Abstract
Background
The MIRUS™ (TIM, Koblenz, Germany) is an electronical gas delivery system, which offers an automated MAC (minimal alveolar concentration)-driven application of isoflurane, sevoflurane, or desflurane, and can be used for sedation in the intensive care unit. We investigated its consumption of volatile anesthetics at 0.5 MAC (primary endpoint) and the corresponding costs. Secondary endpoints were the technical feasibility to reach and control the MAC automatically, the depth of sedation at 0.5 MAC, and awakening times. Mechanically ventilated and sedated patients after major surgery were enrolled. Upon arrival in the intensive care unit, patients obtained intravenous propofol sedation for at least 1 h to collect ventilation and blood gas parameters, before they were switched to inhalational sedation using MIRUS™ with isoflurane, sevoflurane, or desflurane. After a minimum of 2 h, inhalational sedation was stopped, and awakening times were recorded. A multivariate electroencephalogram and the Richmond Agitation Sedation Scale (RASS) were used to assess the depth of sedation. Vital signs, ventilation parameters, gas consumption, MAC, and expiratory gas concentrations were continuously recorded.
Results
Thirty patients obtained inhalational sedation for 18:08 [14:46–21:34] [median 1st–3rd quartiles] hours. The MAC was 0.58 [0.50–0.64], resulting in a Narcotrend Index of 37.1 [30.9–42.4] and a RASS of − 3.0 [− 4.0 to (− 3.0)]. The median gas consumption was significantly lowest for isoflurane ([ml h−1]: isoflurane: 3.97 [3.61–5.70]; sevoflurane: 8.91 [6.32–13.76]; and desflurane: 25.88 [20.38–30.82]; p < 0.001). This corresponds to average costs of 0.39 € h−1 for isoflurane, 2.14 € h−1 for sevoflurane, and 7.54 € h−1 for desflurane. Awakening times (eye opening [min]: isoflurane: 9:48 [4:15–20:18]; sevoflurane: 3:45 [0:30–6:30]; desflurane: 2:00 [1:00–6:30]; p = 0.043) and time to extubation ([min]: isoflurane: 10:10 [8:00–20:30]; sevoflurane: 7:30 [4:37–14:22]; desflurane: 3:00 [3:00–6:00]; p = 0.007) were significantly shortest for desflurane.
Conclusions
A target-controlled, MAC-driven automated application of volatile anesthetics is technically feasible and enables an adequate depth of sedation. Gas consumption was highest for desflurane, which is also the most expensive volatile anesthetic. Although awakening times were shortest, the actual time saving of a few minutes might be negligible for most patients in the intensive care unit. Thus, using desflurane seems not rational from an economic perspective.
Trial registration Clinical Trials Registry (ref.: NCT03860129). Registered 24 September 2018—Retrospectively registered.
Similar content being viewed by others
Background
Although most national and international guidelines do not recommend the use of volatile anesthetics (VA) such as isoflurane (ISO), sevoflurane (SEVO), and desflurane (DES) for sedation in the intensive care unit (ICU), several studies and current German guidelines state that VA might be a feasible alternative compared to intravenous drugs, especially if fast awakening or a quick extubation after deep sedation is intended [1,2,3,4].
The MIRUS™ (TIM, Koblenz, Germany), an electronical gas delivery system introduced in 2013, offers an automated end-expiratory target-controlled application of VA irrespective of the breathing parameters set on the ventilator, which is comparable with the electronic vaporization of some anesthetic machines. This ‘MAC pilot’ is the special feature of the MIRUS™, which consists of a control unit (monitors and controls gas flow, pressure, VA concentration, and VA application), and an ‘exchanger’, which is a VA carbon reflector with filter and heat moisture exchanger. The ‘exchanger’ is inserted between Y-piece of the ICU ventilator and the tracheal tube and connected to the control unit by a special multi-lumen cable. Basically, the MIRUS™ can be used to deliver ISO, SEVO, and DES, but the VA reservoir in the control unit is VA specific [5, 6].
Since cost-effectiveness is of growing interest in ICUs all over the world, the VA consumption and the efficiency of the MIRUS™ is of importance. We defined 0.5 MAC as a target value (age adjusted by MIRUS™) for mechanically ventilated and sedated ICU patients after major surgery and investigated the VA consumption of the system (primary endpoint). Corresponding costs for ISO, SEVO, and DES were calculated. Secondary endpoints were the technical feasibility of the MIRUS™ to reach and maintain the MAC automatically, the depth of sedation at 0.5 MAC judged by the electroencephalography-based Narcotrend® monitor (Narcotrend-Gruppe, Hanover, Germany) and the Richmond Agitation Sedation Scale (RASS), as well as a comparison of awakening times in ISO, SEVO, and DES sedated patients. VA decrement times from 0.5 to 0.25 MAC were additionally recorded.
