Abstract
This study was conducted to evaluate (i) the characteristics of ammonia gas emissions from soybean cultivation soils amended with varying levels of urea and soil water, and (ii) the rate of reduction in ammonia emissions that could be obtained by applying mixed microorganisms (MM) to the urea-treated soils. The ammonia gas emissions from all treatments except the control were highest on day 2 of a laboratory-scale experiment and decreased gradually thereafter. The ammonia gas emissions from the soils increased with increasing urea and soil water contents. However, there were less emissions from soils treated with MM than those from the urea only treatment, and emissions also decreased significantly as the concentration of MM increased. In a field-scale experiment, the total cumulative emissions of ammonia from soil treated with a combination of chemical fertilizers and MM was reduced to 85.8% of that from the soil treated with chemical fertilizers only. Although we infer that MM can be used as an agent to reduce ammonia gas emissions from actual soils used for soybean cultivation, our knowledge of the processes involved in reducing ammonia emissions using microbial treatment is still limited. Consequently, further studies are required to investigate the efficient control of ammonia gas emissions from agricultural soils through the application of microorganisms.
Similar content being viewed by others
Introduction
Over the last several decades, air pollution from rapid industrialization and urbanization has emerged as one of the largest environmental issues facing humanity in the 21st century. Moreover, if urbanization and industrialization accelerate further without additional control measures, air pollution will become even more serious [1]. Air pollutants consist of a complex combination of gaseous and particulate matter (PM) such as dust, aerosols, and smoke. Recently, the World Health Organization [2] provided improved global PM exposure estimates and new information regarding the associated risks. The present risk of PM exposure is much higher than it was in the past, and it is expected to increase annually due to continued industrialization and urbanization [3].
Atmospheric PM is classified according to particle size as PM10 and PM2.5. PM10 includes particles of < 10 μm, which can penetrate the lungs via the respiratory tract and be transported within the body in blood vessels, thereby having a potentially adverse effect on health [4, 5]. PM2.5 includes finer particles, < 2.5 μm in size, that have a relatively larger surface area in comparison to PM10. The risk associated with human exposure to PM2.5 has been found to be higher than that for PM10. This is because many harmful substances in the air can be relatively easily adsorbed by PM2.5 and are potentially transported into the body with the latter [6]. PM2.5 is divided into (i) primary PM2.5 that is emitted directly (e.g., dust from construction sites, roadsides, thermal power plants, and wood burning), and (ii) secondary PM2.5 (~ 72% of the total PM2.5 emitted) produced via the chemical reactions of nitrogen oxides and sulfur oxides in the air [7, 8]. Generally, these oxides are generated during the process of combustion with ammonia in the atmosphere [9, 10].
Accordingly, it is assumed that the goal of reducing PM2.5 can be achieved by controlling the emissions of ammonia gas from various sources. The main sources of ammonia gas in Korea are agriculture (78%), production processes (13%), area source pollution (4%), and road pollution (4%). Those from the agricultural sector are dominated by livestock manure and chemical fertilizer applications [11]. Recently, various technologies and methods have been developed to reduce ammonia generation from the agricultural sector [12]. These include the development of a slow-release fertilizer, organic and natural farming methods, the application of slurry, and treatment with newly developed urease inhibitors. Despite these developments, the methods that are currently used commercially are limited because they are associated with a low economic viability, crop growth reduction, and potential secondary pollution risks [13]. Consequently, many farms still use chemical fertilizers such as urea to improve crop productivity. As such, there is a need for an environmentally friendly method to reduce ammonia while using chemical fertilizers to provide the nutrients required by crops.
Junier et al. [14] reported that microorganisms can be used as an effective agent for removing ammonia because they use nitrogen for various metabolic processes. In addition, many researchers have reported reductions in ammonia emissions after applying microorganisms to livestock manure, composting, and sewage treatment plants [15, 16]. For example, Lee and Lee [17] found that the application of microorganisms to livestock manure processing reduced the emission of ammonia gas from the manure by approximately 60%. However, research into ammonia reduction by microbial agents has so far only focused on livestock manure treatment and sewage treatment facilities, and studies using agricultural soil are very limited. Therefore, the aims of this study are (i) to investigate the characteristics of ammonia emission from soybean cultivation soils amended with varying levels of urea and soil water, and (ii) to evaluate the efficiency of a mixed microbial agent for the reduction of gaseous ammonia emissions from the urea-treated soils.
