Abstract
Background
In Belgium, different routine surveillance systems are in place to follow-up Lyme borreliosis trends. However, accurate data on the disease and monetary burden for the different clinical manifestations are lacking. Despite recommended antibiotic treatment, a proportion of Lyme patients report persisting aspecific symptoms for six months or more (e.g. fatigue, widespread musculoskeletal pain, cognitive difficulties), a syndrome now named “post-treatment Lyme disease syndrome” (PTLDS). Controversy exists on the cause, incidence and severity of PTLDS. This study aims to estimate the incidence of PTLDS in patients with Lyme borreliosis and to quantify the disease burden and economic costs associated with the different clinical manifestations of Lyme borreliosis in Belgium.
Methods
The project is a prospective cohort study in which about 600 patients with an erythema migrans and 100 patients with disseminated Lyme borreliosis will be followed up. Questionnaires, including the SF-36 vitality and pain subscale, the Cognitive Failure Questionnaire and the EQ-5D-5L, will be used to collect information on acute and persisting symptoms and the impact on quality of life. Symptom frequency and severity will be compared with self-reported pre-Lyme health status, a control group and existing Belgian population norms. Additionally, information on the associated costs and possible risk factors for the development of PTLDS will be collected.
Discussion
A study of the health burden will allow evaluation of the relative importance of Lyme borreliosis in Belgium and information on the economic cost will help to formulate cost-effective measures. There are only few prospective studies conducted estimating the incidence of PTLDS and even though discussion exists about the prevalence of subjective symptoms in the general population, a control group of non-Lyme borreliosis participants has often not been included.
Similar content being viewed by others
Background
Lyme borreliosis is a multisystem infectious disease caused by bacteria from the Borrelia burgdorferi sensu lato complex. These spirochetes are transmitted by hard ticks, predominantly by Ixodes ricinus in Europe. Lyme borreliosis is the most prevalent arthropod-borne disease in Europe and North America [1, 2]. Asymptomatic infections are frequent [3,4,5]. If disease develops, it can cause a wide range of symptoms. The most common clinical manifestation is an erythema migrans (EM), a red expanding rash which occurs in 60 to 95% of symptomatic infections, within days to weeks after the tick bite [6,7,8,9,10,11]. In this early disease stage, flu-like symptoms (e.g. fever, headache, fatigue, arthralgia’s, myalgia’s) may accompany EM or appear separately. If an infection is not treated with appropriate antibiotics, it can possibly evolve to disseminated Lyme borreliosis (weeks, months or years after the tick bite) affecting the skin, the nervous system (Lyme neuroborreliosis), the joints (arthritis) or more rarely, the heart or the eyes [1, 12]. Despite adequate antibiotic treatment, it has been reported that some people still suffer from a range of non-specific symptoms after an infection: fatigue, widespread musculoskeletal pain or cognitive difficulties (e.g. memory problems, difficulties concentrating, problems finding words). If these symptoms persist for more than six months after treatment in correctly diagnosed Lyme patients and are of such severity that they influence the patient’s daily life, this is referred to as post-Lyme disease syndrome [2], later named post-treatment Lyme disease syndrome (PTLDS) [13,14,15].
The frequency of subjective symptoms after treatment for Lyme borreliosis reported in the literature varies widely; for early Lyme borreliosis (EM) it ranges between 5 and 20% [1, 13, 16,17,18,19] reaching 36% if included patients present systematic symptoms as well (e.g. flu-like symptoms) [14]. A review by Koedel et al. (2015) reported that PTLDS symptoms developed in 5–54% of Lyme patients that suffered from Lyme neuroborreliosis [16]. There is controversy and concern about the prevalence of these aspecific symptoms in the general population and although it is advised, only few studies included a non-Lyme borreliosis control group [17, 19,20,21,22]. The pathogenesis of the syndrome is still unclear, different possible explanations have been suggested (e.g. persistent infection, post-infective fatigue syndrome, natural response after treatment, autoimmune mechanisms, intercurrent conditions) but no conclusions can be made [15, 17, 23]. Several risk factors for the development of PTLDS have been hypothesized, including dissemination of disease, delayed diagnosis and treatment, more severe symptoms at diagnosis and the elevation of immune mediators (e.g. elevated CCL19) [15, 23,24,25].
It is thought that co-infections with other tick-borne pathogens such as Anaplasma spp. and Babesia spp. can exacerbate the clinical presentation of Lyme borreliosis: they can increase the severity of initial Lyme symptoms, causing unexplained leukopenia, thrombocytopenia or anemia, or high-grade fever after treatment [2, 26]. In the last years, other less known tick-borne pathogens have been found to circulate in Belgian ticks, amongst others, Borrelia miyamotoi, Neoehrlichia mikurensis and Rickettsia spp. [27,28,29]. It is unclear to what extent co-infections with these and other tick-borne pathogens may affect persisting symptoms after treatment of Lyme borreliosis [2, 30,31,32,33,34,35].
