Abstract
Background
The dietary inflammatory index (DII) serves as a tool to assess the inflammatory impact of an individual’s diet. This study aimed to investigate the association between DII and some cardio-metabolic risk indices among patients with T2DM.
Methods
Data from the Hoveyzeh Cohort Study, encompassing 2045 adults with T2DM, were analyzed. DII scores were calculated based on food frequency questionnaires. Anthropometric measurements and biochemical tests were performed to assess cardio-metabolic risk factors.
Results
Higher DII scores were positively associated with elevated triglyceride levels, triglyceride-glucose (TyG) index, lipid accumulation product (LAP), anthropometric indices including a body shape index (ABSI), body roundness index (BRI), body mass index (BMI), hip, waist circumferences (WC), and waist-to-height ratio (all Ptrend < 0.05). Notably, no significant association was observed between DII and fasting blood sugar (FBS) levels (Ptrend > 0.05). Additionally, dietary intake analysis revealed a negative correlation between DII scores and intake of fiber, fruits, vegetables, legumes, fish, seafood, dairy products, magnesium, and vitamins A, C, D, and E (all Ptrend < 0.05). Conversely, higher DII scores were associated with increased consumption of red meat, processed meat, refined cereals, potatoes, and soft drinks (all Ptrend < 0.05).
Conclusion
This study underscores the critical link between dietary inflammation, assessed by the DII score, and a multitude of cardio-metabolic risk factors in patients with T2DM. Notably, while the study did not find a significant association between DII and fasting blood sugar levels, it identified robust associations with novel anthropometric and biochemical indices indicative of cardio-metabolic risk. These findings highlight the potential of dietary interventions as a cornerstone strategy for managing T2DM and mitigating its associated complications.
Similar content being viewed by others
Introduction
Chronic systemic inflammation, serving as the foundation for numerous chronic illnesses, is distinguished by heightened levels of inflammatory indicators [1, 2]. This persistent, low-grade inflammation throughout the body has been linked to various non-communicable diseases such as cancer, rheumatoid arthritis, and diabetes [3,4,5]. Diabetes Mellitus is a global health crisis affecting millions worldwide. The International Diabetes Federation (IDF)’s inaugural Diabetes Atlas in 2000 projected that 151 million adults globally had T2DM [6]. By 2019, this number had tripled to 463 million [7, 8]. This rising prevalence has resulted in a significant economic burden on healthcare systems. In 2007, the direct healthcare expenses associated with T2DM were estimated at US$232 billion, and by 2019, this figure had escalated to US$760 billion [9, 10]. Notably, 80% of individuals with T2DM reside in low- and middle-income countries, with a prevalence of around 14% reported in the Middle East, including Iran [11].
Dietary interventions have proven to be a cornerstone strategy in managing T2DM, demonstrably improving glycemic control and reducing cardiovascular risk [12,13,14]. Chronic inflammation is widely recognized as a key pathophysiological mechanism in the onset of T2DM [15]. The concept of dietary influence on inflammation is well-established, with certain dietary patterns promoting inflammation while others have anti-inflammatory properties. However, the precise impact of dietary manipulation on T2DM outcomes, particularly the role of inflammation, remains to be fully elucidated [16,17,18,19,20]. Therefore, a deeper understanding of the connections between diet, inflammation, and T2DM is crucial [21].
DII, a valuable tool developed by Cavicchia et al. [22] and refined by Shivappa et al. (2014), is designed to assess the overall inflammatory potential of a diet in diverse populations. Its utility lies in assessing the overall inflammatory impact of an individual’s diet, achievable through a 24-hour dietary record interview or the use of food frequency questionnaires (FFQs) [23]. DII score has been linked to markers of systemic inflammation and various chronic conditions associated with inflammation, including cardiovascular disease (CVD) [24], arthritis [25], and certain types of cancers [26].
Both obesity and overweight are well-established risk factors for T2DM, and T2DM itself can further worsen these conditions [27]. Anthropometry, employing simple body measurements, offers a cost-effective and accessible screening tool for cardiovascular disease, metabolic syndrome, and weight issues in individuals and populations [28]. However, while widely used in both clinical and epidemiological settings due to its simplicity, BMI’s limitation lies in its inability to differentiate between muscle and fat mass, leading to potential misclassification of overweight and obesity and underestimation of associated disease risks [29, 30]. This has driven the development of more precise anthropometric indices, such as ABSI, BRI and visceral adiposity index (VAI) to provide a more nuanced assessment [31, 32].