Methods
This monocenter randomized controlled trial was performed in a German University Hospital between February 2016 and Mai 2017. It was approved by the appropriate Institutional Review Board (Ethikkommission der Ruhr-Universität Bochum, 4780-13) and registered at the Clinical Trials Registry (ref.: NCT03860129). Written informed consent was obtained from all patients prior to their inclusion in this study. Measurements complied with the ethical standards of the Declaration of Helsinki.
The MIRUS™ systems were kindly provided by the former marketeer (Pall Medical, Dreieich, Germany).
Study design and setting
We preoperatively enrolled ASA physical status classification I–III patients aged 18–80 years, who were scheduled for major surgery with an expected need for postoperative mechanical ventilation due to respiratory or hemodynamic problems (norepinephrine dose > 0.5 h−1, FiO2 > 0.5, PEEP > 10 mbar, body temperature < 36 °C). Exclusion criteria were ASA physical status classification IV, malignant hyperthermia, any neuromuscular diseases, increased intracranial pressure, autoimmune hepatitis, pregnancy, deafness, language barriers, involvement in other studies, refusal to give informed consent, and an expected tidal volume < 350 ml.
The closed envelop method was used to allocate patients at random to group ISO, SEVO, or DES.
General anesthesia was induced in the operating room with 0.2 µg kg−1 sufentanil and 2 mg kg−1 propofol to facilitate intubation with a cuffed tracheal tube. An epidural analgesia (ropivacaine 2 mg ml−1) was offered to patients without contraindications. Maintenance of anesthesia was with SEVO (1.0 MAC) and sufentanil. At the end of surgery, the SEVO application was stopped and patients obtained propofol (5 mg kg−1 h−1) for the transport from the operating room to the ICU.
Upon arrival in the ICU, patients were equipped with a multivariate electroencephalogram (Narcotrend®) to assess the depth of sedation. The RASS was additionally gathered by the nurses. After at least 1 h of propofol sedation (2.5 mg kg−1 h−1), patients were switched to VA sedation via MIRUS™. The patient’s expiratory VA concentration was monitored by MIRUS™ and automatically adjusted to maintain the target value 0.5 MAC. Sufentanil was administered by a syringe pump (5–30 µg h−1). The minimum VA sedation time was 2 h.
Lung protective mechanical ventilation (tidal volume 6–8 ml kg−1) was performed with a Puritan Bennett 840 ventilator (Covidien, Boulder, Co, USA) on a Bi-Level mode, which allows pressure control and pressure support ventilation. The pressure control mode was solely used until the criteria for a wake-up test were met (norepinephrine < 0.5 h−1, FiO2 < 0.5, PEEP < 10 mbar, body temperature > 36 °C). Then, the respiratory rate was halved, the pressure support mode used, and a spontaneous breathing trail performed (support 5–15 mbar, PEEP 5 mbar, FiO2 0.3). VA was still administered at 0.5 MAC. The sufentanil dose was not changed initially but reduced by 10 µg kg−1 h−1 if the patient did not start breathing within 30 min. An end-expiratory CO2 < 35 mmHg or > 14 breaths min−1 were treated with 5-µg sufentanil. As soon as patients breathed spontaneously (support 5 mbar), the rapid shallow breathing index was calculated (respiratory frequency divided by tidal volume). Once the index was < 105, the MAC was set to 0 with the exchanger within the breathing circuit (start of measuring awakening times). Decrement times from 0.5 to 0.25 MAC were recorded. The rapid shallow breathing index was again calculated immediately before extubation.
RASS was documented twice during propofol sedation, 5, 30, and 60 min after beginning of VA sedation, 60 min before the end and at the end of VA application, as well as after 5, 30, and 60 min in the post-sedation phase.
The Narcotrend® Index (NI), ventilation parameters, VA consumption, MAC, and expiratory VA concentration were continuously provided through the devices. The MAC fraction was evaluated using the formulas MAC1 = 1.47 vol% − (age·0.0071 vol%) for ISO, MAC1 = 2.31 vol% − (age·0.0106 vol%) for SEVO, and MAC1 = 8.43 vol% − (age·0.0428 vol%) for DES according to the manufacturer [7].