In Belgium, it is estimated that, every year, approximately 10.000 people consult a general practitioner (GP) with an erythema migrans and 200–300 people are hospitalized due to disseminated Lyme borreliosis [36, 37]. Although, up to now, no increasing trend in the Lyme borreliosis incidence has been observed (based on the long-term surveillance systems in place), the disease is assumed to represent a significant health and cost burden for the population and the health system in Belgium. This has however not yet been assessed. Lyme borreliosis and especially its possible long-lasting symptoms, even after treatment, remain a subject of controversy in Belgium, as in other parts of the world [2, 38]. In order to provide some answers to this debate, this prospective study is set up.
Study objectives
The aim of the study is to evaluate the incidence of and possible risk factors for the development of post - treatment Lyme disease syndrome (PTLDS) and to estimate the disease burden and economic cost associated with the different clinical manifestations of Lyme borreliosis in Belgium.
Methods
Study design
The core of HUMTICK is a prospective cohort study with follow-up of patients with Lyme borreliosis and a non-Lyme borreliosis control group (in a 1:1 ratio). Questionnaires will be used to collect information on duration and severity of symptoms, health related quality of life, costs and possible risk factors for the development of PTLDS. At the end of the follow-up, a case-control study will be set up within a sub-cohort of EM patients, to analyze tick-borne co-infections as a possible risk factor for the development of PTLDS. In this case-control study, cases are defined as EM patients with PTLDS and controls (in a 1:2 ratio) as patients in which EM resolves without persisting symptoms. To search for the presence of these other tick-borne diseases a blood sample will be collected from all the patients in the EM sub-cohort at the moment of their diagnosis (acute infection). In addition to the prospective study, existing databases will be consulted to obtain supplementary data, necessary for the calculation of the full disease and cost burden of the different clinical manifestations of Lyme borreliosis.
Study population
Two sub-cohorts will be enrolled in the cohort study; one cohort consisting of patients who consult a GP with an erythema migrans and a second consisting of patients with confirmed disseminated Lyme borreliosis (e.g. neuroborreliosis, Lyme arthritis, Lyme carditis) diagnosed by a specialist physician in a hospital. A non-Lyme borreliosis control group, matched for age and gender is added (in a 1:1 ratio) to allow comparison between the cohorts and the general population. Children (<18 years) and pregnant women will be excluded from the study. All participants will sign an informed consent form prior to inclusion. The case definitions for inclusion for cohort 1 and 2 are shown in Table 1.
Participant enrollment
Participants are enrolled since June 2016 and up to August 2018 with follow-up ending no later than February 2019. For cohort 1 (EM), a network of approximately 200 GPs is currently being set up in areas in Belgium that are highly endemic for tick bites and Lyme borreliosis. All patients complying with the case definition are invited to participate, until inclusion of a sufficient sample. Patients with confirmed disseminated Lyme borreliosis (cohort 2) are enrolled through the hospitals linked to the Belgian National Reference Centers (NRC) for Lyme borreliosis: Cliniques Universitaires Saint-Luc-UCL and UZ Leuven (infectious diseases, neurology and rheumatology departments). If this is not sufficient to attain the desired sample size, other hospitals will be involved at a later stage (2017–2018). Participants of the non-Lyme borreliosis control group are selected by the patients in cohorts 1 and 2, among persons in their own environment with the same gender, comparable age (+/− 5 years) and no prior Lyme borreliosis diagnosis. The participants are included in the study after validation of the criteria.
Sample size estimates and power calculation
For the sample size calculation of cohort 1, we assume that 12.5% of EM patients will develop PTLDS (mean of 5–20% [1, 13, 16,17,18,19]) in comparison with 5% [19] of the non-Lyme controls developing the same symptoms. For the sample size calculation of cohort 2, we assume 20% of disseminated Lyme patients developing PTLDS. With an alpha of 0.05, a power of 90 and a cohort-controls ratio of 1:1, this means that we need 285 EM patients in cohort 1 and 93 disseminated Lyme patients in cohort 2 (2 sided-test) to allow detection of a risk ratio (RR) of respectively 2.5 and 4.
However, to analyze the association between PTLDS and co-infections as a risk factor in the case-control study (set-up within cohort 1), we need 74 cases of PTLDS and 148 controls based on an alpha of 0.05, a power of 0.80, a case-controls ratio of 1:2 and the assumption of a less than medium effect size of 0.4 (2-sided test). This means that, if 12.5% of the EM patients develop PTLDS, we need to extend cohort 1 to 592 EM patients in order to have 74 cases in the case-control study. Taking into account a reasonable loss to follow-up (± 25% over 2 years), 780 EM patients and 120 disseminated Lyme patients need to be included in the study.
