Abstract
Background
Most non-communicable diseases (NCDs) are associated to diet and inflammation. The Dietary Inflammatory Index (DII) is a developed and validated self-assessment tool. The study was conducted to assess the association of DII with the hypertension (HTN) and type 2 diabetes mellitus (T2DM).
Methods
This cross-sectional analysis was conducted on 9811 participants aged 35 to 65 years from the Ravansar Non-Communicable Diseases (RaNCD) cohort study’s baseline phase data. The DII was calculated using 31 food frequency questionnaire parameters (FFQ). Univariable and multiple logistic regression was used to derive the estimates.
Results
In healthy participants, the mean DII score was − 2.32 ± 1.60; in participants with T2DM, HTN, or T2DM&HTN, the mean DII score was − 2.23 ± 1.59, − 2.45 ± 1.60 and − 2.25 ± 1.60, respectively (P = 0.011). Males had a significantly higher pro-inflammatory diet than females (P < 0.001). BMI (body mass index), triglyceride, energy intake, smokers were significantly higher and socio-economic status (SES), physical activity and HDL-C were significantly lower in the most pro-inflammatory diet compared to the most anti-inflammatory diet. Participants with T2DM, HTN, and T2DM&HTN had significantly higher mean anthropometry indices (P < 0.001) and lipid profiles than healthy subjects (P < 0.001). After adjusting for age, gender, and physical activity, the probability of developing T2DM was 1.48 (95% CI: 1.19, 1.85) times greater in the fourth quartile of DII than in the first quartile.
Conclusions
The findings of this study showed that an anti-inflammatory diet are associated with HTN, T2DM, and the risk factors associated with these conditions. Modification of diet is recommended to reduce inflammation.
Similar content being viewed by others
Background
Chronic systemic inflammation plays a critical role in the pathogenesis of non-communicable diseases (NCDs), such as cardiovascular disease (CVDs), type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS), Hypertension (HTN), fatty liver, and cancer [1,2,3,4,5]. In type 2 diabetes and HTN, obesity and overweight, insulin resistance, and overexpression of pro-inflammatory proteins including C-reactive protein (CRP) and cytokines (IL-1β, IL-6, and TNF-α) contribute to chronic inflammation [6]. Additionally, environmental, psychosocial, and behavioural factors can lead to inflammation during times of stress [7], where inflammation can be reduced by modifying some of these factors. A diet is a vital part of the human lifestyle, and its modification can help moderate the inflammatory process [8, 9].
Previous research has demonstrated that certain dietary patterns, such as a high fibre, fruit, and vegetable intake and a low fat intake, reduce inflammatory markers and thus the risk of NCDs [10, 11]. Conversely, the Western diet (including processed meats and refined carbohydrates) is associated with elevated inflammatory markers and appears to be a risk factor for certain NCDs [12].
Various foods and food components can affect inflammatory markers in the blood. Recently, a relatively new index called the dietary inflammatory index (DII) had been used to assess the inflammatory potential of an individual’s diet. The DII is based on 45 food parameters. DII measures anti- to pro-inflammatory compounds, and a high value indicates that a person’s diet is inflammatory [13]. Given the diversity of lifestyles and dietary patterns found across geographical regions and ethnic groups, assessing the DII in diverse populations is critical. Given the rising prevalence of NCDs, prevention and control are essential. As a result, identifying and modifying modifiable risk factors for NCDs can help to reduce the disease burden in communities.
According to reports, HTN and its complications account for approximately 9.4 million deaths worldwide each year [14], with Asia being the region with the highest rate of type 2 diabetes [15]. As a result, data from the Ravansar non-communicable diseases cohort study (RaNCD), the only Kurdish cohort study currently being conducted, consists of approximately 10,000 adults. The aim of this study was to determine whether a pro-inflammatory diet as measured by DII was associated with an increased odd of HTN and T2DM in a Kurdish population.
Methods
Study setting and design
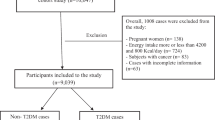
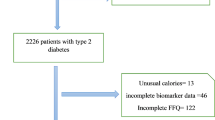
We used baseline data from RaNCD in this cross-sectional study, one of the sub-studies of the national Prospective Epidemiological Research Studies of IrAN (PERSIAN) cohort study [16]. Ravansar is one of Kermanshah Province’s western cities, with a population of approximately 50,000 in western Iran. Comprehensive information about the RaNCD study has been published and is accessible [17]. This study enrolled participants aged 35–65 years’ old who were in the bassline phase of RaNCD (10,000 individuals). Due to the research design, subjects with cancer and pregnant women were excluded. The final population of the study consisted of 9811 adults (Fig. 1).
