Abstract
In this paper, stochastic effect on the spread of the infectious disease with saturated incidence rate and the special transfer from infectious is discussed. The threshold dynamics is explored for the case of relatively small noise. Our results show that large noise will cause the elimination of the disease, which will help suppress the spread of the disease.
Similar content being viewed by others
1 Introduction
Infectious diseases have always threatened the health of human beings and have brought enormous disasters to human beings. In order to protect human life and control the prevalence of infectious diseases, researchers have used various methods, such as pathology, virology, epidemiology, and culture, to study the evolution of infectious diseases [1–4]. Mathematical modeling method is considered as a very useful method which was firstly proposed by D. Bernoulli in 1760 for investigating the spread of smallpox [5]. And in 1906, W.H. Hamer constructed and discussed a mathematical model to explain the recurrent epidemic of measles [6]. In 1911, R.A. Ross proposed a differential equation model to investigate the spread of malaria [7]. In 1927, W.O. Kermack and A.G. McKendrick proposed the classical epidemic model known as SIR model [8], in which the total population size is divided into three disjoint classes, namely the susceptible class (S), the infective class (I), and the recovery class (R). SIR models have been investigated by many scholars, e.g., [9–13]. In the SIR model, the susceptible can be infected, and the infected can be recovered and immunized for life. However, it is well known that for some epidemic diseases, such as meningitis, plague, venereal diseases, malaria, and sleeping sickness [14], the recovery does not produce lifelong immunity, thus the individual is transferred from the susceptible person to the infected person and then returns to the susceptible class upon recovery, then the model is identified as SIS model, e.g., [15–19]. While for some epidemic diseases, such as smallpox, tetanus, cholera, influenza, and typhoid fever, the recovery can produce temporary immunity, recovery may lose immunity after some time and back to the susceptible class. This model is known as SIRS model. SIS and SIRS models have been extensively investigated by many authors, e.g., [20–26].
Recently, Li et al. [27] considered both transfer from the infectious to the susceptible and transfer from the recovery to the susceptible and proposed an SIRS epidemic model with nonlinear transmission rate as follows (see Fig. 1):
where Λ is the birth rate, μ is the natural mortality rate, \(r_{1}\) is the transfer rate from the infective individuals to the susceptible individuals, \(r_{2}\) is the recovery rate of the infective individuals, α is the mortality due to illness, δ is the rate at which the recovered individuals loss of immunity and return to the susceptible individuals. f is a real locally Lipschitz function on \(R^{+} = [0,\infty)\) satisfying (i) \(f(0) = 0\) and \(f(I) > 0\) for \(I > 0\); (ii) \(f(I)/I\) is continuous and monotonously nonincreasing for \(I > 0\) and \(\lim_{I\rightarrow0^{+}}f(I)/I\) exists, denoted by β with \(\beta> 0\).
By using LaSalle’s invariance principle and Lyapunov direct method, a threshold dynamics determined by the basic reproduction number \(R_{0}=\frac{\Lambda\beta}{\mu(\mu+ r_{1} + r_{2} + \alpha)} \) was established, i.e., if \(R_{0}<1\), the infection-free equilibrium \(E_{0} = (\Lambda/\mu, 0, 0)\) is globally asymptotically stable, while if \(R_{0} > 1\), the endemic equilibrium \(E^{*} = (S^{*}, I^{*}, R^{*})\) is globally asymptotically stable.
As we all know, real life is filled with randomness and unpredictability. Stochastic models can better conform to the actual situation, therefore many scholars have done a lot of research on the randomness of biological models; e.g., in references [28–38], the authors considered the stochastic effect in population dynamical system, and in references [39–42], the authors investigated the stochastic stability. Inevitably, the spread of the diseases is affected by random factors. Generally speaking, a stochastic model constructed by different way will have different effect on a population dynamical system. For instance, references [43–49] studied a class of stochastic epidemic systems in which incidence rate β subjects to stochastic white noise disturbance. In references [50–55], the authors considered a class of stochastic systems of infectious diseases in which stochastic interference is assumed to be proportional to the variable. And references [56–58] investigated the stochastic epidemic model thought the Markov transformation.
In this paper, motivated by the previous work, we consider the incidence rate β in model (1.1) subject to stochastic white noise disturbance, i.e., \(\beta\rightarrow\beta+ \sigma\,\mathrm{d}{B}(t)\), and build the following system:
where \(B(t)\) is a standard Brownian motion with intensity \(\sigma^{2}>0\).