Blood gas analyses were performed upon ICU arrival, twice during VA sedation, and after the end of VA application to allow early adjustments of the set ventilator parameters.
A summary of the study design is pictured in Fig. 1.
Study design. After intravenous induction of anesthesia in the operating room, patients obtained a balanced anesthesia with sufentanil and sevoflurane at 1.0 MAC via a standard anesthetic machine during surgery. At the end of surgery, sedation was switched to an intravenous regime with propofol for the transport from the operating room to the intensive care unit (ICU). After a minimum of 1-h intravenous sedation in the ICU, patients obtained isoflurane, sevoflurane, or desflurane at 0.5 MAC via MIRUS™. As soon as the criteria for a wake-up test were met, a spontaneous breathing trail was performed. Once patients passed the test the MAC was set to 0. The dark blue lines mark the course of the MAC throughout the study
Sample size calculation
Sample size calculation was conducted in g power (Heinrich-Heine-University, Dusseldorf, Germany) and is based on a pilot study investigating the VA consumption of MIRUS™ at 1.0 MAC during surgery [8]. Significantly different VA consumption rates of the three gases were reported with an estimated effect size of d = 3.56, which was transferred to the current study design. For an alpha of 0.05 and a power of 0.8, at least 3 patients per group were needed when analyzed via a univariate ANOVA. We enrolled 10 patients per group to detect effect sizes of at least d = 1.2, which displays a strong effect.
Statistical analysis
All variables were tested for normal distribution using the Kolmogorov–Smirnov test and each variables histogram. A non-parametric approach was chosen to analyze data consistently across groups and variables. All metric variables, except age, height and weight, which are displayed as mean ± 1 standard deviation, were presented as median [1st–3rd quartiles].
Differences across the 3 groups were tested for continuous variables with the Kruskal–Wallis test, which was further explored in case of a significant main effect ‘gas’ with the Mann–Whitney test. Age, height and weight were analyzed via a univariate ANOVA and further investigated with independent t-tests. Group-independent comparisons between propofol sedation and VA sedation were analyzed with the Wilcoxon test. Nominal data were analyzed via cross tables and the Chi2-test, or with Fishers exact test.
Effect sizes were calculated in case of significant results, meaning for direct group comparisons (Mann–Whitney tests) and for phase comparisons (Wilcoxon test). This was done via \(r = z/\sqrt {\text{Number of obersations}}\) with r = 0.1 (small effect), r = 0.3 (medium effect), and r = 0.5 (strong effect) [9].
Results
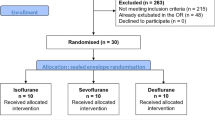
A total of 293 patients underwent major surgeries with expected ICU stay during the study period; 78 of these met the inclusion criteria. Forty-eight patients were already extubated in the operating room, whereas the remaining 30 patients (10 per group) completed the study. Their characteristics are shown in Table 1.
Operating room
Surgery and general anesthesia lasted 5:40 [3:57–7:03] h and 6:27 [4:47–8:18] h (both median; 1st–3rd quartiles), respectively, with no significant difference between the groups (surgery: p = 0.312; anesthesia: p = 0.704).
During surgery, all patients obtained SEVO at 1.12 [1.02–1.20] MAC and 0.17 [0.12–0.22] µg kg−1 h−1 sufentanil (median [1st–3rd quartiles]) without statistically significant group differences (MAC: p = 0.124; sufentanil: p = 0.059).
Intensive care unit
Upon arrival in the ICU, patients obtained propofol sedation for 1:27 [0:59–2:16] h and VA sedation at 0.5 MAC for 18:08 [14:46–21:34] h (both median, 1st–3rd quartiles). Differences between the three groups were not observed (propofol: p = 0.876; VA: p = 0.716). Corresponding ventilation and blood gas parameters are shown in Table 2.
The actual MAC, the corresponding Narcotrend Index as well as the RASS are shown in Table 3.
The median VA consumption [ml h−1; 1st–3rd quartiles] was 3.97 [3.61–5.70] for ISO, 8.91 [6.32–13.76] for SEVO, and 25.88 [20.38–30.82] for DES (all: p < 0.001; ISO vs. DES: p < 0.001, r = 0.845; SEVO vs. DES: p < 0.001, r = 0.761; ISO vs. SEVO: p = 0.004, r = 0.625).