Data collection
Sociodemographic data, such as age, gender, marital status, and residence location, as well as personal habits (smoking status and alcohol consumption), were collected face to face using digital questionnaires.
The socioeconomic status (SES) was determined using 18 items (housing, car price, dishwasher, freezer, washing machine, computer, laptop, internet access, motorcycle, color TV, TV type, bathroom, cell phone, vacuum cleaner, area per capita, room per capita, education level, and residence place); finally, the SES was classified into five groups from poorest to richest using the principal component analysis (PCA) method [18].
A standardized cohort study using a physical activity questionnaire (Including 22 questions) was used to assess participants’ physical activity on a met/hour per week basis. Participants were divided into three groups (light, moderate, high).
Blood samples were collected after 12 h of fasting to measure biochemical markers such as triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (T-C), as well as fasting blood sugar (FBS). A BSM 370 (with 0.1 cm precision) was used to measure the height (Biospace Co, Seoul, Korea).
Weight and other anthropometric indices, including body mass index (BMI), body fat mass (BFM), and visceral fat area (VFA), were measured using a Bio-Impedance Analyzer BIA (with a precision of 0.5 kg) (InBody 770 Biospace, Korea). Standard methods were used to determine waist circumference (WC) and waist to hip ratio (WHR). Blood pressure (BP) was measured via a manometer cuff and stethoscope after resting the arm in the seated position for 10 min.
Assessment of the dietary inflammatory index
Food Frequency Questionnaire (FFQ) items were used to calculate the DII scores. Participants responded to questions about their consumption of various food groups in terms of quantity and frequency. They were shown a photo from the booklet to assist them in estimating portion sizes. At this stage, diet-related data were collected face-to-face to minimize measurement bias.
Shivappa et al. found that 45 foods were associated with one or more of the inflammatory markers Interleukin-1b (IL-1b), Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), C-reactive protein (CRP), anti-inflammatory markers Interleukin-4 (IL-4) and Interleukin-10 (IL-10). Z-scores for each parameter were determined through the method developed by Shivappa et al., using the mean and standard deviation (SD) of global intake. The Z-score was then converted to a percentile. This method was utilized to calculate the inflammatory score for each food parameter, and then the inflammatory scores for all parameters were added to obtain the total DII score. The higher the DII score, the more pro-inflammatory the diet, and the lower the DII score, the more anti-inflammatory the diet [19, 20]. DII scores were classified into four groups (quartiles) to assess associations. The first and fourth quartiles had the lowest and highest DII scores, respectively.
In the current study, DII was calculated using 31 food parameters, including carbohydrate, protein, total fat, trans fat, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), cholesterol, saturated fat, omega-3, omega-6, vitamins A, B6, B12, C, D, E, selenium, zinc, energy, iron, magnesium, niacin, riboflavin, thiamine, beta-carotene, fibre, folic acid, caffeine, garlic, onion, and tea.
Hypertension and type 2 diabetes mellitus assessment
HTN was defined as a systolic blood pressure (SBP) of ≥140 mmHg either-or a diastolic blood pressure (DBP) of ≥90 mmHg either-or as being currently on antihypertensive medication [21]. T2DM was defined as having an FBS (fasting blood sugar) of ≥126 mg/dl either-or being on diabetes medication either-or having diabetes confirmed by a health practitioner [22].
Statistical analysis
The mean ± standard deviation was used for quantitative variables, while the frequency (percentage) was used via DII quartiles for qualitative variables. Additionally, Chi square test and one-way ANOVA compared the frequency (%) and mean ± standard deviation of basic characteristics among the quartiles of DII. Also, one-way ANOVA compared the mean ± standard deviation of anthropometric and biochemical characteristics among the four studied groups (Healthy, T2DM, HTN, T2DM & HTN). Crude and adjusted logistic regression models (Adjusted for potential confounders including age, sex, BMI, BFM, WHR, carbohydrate (%E), protein (%E) and oil/fat (%E) and physical activity) were used to determine the association between DII and hypertension and T2DM. The crude and adjusted odds ratios with 95% confidence interval were reported. P values < 0.05 were considered significant. All analyses were done with STATA software version 14.2 (Stata Corp, College Station, Tex).