While the link between inflammatory biomarkers and inflammation-related chronic diseases is well-established, the association between the DII and T2DM related markers remains relatively underexplored. To address this gap, the present study investigated the association between DII scores and T2DM-related biochemical parameters and novel anthropometric indices in patients with T2DM from the Hoveyzeh Cohort Study.
Methods
Study design and sampling method
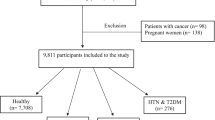
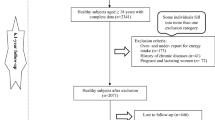
The Hoveyzeh Cohort Study (HCS) investigates non-communicable diseases (NCDs) in southwestern Iran [33]. This population-based cohort study recruited 10,009 adults aged 35–70 years between May 2016 and August 2018. Data collection and measurements followed standardized protocols ensuring consistency across the PERSIAN Cohort Study sites [33, 34]. In the current study, individuals were excluded if they had more than 10% unanswered items on their food frequency questionnaires (n = 122) or if their daily calorie consumption fell outside the range of 600 kcal to 5500 kcal (n = 13), as determined using the standard deviation method proposed by Rosner [35]. Moreover, individuals were excluded if they had incomplete biomarker data or lacked essential information on other covariates deemed significant (n = 46). A final sample of 2045 individuals were encompassed in our study.
Following written informed consent, a standardized, structured questionnaire was administered during in-person interviews to collect demographic information, dietary intake details via a food frequency questionnaire, and past medical history, tobacco use (defined as ever smoking one or more cigarettes daily for at least a month), alcohol consumption (defined as drinking alcohol at least once monthly), occupation, residential history, and other potential confounding variables.
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol and informed consent forms received thorough review and approval from the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (reference number: IR.AJUMS.REC.1398.760). All participants provided written informed consent after receiving a comprehensive explanation of the study’s objectives and methods.
Dietary evaluation
Dietary intake was assessed using a validated semi-quantitative food frequency questionnaire (FFQ) previously employed in the Hoveyzeh Cohort Study [33]. This tool queried participants on their consumption frequency of 130 food items over the past year, with standard portion sizes indicated for each item. Recorded daily food consumption (grams) was then entered into Nutritionist IV software to calculate total energy and nutrient intake.
Determination of DII
Dietary data from the validated FFQ was used to calculate DII scores for each participant. A comprehensive description of the DII method can be found elsewhere [23]. In summary, the corpus of literature pertaining to DII encompasses eligible publications spanning from 1950 to 2010, documenting associations between dietary components and various inflammatory markers such as Interleukin 1 beta (IL-1β), Interleukin 4 (IL-4), Interleukin 6 (IL-6), Interleukin 10 (IL-10), Tumor necrosis factor (TNF-α), and C-reactive protein (CRP). A total of 45 distinct food parameters associated with the six inflammatory biomarkers were identified in the literature review. Each parameter received a “food parameter-specific inflammatory effect score” by tallying the number of studies reporting pro-inflammatory, anti-inflammatory, and neutral effects on one or more of the six inflammatory markers. These scores were adjusted based on study design and the size of the literature for each food parameter’s inflammatory marker relationship. In prior analyses, there was a positive correlation between the DII and the circulating level of high-sensitivity C-reactive protein (hs-CRP) [36, 37]. In this study, DII scores for participants were computed by associating the dietary information with a global database, which furnished average intake values and standard deviations for each nutritional parameter. To adjust for the effect of energy, energy independent variables were calculated for these 45 nutrients. These values were subsequently used as factors to represent an individual’s exposure in relation to the ‘standard global mean,’ expressed as a z-score. This process involved subtracting the reported quantity from the ‘standard global mean’ and dividing the result by the standard deviation. As the data exhibited right-skewness, a typical observation in dietary data, we transformed these values into centered percentile scores. Each individual’s centered percentile score was then multiplied by the specific inflammatory effect score corresponding to the food parameter, resulting in an individualized food parameter-specific DII score. The cumulative sum of these food parameter-specific DII scores was subsequently calculated to derive the overall DII score for each participant in the study [23].
Biochemical test evaluation
Following a 10–12 h fast, trained personnel collected blood and urine samples from participants upon enrollment. Serum was isolated from whole blood by centrifugation (1000 rpm, 15 min) and stored at -80 °C for subsequent analysis. Details regarding the standardized procedures employed can be found in the initial Hoveyzeh Cohort Study publication [33]. Fasting blood glucose was measured by the glucose oxidase method. TC, TG, HDL, BUN, creatinine and liver enzymes were determined by enzymatic kits (Pars Azmoon, Iran).