Our main purpose is to explore the threshold value associated with epidemic spread and try to establish the threshold dynamics of system (1.2). In Sect. 2, we give some notation and auxiliary results. And a completely qualitatively analysis for the threshold dynamics of system (1.2) is showed in Sect. 3. In Sect. 4, we give a brief conclusion and some numerical simulations to verify the main results.
2 Preliminaries
Throughout this paper, we let \(\mathbb{R}^{d}\): the d-dimensional Euclidean space. \(\mathbb{R}^{d}_{+}:= \{x\in\mathbb{R}^{d} : x_{i} > 0, 1\leq i\leq d\}\), i.e., the positive cone.
Let \((\Omega,\mathcal{F}, \mathcal{P})\) be a complete probability space adapted to the filtration \(\{\mathcal{F}\}_{t\geq0}\) and \(\{B_{t}\}_{t\geq 0}\) is a one-dimensional Brownian motion defined on it. \(\mathcal {L}^{1}(\mathbb{R}_{+};\mathbb{R}^{d})\) is the family of all \(\mathbb {R}^{d}\)-valued measurable \(\{\mathcal{F}_{t}\}\)-adapted processes \(g=\{g(t)\}_{t\geq0}\) and
By using the methods from [59] and [60], we can prove the following lemma.
Lemma 2.1
For any given initial value \((S(0),I(0),R(0))\in{R}^{3}_{+}\), system (1.2) has a unique positive solution \((S(t), I(t), R(t))\in{R}^{3}_{+} \) on \(t\geq0\), almost surely.
Lemma 2.2
The region
is a positively invariant set for system (1.2).
Proof
Let \(N(t)=S(t)+I(t)+ R(t)\), by system (1.2), we obtain
This implies that
Then, if we denote \(\Gamma=\{(S(t), I(t), R(t))\in R_{+}^{3}:S(t), I(t), R(t)\leq\frac{\Lambda}{\mu}, t\geq0\}\), we have \(S(t)+I(t)+ R(t)\leq \frac{\Lambda}{\mu}\). Thus, the region Γ is positively invariant. □
By using the methods from Meng et al. [45], we can prove the following lemma.
Lemma 2.3
For any given initial value \((S(0), I(0), R(0))\in R^{3}_{+}\), the solution \((S(t), I(t), R(t))\) of system (1.2) has the following properties:
almost surely.
3 A threshold dynamics
In this section, we try our best to find the threshold that determines the spread of the disease.
3.1 Extinction
Definition 3.1
For system (1.2), the infected individuals \(I(t)\) are said to be extinctive if \(\lim _{t\rightarrow+\infty} I(t)=0\), almost surely.
Let us introduce
Then we have the following.
Theorem 3.2
If \(\sigma^{2}>\max\{\frac{\beta\mu}{\Lambda},\frac{\beta^{2}}{2(\mu +r_{1}+r_{2}+\alpha)}\}\) or \(\sigma^{2}<\frac{\beta\mu}{\Lambda}\) and \({\widetilde{R}_{0}}<1\), then the infected individuals of system (1.2) tend to zero exponentially almost surely.
Proof
Assume that \((S(t), I(t),R(t))\) is a solution of system (1.2) satisfying the initial value \((S(0), I(0), R(0)) \in R^{3}_{+}\). According to Itô’s formula, we have
Integral on both sides of system (3.1) from 0 to t shows
where \(M_{1}(t)=\int_{0}^{t}\frac{\sigma S(\tau)}{1+aI(\tau)}\,\mathrm{d}B(\tau)\) and \(M_{1}(t)\) is the local continuous martingale with \(M_{1}(0)=0\). Next, we have two cases to be discussed, depending on whether \(\sigma^{2}>\frac{\beta\mu}{\Lambda}\).
If \(\sigma^{2}>\frac{\beta\mu}{\Lambda}\), we can easily see from (3.2) that
Dividing both sides of (3.3) by \(t>0\), one gets
Since \(\limsup_{t\rightarrow\infty}\frac{\langle M_{1}(t),M_{1}(t)\rangle _{t}}{t}<\sigma^{2}<\infty\) almost surely, by Lemma 2.3, one can obtain that
almost surely. Then, taking the limit superior on both sides of (3.4), we get
when \(\sigma^{2}>\frac{\beta^{2}}{2(\mu+r_{1}+r_{2}+\alpha)}\), which implies \(\lim_{t\rightarrow+\infty}I(t)=0\) almost surely.