Results
Basic characteristics
The current study entered 9811 participants aged 35–65 years. The baseline characteristics of the study participants according to DII quartiles are summarized in Table 1. Males consumed significantly more pro-inflammatory foods than females (P < 0.001). According to DII score quartiles, subjects with the most pro-inflammatory diets had a significantly higher BMI, higher TG, were heavy smokers, had a lower SES, engaged in less physical activity, and had a lower HDL-C. The DII score was significantly higher in urban residents (P < 0.001) and married subjects (P < 0.001). Increases in DII scores were associated with an increase in the prevalence of T2DM (P = 0.086) and HTN (P = 0.003). Daily energy intake (P < 0.001) and energy from lipids intake (P < 0.001) were significantly higher in subjects on a pro-inflammatory diet than in subjects on an anti-inflammatory diet.
The anthropometric and biochemical characteristics of the participants are listed in Table 2. The mean age was significantly higher in subjects with T2DM, HTN, and comorbidity (T2DM&HTN) than in healthy subjects (P < 0.001). Subjects with T2DM, HTN, and T2DM&HTN had significantly higher mean BMI, WHR, WC, BFM, and VFA than healthy subjects (P < 0.001). In subjects with T2DM, HTN, and T2DM&HTN, the mean lipid profile (LDL-C, TG, and T-C) was significantly higher HTN (P < 0.001).
Association between dietary inflammatory index and T2DM
The crude logistic regression model revealed that the probability of developing T2DM was 1.24 (95% CI: 1.02, 1.51) times greater in subjects with the most pro-inflammatory diet than in subjects with the most anti-inflammatory diet. After adjusting for age and gender, the odds of developing T2DM were 1.54 (95% CI: 1.26, 1.90) times greater in the fourth quartile of DII than in the first quartile (Table 3).
Association between dietary inflammatory index and HTN
After adjusting for age and gender, the odds of HTN were 0.86 (95% CI: 0.73, 1.0), 0.98 (95% CI: 0.84, 1.15) and 1.06 (95% CI: 0.90, 1.24) across all DII score quartiles, respectively. After adjusting for another confounder in model III, we observed that those in the fourth quartile of DII had an approximately 10% higher odds of HTN than those in the first quartile of DII, although this difference was not statistically significant (Table 4). DII was associated with an increased odd of T2DM and HTN in both the crude and adjusted models (Fig. 2).
Discussion
The current study’s findings demonstrate that the odds of developing T2DM was significantly higher in the fourth quartile of DII (indicating a more pro-inflammatory diet) than in the first quartile of DII (indicating the more anti-inflammatory diet). This association persisted after adjusting for confounding variables such as age, gender, physical activity, and energy intake. According to Laouali et al. (2019), a higher anti-inflammatory diet is associated with a lower risk of T2DM in French adults [23]. Denova-Gutiérrez et al. discovered an association between a pro-inflammatory diet and an increased risk of T2DM in adult Mexicans [24]. We found no correlation between DII and the odds of developing HTN. A cohort study of a large population of French people found a weak correlation between DII and HTN incidence [11]. Simultaneously, an Australian study of women discovered that a pro-inflammatory diet was associated with an increased risk of developing HTN [25]. This discrepancy could be explained by differences in how DII is calculated across studies.
The DII score was calculated in this study using 31 dietary parameters. DII has been calculated in some studies using 27 or 25 dietary parameters [24, 25]. Contradictions in the results are due to differences in the studied populations, genetic factors, and dietary patterns throughout the world, contributing to differences in the DII range.
This study demonstrates that males, urban residents, and married subjects had a significantly higher DII score. Furthermore, the most pro-inflammatory diet resulted in a significantly higher BMI, increased TG, lower SES, decreased physical activity, and decreased HDL-C compared to the most anti-inflammatory diet. These factors are associated with a healthy lifestyle; evidence suggests that lifestyle factors contribute to the prevention or initiation of inflammation. Numerous studies have reported an association between lifestyle changes and anti-inflammatory effects, such that increases in fibre intake and moderate to vigorous leisure-time physical activity predicted decreases in either CRP or IL-6 levels [26, 27]. A longitudinal study conducted in the UK demonstrated a linear relationship between CRP levels and weight gain over 9 years [27]. Moreover, some researchers have suggested that obesity plays a role in the relationship between an anti-inflammatory diet and the odds of developing T2DM or HTN [11, 23]. Our findings indicate that an anti-inflammatory diet is positively associated with a high BMI, highlighting the critical role of lifestyle factors in inflammatory markers.