Anthropometric measurement assessment
Trained personnel employed standardized procedures to measure participant height and weight with high precision. Height was assessed using a calibrated SECA 213 stadiometer to the nearest 0.1 cm, while weight was measured on a previously calibrated SECA 874 electronic scale accurate to 0.1 kg. BMI was also calculated as weight divided by height squared. The body shape index was calculated using the following formula [38]: ABSI = WC/[(BMI)ˆ (2/3) × (height)ˆ (1/2)]. The body roundness index was calculated using the following formula [39]: BRI = 365.2 − 365.5 ×\(\sqrt {(1 - (((wc/2\pi )2)/{{[(0.5 \times height)]}^2}))}\). Also, we used from the following formulas for calculation of VAI [40]: Men: VAI = [WC/39.68 + (1.88 × body mass index[BMI])] × [triglycerides(TG)/1.03] × (1.31/HDL); women: VAI = [WC/36.58 + (1.89 × BMI)] × (TG/0.81) × (1.52/HDL). Both TG and HDL levels are expressed in mmol/L. TyG index was calculated with this formula [41]: Ln [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2]. Finally, we used from the following formulas for LAP index calculation: Female: LAP=(WC-58) × TG, Male: LAP= (WC-65) × TG.
Physical activity assessment
Physical activity levels were assessed using the validated short version of the International Physical Activity Questionnaire (s-IPAQ) [42]. This 9-item tool quantifies time spent in moderate-to-intense physical activity sessions lasting at least 10 min, over the preceding seven days. Data analysis followed established IPAQ protocols [43], including conversion of hours to minutes, calculation of weekly frequency averages, and exclusion of participants with missing or ambiguous data (“do not know” or “refused”). Consistent with WHO guidelines, participants were categorized as inactive (< 150 min/week), moderately active (150–299 min/week), or highly active (≥ 300 min/week) based on their self-reported moderate-to-intense activity duration [44]. .
Statistical analysis
Normality of variable distributions was confirmed using the Kolmogorov-Smirnov test. Continuous data were presented as mean ± standard deviation, while categorical data were expressed as percentages and counts. Differences in categorical variables across tertiles of the DII score were assessed with the chi-square test. One-way ANOVA examined significant mean differences in continuous variables across these same tertiles. Post-hoc comparisons using Bonferroni test revealed significant mean differences between DII tertile groups. Linear regression analyses, both crude and multivariable-adjusted, were employed to investigate the associations between the DII score and biochemical and anthropometric indices. The first model controlled for gender, education level, smoking, alcohol and age, while the second additionally included physical activity and calorie intake as covariates, and finally in the model 3, we additionally included waist circumference and BMI. The lowest DII tertile served as the reference category. Statistical significance in this study was set at P < 0.05, with borderline significance considered for P values of 0.05 to 0.07. Analyses were conducted using SPSS version 24 (SPSS Inc., Chicago, IL, USA).
Results
Participant flow
Among 10,009 participants, finally 2045 patients we included in in the final analysis. The flowchart of patient selection process is summarized in Fig. 1.
Baseline characteristics
Table 1 summarizes the baseline characteristics of participants across the DII score tertiles. A total of 2,045 patients were included in the final analysis. The participant population consisted of 36% males and 64% females. The mean age was 53.15 ± 8.93 years, with a significant difference observed across DII score tertiles (p < 0.001). We found significant differences (p < 0.05) in age, BMI, WC, weight, TyG, ABSI, LAP, systolic blood pressure (SBP), hemoglobin A1c (HbA1c), blood urea nitrogen (BUN), aspartate aminotransferase (AST), TG, and HDL levels between DII score tertiles. However, no significant differences were observed in other anthropometric and biochemical variables (P > 0.05). Additionally, 35% of patients reported using oral hypoglycemic agents and there weren’t any significant differences between DII tertiles in term of oral hypoglycemic agents’ usage (P = 0.72).
Dietary factors across different DII score tertiles
Table 2 presents dietary intakes of the study subjects categorized by DII score tertiles. We observed significant differences in carbohydrate and protein intake across DII score tertiles (P < 0.001). Compared to participants in the lower tertile, those in the higher DII tertile consumed significantly lower amounts of fiber, fruits, vegetables, legumes, fish and seafood, dairy products (P < 0.05), and exhibited a higher intake of red meat, processed meat, refined cereals, potatoes, and soft drinks (P < 0.05). Furthermore, significant differences (P < 0.001) were observed in the intake of some vitamins and minerals, with participants in the higher DII tertile consuming lower amounts of magnesium, vitamin A, vitamin C, vitamin E, and vitamin D compared to the lower tertile.
