Abstract
Aryl hydrocarbon receptor (AHR) is a ligand-dependent transcription factor. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a classical exogenous synthetic ligand of AHR that has significant immunotoxic effects. Activation of AHR has beneficial effects on intestinal immune responses, but inactivation or overactivation of AHR can lead to intestinal immune dysregulation and even intestinal diseases. Sustained potent activation of AHR by TCDD results in impairment of the intestinal epithelial barrier. However, currently, AHR research has been more focused on elucidating physiologic AHR function than on dioxin toxicity. The appropriate level of AHR activation plays a role in maintaining gut health and protecting against intestinal inflammation. Therefore, AHR offers a crucial target to modulate intestinal immunity and inflammation. Herein, we summarize our current understanding of the relationship between AHR and intestinal immunity, the ways in which AHR affects intestinal immunity and inflammation, the effects of AHR activity on intestinal immunity and inflammation, and the effect of dietary habits on intestinal health through AHR. Finally, we discuss the therapeutic role of AHR in maintaining gut homeostasis and relieving inflammation.
Graphical Abstract

Similar content being viewed by others
Introduction
Aryl hydrocarbon receptor (AHR) is a critical mediator that modulates the effect of environmental stimuli, such as alterations in the circadian rhythm, oxygen tension, as well as redox potential, on organisms [1]. In the past, exogenous contaminants like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and B(a)P were considered the only ligands of AHR, and TCDD-mediated sustained AHR activation often leads to toxic results such as cell cycle arrest, chloracne and increased atherosclerosis [2,3,4,5]. However, with the discovery of more types of AHR ligands, AHR research has been more focused on elucidating physiologic AHR function than on dioxin toxicity [6].
A majority of the natural exogenous AHR ligands derived from foods and herbal medicines are beneficial for health. For instance, flavonoids are ubiquitous in many fruits and vegetables and have chemopreventive effects against colorectal cancer [7]. In addition to lowering the risk of postoperative myocardial infarction, oral curcumin also decreased the concentrations of plasma indicators for inflammation, oxidation, and damage [8]. A carotenoid-rich diet promotes healthier lives and lower chronic disease mortality [9]. The dietary metabolite indole-3-carbinol (I3C) reduces proinflammatory responses [10]. I3C and 3,3-diindolylmethane (DIM) have been shown to be effective in suppressing carcinogenesis [11,12,13]. Several of the most basic endogenous ligands like indolo [3,2-b] carbazole (ICZ), 6-formylindoleo[3,2-b] carbazole (FICZ)), and 6,12-diformylindolo[3,2-b] carbazole (dFICZ) bind to AHR and function as immunomodulators [14]. Kynurenine (KYN) generated in the tumor microenvironment induces AHR activation, which is related to glioma-associated immunosuppression [15, 16]. AHR activation by bilirubin suppresses experimental colitis, which indicates that bilirubin exerts an AHR-dependent anti-inflammatory effect [17]. Numerous microbial products, including tryptophan metabolites and short-chain fatty acids (SCFAs), induce AHR activation. Tryptophan metabolites are crucial for immune and inflammatory responses [18, 19]. Indole-3-aldehyde (IAld) promotes intestinal homeostasis by activating AHR [20]. SCFAs are required for the intestinal epithelium to maintain homeostasis [21].
AHR is currently considered to be a significant developmental factor and physiological regulator in the intestinal immune system. Proper activation of AHR can promote intestinal immunity and reduce the occurrence of intestinal inflammation. Under normal conditions, persistent organic pollutants alter the metabolic homeostasis of the host and the gut flora by AHR activation [22]. The proinflammatory immune response will be improved when AHR activation is minimal, whereas stronger and sustained activation of AHR disrupts intestinal flora [23]. Under inflammatory conditions, AHR activation decreases cytokine (TNF, IFNγ, IL-7, IL-12, IL-17, and IL-6) production in the intestine [24]. By changing the composition of the intestinal microbiome, AHR activation by natural ligands prevents pathogenic intestinal microbial dysbiosis [25].
Some studies have shown that AHR is crucial for maintaining intestinal health and inhibiting intestinal infections [26]. The regulatory role of AHR in intestinal inflammation has drawn increasing attention [27]. However, there is no systematic understanding of how AHR regulates intestinal immune development or influences intestinal inflammation and immune homeostasis. So, the objective of this review is to discuss the therapeutic role of AHR in maintaining gut homeostasis and relieving inflammation. Specifically, the connection between AHR and intestinal immunity, the ways in which AHR affects intestinal immunity and inflammation, and how dietary habits affect intestinal health via AHR will be discussed.
AHR and ligands
AHR is a ligand-activated transcription factor that belongs to the basic helix-loop-helix-Per-Arnt-Sim homology superfamily (bHLH-PAS) of proteins [28, 29], which consists of a DNA binding domain, a ligand binding domain and a transactivation domain [30]. AHR has been stably expressed in animal cells for 550 million years. The high degree of conservation of AHR implicates it in a variety of physiological processes (cell differentiation, pluripotency, and stemness) [31]. In the 1970s, AHR was first discovered due to its high affinity for TCDD [32]. Studies have shown that environmental chemicals and other xenobiotics, including halogenated aromatic hydrocarbons (HAHs) and polycyclic aromatic hydrocarbons (PAHs), are the major ligands for AHR [33, 34]. With no ligand binding, AHR is found predominantly in the cytoplasm and enters the nucleus following ligand binding [35]. AHR forms a dimer with AHR nuclear transport (ARNT) and then recruits transcription factors (ERAP140, SRC-1, RIP140, etc.) in the nucleus, which in turn regulates gene expression (cytochrome P450 family, COX-2, etc.) and then modulates the immune system and cellular homeostasis of the organism [36, 37]. Several functions of AHR can be achieved by AHR ligands, including regulation of immunity, the cell cycle, cell differentiation, chemical and microbial defense.
Exploring AHR function
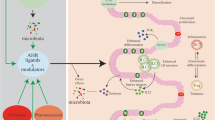
According to the currently available evidence, AHR is crucial for immunity, the cell cycle, cell differentiation, chemical and microbial defense, and tumorigenesis [38, 39] (Fig. 1).
Schematic represents canonical AHR signaling and AHR function. In the absence of ligand, AHR is retained in the cytoplasm in an inactive complex containing chaperone proteins, such as HSP90 and XAP2. Upon ligand binding, AHR translocates into the nucleus, where it dimerizes with ARNT. The AhR/ARNT dimer binds genomic regions containing DRE that regulate gene expression, further exerting a variety of functions. ARNT, AHR nuclear translocator; DRE, dioxin response element; HSP90, heat shock protein 90; XAP2, HBV X-associated protein 2; MDSC, myeloid-derived suppressor cells; Treg, regulatory T cell; T, T cells; B, B cells; NK, natural killer cell; Tr1, Type 1 regulatory T; Th17, T helper cell 17; DC, dendritic cells; ILCs, innate lymphoid cells; SC, stem cell
AHR is vital for the regulation of both innate and adaptive immune cells [40]. AHR regulates the secretion of cytokines from subcellular populations of innate and adaptive immune cells [41, 42]. The expression of AHR in the adaptive immune system is high in Th17 cells and the IL-17/IL-22 subset but low in naive T and B cells [43,44,45]. Furthermore, AHR regulates immune cell differentiation, such as ILCs, Th17, Treg, and Tr1 cells. AHR activation promotes immune tolerance and immune evasion by generating suppressive immune cells (MDSCs and Tregs) [46].
AHR is critical to cell cycle regulation. Low levels of AHR activity are essential for the cell cycle and stem cell self-renewal [47]. Nevertheless, AHR activation at high levels causes cell cycle arrest in the G1/G0 phase, providing conditions for cell differentiation [48]. Furthermore, AHR is crucial for cell differentiation. AHR regulates the differentiation of dendritic cells (DCs) [49]. AHR activation might promote the differentiation of mesenchymal stem cells toward osteoblasts rather than adipocytes [50]. In addition, activation of AHR influences the expression of numerous genes associated with keratinocyte differentiation [51].
AHR is essential in defending against pathogenic microorganisms through chemical and microbial defenses. AHR induces the expression of gene batteries involved in the three phases of drug metabolism, also referred to as the chemical defense, including phase I enzymes (primarily CYPs), phase II conjugate enzymes (UGTs, SULTs, and GSTs), and phase III conjugate transporters (ABCG2) [52,53,54]. Also, AHR is essential for defense against acute and chronic bacterial infections [55]. Studies have demonstrated that AHR-deficient mice are more susceptible to bacterial infection than wild-type mice [56].