Materials and methods
Materials
The soil used in this experiment was collected from the surface layer (up to 15 cm depth) of a soybean cultivation area located in Sacheon, South Korea. The soil was transported to the laboratory and stored at 4 ± 2 °C awaiting analysis. The pH and electrical conductivity (EC) were determined by centrifuging the soil samples and then measuring the supernatant with a pH meter and an EC meter, respectively. The measured pH was 6.1 and the EC was 0.06 dS/m. The collected soil was also air-dried to measure the organic matter (OM) content, total nitrogen (T-N) concentration, and available P2O5 concentration, which were determined to be 26 g/kg, 5.5 g/kg, and 149 mg/kg, respectively. The mixed microorganisms (MM) used for the ammonia reduction studies were purchased from the Ever Miracle Company (South Korea).
Experimental apparatus for collecting ammonia gas
The emission characteristics of ammonia gas under various conditions and its reduction effect from the application of MM were performed at both laboratory and field scales. An ammonia gas collection system was especially designed and constructed according to need and applicability. At the laboratory scale, ammonia gas was captured using an acrylic container with a cover, as illustrated in Fig. 1a. Soil, urea, and MM were placed in the acrylic chamber at the experimental concentrations, and the ammonia gas generated from the chamber was collected via a silicon tube in a gas bubble-trap glass flask containing 0.05 N H2SO4 solution. Air was used as a carrier gas to smoothly move the ammonia gas generated in the chamber into the glass flask using an air pump and gas flow meter. The gas flow rate was adjusted to 1 L/min and the ammonia gas was collected via the outflow method.
At the field scale, ammonia gas was captured by the inflow method (Fig. 1b) because a test of the outflow method revealed that all of the introduced air leaked out through the soil pores; hence, no ammonia gas was collected. The ammonia gas generated from the soil with urea and MM accumulated in the acrylic chamber and was collected in a gas bubble-trap flask containing 0.05 N H2SO4 using the inflow method and a vacuum (Fig. 1b). A silicon tube was installed to introduce external air into the collection chamber, which was installed in the soil to a depth of 20 cm. The inflow rate of the generated ammonia gas was adjusted to 5 L/min.
Methods
To investigate the characteristics of ammonia gas emission according to the degree of nitrogen fertilization, 50 g of soil (water content = 15.1%) was placed into each acrylic pot before being treated with 0.005, 0.01, 0.02, or 0.04 g of urea per g of soil. The injection flow rate of the gas from the air pump was adjusted to 1 L/min by a gas flow meter, and the ammonia gas generated from the acrylic chamber was collected in the gas bubble-trap flask with 0.05 N H2SO4 for 15 days, with measurements taken every 24 h. The concentration of ammonia collected in the flask was measured using a UV–VIS spectrometer (Human Corporation) at a λ of 630 nm by the indophenol method.
To evaluate the effect of the soil water content on the characteristics of the ammonia emissions that were converted from urea, the soil water content in the acrylic chamber containing 50 g of soil and 1 g of urea was adjusted to 2.7, 4.3, 5.5, 8.0, 15.1, 18.6, and 25% using distilled water. The ammonia gas discharged at the various soil water contents was captured and analyzed as described previously.
The ammonia gas emission characteristics under the MM treatments were investigated at the laboratory and field scales. At the laboratory scale, a quantity of MM (1.5 × 109 CFU/mL) were injected into the acrylic chamber (50 g soil + 1 g urea) at 0.001, 0.002, and 0.1 mL per g of soil with water content of 15.1%. The ammonia gas discharged from the acrylic chamber was then collected in the gas bubble-trap flask (0.05 N H2SO4) for 15 days, with measurements taken every 24 h. The long-term emission characteristics were also monitored using a soil and urea-only chamber (‘soil + urea’) and a soil, urea, and MM-treated chamber (‘soil + urea + MM’). The ammonia concentration generated in both chambers was continuously monitored for 80 days.