Association between DII score and biochemical variables
Table 3 presents the associations between DII score and various biochemical variables, including FBS, TG, total cholesterol, LDL cholesterol, HDL cholesterol, BUN, creatinine, ALT, AST, and the TyG index. No significant correlation was observed between DII score and FBS concentration in either the crude (P = 0.33) or fully adjusted model (P = 0.29).
Multivariate linear regression analysis revealed a positive association between DII score and TG concentration (P trend < 0.05). Compared to participants in the first tertile, those in the third tertile of DII exhibited significantly higher TG levels in both the crude model (β = 18.48, SE = 7.43, P = 0.014) and the fully adjusted model (β = 19.62, SE = 7.76, P = 0.01).
Regarding liver enzymes, the present study found a positive correlation between DII score and both ALT and AST levels in the fully adjusted model (P trend < 0.05). Furthermore, the fully adjusted model revealed a positive association between increased DII score and elevated serum creatinine and BUN levels (P trend < 0.05).
Finally, a positive correlation was observed between DII score and both TyG index and LAP levels in both the crude and fully adjusted models (P trend < 0.05). However, no significant association was found between DII score and other biochemical variables, including total cholesterol, LDL cholesterol, and HDL cholesterol (P trend >0.05).
Association between DII score and anthropometric indexes
Table 4 summarizes the associations between DII score and various anthropometric measurements. We observed a significant positive correlation (Ptrend < 0.05) between DII score and ABSI, BRI, BMI, HC, WC and WHtR in both of the crude and full adjusted models (P trend <0.05). Compared to participants in the first tertile, those in the third tertile of DII exhibited significantly higher values in the fully adjusted model for ABSI (β = 0.07, SE = 0.003, P = 0.04), BRI (β = 0.35, SE = 0.09, P < 0.001), BMI (β = 1.46, SE = 0.35, P < 0.001), HC (β = 2.36, SE = 0.54, P < 0.001), WC (β = 2.67, SE = 0.63, P < 0.001) and WHtR(β = 0.015, SE = 0.003, P < 0.001). However, we couldn’t find any significant correlation between DII score with VAI (P trend =0.28) and WHR (P trend >0.05).
Discussion
To our knowledge, this is the first study to investigate the association between the DII and a range of anthropometric and biochemical indices in patients with T2DM. Our key findings revealed significant positive correlations between higher DII scores and several novel indices, including the TyG index, LAP, ABSI, BRI, and WHtR. Additionally, we observed significant associations between higher DII scores and several established variables, including TG, AST, ALT, BUN, BMI, WC, and HC. Notably, no significant associations were identified between DII score and other investigated variables.
Interestingly, while no significant association was observed between DII score and fasting blood sugar FBS in the present study, a positive correlation was identified with other biomarkers that elevate the risk of chronic diseases like chronic kidney disease (CKD) and non-alcoholic fatty liver disease (NAFLD) in patients with T2DM. This finding aligns with previous research emphasizing the connection between inflammation and diabetes [45,46,47,48]. Notably, King et al. conducted a cross-sectional study involving 4434 participants and reported an association between higher DII scores and elevated HbA1c levels [49]. Furthermore, Denova-Gutiérrez et al. reported a positive association between DII and T2DM risk in a separate population-based study conducted in Mexico City [50]. In their study, participants in the highest DII quartile exhibited a higher odds ratio for T2DM compared to those in the lowest quartile. In contrast, the present study population consisted primarily of patients with uncontrolled blood sugar levels, which may explain the lack of a significant association between DII and FBS concentration. It is possible that in patients with established T2DM and chronically elevated blood glucose, the association between DII and glycemic control becomes less direct. Instead, DII might influence factors more closely linked to disease progression, such as renal function (as indicated by BUN and creatinine levels) and anthropometric markers (e.g., BMI, WC) [51].
Our study identified a positive correlation between higher DII scores and TG levels. Additionally, we observed positive associations between DII and novel indices, such as the TyG index and LAP. These findings are noteworthy in the context of the well-established link between insulin resistance (IR) and both prediabetes/diabetes and cardiovascular disease (CVD) [52,53,54,55]. Recent research has emphasized the potential of novel anthropometric and biochemical indices as non-invasive and informative markers of IR [41, 56,57,58,59]. These indices, such as the VAI combining BMI and WC, LAP, TG and WC, and TyG index reflecting both TG and FBS, offer valuable tools for clinical assessment.
