Abstract
This study aimed to select the optimal microbial agents for ammonia gas reduction in Chinese cabbage cultivation and evaluate their ammonia reduction efficiency. By selecting the optimum microorganism to reduce ammonia emissions, the ammonia emission reduction efficiencies of the nitrification microorganisms, Alcaligenes faecalis subsp. faecalis and Brevibacillus sp. were 21 and 31%, respectively, which were superior to those of other microorganisms. The best ammonia emission reduction efficiency of the acid-producing microorganisms was 55%. The optimum mixing ratio of microbial agent for removing ammonia gas emitted from NPK-containing soil was: acid-producing microorganism:Alcaligenes faecalis subsp. faecalis:Brevibaillus sp. = 0.70:0.15:0.15. The optimum treatment amount was 500 L/ha, and the optimum number of microbial agents was basal fertilization (also known as pre-planting fertilization) once and additional fertilization three times, for a total of four times. The reduction efficiency of ammonia emissions from NPK-containing soil under optimum conditions in cabbage cultivation was 27% lower than that of the control (only NPK-containing soil). Therefore, the microbial agent developed in this study can be utilized to effectively reduce the emission of ammonia, a secondary fine particle precursor, while maintaining crop yield in agricultural fields.
Similar content being viewed by others
Introduction
Owing to rapid industrialization and urbanization, various air pollutants have been discharged, and special management is required because of their direct and potential risks to the human body [1]. Public interest in fine dust has increased rapidly since 2013, and it is now recognized as a social problem. Fine dust is classified into PM10 and PM2.5, depending on particle size. In particular, PM2.5, which is ultrafine dust, has a small particle size of < 2.5 μm; therefore, it has a larger surface area than that of PM10 and more harmful substances can be adsorbed and moved [2]. Therefore, PM2.5 penetrates deep into the human body and enters the alveoli and blood vessels, damages body organs and poses a great risk to respiratory health, making it more harmful than PM10 [3]. The International Agency for Research on Cancer (IARC) under the World Health Organization classifies fine dust as a Group 1 carcinogen [4].
PM2.5 is divided into direct emissions from the emission source and indirect emissions secondarily generated through photochemical reactions in the atmosphere [5]. For indirect emissions, precursors such as nitrogen oxides, sulfur oxides, volatile organic compounds, and ammonia in the air undergo photochemical reactions with reactive factors such as ozone and moisture, which generate secondary fine dust, such as organic substances, ammonium sulfate, and ammonium nitrate [6]. Secondary fine dust generated through this route corresponds to approximately 76% of the total [7]. However, unlike direct emission sources, it is challenging to control because it is emitted from various places at irregular concentrations. Therefore, managing the causative substances forming secondary fine dust is more urgent than directly treating and managing secondary fine dust in the air.
Recently, as agricultural land has become a major source of ammonia emissions, the importance of secondary fine dust generated by ammonia has emerged. In Korea, approximately 74% of the ammonia is generated from agriculture [8]. Nitrogen fertilizers are essential in agriculture to increase crop yield and quality. In particular, nitrogen fertilizers such as urea-treated soil emit a large amount of ammonia during decomposition and hydrolysis by microorganisms [9]. Therefore, the key task in reducing the ultrafine dust generated in Korea is to reduce ammonia caused by nitrogen fertilizers such as urea.
Technologies and measures to reduce ammonia emissions from agriculture include treating biochar and urease inhibitors and improving nitrogenous fertilization [10, 11]. Urease decomposes soil-treated urea to form ammonium ions, which can bind to the biochar surface and reduce nitrification and ammonium volatilization. However, biochar has a relatively high pH, and ammonia volatilization may also occur [12]. Because surface fertilization with nitrogen fertilizer increases the amount of ammonia volatilization, deep application of nitrogen fertilizer is performed to reduce the ammonia generated in agricultural land [13]. However, this method requires considerable time and labor. Soil treatment with urease inhibitors can reduce the amount of ammonia volatilization by suppressing urease activity; however, it can affect yields because sufficient nutrients are not supplied to crops [14]. Therefore, it is necessary to develop an eco-friendly ammonia reduction technology that can be easily applied to agricultural land and does not affect crop yield.