The consumption corresponds to average costs of 0.39 € h−1 for ISO, 2.14 € h−1 for SEVO, and 7.54 € h−1 for DES.
Awakening and MAC decreasing times are shown in Fig. 2.
Awakening times. The time needed to decrease the MAC from 0.5 to 0.25 was longest for ISO (pink box) and quickest for DES (blue box). Correspondingly, awakening was quickest after DES sedation, followed SEVO (yellow box) and ISO. Median (horizontal black lines), 1st and 3rd quantile (upper and lower end of the boxes), the 95% interval (horizontal black lines) and statistical outliers (circles: outside the 95% interval) are presented. *DES is significantly quicker than ISO; #SEVO is significantly quicker than ISO
Discussion
In this study, we investigated the VA consumption of the MIRUS™ System at a target value of 0.5 MAC, its technical feasibility to reach and maintain the MAC, corresponding depths of sedation, and awakening times in 30 patients after major surgery in the ICU. Data revealed that an approximately twofold larger amount of SEVO, and a sixfold larger amount of DES compared to ISO are needed to achieve a comparable depth of sedation at 0.5 MAC (judged by NI and RASS). However, recovery times were the longest when using ISO.
Various studies have shown that VAs may be beneficial for sedation of critically ill patients in the ICU because of their minimal systemic metabolism rate, organ protective effects, the rare occurrence of tolerance or ceiling effects, the possibility to control the effective concentration by monitoring the end-tidal VA concentration, as well as due to their rapid onset and offset of action with fast emergence, easy weaning, and extubation [6, 10,11,12,13,14]. However, most physicians still prefer a sedation practice based on intravenous agents. Besides structural (e.g., no air-conditioning) or medical reasons for it, two frequently mentioned concerns about VA sedation in the ICU are the high costs and possible problems during mechanical ventilation due to the device’s dead space of approximately 100 ml.
Indeed, the application of VA by MIRUS™ in the ICU requires technical prerequisites and is, therefore, costly. The VA-specific control unit is the most expansive part of the MIRUS™ System. However, it is not disposable material. The exchanger, which consists of the reflector with HME filter, must be replaced once a week. It costs about 150 € or 1 € h−1. Its long durability is achieved by the fact that the filter is kept separate from the carbon reflector [15]. Furthermore, devices to reduce atmospheric VA pollution must be considered. Studies have shown that an air-conditioning with at least six air changes per hour and a scavenging system should be used to reach mean VA concentrations below 1 ppm in the patient room (the National Institute of Occupational Safety and Health has defined an exposure limit of 2 ppm for ISO, SEVO, and DES in the air) [16, 17].
The VA consumption is of special interest, since it mainly influences the running costs. The hourly consumptions in this study were approximately 4 ml for ISO, 9 ml for SEVO, and 26 ml for DES to reach 0.5 MAC. ISO, the cheapest VA, costs 0.39 € h−1; propofol, in comparison, about 0.41 € h−1 for an infusion rate of 3 mg kg−1 h−1. Although comparable studies are rare, Romagnoli and colleagues reported nearly identical results for SEVO of approximately 8 ml for a slightly lower MAC of 0.45 [17]. Another observation, a case report of an obese woman suffering from acute respiratory distress syndrome after aspiration of gastric contents, demonstrated a twofold higher VA consumption of 53 ml h−1 DES to reach a fraction of 3.3–3.8% (3.21% in this study) [18]. However, it must be noted that the VA consumption depends (besides the MAC) on the loss of VA through the reflector, which is influenced by the respiratory minute volume [19, 20]. Thus, the respiratory minute volume was actually lower in the study of Romagnoli et al. (7.5 l min−1), and higher in the case report (up to 12 l min−1) than in this study (9 l min−1).
Two studies investigated the VA consumption of MIRUS™ at 1.0 MAC. A benchmark study reported a DES consumption of 40 ml per hour to achieve a fraction of 6.0–6.6%, and a clinical trial revealed a threefold higher consumption for ISO and SEVO, as well as a one and a half times higher consumption for DES compared to our results during hip and knee replacement surgery (respiratory minute volume 6–7 l min−1) [5, 8]. These high consumptions can be explained by the fact that the reflection efficiency of the MIRUS™ is highest at expiratory VA fractions of about 0.2–1.0%, and thus, more VA gets lost at higher concentrations [5].