Our findings align with a growing body of evidence supporting the utility of these novel indices. Ahn et al., in a cross-sectional study involving 2,045 German participants, demonstrated that TyG and LAP provide a non-invasive and easily interpretable approach to identifying prediabetes/diabetes [60, 61]. Similarly, Shahavandi et al. observed an inverse correlation between adherence to plant-based dietary patterns and LAP levels in an Iranian population [62, 63]. These studies highlight the potential of dietary interventions to modulate inflammatory markers associated with metabolic health [64,65,66].
However, it is important to acknowledge some conflicting findings. Mirrafiei et al. reported a non-significant association between a meal-specific food-based DII and LAP or TyG levels in Iranian adults [67]. The relationship observed in the current study between DII and the indices related to IR and CVD can indicate the role of consumption of inflammatory diets in increasing the risk of CVD in diabetic patients. Inflammatory cytokines, like IL-1β and IL-6, can dampen the body’s ability to respond to insulin in fat, muscle, and liver. These cytokines are like pieces of a puzzle that explain how inflammation can lead to IR [68, 69]. Prolonged high blood sugar due to IR directly damages blood vessel cells (endothelium, smooth muscle) and immune cells (macrophages), triggering abnormal blood clotting and tissue breakdown. This interplay creates a breeding ground for atherosclerotic plaque formation. Additionally, excess reactive oxygen species and harmful protein-sugar molecules generated by hyperglycemia fuel chronic low-grade inflammation, further amplifying the risk of CVD [70, 71].
We also find a positive correlation between DII with ABSI, BRI and WHtR. Past research has explored WC, BAI, WHR, and WHtR for predicting metabolic risk, but these traditional anthropometric measures lack differentiation between fat and muscle mass [72,73,74]. To address this limitation, recent innovations like ABSI (incorporating WC, weight, and height) and BRI (using height and WC) have been developed to predict total and regional fat percentages and risk of cardio-metabolic disease more effectively [75,76,77]. Xu et al. in cross-sectional study with 17,000 Eastern-China adults found that BRI was significantly associated with high cardio-metabolic risk [31]. Recent studies investigated the effectiveness of two newly proposed indices, ABSI and BRI, in comparison to established measures like BMI and WC for predicting adverse health outcomes. Notably, these studies demonstrated that ABSI and BRI exhibited significantly stronger associations with abdominal fat accumulation, cardiometabolic risk factors, diabetes incidence, and premature mortality risks compared to BMI and WC [20, 78,79,80].
As mentioned, the present study was the first study that examined the relationship between the DII and novel cardio-metabolic risk indices. However, there were some limitations in the present study that should be considered. First, the study’s cross-sectional design limits the ability to establish causality. Second, dietary intake was assessed using a semi-quantitative food frequency questionnaire (FFQ), which may be subject to recall bias and misreporting of dietary intake. Third, While the analysis adjusted for several potential confounders, such as age, gender, education level, smoking, alcohol intake, physical activity, and calorie intake, there may be residual confounding from unmeasured variables (e.g., socioeconomic status, medication use, comorbidities) that could influence the observed associations.
Conclusion
In conclusion, our findings reveal several noteworthy associations between the DII and various anthropometric and biochemical indices. Notably, we observed a positive correlation between higher DII scores and markers of insulin resistance, including triglyceride levels, TyG index, and LAP index. our study underscores the critical role of dietary inflammation in shaping cardio-metabolic health outcomes among individuals with T2DM. Future longitudinal studies incorporating more robust dietary assessment methods are warranted to validate our findings and elucidate the causal relationship between dietary inflammation and cardio-metabolic risk among patients with T2DM.
Data availability
The data that support the findings of this study are available from The Hoveyzeh Cohort Study (HCS), but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available but is available with the corresponding author upon reasonable request.
References
Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101(Suppl 1):S1–45.
Xu Z, Zhang P, Chen Y, Jiang J, Zhou Z, Zhu H. Comparing SARC-CalF with SARC-F for screening Sarcopenia in adults with type 2 diabetes mellitus. Front Nutr. 2022;9:803924.
Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10(5):604.
Zhou Y, Chai X, Yang G, Sun X, Xing Z. Changes in body mass index and waist circumference and heart failure in type 2 diabetes mellitus. Front Endocrinol. 2023;14:1305839.
Yu T, Xu B, Bao M, Gao Y, Zhang Q, Zhang X, Liu R. Identification of potential biomarkers and pathways associated with carotid atherosclerotic plaques in type 2 diabetes mellitus: a transcriptomics study. Front Endocrinol. 2022;13:981100.
Atlas D. International diabetes federation. IDF Diabetes Atlas, 7th edn Brussels, Belgium: International Diabetes Federation. 2015;33(2).