If \(\sigma^{2}<\frac{\beta\mu}{\Lambda}\), similarly, one can have that
Dividing both sides of (3.5) by \(t>0\), we obtain
By taking the superior limit on both sides of (3.6), one can have that
Then when \({\widetilde{R}_{0}}<1\), we get
which implies \(\lim_{t\rightarrow+\infty}I(t)=0\) almost surely. The proof of Theorem 3.2 is completed. □
Remark 3.3
By Theorem 3.2, we get if \(\sigma^{2}>\max\{\frac{\beta\mu}{\Lambda },\frac{\beta^{2}}{2(\mu+r_{1}+r_{2}+\alpha)}\}\), then the infectious disease of system (1.2) goes to extinction almost surely, namely large white noise stochastic disturbance is conducive to control infectious disease. When the white noise is small and \({\widetilde{R}_{0}}<1\), the infectious disease of system (1.2) also goes to extinction almost surely, then \({\widetilde{R}_{0}}\) is the threshold associated with the extinction of infectious disease.
3.2 Persistence in mean
Definition 3.4
For system (1.2), the infected individuals \(I(t)\) are said to be persistent in mean if \(\liminf _{t\rightarrow+\infty}\langle I(t)\rangle>0\), almost surely, where \(\langle I(t)\rangle\) is defined as \(\frac{1}{t}\int_{0}^{t}I(\tau )\,\mathrm{d}\tau\).
Theorem 3.5
If \({\widetilde{R}_{0}}>1\), then the infected individuals \(I(t)\) are persistent in mean, and
almost surely.
Proof
Integral on both sides of system (1.2) from 0 to t yields
Then we obtain
Using Itô’s formula gives
Integral on both sides of system (1.2) from 0 to t and dividing by t (\(t>0\)) yields
where \(M_{2}(t)=\int_{0}^{t}\sigma S(\tau)\,\mathrm{d}B(\tau)\) and \(M_{2}(t)\) is the local continuous martingale with \(M_{2}(0)=0\). From (3.8), we obtain
Since both \(I(t)\leq1\) and \(R(t)\leq1\), then one has \(\lim_{t\rightarrow+\infty}\frac{R(t)}{t}=0\), \(\lim_{t\rightarrow+\infty }\frac{\ln I(t)}{t}=0\) and \(\lim_{t\rightarrow+\infty}\Theta(t)=0\) almost surely. Note that \(\lim_{t\rightarrow+\infty}\frac{M_{2}(t)}{t}=0\) almost surely, we obtain
almost surely. This completes the proof of Theorem 3.5. □
Remark 3.6
Theorems 3.2 and 3.5 show that the condition for the disease to die out or persist depends on the intensity of white noise disturbances strongly and small white noise disturbances will be beneficial to long-term prevalence of the disease; conversely, large white noise disturbances may cause the epidemic disease to die out.
4 Conclusion and numerical simulation
This paper proposed a SIRS model with the special transfer from infectious, stochastic effect to the spread of the infectious disease is discussed. The threshold dynamics is explored when the noise is small. Our results show that dynamics of stochastic system is different with the deterministic case due to the effect of stochastic perturbation, large noise can cause the infectious disease to tend to zero exponentially, which is propitious to epidemic diseases control.
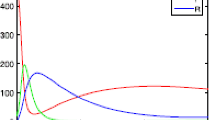
In the following, by employing the Euler–Maruyama (EM) method [61], we make some numerical simulations to illustrate the extinction and persistence of the diseases in the stochastic system and the corresponding deterministic system for comparison. First, we set parameters as \(\Lambda=2\), \(\alpha=0.2\), \(\beta=1\), \(a=1\), \(\mu =1\), \(r_{1}=0.9\), \(r_{2}=0.9\), \(\delta=0.1\) in system (1.1). A simple computation shows that \(R_{0}=0.6667<1\), then system (1.1) has a stable disease-free equilibrium \(E_{0}(2,0,0)\) (see Fig. 2). If we choose \(\beta=2\), in this case, \(R_{0}=1.3333>1\), then system (1.1) has a stable infection equilibrium \(E^{*}(1.7132,0.1421,0.1163)\) (see Fig. 3).