Various microorganisms can be effectively used for ammonia removal because they are involved in nitrogen fixation, ammonia volatilization, nitrification, and denitrification [15]. Studies using microorganisms to reduce ammonia emissions have focused on livestock farming environments and manure treatment, wastewater treatment, compost, fertilizer manufacturing, and waste treatment plants [16]. However, studies directly applying them to farmland are limited. Urea treatment of soil generates ammonia gas through urease decomposition and ammonia volatilization, which are dominantly influenced by the soil environment and microbiological reactions. Therefore, this study hypothesized that applying nitrifying and acid-producing microorganisms could alter the soil environment and microbial community, thereby reducing the ammonia gas discharged from NPK-containing soil. Therefore, this study aimed to select the optimal microbial agents for reducing ammonia gas in Chinese cabbage cultivation and evaluate their ammonia reduction efficiency.
Materials and methods
Soil
The soil used in this study was collected from a farm affiliated with Gyeongsang National University (35°10′84.91"N, 128°11′91.55"E) located in Sacheon, Gyeongsangnam-do; its chemical properties are presented in Table 1.
Microorganism
The nitrification-related microorganisms Pseudomonas fluorescens, Rhodococcus sp., Bacillus subtilis, Acinetobacter junii, Alcaligenes faecalis subsp. faecalis, and Brevibacillus sp. were selected and purchased from KCTC. The 107 acid-producing microorganisms used in this experiment were obtained from a microorganism company located in Jeonju, Jeollabuk-do (Additional file 1: Table S1), including lactic acid bacteria, yeast, actinomycetes, and photosynthetic bacteria.
Pot experiment
Pot experiments were conducted to determine the optimal microbial agents for ammonia reduction. The evaluation of ammonia emission characteristics according to the treatment with nitrifying and acid-producing microorganisms was performed in a Wagner pot with a standard of 1/5000a, and the aforementioned six nitrifying and acid-producing microorganisms were treated at 100 L/ha. N-P2O5-K2O was added at 320–78-198 kg/ha to all treatments except the untreated one, and all treatments were performed in triplicate. The concentration of ammonia gas discharged into the soil treated with nitrifying and acid-producing microorganisms was investigated daily for 20 days. Subsequently, to select the optimal mixing ratio of nitrifying and acid-producing mixed microorganisms, the mixing ratios of acid-producing microorganisms, Alcaligenes faecalis subsp. faecalis, and Brevibacillus sp. were 0.50:0.25:0.25 (SMM1), 0.70:0.15:0.15 (SMM2), and 0.80:0.10:0.10 (SMM3). All subsequent treatments and methods were performed in the same manner as described previously.
Field experiment
The evaluation of ammonia emission characteristics according to microbial agent treatment during cabbage cultivation was conducted at the Gyeongsang National University-affiliated farm located in Sacheon, Gyeongsangnam-do (35°10′84.91"N, 128°11′91.55"E). Changes in climate conditions in the study area are shown in Additional file 1: Fig. S1. Each treatment area was 2 m in width and 3 m in length, with an area of 6 m2, and was arranged in three replicates according to the randomized complete block design. Immediately after basal fertilizer treatment (N-P2O5-K2O = 113–8-78–79 kg/ha), 18 Chinese cabbage (Brassica rapa L.) plants were planted for each treatment. Subsequently, additional fertilizer was supplied three times at 15-day intervals (N-P2O5-K2O = 60–0-26 kg/ha for 1st additional fertilization; N-P2O5-K2O = 73–0-66 kg/ha for the 2nd and 3rd additional fertilizations). Based on previous experimental results, the optimal amount of microbial agent (acid-producing microorganisms:Alcaligenes faecalis subsp. faecalis:Brevibacillus sp = 0.70:0.15:0.15) was divided into 500, 1000, and 2000 L/ha and applied to NPK-treated soil. In addition, to evaluate the amount of ammonia gas discharged from NPK soil according to continuous microbial agent treatment (four times), the microbial agent treatment was applied with the same frequency and timing as that of the fertilizer treatment. The characteristics of the Chinese cabbage grown in each treatment group were investigated by referring to the standard manual for crop growth investigation by the Rural Development Administration.