Data collection procedures
Data collection will start when the patient is diagnosed (T0) with Lyme borreliosis at the consultation with the GP (cohort 1) or in the hospital (cohort 2). During this consultation, the first part of the first paper questionnaire is filled in together with the treating physician (questions on comorbidity, acute symptoms, diagnosis and treatment). The second part of the questionnaire can be filled in by the patients themselves after the consultation (questions on socio-demographic parameters, general health before Lyme borreliosis, tick bite exposure, acute symptoms and costs). The patients’ follow-up questionnaires are completed online (or on paper if preferred by the patient) at different points in time: after 1 month, 3 months, 6 months, 12 months and 24 months. The period of patient follow-up depends on the moment of enrollment and will last maximum 24 months (patients enrolled in early study phase) and minimum 6 months (patients enrolled in final phase of enrollment). Control persons will be requested to fill in a questionnaire at inclusion, after six months and after one year. The blood sample for the patients of cohort 1 (EM) will be collected at T0.
Post-treatment Lyme disease syndrome (PTLDS)
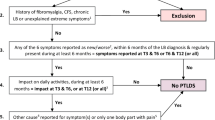
PTLDS is defined (in line with the case definition for post-Lyme disease syndrome proposed by the Infectious Diseases Society of America (IDSA) [2, 13]) as the new onset of fatigue, widespread musculoskeletal pain and/or cognitive difficulties in patients diagnosed with and treated for Lyme borreliosis. Symptoms should occur within six months after the diagnosis, be present for more than six months (continuous or relapsing) and impact the patient’s daily life functioning (quality of life). Patients will be excluded if the symptoms are not new or when there is another explanatory cause for their symptoms.
To apply this case definition, the first patient questionnaire (T0) will assess the presence and severity of the subjective symptoms, as well as the impact on the patient’s life, before the onset of their Lyme borreliosis (during their “pre-Lyme” general health status). The post-treatment presence, severity and impact of the subjective symptoms will be assessed in the same way, in the different follow-up questionnaires (from 3 months onwards). This will allow comparison between the pre- and post-Lyme health status of the patient.
In order to assess the severity and impact more accurately, validated questionnaires will be used:
-
The SF-36 is a self-report 36-item health survey (8 subscales) which has been used widely and was shown to have good reliability and validity [39,40,41]. In our study two subscales are used; the SF-36 vitality subscale (4 items) to assess fatigue and the SF-36 bodily pain subscale (2 items) to assess widespread musculoskeletal pain.
-
The Cognitive Failure Questionnaire (CFQ), a 25-item questionnaire of which psychometric properties have been proven to be good, is used to evaluate cognitive difficulties [42, 43].
-
The EQ-5D-5L, a standardized non-disease specific instrument to evaluate the health-related quality of life, developed by the EuroQol group association [44,45,46], is added to each questionnaire to assess the impact of the symptoms on the patient’s quality of life.
-
The Global Activity Limitation Index (GALI), a single item instrument, is added to the questionnaires from 6 months onwards since it measures the presence of long-lasting (≥ 6 months) health related activity limitations [47,48,49].
The same questionnaires will be used for people from the non-Lyme borreliosis control group, to allow analysis of the incidence of the same symptoms in the general population. Furthermore, currently Belgian population norms exist for the SF-36 vitality, SF-36 bodily pain, the EQ-5D-5L and the GALI, CFQ norms are only available for the Dutch population [50,51,52,53,54].
Finally, the GPs of the patients in cohort 1 (EM) will fill in an online follow-up questionnaire, based on the patient’s medical file, after six and twelve months. Questions are asked about possible supplementary consultations, additional diagnoses, new treatments prescribed and laboratory results if requested. This will allow comparison between the patient’s and GP’s perspectives on the subjective symptoms.
Risk factors for PTLDS
To identify possible risk factors for the development of PTLDS, information on comorbidity, tick bite exposure, severity and duration of symptoms presented, prescribed treatment (type, duration) as well as socio-demographic variables (age, sex, education and employment status) will be collected during the complete study. The blood samples collected at T0 from EM patients will be tested, by means of a multiplex PCR, for the presence of Anaplasma spp., Rickettsia spp., Neoehrlichia mikurensis, Borrelia miyamotoi and Babesia spp. at the Laboratory for Zoonosis and Environmental Microbiology (RIVM), the Netherlands [55].