To investigate the ammonia gas emission characteristics under the MM treatment at the field scale, N, P, and K (3, 3, and 3.2 kg/10 a, respectively) chemical fertilizers were applied to the soybean cultivation soils according to the recommendations of the NIAST [18]. Then, MM were added to the soil at a rate of 20 L/10 a. The gas flow from the air pump was controlled by a gas flow meter at a rate of 5 L/min and the ammonia gas discharged from the chamber was collected in the gas bubble-trap flask (0.05 N H2SO4) for 90 min. Analysis of the collected ammonia was carried out as described previously.
Statistical analysis
All treatments were carried out in triplicate. Statistical analysis was performed using SAS version 8.1 by performing a Duncan’s multiple range test at a 5% confidence interval to identify the differences among the treatments.
Results and discussion
Effects of urea and soil water content on ammonia emission characteristics
The ammonia gas emission characteristics under different levels of urea application are shown in Fig. 2a. Although almost no ammonia gas was generated in the soil without urea, the ammonia emissions from the soils treated with 0.005 and 0.01 g of urea per g of soil increased up to 0.4 and 1.6 mg/kg in 2 days, respectively. However, the emissions subsequently declined sharply and ranged from 0.2 to 0.7 mg/kg/day between days 3 and 16. In the soil treated with 0.04 g of urea per g of soil, the ammonia emissions not only increased rapidly at the beginning of the experiment, but the soils continued to emit large amounts of ammonia irregularly after 2 days. The mean ammonia gas emission from this soil between days 3 and 16 was 8 times higher than that from the soil treated with 0.01 g of urea per g of soil.
Effects of urea levels on ammonia gas emission from soil [a NH3 emission, b accumulated NH3-N, Urea level: 0.005 g/soil g (1/4 N), 0.01 g/soil g (1/2 N), 0.02 g/soil g (N) and 0.04 g/soil g (2 N)]. Error bar indicates standard deviation (n = 3; mean ± SD). Different letters indicate significant difference according to Duncan’s multiple range test (DMRT) (p < 0.05)
Many researchers have found that ammonia gas emitted from soil after the application of N fertilizer is mostly released during a period from a few hours to 12 days after application [19,20,21]; this is consistent with our results. The temporal changes in the emission of ammonia gas from N-fertilized soils are dominated by soil characteristics, climatic factors, and the crop system [22,23,24].
Figure 2b shows that the total cumulative emissions of ammonia from the chamber increased as the amount of urea applied increased, with the highest being 122.8 mg/kg in the soil treated with 0.04 g of urea per g of soil. Rochette et al. [25] evaluated ammonia emissions according to the level of urea applied to soils used for spring barley cultivation and also found that the amount of ammonia gas emitted increased as the amount of urea application increased.
In general, N fertilizers play an important role in increasing crop yields; however, farmers often use much more of the former than crops can absorb [26]. The excess cannot be absorbed due to the low utilization efficiency of crops, and residual N that escapes by denitrification, volatilization, and leaching causes severe pollution to surface water, groundwater, and the atmosphere [27, 28]. As mentioned, our results also demonstrate that ammonia emissions from the soil increased with the increased application of urea. Therefore, the selection of the optimum level of the latter to improve crop productivity should also consider ammonia emission characteristics.
Figure 3a displays the ammonia emission characteristics versus the soil water content. When the soil water content was 2.7–8.0%, the ammonia gas emissions were highest (1.7–3.9 mg/kg) during the first 2 days and subsequently reduced to 0.5–2.0 mg/kg. As the soil water content increased to 15.1%, the ammonia gas emissions on day 2 also increased (6.9 mg/kg) in comparison to that at the lower soil water contents. However, this gradually decreased after 2 days, and after 14 days there was no significant difference compared to the soils with a soil water content of 2.7–8.0%. By comparison, the ammonia gas emissions from the soil with a 18.6% water content increased rapidly to 17.9 mg/kg on day 4 and reached the maximum of 21.97 mg/kg on day 6 before gradually decreasing. The ammonia gas emissions from the soil with a 25.0% water content increased rapidly at the beginning of the experiment and reached a maximum of 27.66 mg/kg on day 4, which was maintained until day 6. After 7 days, the ammonia emissions from the soil decreased slightly in an irregular trend, although the emissions were still higher than those of the other treatments. This confirms that it is possible to generate a large amount of ammonia gas even after 15 days.