Atlas ID. IDF diabetes atlas. International Diabetes Federation (9th editio) Retrieved from http://www.idf.org/about-diabetes/facts-figures. 2019.
Liang D, Cai X, Guan Q, Ou Y, Zheng X, Lin X. Burden of type 1 and type 2 diabetes and high fasting plasma glucose in Europe, 1990–2019: a comprehensive analysis from the global burden of disease study 2019. Front Endocrinol. 2023;14:1307432.
Tinajero MG, Malik VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metabolism Clin. 2021;50(3):337–55.
Zhao X, Zhang Y, Yang Y, Pan J. Diabetes-related avoidable hospitalisations and its relationship with primary healthcare resourcing in China: a cross‐sectional study from Sichuan Province. Health Soc Care Commun. 2022;30(4):e1143–56.
Farmanfarma KK, Ansari-Moghaddam A, Zareban I, Adineh H. Prevalence of type 2 diabetes in Middle–East: systematic review& meta-analysis. Prim Care Diabetes. 2020;14(4):297–304.
Kam J, Puranik S, Yadav R, Manwaring HR, Pierre S, Srivastava RK, Yadav RS. Dietary interventions for type 2 diabetes: how millet comes to help. Front Plant Sci. 2016;7:212107.
Hou Y, Xiang J, Wang B, Duan S, Song R, Zhou W, et al. Pathogenesis and comprehensive treatment strategies of Sarcopenia in elderly patients with type 2 diabetes mellitus. Front Endocrinol. 2024;14:1263650.
Ghanavati M, Rahmani J, Clark CCT, Hosseinabadi SM, Rahimlou M. Pistachios and cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled clinical trials. Complement Ther Med. 2020;52:102513.
Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65(suppl3):S140–6.
Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–40.
Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, Rosenkranz SK. Magnitude and timing of the postprandial inflammatory response to a high-fat meal in healthy adults: a systematic review. Adv Nutr. 2017;8(2):213–25.
Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48(4):677–85.
Rahimlou M, Morshedzadeh N, Karimi S, Jafarirad S. Association between dietary glycemic index and glycemic load with depression: a systematic review. Eur J Nutr. 2018;57:2333–40.
Morvaridzadeh M, Sepidarkish M, Fazelian S, Rahimlou M, Omidi A, Ardehali SH, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and Meta-analysis. Clin Ther. 2020;42(3):e45–63.
Su M, Hu R, Tang T, Tang W, Huang C. Review of the correlation between Chinese medicine and intestinal microbiota on the efficacy of diabetes mellitus. Front Endocrinol. 2023;13:1085092.
Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139(12):2365–72.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96.
Garcia-Arellano A, Ramallal R, Ruiz-Canela M, Salas-Salvadó J, Corella D, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients. 2015;7(6):4124–38.
Amirkhizi F, Hamedi-Shahraki S, Rahimlou M. Dietary total antioxidant capacity is associated with lower disease severity and inflammatory and oxidative stress biomarkers in patients with knee osteoarthritis. J Health Popul Nutr. 2023;42(1):104.
Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer. 2017;141(11):2215–27.
Pillon NJ, Loos RJ, Marshall SM, Zierath JR. Metabolic consequences of obesity and type 2 diabetes: balancing genes and environment for personalized care. Cell. 2021;184(6):1530–44.
Nkwana MR, Monyeki KD, Lebelo SL. Body roundness index, a body shape index, conicity index, and their association with nutritional status and cardiovascular risk factors in South African rural young adults. Int J Environ Res Public Health. 2021;18(1):281.
Kesztyüs D, Lampl J, Kesztyüs T. The weight problem: overview of the most common concepts for body mass and fat distribution and critical consideration of their usefulness for risk assessment and practice. Int J Environ Res Public Health. 2021;18(21):11070.
Zhang H, Wang Z, Wang G, Song X, Qian Y, Liao Z, et al. Understanding the connection between gut homeostasis and psychological stress. J Nutr. 2023;153(4):924–39.
Xu J, Zhang L, Wu Q, Zhou Y, Jin Z, Li Z, Zhu Y. Body roundness index is a superior indicator to associate with the cardio-metabolic risk: evidence from a cross‐sectional study with 17,000 Eastern-China adults. BMC Cardiovasc Disord. 2021;21(1):1–12.
Blus E, Wojciechowska-Kulik A, Majewska E, Baj Z. Usefulness of new indicators of obesity (BAI and VAI) in estimation of weight reduction. J Am Coll Nutr. 2020;39(2):171–7.