Disease burden
The Burden of disease will be expressed in Disability-Adjusted Life Years (DALYs), a summary health measure comprising both mortality and morbidity. DALYs measure the healthy life years lost due to illness and equal the sum of the years lived with disability (YLDs) and the years of life lost due to premature mortality (YLLs). The YLDs are calculated based on the occurrence, severity (disability weights (DW)) and duration of the disease health states [56,57,58]. An incidence approach will be used for the occurrence of the different clinical manifestations of Lyme borreliosis, where the incidences of EM and disseminated Lyme will be estimated based on existing literature and surveillance systems currently in place in Belgium (e.g. sentinel network of general practitioners, network of sentinel laboratories and minimal clinical data) [36, 37]. The cohort study will provide an estimate of the incidence of PTLDS, of the symptom durations for the different clinical manifestations as well as estimates of the group specific disability weights. The latter will be derived from the participants’ EQ-5D-5L responses: following the EQ-5D-5L user guide the EQ-5D-5L scores will first be converted into utilities using a pre-existing preference valuation set for EQ-5D health states of the Belgian (Flemish) adult population [59,60,61]. The resulting utility, a value between 0 (death) and 1 (full health), will then be transformed to DWs by subtracting it from the EQ-5D population averages for the same sex and age-group [52, 62, 63]. Since Lyme related mortality is exceptional and no Lyme related deaths have been reported in Belgium between 1998 and 2014 (only data available), YLLs will equal zero.
Costs
To minimize recall bias between two consecutive questionnaires (recall periods up to 12 months at the end of the follow-up period), patients are invited to keep a cost diary during the complete study period. The patients’ questionnaires assess direct and indirect non-medical costs (e.g. travel costs, absence from work, paid help) and provide information on direct medical costs related to medication use, consultations and hospitalizations (e.g. how many visits to a GP/specialist/…, use of over the counter medication). Additional details on some of these direct medical costs, which can’t be provided by the patients themselves, are collected through the follow-up questionnaires completed by the GPs (e.g. information on the laboratory tests). The standard unit costs (the price of medication, a consultation, a laboratory test, a hospitalization) will be obtained from official sources (e.g. Belgian centre for pharmacotherapeutic information (BCFI), National Institute for Health and Disability Insurance (RIZIV)). Supplementary data on hospitalization costs will be collected through the minimal hospital data (MZG), minimal financial data (MFG) and Belgian health insurance databases.
Statistical analysis
All analyses will be performed in R [64]. A conditional log-binomial regression model with adjustment for comorbid illness will be used to compare the development of the non-specific disease symptoms between the different cohorts and the “matched” non-Lyme borreliosis control group in order to calculate the incidence of PTLDS in patients with Lyme borreliosis [65]. Within cohort 1 & 2, a multivariate log-binomial regression model will be used to calculate exposure risk ratio (RR) of the risk factors for development of PTLDS. For the case-control, the odds ratio (OR) determination for tick-borne co-infections as a risk factor will be done using a multivariate logistic regression. The multivariate models will include adjustment for potential confounding variables (age, gender and comorbid illness).
The burden of disease will be estimated in collaboration with the “Institut de recherche santé et société (IRSS) Université catholique de Louvain (UCL)” using the DALY package [56,57,58, 66]. The total costs (mean, median & confidence intervals) for each of the different clinical manifestations, including PTLDS, will be calculated by combining the different data sources. The cost analysis will be carried out in collaboration with the Centre for Health Economics Research & Modeling Infectious Diseases (CHERMID), University of Antwerp (UA).
Discussion
Lyme borreliosis has been an increasingly “hot topic” in Belgium and the rest of the world, with uncertainties and intensive debates on the burden of the disease and on possible long-lasting (“chronic”) symptoms.
The incidence of PTLDS has been estimated in previous studies, but the reported results vary widely. This is likely related to differences in the inclusion criteria and PTLDS case definitions used, as well as to the variation among study designs [15]. The strength of the presented study lies in the combination of different methodologies which aim at collecting objective data with regard to PTLDS: the prospective study design allows collecting detailed information throughout the complete disease progression and minimizes recall bias; the inclusion of a non-Lyme borreliosis control group allows controlling for the background prevalence of aspecific symptoms in the general population; through the use of validated questionnaires the severity of the aspecific symptoms will be assessed (and afterwards compared) accurately. The availability of Belgian population norms for some of the validated questionnaires is an additional benefit. Furthermore, the use of parallel questionnaires for the patients and their GPs allows comparison between the different perspectives on the persistent aspecific symptoms as well as to obtain detailed information on possible risk factors (e.g. comorbidity, diagnosis, treatment, complications). By looking at both the prevalence and the severity of the symptoms occurring with PTLDS, as well as the impact on the patient’s life, we will be able to assess all components of the PTLDS case definition [13]. Different possible risk factors will be assessed through the patient questionnaires and the analyses of other tick-borne co-infections (in EM patient blood samples) allows collection of innovative data on the importance of co-infections on the clinical presentation and progression of Lyme borreliosis. A better comprehension of this syndrome and its risk factors will allow informing health care providers and patients about what to expect after treatment and, if possible, to early identify those patients at increased risk.