Effects of water contents on ammonia gas emission from soil [a NH3 emission, b accumulated NH3-N, 1 N + 2.7 W (1 g urea + 2.7% Soil Water Content (SWC)), 1 N + 4.3 W (1 g urea + 4.3% SWC), 1 N + 5.5 W (1 g urea + 5.5% SWC), 1 N + 8.0 W (1 g urea + 8.0% SWC), 1 N + 15.1 W (1 g urea + 15.1% SWC), 1 N + 18.6 W (1 g urea + 18.6% SWC), 1 N + 25.0 W (1 g urea + 25.0% SWC)]. Error bar indicates standard deviation (n = 3; mean ± SD). Different letters indicate significant difference according to Duncan’s multiple range test (DMRT) (p < 0.05)
Figure 3b reveals that the cumulative ammonia gas generation increased over 15 days with increasing soil water content. In particular, when the soil water content was above 18.6%, the cumulative ammonia gas generation was 153.31 mg/kg, which was significantly different to that of the soils with a water content of between 2.7% and 8.0%.
The primary reason for the effect of the soil water content on the emission of ammonia gas is understood to be the hydrolysis of urea. In the hydrolysis reaction (Eq. 1), urea is decomposed into NH3 and CH3NO2, which rapidly decomposes into 2NH3 and CO2 [29, 30]. The ammonia gas emissions in the experiments may have increased with an increased soil water content because a higher water content accelerates the hydrolysis of urea.

Bouwmeester et al. [31] also studied the effect of water content on ammonia gas emissions from soils fertilized with urea and reported that emissions increased by 8% when the initial soil water content was increased from 21 to 31%. However, Akiyama et al. [32] reported that the ammonia gas emissions from soils with a water content of 40–80% were not significantly different; this is inconsistent with our results. Based on previous studies, the soil water content range to be used in an experiment is considered an important factor for evaluating characteristics of ammonia emissions. In particular, when the soil water content is high (i.e., 40–80%), it may be difficult to find significant differences in the ammonia gas emissions from urea-treated soils under different soil water contents. It is probable that the application of N fertilizers (e.g., urea) when the soil water content is high (i.e., following heavy rainfall) increases ammonia emissions. Therefore, it is necessary to carefully manage the application of N fertilizers during intensive rainfall and the rainy season.
Reduction of ammonia gas emissions by mixed microorganisms
The emissions of ammonia gas resulting from different concentrations of MM in the soils with and without urea are shown in Fig. 4a. The emissions from soil treated with urea only was 7.9 mg/kg after 2 days, which was considerably higher than the 4.3, 2.5, and 0.5 mg/kg emitted from MM treatments with 0.001, 0.002, and 0.1 mL of MM per g of soil. From day 2 to day 9, the ammonia gas emissions gradually decreased in all treatments except the 0.1 mL of MM per g of soil treatment; the latter generated emissions similar to that of the control (soil only) and showed no specific emission characteristics over the entire experiment. Figure 4b presents the cumulative ammonia gas emissions for the various MM treatments over 15 days. When the concentrations of MM in the soil containing urea were 0.001, 0.002, and 0.1 mL/g of soil, the cumulative ammonia gas emissions were 35.8, 20.3, and 7.6 mg/kg, respectively. These emissions were considerably lower than that from the soil treated only with urea (55.0 mg/kg). The ammonia gas reduction efficiencies were 34.9, 63.1, and 86.2%, respectively, indicating that the reduction of ammonia gas in the soil treated with urea was predominantly affected by the concentration of MM applied.
Effects of mixed microorganism levels on ammonia gas emission from soil [a NH3 emission, b accumulated NH3-N, Mixed microbial (MM) concentration: 0.001 mL/soil g (1/100MM), 0.05 mL/soil g (1/50MM), 0.1 mL/soil g (MM)]. Different letters indicate significant difference according to Duncan’s multiple range test (DMRT) (p < 0.05)
The above results suggest that MM could be used as an agent to effectively reduce the amount of ammonia gas generated from soybean cultivation soils. Previous studies involving MM in urea-treated soils were only conducted for relatively short periods of 15 days; hence, long-term monitoring was required in order to clarify the effectiveness of this method. In particular, it is difficult to predict how ammonia emissions will change after 15 days because MM treatments in soils containing urea can temporarily alter the soil environment as well as the form of N. Consequently, the present study evaluated the ammonia gas emission characteristics for 80 days by studying soil treated only with urea as well as soil treated with urea and MM.