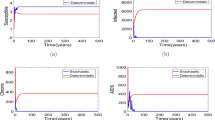
Next, let the intensity of noise \(\sigma=1.2\), a simple computation shows that the condition \(\sigma^{2}>\max\{\frac{\beta\mu}{\Lambda},\frac {\beta^{2}}{2(\mu+r_{1}+r_{2}+\alpha)}\}\) holds, then by Theorem 3.2, the disease dies out under a large white noise perturbation (see Fig. 4). On the other hand, if we reduce the intensity of noise σ to 1, in this case, we have \(\sigma^{2}<\frac{\beta\mu }{\Lambda}\) and \(\widetilde{R}_{0}=0.9067<1\), then by Theorem 3.2, the disease dies out (see Fig. 5). And if we decrease the intensity of noise σ from 1 to 0.2, by calculation we have \(\widetilde{R}_{0}=1.1667>1\), then by Theorem 3.5 the disease is persistent (see Fig. 6).
References
Wang, W., Zhang, T.: Caspase-1-mediated pyroptosis of the predominance for driving CD4+ T cells death: a nonlocal spatial mathematical model. Bull. Math. Biol. 80(3), 540–582 (2018)
Zhang, T., Meng, X., Zhang, T.: Global analysis for a delayed SIV model with direct and environmental transmissions. J. Appl. Anal. Comput. 6(2), 479–491 (2016)
Zhang, T., Meng, X., Zhang, T.: Global dynamics of a virus dynamical model with cell-to-cell transmission and cure rate. Comput. Math. Methods Med. 2015, Article ID 758362 (2015)
Zhang, T., Meng, X., Zhang, T.: SVEIRS: a new epidemic disease model with time delays and impulsive effects. Abstr. Appl. Anal. 2014, Article ID 542154 (2014)
Bernoulli, D.: Essai d’une nouvelle analyse de la mortalité causée par la petite vérole et des avantages de l’inoculation pour la prévenir. Mem. Math. Phys. 1, 1–45 (1760)
Hamer, W.H.: Epidemic disease in England. Lancet 1, 733–739 (1906)
Ross, R.A.: The Prevention of Malaria. Murray, London (1911)
Kermack, W.O., McKendrick, A.G.: A contribution to the mathematical theory of epidemics. Proc. R. Soc., Math. Phys. Eng. Sci. 115(772), 700–721 (1927)
Zhang, T., Meng, X., Zhang, T., Song, Y.: Global dynamics for a new high-dimensional SIR model with distributed delay. Appl. Math. Comput. 218(24), 11806–11819 (2012)
Meng, X., Chen, L., Wu, B.: A delay SIR epidemic model with pulse vaccination and incubation times. Nonlinear Anal., Real World Appl. 11(1), 88–98 (2010)
Xu, R., Ma, Z.: Global stability of a SIR epidemic model with nonlinear incidence rate and time delay. Nonlinear Anal., Real World Appl. 10(5), 3175–3189 (2009)
Miao, A., Zhang, J., Zhang, T., Pradeep, B.G.S.A.: Threshold dynamics of a stochastic SIR model with vertical transmission and vaccination. Comput. Math. Methods Med. 2017, Article ID 4820183 (2017)
Zhou, J., Yang, Y., Zhang, T.: Global stability of a discrete multigroup SIR model with nonlinear incidence rate. Math. Methods Appl. Sci. 40(14), 5370–5379 (2017)
Hethcote, H.W.: Qualitative analyses of communicable disease models. Math. Biosci. 28(3), 335–356 (1976)
Li, J., Ma, Z.: Qualitative analyses of SIS epidemic model with vaccination and varying total population size. Math. Comput. Model. 35, 1235–1243 (2002)
Qi, H., Liu, L., Meng, X.: Dynamics of a nonautonomous stochastic SIS epidemic model with double epidemic hypothesis. Complexity 2017, Article ID 4861391 (2017)
Zhou, Y., Yuan, S., Zhao, D.: Threshold behavior of a stochastic SIS model with jumps. Appl. Math. Comput. 275, 255–267 (2016)
Zhao, D., Yuan, S., Liu, H.: Random periodic solution for a stochastic SIS epidemic model with constant population size. Adv. Differ. Equ. 2018(1), 64 (2018)
Zhao, Y., Zhang, Q., Jiang, D.: The asymptotic behavior of a stochastic SIS epidemic model with vaccination. Adv. Differ. Equ. 2015(1), 328 (2015)
Muroya, Y., Li, H., Kuniya, T.: Complete global analysis of an SIRS epidemic model with graded cure and incomplete recovery rates. J. Math. Anal. Appl. 410(2), 719–732 (2014)
OoRegan, S.M., Kelly, T.C., Korobeinikov, A., OoCallaghan, M.J.A., Pokrovskii, A.V.: Lyapunov functions for SIR and SIRS epidemic models. Appl. Math. Lett. 23(4), 446–448 (2010)