AHR is also critical for tumorigenesis, encompassing both pro- and anti-tumorigenic activities. The function of the ligands (AHR agonist or antagonist), tumor type and the cellular and protein environment all influence the relationship between AHR and tumor [57]. Activation of AHR contributes to tumor initiation through genotoxicity [58]. Formate derived from the microbiome promotes colorectal cancer tumor invasion by activating AHR signaling [59]. Chemical exposure and AHR activation affect mammary gland differentiation processes, increasing the risk of breast cancer [58, 60]. However, the expression of AHR in IECs is a necessary condition for the reduction of premalignant lesions of the colon [61]. AHR agonists such as I3C were used as a chemopreventive therapy for IBD-associated colorectal cancer [62]. In mice, the AHR agonist FICZ inhibited SREBP2 posttranslationally and reversed tumorigenesis [63]. Furthermore, treatment with TCDD blocks the proliferation in human colorectal cancer cells [64, 65].
AHR ligands
AHR ligands are characterized as hydrophobic molecules with aromatic rings [66]. According to their structural characteristics, they are mainly PAHs, HAHs, tryptophan derivatives, indoles and polyphenols [36]. Furthermore, AHR ligands can be classified as exogenous synthetic, natural exogenous, or endogenous ligands (Table 1).
Exogenous synthetic ligands
Exogenous ligands contain synthetic and natural ligands. Exogenous synthetic ligands are mainly derived from municipal and industrial waste incineration, vehicle exhaust, PVC plastics, pesticide production, and steel smelting [67, 68]. These pollutants have significant immunotoxic effects, causing a decrease in humoral and cellular immunity and inducing cancers [69]. Exogenous synthetic ligands mainly include HAHs (dioxins, furans, PCBs) and PAHs (B(a)P, BA) and their congeners [70]. TCDD binds to the AHR protein, and the TCDD/AHR/ARNT heterodimer binds to dioxin response elements in the target genes' regulatory regions, including those encoding dioxin biodegradation enzymes (CYP1A1, CYP1A2, CYP1B1). The increased expression of CYP1A1 is a molecular marker of TCDD action [71]. 2-(4-Amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203) is a high affinity ligand for AHR that antagonizes the induction of CYP1A1 RNA by TCDD [72]. Coplanar PCBs are high affinity AHR ligands. When exposed to PCBs, IEC line monolayers increase permeability and alter junctional protein regulation [73]. PCB 153 causes DNA damage and is genotoxic to IECs. Oral exposure to PCB 153 increased IL-6 expression (15.5-fold, P < 0.01) in proximal small intestine IECs, the driver of this inflammation and increase in permeability is the transcription factor NF-kB, which can become activated through ataxia telangiectasia mutated (ATM) and the NF-KB essential modulator (NEMO) [74]. Benzo(a)pyrene (BaP), an AHR ligand, is one of the components of cigarette smoke. BaP promotes gastric cancer cell proliferation and metastasis via the AHR and ERK signaling pathways [75]. Benz(a)anthracene (BA), as a ligand, has a high binding affinity toward AHR and stimulates downstream signaling cascades that regulate tyrosinase activity and melanin synthesis [76]. VAF347, a small molecular weight compound, binds AHR, induces AHR-driven signal transduction and CYP1A1 expression and exerts anti-inflammatory activity in monocytes [77, 78]. Overall, the exogenous synthetic ligands are the most characteristic high-affinity AHR ligands and AHR agonists.
Natural exogenous ligands
Many natural compounds, such as glucobrassicins, flavonoids, resveratrol, carotenoids, curcumin and berberine, are AHR agonists or partial antagonists [79]. Their anti-inflammatory properties can be explained by the activation or antagonism of AHR. Cruciferous vegetables (broccoli, Brussels sprouts, etc.) contain glucobrassicins, which upon digestion, release I3C, which is a weak AHR agonist [80]. In the stomach, I3C is converted into DIM, ICZ and 2-(indole-3-methane)-3,3'-diindolylmethane (LTr-1), which are AHR agonists. Compared with other natural products, ICZ has a higher affinity toward AHR [6]. Taken together, I3C, LTr-1, DIM and ICZ are natural exogenous AHR ligands.
Dietary flavonoids can act as AHR ligands sourced from tea, fruits, wine, vegetables and cacao, including quercetin, kaempferol and baicalin [81]. Dietary quercetin and kaempferol are AHR ligands that affect CYP1A1 transcription and inhibit TCDD-induced AHR/DRE-driven transactivation. Moreover, quercetin is an AHR agonist that is activated indirectly by inhibiting the degradation of FICZ [82,83,84]. Scutellariae Radix inhibits AHR activity, and baicalin, one of its major active ingredients, effectively blocks the activation of AHR stimulated by cigarette smoke [85]. In addition, baicalin reduces AHR expression, which inhibits the inflammation response to myocardial ischemic injury [85]. Resveratrol, a partial agonist of AHR, induces the expression of CYP1A1 [86]. Resveratrol has anti-inflammatory and antitumor effects by activating AHR [87]. In weaned piglets, dietary supplementation with resveratrol activates AHR and increases CYP1A1 gene expression in the jejunal mucosa [88].
In addition, AHR ligands have been found in other dietary products, such as carotenoids, curcumin and berberine [89]. Carotenoids, as novel antagonists of AHR, are able to affect the AHR signaling pathway, probably through oxidative conversion to retinoids in the body [90]. Retinoids regulate the AHR signaling pathway not only by binding to AHR ligands, but also by regulating the crosstalk between AHR and RAR/RXR signaling [91]. The primary component of turmeric, curcumin, is an agonist and antagonist of AHR and can inhibit the conversion of AHR through its phosphorylation. In the nucleus, curcumin stimulates AHR heterodimerization with ARNT but is unable to induce AHR binding to DRE or CYP1A1 protein expression [92]. Curcumin attenuated AHR signaling by inhibiting AHR-ARNT heterodimerization, which is required for AHR transactivation [89]. Curcumin inhibits TCDD-induced DNA-binding activity of the AHR/ARNT heterodimer [92]. Curcumin reduce AHR nuclear translocation, which is challenged by AHR-agonistic B[a]P [93]. In addition, curcumin may promote ARNT proteasomal degradation by increasing intracellular oxidative stress [94]. Berberine, a quaternary isoquinoline alkaloid found in plants, activates AHR at high concentrations and for short periods [95]. Dietary AHR ligand exposure is common and constant for animals and people, which is beneficial for maintaining intestinal homeostasis under normal conditions.
Endogenous ligands
In addition to exogenous ligands, many endogenous compounds can activate AHR in vivo, such as heme metabolites, arachidonic acid metabolites, tryptophan metabolites, equilenin, indigo and indirubin [18, 96]. Heme metabolites like bilirubin and biliverdin directly activate AHR to induce CYP1A1 gene transcription. Bilirubin-mediated AHR activation is associated with anti-inflammatory signaling [97]. Heme metabolites have comparatively weak affinity toward AHR when compared to TCDD [98]. Arachidonic acid metabolites like lipoxin A4 are competitive substrates for CYP1A1 [99]. Tryptophan metabolites, including ITE, FICZ, kynurenine, cinnabarinic acid (CA), kynurenic acid (KA), and xanthurenic acid (XA), are also endogenous ligands [100,101,102].
The AHR agonist 2-(10H-indole-30-carbonyl)-thiazole-4carboxylic acid methyl ester (ITE) was first isolated from porcine lung [103]. ITE could significantly activate the AHR of placental trophoblast cells, induce the expression of the downstream gene CYP1A1, and inhibit placental trophoblast cell proliferation [104]. ITE binds to the same AHR site as TCDD, and they compete for binding but with only one percent of the affinity of TCDD [105]. Moreover, FICZ, a naturally produced photo-oxidation product of tryptophan, is another high-affinity endogenous agonist of AHR. And a small amount of FICZ is enough to activate AHR [106, 107]. FICZ has been shown to be a superior substrate for enzymes encoded by AHR-regulated genes [108]. AHR ligands, including Kynurenine, KA, XA, and CA, can induce the expression of AHR-dependent gene [109].
Bacterial tryptophan catabolites, including tryptamine, 3-methylindole (skatole), indoleacrylic acid (IA), indole-3-acid-acetic (IAA), indole-3-lactic acid (ILA), IAld, indole-3-acetaldehyde (IAAld), indole-3-carboxaldehyde (3-IAld), and indole-3-propionic acid (IPA), are also endogenous ligands of AHR in the body [20, 110,111,112,113,114]. AHR is activated by tryptamine to regulate intestinal immunity. On the contrary, when intestinal homeostasis is disrupted, AHR regulates tryptamine production [115]. Indigo and indirubin are endogenous AHR activators. In contrast to TCDD, indigo is an equivalent activator of AHR and indirubin is even more potent [116,117,118]. Endogenous ligands, commonly known as AHR agonists, are synthesized in the organism and derive from endogenous/chemical process, photo-oxidation, host metabolism and microbiota metabolism.