The estimation of the burden of disease, quantified by a composite health measure, can be used to compare and prioritize between the different clinical manifestations of Lyme borreliosis but also between different diseases and health interventions, in order to set public health priorities [67]. Some European projects assessed the burden of different communicable diseases but they did not include Lyme borreliosis [67, 68]. A study in the Netherlands did estimate the disease burden for Lyme borreliosis and found that the majority of the substantial burden is caused by persisting symptoms [69]. The additional benefit of our study for the burden estimation is again its prospective study design. In the end, the results will be used to inform the general public, patients associations, policymakers and health care providers on the actual disease burden related to the different stadia of Lyme borreliosis in Belgium.
A few European studies have examined the costs associated with Lyme borreliosis but often only part of the costs were included (e.g. costs of Lyme neuroborreliosis in Sweden [70], cost of testing [71] and of hospital care in Germany [72]). One study in Scotland included both direct and indirect costs for early and late Lyme borreliosis patients and follow-up costs [73]. These studies didn’t include the specific supplementary cost for patients with PTLDS although it is expected to contribute significantly to the overall costs of Lyme borreliosis. A recent American study showed that PTLDS-related diagnoses are associated with notably higher costs and health care utilization ($ 3798 higher costs and 66% more outpatient visits) as compared to Lyme borreliosis without PTLDS-related diagnoses [21, 74]. Through the inclusion of different costs (both direct and indirect, medical and non-medical) for both the patients and the health care system, this study will provide an overall view of the costs associated with Lyme borreliosis, its diagnosis, treatment, follow-up, etc.. The results could be used in future cost-effectiveness analyses of potential interventions.
A first possible limitation of the study is the work–load for the patients at the moment of inclusion (long questionnaire) which might keep them from participating. However, in the context of the current attention for Lyme borreliosis in the media and the debate on existing persisting symptoms, we believe we will find sufficient patients willing to participate to the study. Secondly, as prescribed in the Belgian guidelines for the diagnosis and treatment of Lyme borreliosis, diagnosis of an EM is solely based on clinical symptoms. This implies that the inclusion of EM patients in our study is dependent on correct EM recognition by the participating GPs. As a reminder, the clinical signs of an EM, possible differential diagnoses and corresponding pictures are provided to the participating GPs at the start of the study. The diagnosis of disseminated Lyme borreliosis is based on both clinical symptoms recognized by specialists and laboratory testing. Third, due to project time restrictions, we will only be able to follow up patients during a maximum of two years. It would have been interesting to follow up patients for a longer period. Many patients and patient associations indeed have questions about symptoms after a longer period after treatment. However a longer study period is likely to induce an important loss to follow-up over time. Finally, the study will not allow the collection of data with regard to a group of patients who attribute their persisting aspecific symptoms (fatigue, musculoskeletal pains and cognitive disorders) to Lyme borreliosis, but without having a confirmed diagnosis.
In conclusion, through its multidisciplinary approach, the HUMTICK prospective cohort study allows addressing multiple relevant research questions with regard to Lyme borreliosis; the enrollment of both EM patients (early localized Lyme) and disseminated Lyme borreliosis patients, together with the follow-up over time, will not only allow to estimate the incidence of PTLDS but also to estimate the disease burden and cost associated with the different manifestations of Lyme borreliosis, specifically in Belgium. In addition, the study design allows simultaneous evaluation of risk factors for the development of PTLDS and will so improve the understanding of the evolution of the different clinical manifestations of Lyme borreliosis after treatment.
Abbreviations
- BCFI:
-
Belgian centre for pharmacotherapeutic information
- CFQ:
-
Cognitive failure questionnaire
- CHERMID:
-
Centre for Health Economics Research & Modeling Infectious Diseases
- DALY:
-
Disability-adjusted life year
- DW:
-
Disability weight
- EM:
-
Erythema migrans
- GP:
-
General practitioner
- GALI:
-
Global activity limitation index
- IDSA:
-
Infectious diseases society of America
- MFG:
-
Minimal financial data
- MZG:
-
Minimal hospital data
- RIVM:
-
National Institute for Public Health and Environment
- NRC:
-
National reference center
- OR:
-
Odds ratio
- PCR:
-
Polymerase chain reaction
- PTLDS:
-
Post-treatment Lyme disease syndrome
- RIZIV:
-
National Institute for Health and Disability Insurance
- RR:
-
Risk ratio
- UA:
-
University of Antwerp
- UCL:
-
Université catholique de Louvain
- UZL:
-
University Hospitals Leuven
- WIV-ISP:
-
Scientific Institute of Public Health
- YLD:
-
Years lived with disability
- YLL:
-
Years of life lost
References
Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73.