The results are shown in Fig. 5a and reveal that most of the ammonia gas was emitted within 15 days for the soil treated with urea. Moreover, there was no significant difference in the emissions (0.5–1.6 mg/kg) between days 15 to 47. However, the ammonia emissions from this soil did increased slightly after 47 days, which is considered to have been a temporary effect relating to changes in the soil environment. In the soil treated with urea and MM, the ammonia gas emissions were consistently very low, and ranged from 0.24 to 0.88 mg/kg between days 1 and 80. In terms of the cumulative ammonia gas generation over 80 days, that from the soil treated with urea and MM reduced by approximately 68% compared to that from the soil with urea only (Fig. 5b). Accordingly, the MM treatment of soils with added urea is considered to be both effective for reducing the initial rapid ammonia gas emissions and a method for the stable reduction of ammonia gas emissions over a long period.
Long-term monitoring of ammonia emission from soil with and without mixed microorganisms [(a NH3 emission, b accumulated NH3-N, S + N (Soil + Urea), S + N+MM (Soil + Urea + Mixed microorganism)]. Different letters indicate significant difference according to Duncan’s multiple range test (DMRT) (p < 0.05)
In recent years, most research on the reduction of ammonia gas emissions by MM has been carried out on livestock farms. For example, Choi and Heo [33] reported that ammonia emissions from pig manure with and without MM were 22–42 mg/kg and 8.85–13.18 mg/kg, respectively, and that microbes were effective agents for reducing the emission of ammonia gas from pig manure. In addition, up to 77 days after the MM application, the ammonia gas emissions were found to be lower than those from pig manure without MM, which is consistent with our results. Although livestock farms are already using commercial microbial agents to reduce the generation of ammonia gas, there have been no reports of the use of microbial agents to reduce ammonia gas generation from soybean cultivation.
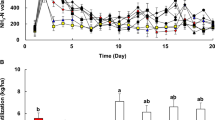
Consequently, MM were applied to soils used for soybean cultivation at the field-scale in the present study to evaluate the ammonia emission characteristics under different treatment times. In the soils that contained N, P, and K chemical fertilizers, the ammonia gas emissions increased rapidly to 714 μg/m2 day after 7 days (Fig. 6a), which was different to the emissions during the laboratory-scale experiment described previously. It should be noted, however, that it is difficult to directly compare the results of laboratory-scale and field-scale experiments because the conditions differ (e.g., the soil and surrounding environment, amount of fertilizer applied, and chamber size). The emissions from the field experiment using chemical fertilizers began to decrease gradually after 8 days and remained at 0.8–2.3 μg/m2 day until day 26. In the soils treated with chemical fertilizers and MM, the ammonia gas emissions remained relatively low (1.6–44.5 μg/m2 day) between days 1 and 26. The total cumulative emission of ammonia from these soils was reduced to 85.8% of that treated with chemical fertilizers only (Fig. 6b). Overall, it is concluded that MM could be used as an agent to reduce ammonia gas emissions from soils used for actual soybean cultivation.
Reduction efficiency of ammonia emission by mixed Microorganisms in field soybean cultivation soil [(a NH3 emission, b accumulated NH3-N, S + F (Soil + NPK), S + F+MM (Soil + NPK + Mixed microorganism)]. Different letters indicate significant difference according to Duncan’s multiple range test (DMRT) (p < 0.05)
Future research
This study evaluated (i) the characteristics of ammonia gas emission from soils with different levels of urea and water content, and (ii) the reduction of ammonia gas emissions from urea-treated soils that could be obtained by applying MM. However, there were 108 species of microorganisms in the MM, and no studies have been conducted regarding which microorganisms are particularly effective in reducing ammonia gas production. Therefore, future studies should separate the MM to isolate and identify each strain, and then apply each one to soil to evaluate the respective ammonia gas reduction efficiency.
