017.1392243.
Shieh Y, Eklund M, Sawaya GF, Black WC, Kramer BS, Esserman LJ. Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol. 2016;13:550–65. https://doi.org/10.1038/nrclinonc.2016.50.
Mascaux C, Peled N, Garg K, Kato Y, Wynes MW, Hirsch FR. Early detection and screening of lung cancer. Expert Rev Mol Diagn. 2010;10:799–815. https://doi.org/10.1586/erm.10.60.
Tian JY, Guo FJ, Zheng GY, Ahmad A. Prostate cancer: updates on current strategies for screening, diagnosis and clinical implications of treatment modalities. Carcinogenesis. 2018;39:307–17. https://doi.org/10.1093/carcin/bgx141.
Greenwald ZR, El-Zein M, Bouten S, Ensha H, Vazquez FL, Franco EL. Mobile Screening Units for the Early Detection of Cancer: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2017;26:1679–94. https://doi.org/10.1158/1055-9965.Epi-17-0454.
Lindquist D, Kvarnbrink S, Henriksson R, Hedman H. LRIG and cancer prognosis. Acta Oncol. 2014;53:1135–42. https://doi.org/10.3109/0284186x.2014.953258.
Sidders B, Zhang P, Goodwin K, O’Connor G, Russell DL, Borodovsky A, et al. Adenosine Signaling Is Prognostic for Cancer Outcome and Has Predictive Utility for Immunotherapeutic Response. Clin Cancer Res. 2020;26:2176–87. https://doi.org/10.1158/1078-0432.Ccr-19-2183.
Poorvu PD, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Collins LC, et al. Prognostic Impact of the 21-Gene Recurrence Score Assay Among Young Women With Node-Negative and Node-Positive ER-Positive/HER2-Negative Breast Cancer. J Clin Oncol. 2020;38:725–33. https://doi.org/10.1200/jco.19.01959.
Cobain EF, Hayes DF. Indications for prognostic gene expression profiling in early breast cancer. Curr Treat Options Oncol. 2015;16:23. https://doi.org/10.1007/s11864-015-0340-x.
Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol. 2014;25:61–8. https://doi.org/10.1016/j.semcancer.2014.02.006.
Yu T, Wang Y, Fan Y, Fang N, Wang T, Xu T, et al. CircRNAs in cancer metabolism: a review. J Hematol Oncol. 2019;12:90. https://doi.org/10.1186/s13045-019-0776-8.
Hua JT, Chen S, He HH. Landscape of Noncoding RNA in Prostate Cancer. Trends Genet. 2019;35:840–51. https://doi.org/10.1016/j.tig.2019.08.004.
Acknowledgements
Figures in this manuscript were created with BioRender.com.
Funding
This work was funded by the National Key Research and Development Program of China (2021YFC2301800), the National Nature Science Foundation of China (U20A20343), and Zhejiang University Academic Award for Outstanding Doctoral Candidates (2020055).
Author information
Authors and Affiliations
Contributions
Lanjuan Li and Juan Lu designed and guided the review. Chen Xue, Ganglei Li, and Qiuxian Zheng wrote and edited the manuscript. Xinyu Gu and Zhengyi Bao helped with reference collection and draw the figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.