On the other hand, following a Mediterranean diet low in inflammation has been shown to be a protective factor against T2DM (49% risk reduction) and lower TNF-α, CRP, and IL-6 levels [28]. Single-nutrient studies evaluated the polyphenols in grapes and raisins, both of which have been shown to reduce plasma TNF-α levels [29, 30]. Dietary patterns analysis revealed that subjects who consumed a significant amount of red meat, low-fiber bread and cereals, dried beans, fried potatoes, tomato vegetables, eggs, cheese and drank little wine had a nearly 4.5-fold increased risk of T2DM [31]. Additionally, a significant association between high-salt and high-fat diets and the risk of T2DM was discovered [32]. After 1 year on the Mediterranean diet, CPR decreased by 37%, and adiponectin increased by 43% in a randomized controlled trial (RCT) [33].
Moreover, most high-consumption foods found in the most pro-inflammatory diets, such as red and processed meats, refined grains, and soft drinks, have been linked to elevated inflammatory markers and an increased risk of T2DM [34]. Additionally, CRP levels can be used to predict mortality risk in adults with T2DM [35]. Increased production of the IL-1 family cytokines, IL-1β and IL-18, has been linked to an increased risk of HTN [36, 37]. As a result, it can be concluded that inflammatory markers play a role in developing T2DM and HTN and that diet is a critical factor in increasing or decreasing inflammatory marker levels. The DII is an accurate tool for examining these correlations.
We calculated DII using a validated dietary questionnaire; thus, diet information was collected face-to-face, minimizing measurement error. However, diets may change over time, and we cannot quantify these changes, longitudinal studies examining these associations are required to establish causality. The main limitation of current study was its cross-sectional design, limiting causal inference of the observed associations. The study’s advantages include large sample size and the use of data from a well-designed cohort study, as well as the ability to adjust for potentially confounding variables. Additionally, the first study is based on a sizable population of Kurdish subjects, allowing for comparisons with other ethnic groups worldwide. The results of this study can be generalized to adults of Kurdish ethnicity because of the similarity of diet in the Kurdish ethnic groups of Iran and neighboring countries.
Conclusion
In conclusion, we found a significant association between a pro-inflammatory diet and the odds of developing T2DM, but only a weak association between a pro-inflammatory diet and HTN. The subjects who consumed the most pro-inflammatory diet had a significantly higher BMI, TG, lower SES, physical activity, and HDL-C than those who consumed a primarily anti-inflammatory diet. As a result of the current study’s findings, an anti-inflammatory diet may help prevent the development of HTN, T2DM, and their associated risk factors. Modification of diet is recommended to reduce inflammation.
Availability of data and materials
The data sets generated during this study are available from the correspondence author on reasonable request via email.
Abbreviations
- NCDs:
-
Non-communicable diseases
- CVDs:
-
Cardiovascular disease
- T2DM:
-
Type 2 diabetes mellitus
- MetS:
-
Metabolic syndrome
- CRP:
-
C-reactive protein
- DII:
-
Dietary inflammatory index
- RaNCD:
-
Ravansar Non-Communicable Diseases
- FFQ:
-
Food frequency questionnaire
- SES:
-
Socio-economic status
- PERSIAN:
-
Prospective Epidemiological Research Studies in IrAN
- PCA:
-
Principal component analysis
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein Cholesterol
- HDL-C:
-
High-density lipoprotein Cholesterol
- T-C:
-
Total cholesterol
- FBS:
-
Fasting blood sugar
- BMI:
-
Body mass index
- BFM:
-
Body fat mass
- VFA:
-
visceral fat area
- WC:
-
Waist circumference
- WHR:
-
Waist to hip ratio
- BP:
-
Blood pressure
- DBP:
-
Diastolic Blood Pressure
- SBP:
-
Systolic Blood Pressure
- Il-1β :
-
Interleukin-1β
- Il-4:
-
Interleukin 4
- Il-6:
-
Interleukin 6
- Il-10 :
-
Interleukin 10
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- RCT:
-
Randomized controlled trial
- CI:
-
Confidence interval
- SD:
-
Standard deviation
References
Darbandi M, Hamzeh B, Ayenepour A, Rezaeian S, Najafi F, Shakiba E, et al. Anti-inflammatory diet consumption reduced fatty liver indices. Sci Rep. 2021;11(1):1–8.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. https://doi.org/10.1016/j.diabres.2014.04.006.
Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660–7. https://doi.org/10.1161/HYPERTENSIONAHA.117.07802.
Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013;13(3):435–44. https://doi.org/10.1007/s11892-013-0375-y.
Ayeneh Pour A, Moradinazar M, Samadi M, Hamzeh B, Najafi F, Karimi S, et al. Association of Dietary Inflammatory Index with cardiovascular disease in Kurdish adults: results of a prospective study on Ravansar non-communicable diseases. BMC Cardiovasc Disord. 2020;20(1):1–8.
Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–805. https://doi.org/10.2337/diabetes.52.7.1799.
Kang D-H, Rice M, Park N-J, Turner-Henson A, Downs C. Stress and inflammation: a biobehavioral approach for nursing research. West J Nurs Res. 2010;32(6):730–60. https://doi.org/10.1177/0193945909356556.
Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6(6):738–47. https://doi.org/10.3945/an.115.009415.
Cruz-Teno C, Pérez-Martínez P, Delgado-Lista J, Yubero-Serrano EM, García-Ríos A, Marín C, et al. Dietary fat modifies the postprandial inflammatory state in subjects with metabolic syndrome: the LIPGENE study. Mol Nutr Food Res. 2012;56(6):854–65. https://doi.org/10.1002/mnfr.201200096.
Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: a randomized controlled clinical trial. Horm Metab Res. 2015;47(03):232–8. https://doi.org/10.1055/s-0034-1376990.
MacDonald C-J, Laouali N, Madika A-L, Mancini FR, Boutron-Ruault M-C. Dietary inflammatory index, risk of incident hypertension, and effect modification from BMI. Nutr J. 2020;19(62):1–8. https://doi.org/10.1186/s12937-020-00577-1.
Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. https://doi.org/10.1111/nure.12035.
Shivappa N, Hébert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr. 2015;113(4):665–71. https://doi.org/10.1017/S000711451400395X.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–60. https://doi.org/10.1016/S0140-6736(12)61766-8.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88. https://doi.org/10.1038/nrendo.2017.151.
Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN cohort study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–55. https://doi.org/10.1093/aje/kwx314.
Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, et al. Cohort profile: Ravansar non-communicable disease cohort study: the first cohort study in a Kurdish population. Int J Epidemiol. 2019;48(3):682–3. https://doi.org/10.1093/ije/dyy296.
Najafi F, Pasdar Y, Hamzeh B, Rezaei S, Nazar MM, Soofi M. Measuring and decomposing socioeconomic inequalities in adult obesity in Western Iran. J Prev Med Public Health. 2018;51(6):289–97. https://doi.org/10.3961/jpmph.18.062.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96.
Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MA. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. 2016;17(8):1265.
Rajati F, Hamzeh B, Pasdar Y, Safari R, Moradinazar M, Shakiba E, et al. Prevalence, awareness, treatment, and control of hypertension and their determinants: results from the first cohort of non-communicable diseases in a Kurdish settlement. Sci Rep. 2019;9(1):1–10. https://doi.org/10.1038/s41598-019-8232-y.
Safari-Faramani R, Rajati F, Tavakol K, Hamzeh B, Pasdar Y, Moradinazar M, et al. Prevalence, awareness, treatment, control, and the associated factors of diabetes in an Iranian Kurdish population. J Diabetes Res. 2019;2019. https://doi.org/10.1155/2019/5869206.
Laouali N, Mancini FR, Hajji-Louati M, El Fatouhi D, Balkau B, Boutron-Ruault M-C, et al. Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: the mediating role of BMI. Diabetologia. 2019;62(12):2222–32. https://doi.org/10.1007/s00125-019-4972-0.
Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N, Hébert JR, Tolentino-Mayo L, Batis C, et al. Dietary inflammatory index and type 2 diabetes mellitus in adults: the diabetes mellitus survey of Mexico City. Nutrients. 2018;10(4):385. https://doi.org/10.3390/nu10040385.