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134.
Hofhuis A, Herremans T, Notermans DW, Sprong H, Fonville M, van der Giessen JW, et al. A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in The Netherlands. PLoS One. 2013;8:e64361.
Huegli D, Moret J, Rais O, Moosmann Y, Erard P, Malinverni R, et al. Prospective study on the incidence of infection by Borrelia burgdorferi sensu lato after a tick bite in a highly endemic area of Switzerland. Ticks Tick Borne Dis. 2011;2:129–36.
Wilhelmsson P, Fryland L, Lindblom P, Sjowall J, Ahlm C, Berglund J, et al. A prospective study on the incidence of Borrelia burgdorferi sensu lato infection after a tick bite in Sweden and on the Aland Islands, Finland (2008-2009). Ticks Tick Borne Dis. 2016;7:71–9.
Letrilliart L, Ragon B, Hanslik T, Flahault A. Lyme disease in France: a primary care-based prospective study. Epidemiol Infect. 2005;133:935–42.
Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16
Berglund J, Eitrem R, Ornstein K, Lindberg A, Ringer A, Elmrud H, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–27.
Wilking H, Stark K. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009-2012. Ticks Tick Borne Dis. 2014;5:219–24.
Hofhuis A, Harms M. Bennema S, van den Wijngaard CC, van PW: physician reported incidence of early and late Lyme borreliosis. Parasit Vectors. 2015;8:161.
Vandenesch A, Turbelin C, Couturier E, Arena C, Jaulhac B, Ferquel E, et al. Incidence and hospitalisation rates of Lyme borreliosis, France, 2004 to 2012. Euro Surveill. 2014:19.
Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79.
Aucott JN, Crowder LA, Kortte KB. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int J Infect Dis. 2013;17:e443–9.
Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Qual Life Res. 2013;22:75–84.
Aucott JN. Posttreatment Lyme disease syndrome. Infect Dis Clin N Am. 2015;29:309–23.
Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol. 2015;11:446–56.
Marques A. Chronic Lyme disease: a review. Infect Dis Clin N Am. 2008;22:341–60.
Wormser GP, Ramanathan R, Nowakowski J, McKenna D, Holmgren D, Visintainer P, et al. Duration of antibiotic therapy for early Lyme disease. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:697–704.
Cerar D, Cerar T, Ruzic-Sabljic E, Wormser GP, Strle F. Subjective symptoms after treatment of early Lyme disease. Am J Med. 2010;123:79–86.
Cairns V, Godwin J. Post-Lyme borreliosis syndrome: a meta-analysis of reported symptoms. Int J Epidemiol. 2005;34:1340–5.
Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS One. 2015;10:e0116767.
Seltzer EG, Gerber MA, Cartter ML, Freudigman K, Shapiro ED. Long-term outcomes of persons with Lyme disease. JAMA. 2000;283:609–16.
Aucott JN, Soloski MJ, Rebman AW, Crowder LA, Lahey LJ, Wagner CA, et al. CCL19 as a Chemokine risk factor for post-treatment Lyme disease syndrome: a prospective clinical cohort study. Clin Vaccine Immunol. 2016;23:757–66.
Nowakowski J, Nadelman RB, Sell R, McKenna D, Cavaliere LF, Holmgren D, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. 2003;115:91–6.
Eikeland R, Mygland A, Herlofson K, Ljostad U. Risk factors for a non-favorable outcome after treated European neuroborreliosis. Acta Neurol Scand. 2013;127:154–60.
Krause PJ, Telford SR III, Spielman A, Sikand V, Ryan R, Christianson D, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–60.
Cochez C, Heyman P, Heylen D, Fonville M, Hengeveld P, Takken W, et al. The presence of Borrelia miyamotoi, a relapsing fever Spirochaete, in questing Ixodes ricinus in Belgium and in The Netherlands. Zoonoses Public Health. 2015;62:331–3.
Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from north-west Europe. Parasit Vectors. 2012;5:74.
Claerebout E, Losson B, Cochez C, Casaert S, Dalemans AC, De CA, et al. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit Vectors. 2013;6:183.
Borgermans L, Goderis G, Vandevoorde J, Devroey D. Relevance of chronic lyme disease to family medicine as a complex multidimensional chronic disease construct: a systematic review. Int J Family Med. 2014;2014:138016.
Berghoff W. Chronic Lyme disease and co-infections: differential diagnosis. Open Neurol J. 2012;6:158–78.
Lantos PM, Wormser GP. Chronic coinfections in patients diagnosed with chronic lyme disease: a systematic review. Am J Med. 2014;127:1105–10.