AHR is utilized and activated by ligands
AHR was first discovered during research on TCDD. As a high-affinity ligand, TCDD can lead to persistent AHR activation [119]. With industrial advancement, various exogenous AHR ligands have been synthesized, including PAHs, polybrominated dibenzo-p-dioxins, polybrominated diphenyl ethers, and other major classes of known and unknown toxicant compounds [120]. Extractable organic matter from PM2.5, lipophilic components of diesel exhaust extract and cigarette smoke extract all have negative impacts on organisms by activating AHR [121,122,123,124].
However, AHR is not a “dioxin receptor” but rather a factor in the maintenance of individual development and normal immune function [125]. Before these exogenous substances were synthesized, AHR was stably expressed in animal cells for 550 million years, and its high degree of conservation is consistent with AHR having essential roles in physiological and toxicological processes [126]. Hence, we speculated that AHR was being utilized by exogenous synthetic ligands, not acting as a dioxin receptor. In addition to toxic effects, TCDD was proven to have a positive impact on organisms by activating AHR, such as relieving colitis symptoms, decreasing the viral load and the levels of proinflammatory cytokines, and suppressing experimental autoimmune encephalitis [14, 127, 128].
Why does AHR have a positive effect on organisms in response to exogenous synthetic ligands? One explanation is that the AHR signaling pathway might alleviate the toxicity of exogenous toxic substances in the body by inducing their metabolism or inducing immunoreactions [129]. When exposed to exogenous toxic ligands, AHR activation induces host immunity and maintains the steady state of the organism [130]. Moreover, TCDD and some exogenous toxicants have strong oncogenic effects [131]. AHR has been identified as a tumor-associated protein and therapeutic target molecule in recent years [57]. Thus, AHR has the dual role of both suppressing toxic reactions and acting as a pattern recognition receptor to detect these risk-associated molecules and induce defenses against their general toxic effects on the body.
How can the activation of AHR be regulated? AHR activity can be regulated in multiple ways. Treatment with AHR ligands is the traditional approach for activating AHR [132]. This results in the formation of the AHR/ARNT complex, which then mediates the transcriptional activation of several genes encoding drug-metabolizing enzymes, most notably CYP1A1 and CYP1B1 [133]. Thus, the effect of the amount of ligand on AHR may be easier to understand. Aside from the amount of available AHR ligands, the characteristics of AHR ligands can influence the activity of AHR and further modulate intestinal immunity. The characteristics of AHR ligands can be defined as the following two main aspects: (1) Activity of the ligand in binding to AHR. (2) Its properties of being metabolized by the organism.
-
(1)
Activity of the ligand in binding to AHR
Different types of ligands have distinct effects on AHR activation. Exogenous ligands such as TCDD, BaP and other environmental pollutants have a high affinity for AHR, which can activate AHR constantly [134]. There are significant differences in activation properties among endogenous ligands, such as FICZ and ITE, which have a low content in the body but have a high affinity for AHR. However, some other endogenous ligands, such as KYNA and L-KYN, are weak agonists of AHR [100, 135]. Chewing cruciferous vegetables like broccoli and Brussels sprouts promotes the enzymatic cleavage of glucosinolates by myrosinase, producing I3C and indole-3-acetonitrile (I3ACN), which both have the ability to bind and activate AHR, albeit weakly. However, ICZ, as a derivative of I3C, potently binds and activates AHR in a manner comparable to TCDD [136]. Endogenous ligands are molecules necessary for physiological activity, and there is a negative feedback regulatory system controlling their concentration in the body [137]. Dietary-derived ligands serve important physiological functions, although they are rapidly metabolized in vivo [18]. Most dietary-derived ligands are weak AHR agonists and activate AHR at low concentrations, but at high concentrations, they act as AHR antagonists [138]. In addition, dietary-derived ligands have more significant agonistic or antagonistic effects when interacting with high-affinity ligands such as TCDD and FICZ [80].
-
(2)
The properties of being metabolized by the organism
The properties of ligands been metabolized by the organism also influence the activity of AHR [89]. Some AHR ligands are difficult for the body to metabolize, such as TCDD and PCBs. The half-life of dioxin is about 7 years, so TCDD is exceptionally stable in biological systems [139]. Polychlorinated biphenyls (PCBs) are chlorinated compounds that are hydrophobic and lipophilic. This compound is relatively stable, difficult to degrade, accumulates in organisms for a long time, and widely exists in the environment [140]. The body can metabolize natural exogenous ligands more quickly than TCDD. After oral administration, the half-lives of quercetin, curcumin and resveratrol are 11–28 h, 28.1 h, and 9.2 h, respectively. The half-lives of I3C and DIM are 12–24 h [141,142,143,144,145].
Most endogenous ligands can be metabolized by organisms, such as arachidonic acid, heme, tryptophan, and other molecules [146]. Lipoxin A4 (LXA4) is a metabolite of arachidonic acid, which has no aromatic ring and complete planar structure. The metabolism of LXA4 is autoregulated by activated AHR to regulate CYP1A1 expression [99]. Tryptophan can be catabolized through a variety of pathways in vivo, such as KYN and 5-HT [147, 148]. Tryptophan can also be converted by the gut microbiota into a variety of AHR ligands, including indole and its derivatives [96]. Thus, the activation effect of these ligands is not continuous. Their metabolic rate and the metabolites of the ligands determine the AHR activation. Dietary-derived ligands are also rapidly metabolized in vivo. Strikingly, AHR-regulated cytochrome P450 enzymes are capable of efficiently metabolizing ITE, FICZ, I3C, and curcumin [149,150,151].
Additionally, AHR activity can be modulated independently of ligands. For instance, in the absence of ligands, AHR may undergo nucleocytoplasmic shuttling, causing the activation of its target gene CYP1A1 [152, 153]. Sulindac directly binds AHR and stimulates AHR: ARNT heterodimerization to drive CYP1A1 expression [154]. Both sunitinib, a tyrosine kinase inhibitor, and omeprazole induce CYP1A1 gene expression through ligand-independent AHR activation [155,156,157]. Furthermore, in the absence of ligand, AHR activation is also affected by metabolic conversion of molecules into ligands or various compounds’ ability to affect other cellular pathways. In lymphocytes, RORgt can also form a complex with AHR to modulate IL-22 expression upon AHR activation [158]. Through redox modification of AHR, 1-(4-Chlorophenyl)-benzo-2,5-quinone activates an AHR-dependent but ligand-independent signaling pathway, thereby promoting AHR's nuclear translocation and activation of the target gene, CYP1A1 [159].
AHR, intestinal immunity and inflammation
AHR and intestinal immunity, friend, foe or both?
AHR is widely expressed in immune cells [28]. It regulates innate and adaptive immune cell development and function in the gut [61]. AHR is essential for maintaining the homeostasis of the intestinal epithelium and associated immune cells and for generating appropriate responses to epithelial injury and invading pathogens [160,161,162,163,164,165]. The maintenance of AHR-dependent intraepithelial lymphocytes (IELs) contributes to intestinal epithelial cell (IEC) homeostasis. IECs play a vital role in integrating extra intestinal and internal signals and coordinating the ensuing subsequent response [166, 167].
Is the relationship between AHR and intestinal immunity a friend, foe or both? Sometimes, AHR regulates intestinal immunity via ligands, thereby maintaining immune homeostasis [164]. Proper activation of AHR can promote intestinal immunity and reduce the occurrence of intestinal inflammation [168]. However, excessive activation of AHR can impair intestinal immunity and promote intestinal inflammation and even intestinal cancer [28]. The relationship between AHR and intestinal immunity is dynamic and depends on the activity of AHR, its ligands and the physiological state of the body [169]. Accordingly, for the purpose of mediating the desired treatment outcome while minimizing the inherent risk of prolonged receptor activity, further discussion is required to determine the ligand dose-dependent level of AHR signaling.
Effects of AHR activity on intestinal immunity and inflammation
Different levels of AHR activation generate distinct effects on intestinal immunity. Moderation is necessary to maintain homeostasis, and if the AHR activation level is lower or higher than optimal, intestinal disorders appear (Fig. 2).