Capture and analysis of ammonia gas
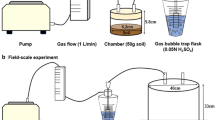
The static chamber method was used to capture the ammonia emitted during cabbage cultivation; the diameter and height of the chamber were 12.5 cm and 30 cm, respectively. The chamber was installed at a 15 cm depth at the center of each pot and treatment area. Ammonia gas discharged from the soil treated with fertilizers and/or microbial agents was collected for 24 h after chamber installation and monitored for 20 and 69 days in the pot and field experiments, respectively. Two sponges were installed in the chamber: the lower sponge was used to collect the ammonia generated from the soil, and the upper sponge blocked the ammonia flowing in from the atmosphere. These sponges were immersed in 30 mL of a 1:1 mixture of 1 M H3PO4 and 1% glycerol to collect ammonia, and then installed in the chamber. After 24 h of ammonia collection, the sponge was separated from the chamber and immersed in 150 mL of 2 M KCl to leach the ammonia. The extracted ammonia was analyzed using the indophenol method, and the ammonia in the solution was quantified at a wavelength of 630 nm using a UV–VIS Spectrophotometer (PerkinElmer, MA, USA). The ammonia emissions per unit area were calculated using the following equation:
NH3-N (mg/m2/day): (C × V)/(t × A) × (14.0067)/[14.0067 + (1.00794 × 4)] × p × 273/(273 + T).
Soil microbial community analysis
To analyze the soil microbial community, soil was collected immediately, 30, and 60 days after treatment with fertilizer and a microbial agent, and NucleoSpin Soil (MACHEREY–NAGEL, Nordrhein-Westfalen, Germany) kit was used to extract the DNA of soil microorganisms. The microbial community was commissioned by Cheonlab Co., Ltd. and analyzed with MiSeq (Illumina, CA, USA) equipment, using 318F and 80R primers that amplify the V3–V4 region of the 16S rRNA gene and forward through paired-end sequencing. A pair of 300 bp-long nucleotide sequences of the reverse and reverse sides were merged into one to produce a nucleotide sequence of ≥ 400 bp and then analyzed.
Statistical analysis
All treatments were performed in triplicate. Statistical analysis was performed using SAS version 8.1 by performing Duncan’s multiple range test at a 5% confidence interval to identify the differences among the treatments.
Results and discussions
Selection of optimal microorganisms to reduce ammonia emissions in pot experiments
Effects of nitrifying microorganisms
Ammonia emission characteristics were investigated according to the treatment of microorganisms with nitrification functions in soil containing NPK (Fig. 1). Ammonia gas discharged from the NPK-containing soil with or without microbial treatment increased rapidly on the 2nd day after treatment and thereafter decreased gradually. Ammonia emissions from soil containing only NPK (Control) were 619.7 g/ha/day on the 2nd day after treatment, and then discharged irregularly in the range of 99–288.3 g/ha/day. When Alcaligenes faecalis subsp. faecalis (SM1) and Brevibacillus sp. (SM2) were added to the NPK-containing soil, the ammonia gas production was 548.8 and 585.7 g/ha/day, respectively, on the 2nd day, which was significantly lower than that treated with only NPK. In contrast, ammonia emissions from NPK-containing soil treated with Rhodococcus sp., Bacillus subtilis, Acinetobacter junii, and Pseudomonas fluorescens increased compared to the control on the 2nd day. Ammonia gas discharged from the NPK-containing soil was generated immediately after treatment for 7 days regardless of the presence of microorganisms, accounting for > 50% of the total amount of ammonia gas discharged during the investigation. Cantarella et al. [17] reported that the hydrolysis reaction of urea locally increases the soil pH by consuming H+, which has a decisive effect on the volatilization of NH3 from the soil surface immediately after urea treatment. Soares et al. [18] reported that 20% of the N applied was lost as NH3 within 2 days after applying urea fertilizer. In particular, ammonia gas generated from urea-applied soil has a dominant effect on environmental factors, such as soil pH, buffering capacitance, temperature, moisture content, and microbial community [19]. Therefore, to effectively reduce the ammonia gas generated from urea-containing soil, improving the above-mentioned soil environment is necessary immediately after fertilizer treatment.