Lantos PM. Chronic Lyme disease. Infect Dis Clin N Am. 2015;29:325–40.
Wang TJ, Liang MH, Sangha O, Phillips CB, Lew RA, Wright EA, et al. Coexposure to Borrelia burgdorferi and Babesia microti does not worsen the long-term outcome of lyme disease. Clin Infect Dis. 2000;31:1149–54.
Ramsey AH, Belongia EA, Gale CM, Davis JP. Outcomes of treated human granulocytic ehrlichiosis cases. Emerg Infect Dis. 2002;8:398–401.
Bleyenheuft C, Lernout T, Berger N, Rebolledo J, Leroy M, Robert A, et al. Epidemiological situation of Lyme borreliosis in Belgium, 2003 to 2012. Arch Public Health. 2015;73:33.
Vanthomme K, Bossuyt N, Boffin N, Van C. V: incidence and management of presumption of Lyme borreliosis in Belgium: recent data from the sentinel network of general practitioners. Eur J Clin Microbiol Infect Dis. 2012;31:2385–90.
Lantos PM. Chronic Lyme disease: the controversies and the science. Expert Rev Anti-Infect Ther. 2011;9:787–97.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83.
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–68.
Razavi D, Gandek B. Testing Dutch and French translations of the SF-36 health survey among Belgian angina patients. J Clin Epidemiol. 1998;51:975–81.
Vom Hofe A, Mainemarre G, Vannier L-C. Sensitivity to everyday failures and cognitive inhibition: are they related? European Review of Applied Psychology / Revue Européenne de Psychologie Appliquée. 1998;48:49–56.
Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16.
EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990, 16: 199-208.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36.
Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22:1717–27.
Robine JM, Jagger C. Creating a coherent set of indicators to monitor health across Europe: the Euro-REVES 2 project. Eur J Pub Health. 2003;13:6–14.
Van OH, Van der Heyden J, Perenboom R, Jagger C. Monitoring population disability: evaluation of a new Global activity limitation indicator (GALI). Soz Praventivmed. 2006;51:153–61.
Berger N, Van OH, Cambois E, Fouweather T, Jagger C, Nusselder W, et al. Assessing the validity of the Global activity limitation indicator in fourteen European countries. BMC Med Res Methodol. 2015;15:1.
Gisle L. Santé mentale. Dans : Van der Heyden J, Charafeddine R (éd.). Enquête de santé 2013. Rapport 1 : Santé et Bien-être. WIV-ISP, Bruxelles. 2014. https://his.wiv-isp.be/fr/Documents%20partages/WB_FR_2013.pdf. Accessed 27 Apr 2017.
Drieskens S. Douleur physique. Dans : Van der Heyden J, Charafeddine R (éd.). Enquête de santé 2013. Rapport 1 : Santé et Bien-être. WIV-ISP, Bruxelles. 2014. https://his.wiv-isp.be/fr/Documents%20partages/PI_FR_2013.pdf. Accessed 27 Apr 2017.
Charafeddine R. Qualité de vie liée à la santé. Dans : Van der Heyden J, Charafeddine R (éd.). Enquête de santé 2013. Rapport 1 : Santé et Bien-être. WIV-ISP, Bruxelles. 2014. https://his.wiv-isp.be/fr/Documents%20partages/QL_FR_2013.pdf. Accessed 27 Apr 2017.
Tafforeau J. Santé subjective. Dans : Van der Heyden J, Charafeddine R (éd.). Enquête de santé 2013. Rapport 1 : Santé et Bien-être. WIV-ISP, Bruxelles. 2014. https://his.wiv-isp.be/fr/Documents%20partages/SH_FR_2013.pdf. Accessed 27 Apr 2017.
Ponds R, van Boxtel M, Jolles J. De "cognitive failure questionnaire" als maat voor subjectief functioneren. Tijdschrift voor neuropsychologie. 2006;1(2):37–42.
Jahfari S, Hofhuis A. Fonville M, van der Giessen J, van PW, Sprong H: molecular detection of tick-borne pathogens in humans with tick bites and Erythema Migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10:e0005042.
Devleesschauwer B, Havelaar AH, Maertens de NC, Haagsma JA, Praet N, Dorny P, et al. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health. 2014;59:565–9.
Devleesschauwer B, Havelaar AH, Maertens de NC, Haagsma JA, Praet N, Dorny P, et al. DALY calculation in practice: a stepwise approach. Int J Public Health. 2014;59:571–4.
Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–45.
The EuroQol Group. EQ-5D-5L user guide. 2015. https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf. Accessed 9 June 2017.
van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–15.
Cleemput I. A social preference valuations set for EQ-5D health states in Flanders, Belgium. Eur J Health Econ. 2010;11:205–13.