At present, no studies have been undertaken regarding the mechanisms by which microorganisms reduce ammonia gas emissions from soils. In order to efficiently utilize microorganisms to control ammonia gas emissions from agricultural soils, it will be necessary to study these mechanisms. Such studies will involve investigating the changes in the physicochemical properties of given soils, including urea mineralization and microbial community changes using polymerase chain reaction, both before and after the application of microorganisms.
Availability of data and materials
All data is available in the main text.
Change history
28 January 2021
An amendment to this paper has been published and can be accessed via the original article.
References
Zhang R, Wang G, Guo S, Zamora ML, Ying Q, Lin Y, Wang W, Hu M, Wang Y (2015) Formation of urban fine particulate matter. Chem Rev 115:3803–3855
WHO (2014) Burden of Disease from ambient air pollution for 2012, description of method. Version 1.3. WHO, Genava
Karagulian F, Belis CA, Dora CFC, Pruss-Ustun AM, Bonjour S, Adair-Rohani H, Amann M (2015) Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ 120:475–483
Shahid I, Kistler M, Mukhtar A, Ghauri BM, Cruz CR, Bauer H, Puxbaum H (2016) Chemical characterization and mass closure of PM10 and PM2.5 at an urban site in Karachi–Pakistan. Atmos Environ 128:114–123
Wu Y, Gu B, Erisman JW, Reis S, Fang Y, Lu X, Zhang X (2016) PM2.5 pollution is substantially affected by ammonia emissions in China. Environ Pollut 218:86–94
Yunesian M, Rostami R, Zarei A, Fazlzadeh M, Janjani H (2019) Exposure to high levels of PM2.5 and PM10 in the metropolis of Tehran and the associated health risks during 2016–2017. Microchem J 150:104174
Boningari T, Smirniotis PG (2016) Impact of nitrogen oxides on the environment and human health: mn-based materials for the NOx abatement. Curr Opin Chem Eng 13:133–141
Tang L, Nagashima T, Hasegawa K, Ohara T, Sudo K, Itsubo N (2018) Development of human health damage factors for PM2.5 based on a global chemical transport model. Int J Life Cycle Ass 23:2300–2310
Tao J, Gao J, Zhang L, Zhang R, Che H, Zhang Z, Lin Z, Jing J, Cao J, Hsu SC (2014) PM2.5 pollution in a megacity of southwest China: source apportionment and implication. Atmos Chem Phys 14:8679–8699
Wu W, Jin Y, Carlsten C (2018) Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol 141:833–844
Choi H, Hyun J, Kim YJ, Yoo G (2019) Improvement of ammonia emission inventory estimation methodology for fertilizer application in the agricultural sector. J Clim Change Res 10:237–242
Wang H, Hegazy AM, Jiang X, Hu Z, Lu J, Mu J, Zhang X, Zhu X (2016) Suppression of ammonia volatilization from rice-wheat rotation fields amended with controlled-release urea and urea. Agron J 108:1214–1224
Guo C, Ren T, Li P, Wang B, Zou J, Hussain S, Cong R, Wu L, Lu J, Li X (2019) Producing more grain yield of rice with less ammonia volatilization and greenhouse gases emission using slow/controlled-release urea. Environ Sci Pollut Res 26:2569–2579
Junier P, Molina V, Dorador C, Hadas O, Kim OS, Junier T, Witzel KP, Imhoff JF (2010) Phylogenetic and functional marker genes to study ammonia-oxidizing microorganisms (AOM) in the environment. Appl Microbiol Biotechnol 85:425–440
Yamamoto N, Otawa K, Nakai Y (2010) Diversity and abundance of ammonia-oxidizing bacteria and ammonia-oxidizing archaea during cattle manure composting. Microb Eco 60:807–815
Wang M, Awasthi MK, Wang Q, Chen H, Ren X, Zhao J, Li R, Zhang Z (2017) Comparison of additives amendment for mitigation of greenhouse gases and ammonia emission during sewage sludge co-composting based on correlation analysis. Bioresour Technol 243:520–527
Lee SJ, Lee EY (2009) Screening and isolation of ammonia removal microorganism for the improvement of livestock environment. Kor. J. Microbiol. Biotechnol. 37:408–412
NIAST (2006) Fertilizer recommendation for crops. National Institute of Agricultural Science and Technology, RDA, Suwon