Different effects on intestinal immunity between persistent activation and defective AHR activation. a. persistent activation by AHR ligands has deleterious consequences. b. defective AHR activation is detrimental to the maintenance of intestinal homeostasis. This balance of intestinal homeostasis requires appropriate level of AHR activation. I3C, indole-3-carbinol; IEL, intraepithelial lymphocytes; ILC3, intraepithelial lymphocytes and type 3 innate lymphoid cells
Persistent activation by AHR ligands has a detrimental effect on intestinal health. Sustained TCDD-induced AHR activation usually results in rapid protein ubiquitination that targets the receptor for degradation by the proteasome [170,171,172], leading to toxic results such as cell cycle arrest, chloracne or increased atherosclerosis [2]. Sustained activation of AHR encourages tumor growth and affects immune defense [173]. Furthermore, excessive AHR signaling activation aggravates abnormalities in the gastric mucosa [174]. When compared to AHR homozygous mice, symptoms of colitis were dramatically ameliorated in AHR heterozygous mice, indicating that overstimulation of AHR may contribute to the development of colitis [175]. I3C, an AHR ligand, limits proinflammatory responses by suppressing nuclear factor kB signaling pathways [10]. In contrast, I3C administration can hasten the development of colonic lesions. Long-term in vivo treatment with I3C may be carcinogenic [176]. Negative feedback regulation is essential for AHR activation because prolonged stimulation is harmful. Excessive AHR activation can result in intestinal immune dysregulation and even intestinal diseases.
However, defective AHR activation is also detrimental to the maintenance of intestinal homeostasis. In the gut immune system, AHR deficiency in hematopoietic cells leads to IELs and type 3 innate lymphoid cells (ILC3) vanish [162, 177,178,179]. In the intestine, reduced AHR ligand availability results in defective AHR activation, which disrupts the intestinal barrier, alters immune responses and amplifies dysbiosis in chronic inflammation and consequently leads to IBD [167]. AHR ligand degradation has a detrimental impact on intestinal immune functions, which can be counterbalanced by increasing dietary intake of AHR ligands [180]. Therefore, an appropriate level of AHR activation is important to maintain intestinal homeostasis and maintain the intestine in a healthy state.
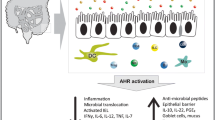
In terms of affecting intestinal inflammation, AHR activation could significantly inhibit the intestinal inflammatory response and alleviate the symptoms of colitis in experimental animals [161, 181, 182] (Fig. 3). AHR is a susceptibility locus for intestinal inflammation. Compared to healthy ones, AHR expression was decreased in the inflammatory tissues of Crohn’s disease patients, and the amount of natural AHR ligands was significantly lower in the feces of ulcerative colitis patients [183]. AHR activation regulates IEL and anti-inflammatory factors for the future treatment of intestinal inflammation. The AHR ligand FICZ ameliorates experimental colitis by lowering the number of activated IELs and reducing IL-7 production [28]. Additionally, AHR ligands or ligand precursors found in foods may prevent chemically induced intestinal inflammation. I3C or DIM supplementation reduces DSS-mediated colonic inflammation and disease severity [184]. AHR activation and the subsequent production of anti-inflammatory factors are important for the clearance of intestinal pathogenic microorganisms [180, 185]. Knockout studies have shown that AHR is indispensable for the treatment of intestinal inflammation. Citrobacter rodentium is commonly used to mimic the intestinal inflammation arising from enteropathogenic and enterohemorrhagic E. coli [186]. AHR-deficient mice exhibit increased sensitivity to Citrobacter rodentium due to their inability to produce IL-22 [187]. Higher sensitivity to Citrobacter rodentium was also observed in R26Cyp1a1 mice [180].
Possible mechanisms of AHR effects on Intestinal Immunity and Inflammation
AHR is required for the regulation of intestinal immunity and inflammation (Fig. 4). The first physical barrier against pathogens and poisons entering the body through the digestive system is the intestinal mucosal immune system. It is primarily composed of innate lymphoid cells (ILCs), IELs and IECs [41]. The widespread expression of AHR in IELs and ILCs reveals its important function in the regulation of the intestinal immune system. Activation of AHR by ligands from dietary sources is crucial for regulating intestinal mucosal immunity and intestinal barrier function [181, 188]. AHR affects intestinal immunity in two main ways (Fig. 5): (1) AHR acts directly on IECs. AHR is necessary for the development and self-renewal of IECs derived from local stem cells [189, 190]. AHR is activated in IECs to enhance intestinal barrier function, alleviate inflammation, and maintain overall mucosal homeostasis [191, 192]. In addition, AHR slowed down the repositioning of zonula occludens-1 (ZO-1), increased the integrity of the intestinal epithelium, ameliorated hypoxia-induced changes in intestinal permeability, and maintained a normal intestinal barrier [188, 193]. Deficiency of AHR in IECs hampered the ability of intestinal stem cells to restore and differentiate in response to cell injury, having a significant impact on infection resistance and colorectal cancer development [194]. Furthermore, in the intestinal epithelium, AHR deficiency enhanced IEC apoptosis, impaired the proliferation of colonic crypt stem cells, and made mice more sensitive to DSS-induced intestinal inflammation [195, 196].
Schematic represents the effects of AHR on gut immunity and inflammation in intestinal mucosal immune system. Activation of AHR plays an important role in regulating intestinal immunity (on left). the deficiency of AHR results in intestinal inflammation (on right). ILCs, innate lymphoid cells; IELs, intraepithelial lymphocytes; IECs, intestinal epithelial cells; AHR, aryl hydrocarbon receptor; ROR γt + , RORγt + ILCs; Notch1, tight junction protein Notch1; ZO-1, tight junction proteins zonula occludens-1
AHR affects intestinal immunity in two main ways: (1) AHR acts directly on intestinal epithelial cells (IECs). (2) AHR affects intestinal immunity through regulating intestinal immune cells. N, neutrophils; M, macrophages; DC, dendritic cells; T, T cells; B, B cells; IECs, intestinal epithelial cells; FICZ, 6-formylindoleo[3,2-b] carbazole; AHR, aryl hydrocarbon receptor
(2) AHR affects intestinal immunity by regulating intestinal immune cells. Intestinal immune cells mainly include IELs, Th17 cells, ILCs, neutrophils, macrophages, and dendritic cells [197,198,199]. These immune cells secrete various cytokines that regulate gut immune homeostasis, including proinflammatory factors (IL-1β, IL-6, IL-23, TNF-α) and anti-inflammatory factors (TGF-β, IL-10) [200, 201]. The balance of proinflammatory and anti-inflammatory factors is critical in preventing intestinal inflammatory diseases. In addition, it was shown that AHR affects intestinal immunity via interactions with numerous intestinal immune cells and cytokines [167, 180].
IELs are essential for intestinal immune development and intestinal inflammation. AHR is highly expressed on the IEL surface and influences the maintenance and differentiation of IELs [202, 203]. Also, AHR is an essential regulator for maintaining the number of IEL in the intestine. AHR activation can influence the subsets and distribution of IELs, which induce the upregulation of the anti-inflammatory factors IL-10 and IL-22 and the downregulation of the proinflammatory factors IFN-γ and TNF-α [181, 182, 204]. Instead, when the AHR is absent, the number of IELs is decreased, and the burden of intestinal bacteria is increased, which promotes dextran sulfate sodium (DSS)-induced colitis [205]. Collectively, AHR controls the number and homeostasis of IELs and further regulates intestinal immunity.
ILCs mediate the regulatory properties of AHR on the intestinal immune system [206]. ILCs are essential for the development of gut lymphoid follicles, epithelial homeostasis, defense against intestinal pathogens, and protection from inflammatory disorders [207, 208]. AHR is expressed in RORγt+ ILCs, and AHR signaling is considered necessary for these cells to expand and maintain homeostasis in the intestine [162]. ILCs and CD4 + T cells produce IL-22, which protect against intestinal inflammation [209]. IL-22 promotes IEC regeneration by increasing the production of anti-microbial peptides and mucins and enhancing the integrity of the mucosal barrier [210]. However, in mice exposed to DSS, the absence of IL-22 resulted in more severe colitis [211,212,213]. Thus, ILCs and IL-22 maintain the balance of intestinal immune homeostasis and protect against inflammation by modulating AHR.