Effect of ammonia emission using nitrifying microorganism [A NH3 emission, B Accumulated NH3-N, Control: NPK, SM1: NPK + Brevibacillus sp., SM2: NPK + Alcaligenes faecalis subsp. faecalis, SM3: NPK + Rhodococcus sp., SM4: NPK + Bacillus subtilis, SM5: NPK + Acinetobacter junii, SM6: NPK + Pseudomonas fluorescens]
The cumulative ammonia gas discharged from the NPK-containing soil treated with the six microorganisms with nitrifying functions is shown in Fig. 1B. The cumulative ammonia emissions from soils containing only NPK were 5.6 kg/ha, whereas those from Brevibacillus sp. and Alcaligenes faecalis subsp. faecalis-treated soils containing NPK were 4.4 and 3.8 kg/ha, respectively. This indicated that Brevibacillus sp. and Alcaligenes faecalis subsp. faecalis reduced the cumulative ammonia gas by 21% and 31%, respectively, compared to the control. Brevibacillus sp. and Alcaligenes faecalis subsp. faecalis are involved in the ammonia oxidation-denitrification process [20, 21]. In particular, Alcaligenes faecalis subsp. faecalis can simultaneously perform nitrification and denitrification under aerobic conditions. Its growth is not inhibited even in high-concentration ammonia environments and substantially affects ammonia emission reduction. Brevibacillus sp. is an ammonia oxidizing microorganism that oxidizes ammonium to nitrite during nitrification. Therefore, Brevibacillus sp. oxidizes the decomposed NH4+ ions from urea and converts them to NO2−, which reduces the amount of ammonia gas discharged from NPK-containing soil. Therefore, Brevibacillus sp. and Alcaligenes faecalis subsp. faecalis can be used as effective microorganisms to reduce ammonia discharge from the soil.
Effects of acid-producing mixed microorganisms
In general, ammonia volatilization in soil is directly related to soil pH. Therefore, the application of acid-producing microorganisms maintained a low soil pH, which reduced the amount of ammonia volatilization. The amount of ammonia gas generated after treating NPK-containing soil with a mixed microbial agent containing a large number of acid-producing microorganisms is shown in Fig. 2. The emission of ammonia gas from soil containing only NPK was 619.7 g/ha/day on the 2nd day, whereas it was significantly reduced to 226.3 g/ha/day by acid-producing microorganisms. In particular, it was confirmed that treating acid-producing microorganisms in NPK-containing soil suppressed the rapid discharge of ammonia in the early stage, which further increased the ammonia reduction efficiency. Andreev et al. [22] reported that soil pH decreased after applying lactic acid bacteria to the soil, which directly reduced ammonia gas emissions. The cumulative ammonia emission for 20 days in the NPK-containing soil treated with acid-producing microorganisms was 2.5 kg/ha, which was 55% lower than that in the soil containing only NPK.
Selecting the optimal mixing ratio of microorganisms to reduce ammonia emissions
The reduction efficiency of ammonia gas discharged from NPK-containing soil could be maximized if nitrifying microorganisms and acid-producing microorganisms were mixed at an appropriate ratio. Therefore, this study investigated ammonia emissions in NPK-containing soil treated with a mixture of nitrifying and acid-producing microorganisms at different ratios (Fig. 3). Regardless of the mixing ratio, the amount of ammonia gas emitted from the NPK-containing soil treated with mixed microorganisms was lower than that from the soil treated with nitrifying microorganisms alone.