Cheraghian B, Hashemi SJ, Hosseini SA, Poustchi H, Rahimi Z, Sarvandian S, et al. Cohort profile: the Hoveyzeh cohort study (HCS): a prospective population-based study on non-communicable diseases in an arab community of Southwest Iran. Med J Islamic Repub Iran. 2020;34:141.
Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN cohort study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–55.
Rosner B, Willett WC. Interval estimates for correlation coefficients corrected for within-person variation: implications for study design and hypothesis testing. Am J Epidemiol. 1988;127(2):377–86.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 2014;17(8):1825–33.
Hebert JR, Ockene IS, Hurley TG, Luippold R, Well AD, Harmatz MG. Development and testing of a seven-day dietary recall. J Clin Epidemiol. 1997;50(8):925–37.
Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–71.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Martinez SM, Ainsworth BE, Elder JP. A review of physical activity measures used among US latinos: guidelines for developing culturally appropriate measures. Ann Behav Med. 2008;36(2):195–207.
Group I. International physical activity questionnaire guidelines for the data processing and analysis of the international physical activity questionnaire: short and long forms. Palo Alto, CA, USA: IPAQ Group; 2012.
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62.
Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65(suppl3):S253–9.
Calle M, Fernandez M. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38(3):183–91.
Yao H, Zhang A, Li D, Wu Y, Wang C-Z, Wan J-Y, Yuan C-S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis. BMJ. 2024;384.
Zheng W, Yang L, Li C, Gu J, Gao X, Qin L, Robinson BO. LCZ696 ameliorates cardiac function via the Akt/FoxO1 signaling pathway in rats with diabetic cardiomyopathy. J Biol Reg Homeos Ag. 2023;37(12):6969–77.
King DE, Xiang J. The dietary inflammatory index is associated with diabetes severity. J Am Board Family Med. 2019;32(6):801–6.
Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N, Hébert JR, Tolentino-Mayo L, Batis C, Barquera S. Dietary inflammatory index and type 2 diabetes mellitus in adults: the diabetes mellitus survey of Mexico City. Nutrients. 2018;10(4):385.
Karimi A, Azar PS, Reshadatjoo M, Vajdi M, Bahrami A, Najafipour F, Tutunchi HA, Double-Blind. Randomized, clinical trial of the effects of Fumaria parviflora supplementation on metabolic parameters, anthropometric indices and serum levels of leptin, adiponectin and resistin in patients with type 2 diabetes. J Biol Reg Homeos Ag. 2023;37(9):4825–36.
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94.
Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep. 2014;16:1–8.
Zhang X, Li H, Wang H, Zhang Q, Deng X, Zhang S et al. Iron/ROS/Itga3 mediated accelerated depletion of hippocampal neural stem cell pool contributes to cognitive impairment after hemorrhagic stroke. Redox Biol. 2024:103086.
Ge H, Zhou T, Zhang C, Cun Y, Chen W, Yang Y, et al. Targeting ASIC1a promotes neural progenitor cell migration and neurogenesis in ischemic stroke. Research. 2023;6:0105.
Ji B, Qu H, Wang H, Wei H, Deng H. Association between the visceral adiposity index and homeostatic model assessment of insulin resistance in participants with normal waist circumference. Angiology. 2017;68(8):716–21.
Oh JY, Sung YA, Lee HJ. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity. 2013;21(8):1690–4.
Khan H. The lipid accumulation product is better than BMI for identifying diabetes. Diabetes Care. 2006;29(1):151–3.
Vasques ACJ, Novaes FS, de Oliveira MS, Souza JRM, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100.
Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9(1):9693.
Morshedzadeh N, Ramezani Ahmadi A, Behrouz V, Mir E. A narrative review on the role of hesperidin on metabolic parameters, liver enzymes, and inflammatory markers in nonalcoholic fatty liver disease. Food Sci Nutr. 2023;11(12):7523–33.
Shahavandi M, Djafari F, Shahinfar H, Davarzani S, Babaei N, Ebaditabar M, et al. The association of plant-based dietary patterns with visceral adiposity, lipid accumulation product, and triglyceride-glucose index in Iranian adults. Complement Ther Med. 2020;53:102531.
Parastouei K, Rostami H, Ramezani AA, Tavakoli H, Alipour M. Gene-diet interaction of FTO-rs9939609 gene variant and hypocaloric diet on glycemic control in overweight and obese adults: a systematic review and meta-analysis of clinical trials. Chin Med J. 2020;133(03):310–7.
Guo W, Zhang Z, Li L, Liang X, Wu Y, Wang X, et al. Gut microbiota induces DNA methylation via SCFAs predisposing obesity-prone individuals to diabetes. Pharmacol Res. 2022;182:106355.