Th17 cells and Tregs are both derived from CD4 + T cells [214]. AHR is highly expressed in Th17 cells and weakly expressed in Foxp3 + Treg cells, and its activation by FICZ enhances Th17 cell differentiation and promotes IL-22 production [44, 215]. In addition, AHR regulates Treg and TH17 cell differentiation in a ligand-specific manner [216]. I3C and DIM induce Tregs while suppressing Th17 cells, whereas FICZ has the opposite effect [217]. AHR regulates Tr1 cell metabolism and decreases HIF1α cellular levels, participating in the late stages of Tr1 cell differentiation [218]. AHR may be important in modulating TH17 cell function by inhibiting TH17 cell conversion to a TH1 cell-like profile and instead promoting TH17 cell transition to a Tr1 cell-like state [219]. At early stages of Th17 cell differentiation, AHR activation might convert Th17 cells into IL-10-producing immunosuppressive Tr1 cells [219, 220]. Tregs suppress inflammation and maintain immune tolerance by secreting IL-4, IL-10, and TGF-β [221]. Baicalein and DIM regulates Th17/Treg differentiation via AHR activation, thereby protecting against DSS-induced colitis in mice [222, 223]. Collectively, modulation of Th17/Treg homeostasis by ligand-activated AHR can improve intestinal immunity and relieve inflammation.
Th17 cells secrete interleukin-7 (IL-7), which is essential for T and B cell development as well as IEL differentiation and maturation [224,225,226,227]. Furthermore, IL-7, a novel target of the AHR pathway in the intestine, is a critical cytokine for triggering mucosal inflammation in IBD [203]. In DSS-induced colitis mice, AHR activation downregulates IL-7 and reduces inflammation [228]. Specifically, FICZ, as an endogenous ligand of AHR, reduces epithelial cell-derived IL-7 expression, concomitant with the amelioration of experimental colitis by reducing the frequency of activated IELs [181]. In short, IL-7 blockade mediates the favorable impacts of AHR pathway activation on colitis.
AHR is a key cofactor involved in IL-10 production by NK cells [229]. IL-10 can also be secreted T cells, B cells, macrophages, dendritic cells, eosinophils and neutrophils [230]. As an anti-inflammatory cytokine, IL-10 is essential for maintaining gut immune homeostasis and the intestinal mucosal barrier [231]. The AHR-Src-STAT3 pathway is required for inflammatory macrophages to produce IL-10 [232]. In addition, kynurenine activates AHR, resulting in the upregulation of IL-10R1 in IECs and reducing mucosal inflammation [233, 234]. In brief, immune cells and cytokines provide a regulatory immunological and inflammatory response via AHR activation.
AHR, a potential target for maintaining intestinal health
AHR, a potential target to maintain intestinal homeostasis
Since AHR plays a vital role in intestinal health, it is feasible to improve intestinal homeostasis by regulating this potential target. AHR promotes immune homeostasis through a variety of mechanisms, including T-cell differentiation and Th17 development, as well as increased IL-22 production [161, 218, 235]. After binding to a ligand, the AHR can interact with DREs in the promoter regions of IL-10 or IL-22, promoting their production in gut ILCs, DCs, and Treg cells [236, 237]. Elevated IL-10 can promote the generation of tolerogenic DCs and Treg cells while inhibiting Th17 cell differentiation, resulting in a decrease in proinflammatory cytokines that mediate gut microbial composition and host homeostasis [238].
The microbiota has a crucial function in gut homeostasis. Microbial dysbiosis, which results in altered L-tryptophan metabolism, decreased AHR activation, and insufficient IL-22 levels, can promote a vicious cycle that promotes gastrointestinal homeostasis loss. Supplemental IL-22 may reverse this process, remodeling the microbiome to increase AHR activity and enhance a virtuous cycle to help regain homeostasis [239]. The intestinal microflora produces tryptophan/indole metabolites that act as AHR ligands. Indole metabolites enhance intestinal homeostasis through AHR-mediated regulation of the IEC IL-10R1 [110]. Indoles, as AHR ligands, have anti-inflammatory activities, maintaining intestinal homeostasis [240]. Lactobacillus spp. can convert tryptophan to IAld, which are AHR ligands. They can activate AHR and further encourage intestinal homeostasis by inducing IL-22 [20]. The SCFA butyrate as an AHR ligand, which is produced by the intestinal flora, contributes to the maintenance of intestinal immune homeostasis via encouraging the differentiation of IL-10-producing Treg and Tr1 cells [241,242,243]. Therefore, the intake of these AHR ligands may be a strategy for maintaining intestinal health.
Under normal conditions, persistent organic pollutants alter gut microbiota-host metabolic homeostasis via AHR overactivation [22]. Sustained potent activation by exogenous synthetic AHR ligands may cause intestinal dysfunction. After 5 days of exposure to the strong and long-lasting xenobiotic pollutant 2,3,7,8-tetrachlorodibenzofuran, mice developed microbial imbalance and disease [22]. Furthermore, high-dose PCB126 and B(a)P exposure adversely affected the microbiota community structure [244, 245]. Hence, minimizing the intake of exogenous synthetic AHR ligands is important to prevent intestinal dysfunction caused by overactivation of AHR.
The consumption of AHR ligands is crucial for the effects of AHR on intestinal homeostasis [246]. Appropriate levels of AHR activated by some exogenous ligands (vegetables or other beneficial ligands) can effectively maintain intestinal homeostasis. I3C and DIM maintain the number of lymphocytes in the intestinal epithelium and protect the intestinal mucosal barrier [205]. A lower level of AHR activation by endogenous ligands facilitates the maintenance of intestinal homeostasis [167].
Moderate AHR activity is essential for maintaining proper intestinal immune homeostasis under normal conditions. Accordingly, the intake of beneficial dietary ligands should be increased, and the intake of harmful ligands should be reduced, which can enhance epithelial barrier function, protect against intestinal challenges.
A potential therapeutic target in intestinal inflammation
Studies have proven a positive correlation between the degree of inflammation and the level of AHR activation [247]. Dysregulated AHR activity adversely affect intestinal infection and inflammation through intestinal intraepithelial lymphocytes loss [248]. AHR ligands containing xenobiotics (TCDD), endogenous substances (FICZ, norisoboldine) and dietary products (soy isoflavones, arachidonic acid, quercetin and baicalein) can activate AHR, suppress inflammatory responses and alleviate the symptoms of colitis [161, 249, 250]. TCDD ameliorates the symptoms of colitis by inhibiting Th17 cell differentiation and decreasing the expression of IL-17 and IFN-γ [251]. FICZ, a tryptophan photoproduct, can alleviate both DSS-induced enterocolitis and trinitrobenzene sulfonic acid or T-cell transfer-induced colitis by reducing the production of proinflammatory cytokines and increasing the production of anti-inflammatory IL-22 by Th17 cells [175, 204, 252]. Norisoboldine stimulates Treg differentiation and suppresses the NLRP3 inflammasome to alleviate the symptoms of colitis [253]. In mouse models with gastrointestinal inflammation, AHR ligand provision can directly attenuate inflammatory signaling [254]. When Qing-Dai was administered, the inflammatory responses of colonic macrophages as well as the generation of TNF-α, IL-1β, and IL-6 in colonic tissue were suppressed [255]. The AHR agonist β-naphthoflavone was administered orally and reduced DSS-induced colitis [256]. I3C produced from vegetables was administered to suppress the development of acute colitis brought on by DSS [177]. When glucosinolate-rich cabbage was consumed, the AHR target gene CYP1A1 was substantially upregulated in the colon, which also reduced colitis [257]. Mice lacking AHR had more severe colitis, whereas those treated with AHR agonists had attenuated disease progression [28]. Kurarinone (KAR) has a therapeutic role in irritable bowel syndrome (IBS) by modulating macrophage functions via stimulating AHR signaling. When AHR deficiency in macrophages, the effect of KAR in IBS mice was weakened [258].
Some bacteria have immunoregulatory effects in the gut by activating the AHR pathway, which culminates in anti-inflammatory responses [259, 260]. A probiotic bacterium can control intestinal inflammation via AHR in the presence of tryptophan [28]. Lactobacillus bulgaricus strain OLL1181 alleviated DSS-colitis by activating AHR signaling and increasing the expression of CYP1A1 [261]. Oral administration of 1,4-dihydroxy-2-naphthoic acid (DHNA), an AHR activator derived from the cheese bacteria Propionibacterium freudenreichii ET-3, induced anti-microbial peptides in the intestine, further controlling inflammation in DSS-colitis [262].
Thus, AHR has the potential as a drug target for the treatment of colitis. Furthermore, the intestinal environment is extremely complex and uncertain. The variables affecting the production and regulation of AHR ligands are numerous and complex, including changes in diet and gut microbes. Hence, the impact of AHR ligands on intestinal immunity in this complex environment should be considered before treating intestinal diseases. More research is needed to accurately evaluate ligands and activation effects in the complex intestinal environment.
Dietary habits affect intestinal health through AHR
Dietary habits can directly affect intestinal immunity. Vegetable, tryptophan and microbial metabolism, SCFA, and natural plant extracts are the largest sources of AHR ligands [241] (Table 2). Dietary AHR ligands have a transient effect on intestinal health that varies greatly depending on the ligand types, bioavailability, and half-life.