Effect of ammonia emission using nitrifying and mixed microorganism [A NH3 emission, B Accumulated NH3-N, Control: NPK, SM1: NPK + Brevibacillus sp., SM2: NPK + Alcaligenes faecalis subsp. faecalis, M1: NPK + acid-producing mixed microorganisms, SMM1: NPK + 0.50 M:0.25AF:0.25BB, SMM2: NPK + 0.70 M:0.15AF:0.15BB, SMM3:NPK + 0.80 M:0.10AF:0.10AF]
In particular, when only acid-producing microorganisms were treated with NPK-containing soil, the cumulative ammonia emission for 7 days was 1091.6 g/ha. In contrast, the ammonia gases released from the NPK-containing soil after treatment with the acid-producing microorganisms, Alcaligenes faecalis subsp. faecalis, and Brevibacillus sp. at mixing ratios of 0.5:0.25:0.25 (SMM1), 0.70:0.15:0.15 (SMM2), and 0.80:0.10:0.10 (SMM3) were 1191.2, 1010.4, and 1009.2 g/ha, respectively, which was similar to that treated with acid-producing microorganisms alone. However, from 7 to 20 days after treatment, the cumulative ammonia emission in NPK-containing soil treated with only acid-producing microorganisms was 1420.3 g/ha, whereas that of the NPK-containing soil treated with SSM2 was 958.8 g/ha, which was lower than that of the soil treated with only acid-producing microorganisms. This because NH4+ was not converted to NH3, owing to the simultaneous action of pH control and nitrification caused by mixed microorganisms in the NPK-containing soil. Cumulative ammonia emissions from NPK-containing soils according to the mixing ratio of the two nitrifying and acid-producing microorganisms were in the order of SMM1 (2.7 g/ha) > SMM3 (2.4 g/ha) > SMM2 (2.0 g/ha), and the ammonia reduction efficiency compared to NPK-containing soil was in the order of SMM2 (65%) > SMM3 (57%) > SMM1 (51%). Therefore, SMM2 was the optimum microbial mixing condition for reducing ammonia gas in NPK-treated soil.
Application of microbial agents in Chinese cabbage cultivation
Ammonia reduction efficiency according to application of microbial agent
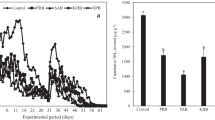
The ammonia emission reduction efficiency was evaluated for 3 months by applying the optimal microorganisms based on the aforementioned pot experiment results to actual cabbage cultivation (Fig. 4). Ammonia emissions remained relatively low after basal fertilization in all treatments, but tended to increase rapidly after additional fertilization. In the NPK treatment without microbial agents, ammonia emissions increased rapidly to 1471 g/ha/day 7 days after the first additional fertilization, and thereafter gradually decreased to 474.3 g/ha/day. After 5 days of the 2nd additional fertilization, the ammonia emissions increased rapidly to 2852.2 g/ha/day and then gradually decreased, reaching 216.5 g/ha/day after 14 days. After the 3rd additional fertilization, ammonia emissions from the NPK-containing soils without microorganisms were maintained from 270.2 g/ha/day to 792.1 g/ha/day. Ammonia gas emissions in the NPK-containing soil treated with the microbial agent were significantly lower than those in the non-treated soil. However, ammonia emissions tended to increase as the number of microbial agents increased. In particular, the ammonia emissions rapidly discharged from the NPK-containing soil after the first additional fertilizer application were the lowest at 500 L/ha, which was the treatment with the fewest microbial agents. It is believed that the water content in the soil increases with increasing amounts of microbial agent treated in liquid form, which accelerates urea decomposition and increases the amount of ammonia volatilization. Bouwmeester et al. [23] and Park et al. [24] reported that the amount of ammonia gas emitted from urea-treated soil increased with increasing soil moisture content, similar to our results.
Figure 4B shows the cumulative ammonia emissions from the cabbage culture according to the optimal microbial agent treatment amount. When 500, 1000, and 2000 L/ha of microbial agents were applied to NPK-containing soils, the cumulative amounts of ammonia released were 25.9, 27.7, and 33.7 kg/ha, respectively, which were lower than those of NPK-containing soils not treated with microorganisms.