Zhao, J., Wang, L., Han, Z.: Stability analysis of two new SIRS models with two viruses. Int. J. Comput. Math. 95(10), 2026–2035 (2018). https://doi.org/10.1080/00207160.2017.1364369
Zhang, S., Meng, X., Wang, X.: Application of stochastic inequalities to global analysis of a nonlinear stochastic SIRS epidemic model with saturated treatment function. Adv. Differ. Equ. 2018(1), 50 (2018)
Ji, C., Jiang, D.: The extinction and persistence of a stochastic SIR model. Adv. Differ. Equ. 2017(1), 30 (2017)
Leng, X., Feng, T., Meng, X.: Stochastic inequalities and applications to dynamics analysis of a novel SIVS epidemic model with jumps. J. Inequal. Appl. 2017(1), 138 (2017)
Han, Q., Jiang, D., Lin, S., Yuan, C.: The threshold of stochastic SIS epidemic model with saturated incidence rate. Adv. Differ. Equ. 2015(1), 22 (2015)
Li, T., Zhang, F., Liu, H., Chen, Y.: Threshold dynamics of an SIRS model with nonlinear incidence rate and transfer from infectious to susceptible. Appl. Math. Lett. 70, 52–57 (2017)
Liu, G., Wang, X., Meng, X., Gao, S.: Extinction and persistence in mean of a novel delay impulsive stochastic infected predator–prey system with jumps. Complexity 2017, Article ID 1950970 (2017)
Zhang, S., Meng, X., Feng, T., Zhang, T.: Dynamics analysis and numerical simulations of a stochastic non-autonomous predator–prey system with impulsive effects. Nonlinear Anal. Hybrid Syst. 26, 19–37 (2017)
Lv, X., Wang, L., Meng, X.: Global analysis of a new nonlinear stochastic differential competition system with impulsive effect. Adv. Differ. Equ. 2017(1), 296 (2017)
Feng, T., Meng, X., Liu, L., Gao, S.: Application of inequalities technique to dynamics analysis of a stochastic eco-epidemiology model. J. Inequal. Appl. 2016(1), 327 (2016)
Liu, L., Meng, X.: Optimal harvesting control and dynamics of two-species stochastic model with delays. Adv. Differ. Equ. 2017(1), 18 (2017)
Yu, X., Yuan, S., Zhang, T.: Persistence and ergodicity of a stochastic single species model with Allee effect under regime switching. Commun. Nonlinear Sci. Numer. Simul. 59, 359–374 (2018)
Zhao, Y., Yuan, S., Zhang, T.: Stochastic periodic solution of a non-autonomous toxic-producing phytoplankton allelopathy model with environmental fluctuation. Commun. Nonlinear Sci. Numer. Simul. 44, 266–276 (2017)
Meng, X., Wang, L., Zhang, T.: Global dynamics analysis of a nonlinear impulsive stochastic chemostat system in a polluted environment. J. Appl. Anal. Comput. 6(3), 865–875 (2016)
Chi, M., Zhao, W.: Dynamical analysis of multi-nutrient and single microorganism chemostat model in a polluted environment. Adv. Differ. Equ. 2018(1), 120 (2018)
Zhang, T., Liu, X., Meng, X., Zhang, T.: Spatio-temporal dynamics near the steady state of a planktonic system. Comput. Math. Appl. 75(12), 4490–4504 (2018)
Bian, F., Zhao, W., Song, Y., Yue, R.: Dynamical analysis of a class of prey–predator model with Beddington–Deangelis functional response, stochastic perturbation, and impulsive toxicant input. Complexity 2017, Article ID 3742197 (2017)
Li, X., Lin, X., Lin, Y.: Lyapunov-type conditions and stochastic differential equations driven by g-Brownian motion. J. Math. Anal. Appl. 439(1), 235–255 (2016)
Lin, X., Zhang, R.: H-∞ control for stochastic systems with Poisson jumps. J. Syst. Sci. Complex. 4, 683–700 (2011)
Zong, Z.J.: A comonotonic theorem for backward stochastic differential equations in and its applications. Ukr. Math. J. 64(6), 857–874 (2012)
Guo, Y.: Mean square exponential stability of stochastic delay cellular neural networks. Electron. J. Qual. Theory Differ. Equ. 2013, 34 (2013)
Gray, A., Greenhalgh, D., Hu, L., Mao, X., Pan, J.: A stochastic differential equation SIS epidemic model. SIAM J. Appl. Math. 71(3), 876–902 (2011)
Tornatore, E., Buccellato, S.M., Vetro, P.: Stability of a stochastic SIR system. Phys. A, Stat. Mech. Appl. 354, 111–126 (2005)