Vissers LE, Waller M, van der Schouw YT, Hebert JR, Shivappa N, Schoenaker D, et al. A pro-inflammatory diet is associated with increased risk of developing hypertension among middle-aged women. Nutr Metab Cardiovasc Dis. 2017;27(6):564–70. https://doi.org/10.1016/j.numecd.2017.03.005.
Herder C, Peltonen M, Koenig W, Sütfels K, Lindström J, Martin S, et al. Anti-inflammatory effect of lifestyle changes in the Finnish diabetes prevention study. Diabetologia. 2009;52(3):433–42. https://doi.org/10.1007/s00125-008-1243-1.
Fogarty AW, Glancy C, Jones S, Lewis SA, McKeever TM, Britton JR. A prospective study of weight change and systemic inflammation over 9 y. Am J Clin Nutr. 2008;87(1):30–5. https://doi.org/10.1093/ajcn/87.1.30.
Koloverou E, Esposito K, Giugliano D, Panagiotakos D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: a meta-analysis of 10 prospective studies and 136,846 participants. Metabolism. 2014;63(7):903–11. https://doi.org/10.1016/j.metabol.2014.04.010.
Zern T, Wood R, Greene C, West K, Liu Y, Aggarwal D, et al. Grape polyphenols lower plasma lipids and apolipoproteins associated with increased risk for cardiovascular disease in pre and post-menopausal women. J Nutr. 2005;135:1911–47 https://doi.org/10.1093/jn/135.8.1911
Puglisi MJ, Vaishnav U, Shrestha S, Torres-Gonzalez M, Wood RJ, Volek JS, et al. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. 2008;7(1):1–9. https://doi.org/10.1186/476-511X-7-14.
Liese AD, Weis KE, Schulz M, Tooze JA. Food intake patterns associated with incident type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32(2):263–8. https://doi.org/10.2337/dc08-1325.
Fang M, Feng L-J. Association between dietary pattern and the risk of type 2 diabetes mellitus in Zhejiang Province, China: a case-control study. Asia Pac J Clin Nutr. 2020;29(4):821–6. https://doi.org/10.6133/apjcn.202012_29(4).0018.
Maiorino MI, Bellastella G, Petrizzo M, Scappaticcio L, Giugliano D, Esposito K. Mediterranean diet cools down the inflammatory milieu in type 2 diabetes: the MÉDITA randomized controlled trial. Endocrine. 2016;54(3):634–41. https://doi.org/10.1007/s12020-016-0881-1.
Schulze MB, Hu FB. Primary prevention of diabetes: what can be done and how much can be prevented? Annu Rev Public Health. 2005;26:445–67 https://doi.org/10.1146/annurev.publhealth.26.021304.144532.
Cox A, Agarwal S, Herrington DM, Carr J, Freedman B, Bowden D. C-reactive protein concentration predicts mortality in type 2 diabetes: the diabetes heart study. Diabet Med. 2012;29(6):767–70. https://doi.org/10.1111/j.464-5491.2011.03560.x.
Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR. IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br J Pharmacol. 2014;171(24):5589–602. https://doi.org/10.1111/bph.12876.
Pioli MR, de Faria AP. Pro-inflammatory cytokines and resistant hypertension: potential for novel treatments? Curr Hypertens Rep. 2019;21(12):1–8. https://doi.org/10.1007/s11906-019-1003-2.
Acknowledgements
This study was extracted from MSc thesis of Nutrition Sciences (Ms. Samira Arbabi Jam), in the School of Nutritional Science and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Funding
This research was supported by Kermanshah University of Medical Sciences (grant number: 92472).
Author information
Authors and Affiliations
Contributions
YP, SA and BH designed the study. FH and MM collaborated in data collection. SHR and MD conducted data analyses and interpreted the results. SA and YP drafted the manuscript. FN, SE and ESH edited the drafted the manuscript. All authors revised it critically for important intellectual content and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Kermanshah University of Medical Sciences (KUMS.REC.1399.1050). From all participants was taken oral and written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jam, S.A., Rezaeian, S., Najafi, F. et al. Association of a pro-inflammatory diet with type 2 diabetes and hypertension: results from the Ravansar non-communicable diseases cohort study. Arch Public Health 80, 102 (2022). https://doi.org/10.1186/s13690-022-00839-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-022-00839-w