Therefore, it can be concluded that treating microorganisms in cabbage cultivation can effectively control the ammonia discharged from NPK-containing soils. However, owing to the growth characteristics of Chinese cabbage, if additional fertilizer is supplied for a long period, microorganism activity is reduced, which negatively affects ammonia reduction efficiency. Therefore, the microbial agent was treated in the same manner as the number of fertilizer treatments (Fig. 4). The amount of ammonia discharged from the NPK-containing soil was significantly reduced compared to one treatment with the microbial agent after the same number of fertilizer treatments and treatments with the microbial agent. In particular, the amount of ammonia gas rapidly discharged from the NPK-containing soil after each additional fertilizer application was significantly reduced by the continuous microbial agent treatment.
Growth characteristics of Chinese cabbage according to application of microbial agent
The growth characteristics of Chinese cabbage according to the NPK and microbial treatment levels are shown in Table 2. The untreated and NPK cabbage yields were 27.1 and 93.9 Mg/ha, respectively, and the yield of Chinese cabbage treated with NPK was more than three times higher than that of untreated cabbage. When 500, 1000, and 2000 L/ha of the microbial agent were applied to the NPK-containing soil, cabbage yields were 94.2, 96.9, and 97.2 Mg/ha, respectively, which were not significantly different from those in the soil treated with only NPK. Santos et al. [25] reported that a mixture of microorganism strains or species with different functions could maximize the benefits and improve crop yield. Huang et al. [26] reported that plant growth was promoted when Bacillus and Pseudomonas were added to the soil, which was also included in the mixed microbial agents used in this study. However, in this study, the mixed strain effectively reduced ammonia gas discharged from NPK-containing soil but had no significant effect on Chinese cabbage growth. When the microbial treatment was applied at the same frequency and timing as the fertilizer treatment, the Chinese cabbage yield was 98.1 Mg/ha. This did not differ significantly from that of the soil treated with NPK and microbial agent once.
Soil microbial communities according to the application of microbial agents
This study analyzed the distribution of microbial communities in the soil with and without microbial agent treatment during cabbage cultivation (Additional file 1: Fig. S2 and Fig. 5). Analysis of the distribution of soil microorganisms immediately after NPK treatment showed that the distribution ratios of Proteobacteria, Acidobacteria, and Bacteroidetes were 53.9, 16.9, and 11.9%, respectively, with Proteobacteria having the highest distribution ratio. Furthermore, despite the lapse of 30 and 65 days after NPK treatment, there was no significant difference in the distribution ratio of soil microorganisms.
The distribution ratio of Proteobacteria immediately after the microbial agent and NPK were applied to the soil was 53.9%; however, it decreased to 35.3% after 30 days of treatment. However, after 65 days of treatment, the distribution ratio of Proteobacteria increased to 45.0%. The distribution ratios of Acidobacteria were 16.9%, 23.1%, and 19.8% immediately after NPK and microbial agent treatment and 30 and 65 days after treatment, respectively. The phylum Acidobacteria contains many acid-producing microorganisms that regulate pH in the soil [27]. This study confirmed that the ammonia gas emitted from NPK-containing soil was reduced because of the increase in Acidobacteria upon treatment with the microbial agent. Bacteroidetes showed a trend similar to that of Acidobacteria. Bacteroidetes is a phylum in which a large number of various bacteria, such as ammonia-oxidizing and nitrifying microorganisms, are distributed [28]. It is considered that the distribution ratio in the soil increased owing to the influence of nitrifying microorganisms included in the microbial agent in our study. The distribution ratios of Firmicutes were 0.3%, 2.9%, and 1.4% immediately after treatment with fertilizer and microbial agent, and 30 and 65 days after treatment, respectively, and were the highest 30 days after treatment with microbial agent and fertilizer. Firmicutes are a phylum to which lactic acid bacteria belong [29], and they play an important role in regulating soil pH [30]. These phyla were included in the microbial agents used in this experiment, and their distribution ratio in the soil increased after the treatment. However, the distribution ratio of each phylum 65 days after soil treatment with fertilizer and microbial agents showed a similar tendency to that immediately after fertilizer and microbial agent treatment. These results indicate that the treated microorganisms proliferated in the soil for a short period rather than proliferating and dominating for a long period, thereby reducing ammonia emissions. Therefore, to effectively control the ammonia gas discharged from NPK-treated soil using a microbial agent, it is necessary to monitor changes in the soil microbial community and apply continuous microbial agent treatment.