Meng, X., Zhao, S., Feng, T., Zhang, T.: Dynamics of a novel nonlinear stochastic SIS epidemic model with double epidemic hypothesis. J. Math. Anal. Appl. 433(1), 227–242 (2016)
Yu, J., Jiang, D., Shi, N.: Global stability of two-group SIR model with random perturbation. J. Math. Anal. Appl. 360(1), 235–244 (2009)
Zhao, Y., Jiang, D.: The threshold of a stochastic SIRS epidemic model with saturated incidence. Appl. Math. Lett. 34, 90–93 (2014)
Miao, A., Wang, X., Zhang, T., Wang, W., Sampath Aruna Pradeep, B.: Dynamical analysis of a stochastic SIS epidemic model with nonlinear incidence rate and double epidemic hypothesis. Adv. Differ. Equ. 2017(1), 226 (2017)
Li, F., Meng, X., Wang, X.: Analysis and numerical simulations of a stochastic SEIQR epidemic system with quarantine-adjusted incidence and imperfect vaccination. Comput. Math. Methods Med. 2018, Article ID 7873902 (2018)
Zhao, Y., Jiang, D.: The threshold of a stochastic SIS epidemic model with vaccination. Appl. Math. Comput. 243, 718–727 (2014)
Lahrouz, A., Settati, A., Akharif, A.: Effects of stochastic perturbation on the SIS epidemic system. J. Math. Biol. 74(1), 469–498 (2017)
Liu, Q., Jiang, D., Shi, N., Hayat, T., Alsaedi, A.: Stationary distribution and extinction of a stochastic SIRS epidemic model with standard incidence. Phys. A, Stat. Mech. Appl. 469, 510–517 (2017)
Cai, Y., Kang, Y., Banerjee, M., Wang, W.: A stochastic epidemic model incorporating media coverage. Commun. Math. Sci. 14(4), 893–910 (2016)
Dieu, N.T., Nguyen, D.H., Du, N.H., Yin, G.: Classification of asymptotic behavior in a stochastic SIR model. SIAM J. Appl. Dyn. Syst. 15(2), 1062–1084 (2016)
Cai, Y., Kang, Y., Banerjee, M., Wang, W.: A stochastic SIRS epidemic model with infectious force under intervention strategies. J. Differ. Equ. 259(12), 7463–7502 (2015)
Gray, A., Greenhalgh, D., Mao, X., Pan, J.: The SIS epidemic model with Markovian switching. J. Math. Anal. Appl. 394(2), 496–516 (2012)
Zhang, X., Jiang, D., Alsaedi, A., Hayat, T.: Stationary distribution of stochastic SIS epidemic model with vaccination under regime switching. Appl. Math. Lett. 59, 87–93 (2016)
Tuckwell, H.C., Williams, R.J.: Some properties of a simple stochastic epidemic model of SIR type. Math. Biosci. 208(1), 76–97 (2007)
Mao, X.: Stochastic Differential Equations and Applications, 2nd edn. Horwood, Chichester (2007)
Lahrouz, A., Omari, L.: Extinction and stationary distribution of a stochastic SIRS epidemic model with non-linear incidence. Stat. Probab. Lett. 83(4), 960–968 (2013)
Kloeden, P.E., Platen, E.: Higher-order implicit strong numerical schemes for stochastic differential equations. J. Stat. Phys. 66(1), 283–314 (1992)
Acknowledgements
We thank the referees and the editor for their careful reading of the original manuscript and many valuable comments and suggestions that greatly improved the presentation of this paper.
Funding
YS, TZ, and XW are supported by the National Natural Science Foundation of China (No.11371230) and Shandong Provincial Natural Science Foundation of China (No.ZR2015AQ001). TZ and XW are supported by Research Funds for Joint Innovative Center for Safe and Effective Mining Technology and Equipment of Coal Resources by Shandong Province and SDUST(2014TDJH102). AM and TZ are supported by SDUST Innovation Fund for Graduate Students (SDKDYC180348).
Author information
Authors and Affiliations
Contributions
All authors worked together to produce the results and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Song, Y., Miao, A., Zhang, T. et al. Extinction and persistence of a stochastic SIRS epidemic model with saturated incidence rate and transfer from infectious to susceptible. Adv Differ Equ 2018, 293 (2018). https://doi.org/10.1186/s13662-018-1759-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13662-018-1759-8