Vegetable intake and associated AHR ligands
Consuming Brassica vegetables, which are known to promote AHR activation, is linked to decreased inflammatory signaling and a lower risk of colon cancer [257] (Fig. 6a). Several studies have reported that indolyl glucosinolates, I3C, LTr-1, DIM and ICZ, which are abundant in cruciferous vegetables, are AHR ligands [263]. I3C or glucobrassicin supplementation in rodents' diets can prevent DSS-induced colitis [177, 254, 264]. Additionally, the administration of broccoli could change the resident microflora and reduce intestinal inflammation [265]. Notably, the therapeutic effect of maintaining homeostasis in the gut after broccoli eating is mediated by AHR activation. Overall, consuming vegetables can enhance AHR signaling and maintain intestinal barrier homeostasis, which is beneficial to gut health. As a result, selecting vegetable cultivars with higher levels of glucosinolates, which produce AHR ligands, may have greater health benefits.
Dietary habits affect intestinal health through AHR. a Consumption of Brassica vegetables lead to the formation of AHR ligands, which affect intestinal health. b Tryptophan affect intestinal health through AHR. c Dietary fibers influence intestinal health through AHR. d Natural plant extracts affect intestinal health through AHR. I3C, indole-3-carbinol; DIM,3,30- diindolylmethane; ICZ, indolo[3,2-b] carbazole; LTr-1, 2-(indole-3-methane)-3, 3'-diindolylmethane; IAld, indole-3-aldehyde; IAA, indole-3-acid-acetic; IPA, indole-3-propionic acid; ILA, indolelactic acid; IAAld, indole-3-acetaldehyde; IA, indoleacrylic acid; skatole, 3-methylindole; HIF1α, hypoxia- inducible factor 1α; MFH,medicine food homology; M, macrophage; En, enterochromaffin cells; Treg, regulatory T cell; Th17, T helper cell 17; SCFAs, Short-chain fatty acids; ZO-1, tight junction proteins zonula occludens-1; KAR, kurarinone
Tryptophan and microbial metabolism
AHR is essential for linking tryptophan catabolism between microbial communities and hosts (Fig. 6b). Dietary tryptophan can improve the function of the intestinal mucosal barrier, alleviate acute colitis, and maintain epithelial homeostasis via AHR [115, 266]. Intestinal microorganisms directly convert tryptophan to indole and its derivatives. Many indole derivatives, including IAld, IAA, IPA, ILA, IAAld and IA, are AHR ligands [110]. Likewise, tryptamine and skatole also act as ligands of AHR.
Recently, several studies have demonstrated that intestinal microorganisms produce various tryptophan catabolites. For instance, Clostridium sporogenes, Peptostreptococcus spp. and Lactobacillus spp. can convert tryptophan into tryptamine, ILA, IA, IAld and IPA [20, 114, 267,268,269]. Skatole, a common intestinal metabolite, is produced by decarboxylation of IAA from Bacteroides spp. and Clostridium spp. [270,271,272,273]. How do tryptophan metabolites affect intestinal health? Skatole induces the activation of AHR, thereby regulating IEC death [274]. IA can attenuate the inflammatory response and repair barrier function by promoting the formation of mucus and the differentiation of goblet cells by activating AHR [275, 276]. ILA protects cultured intestinal epithelial cells by activating the AHR and Nrf2 pathways [277]. In mice fed a high-fat diet, IPA reduces intestinal permeability [278]. Both oral indole and IPA can improve colonic inflammation in mice [279, 280]. AHR activation by IAld induces IL-22, thereby maintaining intestinal homeostasis [20]. Tryptophan can be metabolized to numerous AHR ligands via a variety of metabolic pathways. These AHR ligands can activate AHR and the expression of downstream target genes like IL-22 and IL-17, which are beneficial to maintaining intestinal homeostasis.
SCFAs
SCFAs are derived from the bacterial metabolism of ingested fibers, including acetate, propionate, and butyrate [281]. SCFAs regulate the intestinal immune system in a variety of ways by inducing and regulating T cells and constraining cytokine responses [242, 282,283,284] (Fig. 6c).
In the intestine, SCFAs regulate AHR and its target genes [285]. Among the SCFAs, butyrate, as a ligand, activates AHR in human IECs and inhibits proinflammatory responses by intestinal macrophages [286]. The butyrate-serotonin-AHR axis influences intestinal immune homeostasis. Butyrate via AHR activation promotes 5-HT release from neural enterochromaffin cells to regulate intestinal homeostasis and peristalsis [287]. 5-HT induces the expression of CYP1A1 in IECs via AHR activation [288]. In addition, the AHR signaling pathway can affect the composition of small intestinal flora, thus regulating intestinal flora balance and maintaining intestinal health [24].
Dietary habits influence SCFA levels in the intestine. A Mediterranean diet high in fruits, vegetables, and legumes is linked to an increase in fecal SCFA levels [289, 290]. A high-fiber diet led to an obvious rise in gut SCFAs [291]. In addition, SCFAs upregulate IL-22 production by encouraging the expression of AHR and hypoxia-inducible factor 1α to maintain intestinal homeostasis [292]. Fermentable dietary fibers influence the production of SCFAs, and SCFAs exert important regulatory functions on intestinal health by regulating the AHR pathway. Due to this, the Mediterranean diet and fermentable dietary fiber may maintain intestinal homeostasis partially via modulation of AHR.
Natural plant extracts
Natural plant extracts, including berberine, resveratrol, mangosteen ketones, flavonoids, indigo naturalis and more, can treat intestinal inflammation to regulate intestinal immunity through AHR. Among these, berberine, baicalin, alpinetin, quercetin and indigo are all AHR ligands (Fig. 6d).
Berberine, a protoberberine alkaloid from several plant species, is an AHR ligand [293, 294]. Berberine has significant therapeutic potential against IBD. It may reduce gut epithelial barrier dysfunction via increasing the expression of ZO-1 and treat DSS-induced colitis by modulating the activation of AHR by tryptophan metabolites associated with the intestinal microbiota [295,296,297,298,299,300]. Resveratrol, a known AHR antagonist, is a natural polyphenol found in grape skin and red wine [301]. In weaned pigs exposed to diquat, resveratrol modulates the AHR/Nrf2 pathways to lessen intestinal inflammation and protect intestinal integrity [88].
Mangosteen ketones from the tropical fruit mangosteen (Garcinia mangostana) showed anti-inflammatory and antioxidant activities. Garcinone D, one of the seven acylated xanthones contained in mangosteen, can activate AHR, significantly upregulate AHR/CYP1A1 protein expression and enhance ZO-1 and occludin protein levels. Additionally, Garcinone D prevented intestinal epithelial barrier dysfunction brought on by oxidative stress [302].
Flavonoids have anti-inflammatory and chemopreventive properties [303, 304]. Specific examples of flavonoids include baicalin, alpinetin, quercetin and KAR. Baicalin, isolated from Radix scutellariae, is a novel AHR ligand that restores the balance of Th17/Treg cells via AHR to alleviate colitis [222]. Baicalein strengthens the intestinal epithelial barrier through the AHR/IL-22 pathway in ILC3s, thereby reducing the symptoms of ulcerative colitis [305]. As a potential AHR activator, alpinetin regulates Treg differentiation, thereby reducing the symptoms of colitis [306,307,308]. Quercetin, a clear AHR agonist, could shorten the course of chronic DSS colitis in an AHR-dependent manner [309] KAR, a flavonoid derived from Sophora flavescens, is an effective treatment of visceral hypersensitivity in IBS, and it regulates the development of IBS through macrophage-intrinsic AHR [258].
Indigo Naturalis is derived from indigoferous plants [310, 311]. It has been demonstrated that Indigo, one of the main components of Indigo Naturalis, is an AHR ligand. Indigo alleviated murine colitis by activating AHR signaling [118, 195, 312]. Indigo promotes colon epithelial cell proliferation and migration by activating AHR in intestinal epithelia, thereby exerting its erosion-healing effects [313]. Moreover, adding Indigo Naturalis to DSS-induced colitis mice can promote the expression of IL-10 and IL-22 in colonic lamina propria lymphocytes but not in AHR-deficient mice, which may be related to reduced production of regulatory cytokines [314].