Availability of data and materials
All data is available in the main text.
References
Shi Y, Bilal M, Ho HC, Omar A (2020) Urbanization and regional air pollution across South Asian developing countries—a nationwide land use regression for ambient PM2.5 assessment in Pakistan. Environ Pollut. 266:115145
Tatarko J, Kucharski M, Li H, Li H (2020) PM2.5 and PM10 emissions by abrasion of agricultural soils. Soil Tillage Res 200:104601
Wang W, Lin Y, Yang H, Ling W, Liu L, Zhang W, Lu D, Liu Q, Jiang G (2022) Internal exposure and distribution of airborne fine particles in the human body: methodology, current understandings, and research needs. Environ Sci Technol 56(11):6857–6869
Behrooz RD, Kaskaoutis DG, Grivas G, Mihalopoulos N (2021) Human health risk assessment for toxic elements in the extreme ambient dust conditions observed in Sistan. Iran Chemosphere 262:127835
Baker KR, Foley KM (2011) A nonlinear regression model estimating single source concentrations of primary and secondarily formed PM2.5. Atmos Environ 45(22):3758–3767
Pei C, Ou Q, Yu T, Pui DYH (2019) Loading characteristics of nanofiber coated air intake filter media by potassium chloride, ammonium sulfate, and ammonium nitrate fine particles and the comparison with conventional cellulose filter media. Sep Purif Technol 228:115734
Choi H, Hyun J, Kim Y, Yoo G (2019) Improvement of ammonia emission inventory estimation methodology for fertilizer application in the agricultural sector. J Climate Change Res 10(3):237–242
Park J, Jeong H, Lee SY, Choi LY, Hong SW (2021) An investigation of emission of particulate matters and ammonia in comparison with animal activity in Swine Barns. J Korean Soc Agric Eng 63(6):117–129
Mira AB, Cantarella H, Souza-Netto GJM, Moreira LA, Kamogawa MY, Otto R (2017) Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric Ecosyst Environ 248:105–112
Abdo AI, Xu Y, Shi D, Li J, Li H, El-Sappah AH, Elrys AS, Alharbi SA, Zhou C, Wang L, Kuzyakov Y (2022) Nitrogen transformation genes and ammonia emission from soil under biochar and urease inhibitor application. Soil Tillage Res 223:105491
Li L, Wu T, Li Y, Hu X, Wang Z, Liu J, Qin W, Ashraf U (2022) Deep fertilization improves rice productivity and reduces ammonia emissions from rice fields in China: a meta-analysis. Field Crops Res 289:108704
Feng Y, Sun H, Xue L, Liu Y, Gao Q, Lu K, Yang L (2017) Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 168:1277–1284
Pan S, Wen X, Wang Z, Ashraf U, Tian H, Duan M, Mo Z, Fan P, Tang X (2017) Benefits of mechanized deep placement of nitrogen fertilizer in direct-seeded rice in South China. Field Crops Res 203:139–149
Sanz-Cobena A, Sanchez-Martin L, Garcia-Torres L, Vallejo A (2012) Gaseous emissions of N2O and NO and NO3- leaching from urea applied with urease and nitrification inhibitors to a maize (Zea mays) crop. Agric Ecosyst Environ 149:64–73
Wang X, Zhu H, Yan B, Chen L, Shutes B, Wang M, Lyu J, Zhang F (2023) Ammonia volatilization, greenhouse gas emissions and microbiological mechanisms following the application of nitrogen fertilizers in a saline-alkali paddy ecosystem. Geoderma 433:116460
Kim SI, Heo W, Lee SJ, Han BY, Lee HG, Kim YJ (2021) Characteristics of Saccharomyces boulardii for reducing ammonia emission from livestock manure. Appl Biol Chem 64:30
Cantarella H, Otto R, Soares JR, Silva AGB (2018) Agronomic efficiency of NBPT as a urease inhibitor: a review. J Adv Res 13:19–27
Soares JR, Cantarella H, Menegale MLC (2012) Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol Biochem 52:82–89