Zhang X, Wang Q, Xu J, Gilliam FS, Tremblay N, Li C (2015) In situ nitrogen mineralization, nitrification, and ammonia volatilization in maize field fertilized with urea in Huanghuaihai region Northern China. PLoS ONE 10:1–15
Cui ZL, Zhang F, Chen X, Dou Z, Li J (2010) In-season nitrogen management strategy for winter wheat: maximizing yields, minimizing environmental impact in an over-fertilization context. Field Crops Res 116:140–146
Sommer SG, Schjoerring JK, Denmead OT (2004) Ammonia emission from mineral fertilizers and fertilized crops. Adv Agron 82:557–622
Roelle PA, Aneja VP (2002) Characterization of ammonia emissions from soils in the upper coastal plain, North Carolina. Atmos Environ 36:1087–1097
Sommer SG, Genermont S, Cellier P, Hutchings NJ, Olesen JE, Morvan T (2003) Processes controlling ammonia emission from livestock slurry in the field. Eur J Agron 19:465–486
Martines AM, Nogueira MA, Santos CA, Nakatani AS, Andrade CA, Coscione AR, Cantarella H, Sousa JP, Cardoso EJBN (2010) Ammonia volatilization in soil treated with tannery sludge. Bioresour Technol 101:4690–4696
Rochette P, Angers DA, Chantigny MH, Gasser MO, MacDonald JD, Pelster DA, Bertrand N (2013) Ammonia volatilization and nitrogen retention: how deep to incorporate urea? J Environ Qual 42:1635–1642
Peng SB, Buresh RJ, Huang JL, Yang JC, Zou YB, Zhong XY, Wang GH, Zhang FS (2006) Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crops Res 96:37–47
Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, Zhang FS (2009) Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc Natl Acad Sci 106:3041–3046
Xu J, Peng S, Yang S, Wang W (2012) Ammonia volatilization losses from a rice paddy with different irrigation and nitrogen management. Agric Water Manag 104:184–192
Perez-Fortes M, Bocin-Dumitriu A, Tzimas E (2014) CO2 utilization pathways: techno-economic assessment and market opportunities. Energy Procedia 63:7968–7975
Voronin GF, Genkin MV, Kutsenok IB (2015) Virial equations of state for gaseous ammonia, water, carbon dioxide, and their mixtures at elevated temperatures and pressures. Russ J Phys Chem A 89:1958–1970
Bouwmeester RJB, Velk PLG, Stumpe JM (1985) Effect of environmental factors on ammonia volatilization from a urea-fertilized soil. Soil Sci Soc Am J 49:376–381
Akiyama H, McTaggart IP, Ball BC, Scott A (2004) N2O, NO, and NH3 emission from soil after the application of organic fertilizers, urea and water. Water Air Soil Poll 156:113–129
Choi YJ, Heo JY (2019) Odor reduction in swine farms during fattening period using probiotics. J Odor Indoor Environ 18:167–176
Acknowledgements
This study was carried out with the support of “Research Program for Agricultural Science & Technology Development (Project No. PJ014253042020)”, National Academy of Agricultural Science, Rural Development Administration, Republic of Korea. This work was also supported the Ministry of Science and ICT (MSIT) through the National Research Foundation (NRF) of Korea [NRF-2019R1C1C1004572; NRF-2019R1A4A1029125]. This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Animal Disease Management Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (319078-2).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
J-HP, S-LL and D-CS designed and conducted the experiment as well as wrote the manuscript. S-WH, J-HE, S-HK, and S-WK conducted characteristic analysis and interpretation of soil used. J-SC inspired the overall work and revised the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The project number in Acknowledgment section was incorrect.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, JH., Lee, SL., Hwang, SW. et al. Characteristics of ammonia gas emissions from soybean cultivation soils treated with mixed microorganisms. Appl Biol Chem 63, 20 (2020). https://doi.org/10.1186/s13765-020-00503-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-020-00503-3