Rebreathing and hypercarbia could be adverse effects when using the MIRUS™ because of its dead space. Data demonstrated that etCO2 increases significantly during VA sedation compared to intravenous sedation, although the respiratory minute volume had been raised by 0.8 l min−1. However, on the one hand, the pH was still within a normal range (it even increased), and on the other hand a lot of different factors could have caused these changes.
Awakening times and mental recovery were significantly shortest after DES sedation, although patients in this group obtained the highest amount of sufentanil. Moreover, all patients opened their eyes within 10 min after VA application had stopped, even though sedation lasted 18 h on average and we did not remove the reflector (it is to be expected that VA concentrations decrease more quickly without reflector; however, we did not remove it to monitor the VA concentration, pressure and flow, as well as to collect these data automatically for later analysis). This quick awakening is consistent with previous observations and can be attributed to the VAs’ characteristics (quick washout times, small risk of oversedation due to real-time feedback, low metabolic rates) [4, 21]. Furthermore, it is of advantage since it has been shown that a quick interruption of sedation accompanied by early awakening and extubation, as well as the control of the depth of sedation improves clinical outcomes in postoperative ICU patients [22, 23]. However, DES is not rational from an economic point of view, especially because the time saving of a few minutes compared to ISO is negligible for most ICU patients. Thus, ISO should be preferred.
To our knowledge, this is the first study using an automated target-controlled MAC and not an expiratory VA fraction to perform sedation in the ICU. The target value was reliably reached by MIRUS™, and the NI was comparable in all groups. Furthermore, the MAC was kept constant during hyperventilation and hypoventilation, since it is independent of the respiratory minute volume (no inadequate VA application).
This study lacks a common intravenous control group, which is a limitation. However, we performed case–control-group comparisons within the study population. This approach enabled us to compare ventilation and blood gas parameters, NI, and RASS during intravenous and VA sedation within the same patient. Awakening times could obviously not be determined for propofol; however, the comparison between propofol and VA has already been investigated by others [3, 21]. Another limitation is the study’s small sample size. This investigation was designed to detect strong effects only; however, the described effects must be considered significant. Furthermore, we did not focus on clinical effects or outcome parameters, respectively. This must be elucidated in follow-up studies. Lastly, we focused on a well-selected patient group without severe organ dysfunctions. It remains questionable whether such patients must receive sedation in the postoperative period. Thus, our results must be verified in a broader ICU population of critically ill patients.
Conclusions
In summary, a target-controlled, MAC-driven automated VA application of 0.5 MAC for sedation in the ICU is technically feasible and enables an adequate depth of sedation. The gas consumption is highest for DES, which is also the most expansive VA, and the actual time savings is only a few minutes, which is clinically insignificant for most ICU patients. Thus, the use of DES seems not rational from an economic perspective. Follow-up studies are needed to elucidate outcome effects of VA sedation in the ICU.
Availability of data and materials
A limited de-identified dataset is available from the corresponding author on reasonable request.
Abbreviations
- ASA Class:
-
American Society of Anesthesiologists Classification
- DES:
-
desflurane
- ICU:
-
intensive care unit
- ISO:
-
isoflurane
- MAC:
-
minimum alveolar concentration
- NI:
-
Narcotrend Index
- PEEP:
-
positive end-expiratory pressure
- RASS:
-
Richmond Agitation Sedation Scale
- SEVO:
-
sevoflurane
- VA:
-
volatile anesthetic
References
Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73.
Baron R, Binder A, Biniek R, Braune S, Buerkle H, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Taskforce 2015)—short version. Ger Med Sci. 2015;13:19.
Mesnil M, Capdevila X, Bringuier S, Trine PO, Falquet Y, Charbit J, et al. Long-term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med. 2011;37:933–41.
Jerath A, Panckhurst J, Parotto M, Lightfoot N, Wasowicz M, Ferguson ND, et al. Safety and efficacy of volatile anesthetic agents compared with standard intravenous midazolam/propofol sedation in ventilated critical care patients: a meta-analysis and systematic review of prospective trials. Anesth Analg. 2017;124:1190–9.
Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane—the Mirus. Anaesthesia. 2014;69:1241–50.
Herzog-Niescery J, Seipp HM, Weber TP, Bellgardt M. Inhaled anesthetic agent sedation in the ICU and trace gas concentrations: a review. J Clin Monit Comput. 2018;32:667–75.
Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91:170–4.
Bellgardt M, Drees D, Vinnikov V, Procopiuc L, Meiser A, Bomberg H, et al. Use of the MIRUS™ system for general anaesthesia during surgery: a comparison of isoflurane, sevoflurane and desflurane. J Clin Monit Comput. 2018;32:623–7.