Chien SH, Prochnow LI, Cantarella H (2009) Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron 102:267–322
Jung TK, Ra CS, Joh KS, Song HG (2016) Characterization of heterotrophic nitrification and aerobic denitrification by Alcaligenes faecalis NS13. Korean J Microbiol 52(2):166–174
Matsuno T, Horii S, Sato T, Matsumiya Y, Kubo M (2012) Analysis of nitrification in agricultural soil and improvement nitrogen circulation with autotrophic ammonia-oxidizing bacteria. Appl Biochem Biotechnol 169(3):795–809
Andreev N, Ronteltap M, Boincean B, Wernli M, Zubcov E, Bagrin N, Borodin N, Lens PNL (2017) Lactic acid fermentation of human urine to improve its fertilizing value and reduce odour emissions. J Environ Manage 198:63–69
Bouwmeester RJB, Velk PLG, Stumpe JM (1985) Effect of environmental factors on ammonia volatilization from a urea-fertilized soil. Soil Sci Soc Am J 49:376–381
Park JH, Lee SL, Hwang SW, Eom JH, Kim SH, Kang SW, Cho JS, Seo DC (2020) Characteristics of ammonia gas emissions from soybean cultication soils treated with mixed microorganisms. Appl Biol Chem 63:20
Santos MS, Nogueira MA, Hungria M (2019) Microbial inoculants : reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Expr 9:205
Huang P, de-Bashan L, Crocker T, Kloepper JW, Bashan Y (2017) Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting(rhizo)bacteria on growth promotion of crop plants. Biol Fertil Soils 53:199–208
Kalam S, Basu A, Ahmad I, Sayyed RZ, El-Enshasy HA, Dailin DJ, Suriani NL (2020) Recent understanding of soil Acidobacteria and their ecological significance: a critical review. Front Microbiol 11:580024
Sun H, Narihiro T, Ma X, Zhang XX, Ren H, Ye L (2019) Diverse aromatic-degrading bacteria present in a highly enriched autotrophic nitrifying sludge. Sci Total Environ 666:245–251
Minervini F, Celano G, Lattanzi A, Tedone L, De Mastro G, Gobbetti M, De Angelis M (2015) Latic acid bacteria in durum wheat flour are endophytic components of the plant during its entire life cycle. Appl Environ Microbiol 81:19
Bai B, Qiu R, Wang Z, Liu Y, Bao J, Sun L, Liu T, Ge G, Jia Y (2023) Effects of cellulose and lactic acid bacteria on ensiling performance and bacterial community of Caragana korshinskii silage. Microorganisms 11(2):337
Acknowledgements
This work was supported, in part, Biomaterials Specialized Graduate Program through the Korea Environmental Industry & Technology Institute (KEITI) funded by the Ministry of Environment (MOE), by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) [NRF-2022R1C1C1007804], and by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Livestock Industrialization Technology Development Program (121034-03) and Technology Commercialization Support Program (821007-03), funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
J-HP, S-HK, S-WK and D-CS designed and conducted the experiment as well as wrote the manuscript. S-LL, J-HL, J-SR and J-ML conducted characteristic analysis and interpretation of soil, gas, and plant in Chinese cabbage cultivation field. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Acid-producing microorganisms. Fig. S1. Changes in climate conditions in cabbage cultivation areas. Fig. S2. Alpha and beta diversity of microbial communities in Chinese cabbage cultivation soil with chemical fertilizer and microbial.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, JH., Lee, SL., Lee, JH. et al. Reduction of ammonia gas by microbial agent treatment in Chinese cabbage cultivation. Appl Biol Chem 66, 91 (2023). https://doi.org/10.1186/s13765-023-00847-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00847-6