Li M, Li L, Tao X, Xie Z, Xie Q, Yuan J. Boosting healthiness exposure in category-constrained Meal recommendation using nutritional standards. ACM Transactions on Intelligent Systems and Technology; 2024.
Ahmadi Taheri S, Ramazani Ahmadi AH, Javadi M, Barikani A. Comparison of dietary patterns during pregnancy in the mothers of the infants with low birth weight and normal weight. Iran J Obstet Gynecol Infertility. 2018;21(1):80–9.
Mirrafiei A, Ansari S, Jayedi A, Lesani A, Djafarian K, Shab-Bidar S. The association of meal-specific food-based dietary inflammatory index with cardiovascular risk factors and inflammation in a sample of Iranian adults. BMC Endocr Disorders. 2023;23(1):1–12.
Yang W, Ding N, Luo R, Zhang Q, Li Z, Zhao F, et al. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact Mater. 2023;27:1–14.
Yang Y-Y, Chen Z, Yang X-D, Deng R-R, Shi L-X, Yao L-Y, Xiang D-X. Piperazine ferulate prevents high–glucose–induced filtration barrier injury of glomerular endothelial cells. Experimental Therapeutic Med. 2021;22(4):1–10.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Reviews Endocrinol. 2014;10(5):293–302.
Li J-M, Li X, Chan LW, Hu R, Zheng T, Li H, Yang S. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 2023;66(12):2368–86.
Jiang CQ, Xu L, Zhang WS, Jin YL, Zhu F, Cheng KK, Lam TH. Adiposity and mortality in older Chinese: an 11-year follow-up of the Guangzhou Biobank cohort study. Sci Rep. 2020;10(1):1924.
Hsieh SD, Muto T, Tsuji H, Arase Y, Murase T. Clustering of other metabolic risk factors in subjects with metabolic syndrome. Metabolism. 2010;59(5):697–702.
Jenkins DA, Bowden J, Robinson HA, Sattar N, Loos RJF, Rutter MK, Sperrin M. Adiposity-Mortality relationships in type 2 diabetes, coronary heart disease, and cancer subgroups in the UK Biobank, and their modification by smoking. Diabetes Care. 2018;41(9):1878–86.
Zhao Q, Zhang K, Li Y, Zhen Q, Shi J, Yu Y, et al. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabet Med. 2018;35(11):1580–7.
Geraci G, Zammuto M, Gaetani R, Mattina A, D’Ignoto F, Geraci C, et al. Relationship of a body shape index and body roundness index with carotid atherosclerosis in arterial hypertension. Nutr Metab Cardiovasc Dis. 2019;29(8):822–9.
Khader Y, Batieha A, Jaddou H, El-Khateeb M, Ajlouni K. The performance of anthropometric measures to predict diabetes mellitus and hypertension among adults in Jordan. BMC Public Health. 2019;19(1):1416.
Gu Z, Li D, He H, Wang J, Hu X, Zhang P, et al. Body mass index, waist circumference, and waist-to-height ratio for prediction of multiple metabolic risk factors in Chinese elderly population. Sci Rep. 2018;8(1):385.
Chang Y, Guo X, Li T, Li S, Guo J, Sun Y. A body shape index and body roundness index: two new body indices to identify left ventricular hypertrophy among rural populations in Northeast China. Heart Lung Circ. 2016;25(4):358–64.
Zhang N, Chang Y, Guo X, Chen Y, Ye N, Sun Y. A body shape index and body roundness index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur J Intern Med. 2016;29:32–6.
Acknowledgements
Not applicable.
Funding
This work was supported by the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Author information
Authors and Affiliations
Contributions
SAH conceived the study, MR, GhB, SSGh, ZR, ShN, NBJ and BCH collected the data. ARA, and SJH analyzed data. SAH and ZR interpreted the statistical analyses and MR wrote the first draft of the manuscript. SAH contributed to the manuscript revising and editing. All of the authors critically revised the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was carried out in accordance with the Declaration of Helsinki, and the research protocol received approval from the of Ahvaz Jundishapur University of Medical Sciences under the reference number (IR.AJUMS.REC.1398.760). Prior to collecting data, participants were provided with a detailed explanation of the study’s objectives and methods, and subsequently informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahimlou, M., Ahmadi, A.R., Cheraghian, B. et al. The association between dietary inflammatory index with some cardio-metabolic risk indices among the patients with type 2 diabetes from Hoveyzeh cohort study: a cross-sectional study. BMC Endocr Disord 24, 91 (2024). https://doi.org/10.1186/s12902-024-01624-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01624-2