Field A. Discovering statistic using SPSS. 4th ed. London: SAGE Publications Ltd; 2014.
Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993;79:795–807.
Kunst G. From coronary steal to myocardial, renal, and cerebral protection: more questions than answers in anaesthetic preconditioning? Br J Anaesth. 2014;112:958–60.
Bellgardt M, Bomberg H, Herzog-Niescery J, Dasch B, Vogelsang H, Weber TP, et al. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: retrospective analysis. Eur J Anaesthesiol. 2016;33:6–13.
Meiser A, Groesdonk HV, Bonnekessel S, Volk T, Bomberg H. Inhalation sedation in subjects with ARDS undergoing continuous lateral rotational therapy. Respir Care. 2018;63:441–7.
Rand A, Zahn PK, Schildhauer TA, Waydhas C, Hamsen U. Inhalative sedation with small tidal volumes under venovenous ECMO. J Artif Organs. 2018;21:201–5.
Jerath A, Parotto M, Wasowicz M, Ferguson ND. Opportunity knocks? the expansion of volatile agent use in new clinical settings. J Cardiothorac Vasc Anesth. 2018;32:1946–54.
Herzog-Niescery J, Vogelsang H, Gude P, Seipp HM, Uhl W, Weber TP, Bellgardt M. Environmental safety: air pollution while using MIRUS™ for short-term sedation in the ICU. Acta Anaesthesiol Scand. 2019;63:86–92.
Romagnoli S, Chelazzi C, Villa G, Zagli G, Benvenuti F, Mancinelli P, et al. The new MIRUS system for short-term sedation in postsurgical ICU patients. Crit Care Med. 2017;45:925–31.
Bomberg H, Groesdonk HV, Bellgardt M, Volk T, Meiser A. AnaConDa™ and Mirus™ for intensive care sedation, 24 h desflurane versus isoflurane in one patient. SpringerPlus. 2016;5:420.
Meiser A, Bellgardt M, Belda J, Röhm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device—a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23:11–9.
Bomberg H, Wessendorf M, Bellgardt M, Veddeler M, Wagenpfeil S, Volk T, et al. Evaluating the efficiency of desflurane reflection in two commercially available reflectors. J Clin Monit Comput. 2018;32:605–14.
Meiser A, Sirtl C, Bellgardt M, Lohmann S, Garthoff A, Kaiser J, et al. Desflurane compared with propofol for postoperative sedation in the intensive care unit. Br J Anaesth. 2003;90:273–80.
Chanques G, Conseil M, Roger C, Constantin JM, Prades A, et al. Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med. 2017;5:795–805.
Eikermann M, Sarge TW. Postoperative interruption of sedation on arrival in the surgical ICU: a new standard of care. Lancet Respir Med. 2017;5:764–5.
Acknowledgements
We thank Dr. Magdalene Ortmann for statistical advice and revision.
Parts of this manuscript were presented at the International Symposium on Intensive Care and Emergency Medicine, Brussels, November 2018 and at the LIVES Congress of the European society of intensive medicine, Paris, October 2018.
Financial support
The MIRUS™ systems were kindly provided by the marketeer (Pall Medical, Dreieich, Germany).
Funding
Departmental funding only.
Author information
Authors and Affiliations
Contributions
MB, AG, MK and JH-N had full access to all data and are responsible for the integrity and the accuracy of data analysis. They made substantial contributions to conception and design of the study, acquisition, analysis and interpretation of data. MB and JH-N wrote the manuscript. DD, AM, PG, HV, and TW made contributions to conception and design, literature search and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective study was performed in a German University Hospital between February 2016 and Mai 2017. It was approved by the appropriate Institutional Review Board (Ethikkommission der Ruhr-Universität Bochum, 4780-13) and registered at the Clinical Trials Registry (ref.: NCT03860129). Written informed consent was obtained from all patients prior to their inclusion in this study. Measurements complied with the ethical standards of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
MB, AM, and JH-N received speakers’ honoraria from Pall Medical, Dreieich, Germany, the former owner of the MIRUS System in 2017 (1200 € each). The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bellgardt, M., Georgevici, A.I., Klutzny, M. et al. Use of MIRUS™ for MAC-driven application of isoflurane, sevoflurane, and desflurane in postoperative ICU patients: a randomized controlled trial. Ann. Intensive Care 9, 118 (2019). https://doi.org/10.1186/s13613-019-0594-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-019-0594-8