Maertens de NC, Devleesschauwer B, Gielens L, Plasmans MHD, Haagsma JA, Speybroeck N. Mapping EQ-5D utilities to GBD 2010 and GBD 2013 disability weights: results of two pilot studies in Belgium. Arch Public Health. 2017;75:6.
Bilcke J, Hens N, Beutels P. Quality-of-life: a many-splendored thing? Belgian population norms and 34 potential determinants explored by beta regression. Qual Life Res. 2017;
R Core Team. R: A language and environment for statistical computing. 2015. R Foundation for Statistical Computing https://www.r-project.org/. Accessed 26 Sept 2016.
McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–3.
Brecht Devleesschauwer, Scott McDonald, Juanita Haagsma, Nicolas Praet, Arie Havelaar, Niko Speybroeck. DALY: DALY Calculator - A GUI for stochastic DALY calculation in R. 2014. https://cran.r-project.org/web/packages/DALY/index.html. Accessed 26 Sept 2016.
Kretzschmar M, Mangen MJ, Pinheiro P, Jahn B, Fevre EM, Longhi S, et al. New methodology for estimating the burden of infectious diseases in Europe. PLoS Med. 2012;9:e1001205.
van Lier A, SA MD, Bouwknegt M, Kretzschmar ME, Havelaar AH, Mangen MJ, et al. Disease burden of 32 infectious diseases in the Netherlands, 2007-2011. PLoS One. 2016;11:e0153106.
van den Wijngaard CC, Hofhuis A, Harms MG, Haagsma JA, Wong A, de Wit GA, et al. The burden of Lyme borreliosis expressed in disability-adjusted life years. Eur J Pub Health. 2015;
Henningsson AJ, Malmvall BE, Ernerudh J, Matussek A, Forsberg P. Neuroborreliosis--an epidemiological, clinical and healthcare cost study from an endemic area in the south-east of Sweden. Clin Microbiol Infect. 2010;16:1245–51.
Muller I, Freitag MH, Poggensee G, Scharnetzky E, Straube E, Schoerner C, et al. Evaluating frequency, diagnostic quality, and cost of Lyme borreliosis testing in Germany: a retrospective model analysis. Clin Dev Immunol. 2012;2012:595427.
Lohr B, Muller I, Mai M, Norris DE, Schoffski O, Hunfeld KP. Epidemiology and cost of hospital care for Lyme borreliosis in Germany: lessons from a health care utilization database analysis. Ticks Tick Borne Dis. 2015;6:56–62.
Joss AW, Davidson MM, Ho-Yen DO, Ludbrook A. Lyme disease--what is the cost for Scotland? Public Health. 2003;117:264–73.
Zhang X, Meltzer MI, Pena CA, Hopkins AB, Wroth L, Fix AD. Economic impact of Lyme disease. Emerg Infect Dis. 2006;12:653–60.
Acknowledgements
We gratefully acknowledge the physician specialists from the participating hospitals who will include patients with disseminated Lyme borreliosis: Dr. Leïla Belkhir (UCL), Dr. Paul de Munter (UZL), Prof. Dr. Benedicte Dubois (UZL) and Prof. Dr. Rene Westhovens (UZL) as well as all the GPs participating in the GP network to include patients with an erythema migrans.
Funding
The survey is funded by the WIV-ISP with a budget for a period of four years.
Availability of data and materials
Not applicable.
Authors’ contributions
KT, TL, LG conceived the study design, KT and TL coordinate the execution of the project by LG. LG wrote this manuscript in collaboration with KT and TL. NS is promoter of the study, KT is co-promoter. All partners of the HUMTICK project contributed to the elaboration of the study design and took part in reviewing the questionnaires, each member contributed specifically to the parts of the study corresponding with their own expertise. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study will be conducted in compliance with the principles of the Declaration of Helsinki (2008) and all of the applicable regulatory requirements. Ethical approvals were obtained from the “Comité d’Ethique Hospitalo-Facultaire Saint-Luc UCL” (ref.: 2016/13AVR/166) and the “Commissie Medische Ethiek UZ KU Leuven” (ref.: S59379/ B403201628482). Informed consent will be obtained from all study participants. The study has been approved by the Commission to Protect the Personal Privacy (Beraadslaging Nr. 16/038, reference: SCSZG/16/144).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Specific description of the case definitions for confirmed cases of disseminated Lyme borreliosis which will be included in the HUMTICK study. (DOCX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Geebelen, L., Lernout, T., Kabamba-Mukadi, B. et al. The HUMTICK study: protocol for a prospective cohort study on post-treatment Lyme disease syndrome and the disease and cost burden of Lyme borreliosis in Belgium. Arch Public Health 75, 42 (2017). https://doi.org/10.1186/s13690-017-0202-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-017-0202-z