The components of medicine food homology (MFH) are complex and can treat intestinal diseases by exerting anti-inflammatory and antioxidant effects, as well as regulating the intestinal flora [315]. In addition, a growing body of research has demonstrated that TCM has great potential in treating intestinal diseases by regulating intestinal immunity and inflammation through the AHR pathway [316]. Eating MFH will have a beneficial effect on intestinal health. IBD is relieved by Hericium erinaceus, a traditional edible mushroom. It regulates intestinal bacteria and the immune system [317]. The major traditional uses of berberine or berberine-containing plants have been for treating intestinal infections such as gastroenteritis, cholera, and dysentery. Extracts of barberry and Oregon grape are used for balancing the intestinal flora in the digestive tract [318]. Phytochemicals derived from natural products, including alpinetin, baicalin, curcumin, resveratrol, indirubin, berberine, and norisoboldine, are effective Th17/Treg regulators and have anti-inflammatory properties in the colon [319].
Both cardamonin and norisoboldine (NOR) from Tilia can alleviate TNBS-induced colitis in mice by activating AHR and eventually inhibiting the activation of colonic NLRP3 inflammatory vesicles [250, 320]. The anti-inflammatory effect of Magnolol on DSS-induced colitis in mice was primarily due to the restoration of colitis serum tryptophan metabolites KA, 5-HIAA, IAA, and indoxylsulfuric acid, all of which are AHR ligands [321]. Galangal ameliorated colitis in mice by activating AHR, upregulating Foxp3 expression, and restoring Th17/Treg balance [322]. Naringenin activates AHR, causing naive T cells to differentiate into Treg cells while inhibiting differentiation into Th17 and Th1 cells, increasing the ratio of Treg cells in the peripheral blood of mice with colitis and thus alleviating colitis [323, 324]. In addition, Qing Dai and indigo may improve colitis by activating AHR to upregulate IL-10 and IL-22 [325].
Altogether, berberine, resveratrol, mangosteen ketones, flavonoids and indigo naturalis promote intestinal self-healing by activating the AHR, lowering intestinal inflammation, altering the ecology of intestinal microorganisms, and mediating immunomodulation. Additionally, natural plant extracts regulate intestinal flora and organismal metabolism. The effect of microbial metabolites on AHR requires further elucidation, which may guide the development of AHR-targeted immunomodulators, further benefiting the healthy development of the intestinal tract.
Conclusion and perspectives
AHR regulates different types of cells in the gut, including ILCs, IELs and IECs, and it is essential for maintaining intestinal immune homeostasis. In addition, the sources and structures of AHR ligands are diverse, and they act differently. AHR ligands are derived from diet, gut microbial metabolites and the environment. They are enriched in the gastrointestinal tract and activate AHR in the gut. Dietary AHR agonists, indole derivatives and/or probiotics that produce AHR ligands have significant potential for preventive and therapeutic interventions against intestinal inflammation. Under normal conditions, moderate activation of AHR regulates intestinal immune homeostasis. When the activation of AHR is excessive or insufficient, it can affect intestinal immune disorders and result in the formation of intestinal diseases and even cancer. Furthermore, the AHR pathway is a very promising therapeutic target for the regulation of intestinal inflammation, but there are still some scientific challenges to be addressed.
The side effects of numerous AHR ligands are challenges to the therapeutic effect of AHR. Many AHR agonists, such as FICZ and TCDD, have been shown to alleviate colitis symptoms [14, 127, 128]. Unfortunately, long-term use of these compounds causes severe side effects [2,3,4,5, 326, 327], limiting their use as therapeutic agents in animals or humans. New or existing AHR-targeting drugs with high efficiency and minimal side effects should be filtered for better therapeutic effect.
In addition, accurately evaluating ligand and activation effects in the complex intestinal environment also poses challenges for the therapeutic potential of AHR. Dietary and medicinal plant extracts have been shown to suppress inflammatory responses and alleviate the symptoms of colitis [99, 222, 305]. However, since the background content of AHR ligands is not low in the intestinal environment, activation of AHR signals itself cannot be dismissed. So, more research is needed to determine how to eliminate the influence of preexisting ligands in the intestine to evaluate the activational effect of a single ligand or assess the levels of background AHR activation before treatment with AHR ligands.
Availability of data and materials
Not applicable.
Abbreviations
- AHR:
-
Aryl hydrocarbon receptor
- TCDD:
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- I3C:
-
Indole-3-carbinol
- DIM:
-
3, 3-Diindolylmethane
- ICZ:
-
Indolo[3,2-b] carbazole
- FICZ:
-
6-Formylindoleo[3,2-b] carbazole
- dFICZ:
-
6,12-Diformylindolo[3,2-b] carbazole
- KYN:
-
Kynurenine
- SCFAs:
-
Short-chain fatty acids
- IAld:
-
Indole-3-aldehyde
- HAHs:
-
Halogenated aromatic hydrocarbons
- PAHs:
-
Polycyclic aromatic hydrocarbons
- ARNT:
-
AHR nuclear transport
- DC:
-
Dendritic cells
- BaP:
-
Benzo(a)pyrene
- BA:
-
Benz(a)anthracene
- LTr-1:
-
2-(Indole-3-methane)-3, 3'-diindolylmethane
- CA:
-
Cinnabarinic acid
- KA:
-
Kynurenic acid
- XA:
-
Xanthurenic acid
- ITE:
-
2-(10H-indole-30-carbonyl)-thiazole-4carboxylic acid methyl ester
- 3-IAld:
-
Indole-3-carboxaldehyde
- skatole:
-
3-Methylindole
- IAA:
-
Indole-3-acid-acetic
- IPA:
-
Indole-3-propionic acid
- IAAld:
-
Indole-3-acetaldehyde
- IA:
-
Indoleacrylic acid
- ILA:
-
Indolelactic acid
- IELs:
-
Intraepithelial lymphocytes
- IECs:
-
Intestinal epithelial cells
- ILCs:
-
Innate lymphoid cells
- IL-7:
-
Interleukin-7
- CYP1A1 :
-
Cytochrome P450 1A1
- CYP1B1 :
-
Cytochrome P450 1B1
- I3ACN:
-
Indole-3-acetonitrile
- PCBs:
-
Polychlorinated biphenyls
- LXA4:
-
Lipoxin A4
- ILC3:
-
Intraepithelial lymphocytes and type 3 innate lymphoid cells
- KAR:
-
Kurarinone
- IBS:
-
Irritable bowel syndrome
- APC:
-
Adenomatous polyposis coli
- ZO-1:
-
Zonula occludens-1
- TCM:
-
Traditional Chinese medicines
- MFH:
-
Medicine food homology
References
Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36(2):189–204. https://doi.org/10.1016/S1357-2725(03)00211-5.
Bock KW. Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol. 2019;168:65–70. https://doi.org/10.1016/j.bcp.2019.06.015.
Xiao L, Zhang Z, Luo X. Roles of xenobiotic receptors in vascular pathophysiology. Circ J. 2014;78(7):1520–30. https://doi.org/10.1253/circj.CJ-14-0343.
Reynolds LM, Wan M, Ding J, et al. DNA methylation of the aryl hydrocarbon receptor repressor associations with cigarette smoking and subclinical atherosclerosis. Cir Cardiovasc Genet. 2015;8(5):707–16. https://doi.org/10.1161/CIRCGENETICS.115.001097.
Kerley-Hamilton JS, Trask HW, Ridley CJA, et al. Inherent and Benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391–404. https://doi.org/10.1093/toxsci/kfs002.
Schanz O, Chijiiwa R, Cengiz SC, et al. Dietary AhR ligands regulate AhRR expression in intestinal immune cells and intestinal microbiota composition. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21093189.
Theodoratou E, Kyle J, Cetnarskyj R, et al. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Prev Biomarkers. 2007;16(4):684–93.
Wongcharoen W, Jai-Aue S, Phrommintikul A, et al. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol. 2012;110(1):40–4.
Diplock A, Charuleux J-L, Crozier-Willi G, et al. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80(S1):S77–112.
Safa M, Tavasoli B, Manafi R, et al. Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumor Biol. 2015;36(5):3919–30.
Bradlow HL, Michnovicz JJ, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12(9):1571–4.
Grubbs C, Steele V, Casebolt T, et al. Chemoprevention of chemically-induced mammary carcinogenesis by indole-3-carbinol. Anticancer Res. 1995;15(3):709–16.
Manson MM, Hudson EA, Ball HW, et al. Chemoprevention of aflatoxin B1-induced carcinogenesis by indole-3-carbinol in rat liver–predicting the outcome using early biomarkers. Carcinogenesis. 1998;19(10):1829–36.
Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev. 2013;71(6):353–69. https://doi.org/10.1111/nure.12024.
Gabriely G, Wheeler MA, Takenaka MC, Quintana FJ. Role of AHR and HIF-1α in glioblastoma metabolism. Trends Endocrinol Metab. 2017;28(6):428–36.
Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol. 2016;37(7):427–39.
Longhi MS, Vuerich M, Kalbasi A, et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight. 2017. https://doi.org/10.1172/jci.insight.92791.
Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to ah receptor activation. Drug Metab Dispos. 2015;43(10):1522–35. https://doi.org/10.1124/dmd.115.064246.
Ma N, He T, Johnston LJ, Ma X. Host–microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes. 2020;11(5):1203–19.
Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85.
Nicholson JK, Holmes E, Kinross J, et al. Host-Gut Microbiota Metabolic Interactions. Science. 2012;336(6086):1262–7. https://doi.org/10.1126/science.1223813.
Zhang L, Nichols RG, Correll J, et al. Persistent organic pollutants modify gut microbiota–host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ Health Perspect. 2015;123(7):679–88.
Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol Sci. 2018;161(2):310–20.
Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The Aryl Hydrocarbon Receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol. 2020. https://doi.org/10.1007/s12016-020-08789-3.
Singh R, Zogg H, Wei L, et al. Gut microbial dysbiosis in the pathogenesis of gastrointestinal dysmotility and metabolic disorders. J Neurogastroenterol Motility. 2021;27(1):19.
Rannug A. How the AHR became important in intestinal homeostasis—a diurnal FICZ/AHR/CYP1A1 feedback controls both immunity and immunopathology. Int J Mol Sci. 2020;21(16):5681.
Li X, Zhang ZH, Zabed HM, Yun J, Zhang G, Qi X. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol Nutr Food Res. 2021;65(5):2000461.
Pernomian L, Duarte-Silva M, de Barros Cardoso CR. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin Rev Allergy Immunol. 2020;59:382–90.
Wang X-S, Cao F, Zhang Y, Pan H-F. Therapeutic potential of aryl hydrocarbon receptor in autoimmunity. Inflammopharmacology. 2020;28:63–81.
Yi T, Wang J, Zhu K, et al. Aryl hydrocarbon receptor: a new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int. 2018. https://doi.org/10.1155/2018/6058784.
Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45. https://doi.org/10.3389/fcell.2016.00045.
Kawajiri K, Fujii-Kuriyama Y. The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Exp Anim. 2017;66(2):75–89.
Kim JB, Zhao Q, Nguyen T, Pjanic M, Cheng P, Wirka R, Travisano S, Nagao M, Kundu R, Quertermous T. Environment-sensing aryl hydrocarbon receptor inhibits the chondrogenic fate of modulated smooth muscle cells in atherosclerotic lesions. Circulation. 2020;142(6):575–90. https://doi.org/10.1161/CIRCULATIONAHA.120.045981.
Bock KW. Human and rodent aryl hydrocarbon receptor (AHR): from mediator of dioxin toxicity to physiologic AHR functions and therapeutic options. Biol Chem. 2017;398(4):455–64. https://doi.org/10.1515/hsz-2016-0303.
Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45. https://doi.org/10.3389/fcell.2016.00045.
Tian J, Feng Y, Fu H, Xie HQ, Jiang JX, Zhao B. The aryl hydrocarbon receptor: a key bridging molecule of external and internal chemical signals. Environ Technol. 2015;49(16):9518–31. https://doi.org/10.1021/acs.est.5b00385.
Jackson DP, Joshi AD, Elferink CJ. Ah receptor pathway intricacies; signaling through diverse protein partners and DNA-motifs. Toxicol Res. 2015;4(5):1143–58. https://doi.org/10.1039/c4tx00236a.
Larigot L, Juricek L, Dairou J, Coumoul X. AhR signaling pathways and regulatory functions. Biochimie open. 2018;7:1–9.
Bock KW. From TCDD-mediated toxicity to searches of physiologic AHR functions. Biochem Pharmacol. 2018;155:419–24. https://doi.org/10.1016/j.bcp.2018.07.032.
Zhou L. AHR function in lymphocytes: emerging concepts. Trends Immunol. 2016;37(1):17–31. https://doi.org/10.1016/j.it.2015.11.007.
Wang H, Wei Y, Yu D. Control of lymphocyte homeostasis and effector function by the aryl hydrocarbon receptor. Int Immunopharmacol. 2015;28(2):818–24. https://doi.org/10.1016/j.intimp.2015.03.046.
Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–79.
Marcus RS, Holsapple MP, Kaminski NE. Lipopolysaccharide activation of murine splenocytes and splenic B cells increased the expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator. J Pharmacol Exp Ther. 1998;287(3):1113–8.
Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH 17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–9.
Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–30.
Stockinger B, Meglio PD, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32.
Ma Q, Whitlock JP Jr. The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16(5):2144–50.
Mitchell KA, Elferink CJ. Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol. 2009;77(6):947–56.
Hwang W-B, Kim D-J, Oh G-S, Park J-H. Aryl hydrocarbon receptor ligands indoxyl 3-sulfate and indole-3-carbinol inhibit FMS-like tyrosine kinase 3 ligand-induced bone marrow-derived plasmacytoid dendritic cell differentiation. Immune Netw. 2018;18(5):e35. https://doi.org/10.4110/in.2018.18.e35.
Fader KA, Nault R, Raehtz S, McCabe LR, Zacharewski TR. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin dose-dependently increases bone mass and decreases marrow adiposity in juvenile mice. Toxicol Appl Pharmacol. 2018;348:85–98.
Sutter CH, Rainwater HM, Sutter TR. Contributions of nitric oxide to AHR-ligand-mediated keratinocyte differentiation. Int J Mol Sci. 2020;21(16):5680.
Kress S, Reichert J, Schwarz M. Functional analysis of the human cytochrome P4501A1 (CYP1A1) gene enhancer. Eur J Biochem. 1998;258(2):803–12. https://doi.org/10.1046/j.1432-1327.1998.2580803.x.
Ueda R, Iketaki H, Nagata K, et al. A common regulatory region functions bidirectionally in transcriptional activation of the human CYP1A1 and CYP1A2 genes. Mol Pharmacol. 2006;69(6):1924–30. https://doi.org/10.1124/mol.105.021220.
Jorge-Nebert LF, Jiang Z, Chakraborty R, et al. Analysis of human CYP1A1 and CYP1A2 genes and their shared bidirectional promoter in eight world populations. Hum Mutat. 2010;31(1):27–40. https://doi.org/10.1002/humu.21132.
Fernandez-Salguero P, Pineau T, Hilbert DM, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268(5211):722–6.
Lawrence BP, Vorderstrasse BA. New insights into the aryl hydrocarbon receptor as a modulator of host responses to infection. Semin Immunopathol. 2013;35(6):615–26. https://doi.org/10.1007/s00281-013-0395-3.
Paris A, Tardif N, Galibert M-D, Corre S. AhR and cancer: from gene profiling to targeted therapy. Int J Mol Sci. 2021;22(2):752.
Wang Z, Monti S, Sherr DH. The diverse and important contributions of the AHR to cancer and cancer immunity. Curr Opin Toxicol. 2017;2:93–102.
Ternes D, Tsenkova M, Pozdeev VI, et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab. 2022;4(4):458–75.
Wang Z, Snyder M, Kenison JE, et al. How the AHR became important in cancer: the role of chronically active AHR in cancer aggression. Int J Mol Sci. 2020;22(1):387.
Garcia-Villatoro EL, DeLuca JAA, Callaway ES, et al. Effects of high-fat diet and intestinal aryl hydrocarbon receptor deletion on colon carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G451–63. https://doi.org/10.1152/ajpgi.00268.2019.
Díaz-Díaz CJ, Ronnekleiv-Kelly SM, Nukaya M, et al. The aryl hydrocarbon receptor is a repressor of inflammation-associated colorectal tumorigenesis in mouse. Ann Surg. 2016;264(3):429–36.
Chen W, Wen L, Bao Y, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci. 2022;119(52):e2203894119.
Yamaguchi M, Hankinson O. 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin suppresses the growth of human colorectal cancer cells in vitro: Implication of the aryl hydrocarbon receptor signaling. Int J Oncol. 2019;54(4):1422–32.
Garcia-Villatoro EL, DeLuca JA, Callaway ES, et al. Effects of high-fat diet and intestinal aryl hydrocarbon receptor deletion on colon carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G451–63.
Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact. 2002;141(1–2):3–24.
Baker JR, Sakoff JA, McCluskey A. The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med Res Rev. 2020;40(3):972–1001. https://doi.org/10.1002/med.21645.
Boule LA, Burke CG, Jin GB, Lawrence BP. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci Rep. 2018;8(1):1826. https://doi.org/10.1038/s41598-018-20197-4.
Ferdinand R, Yang RJ, Takahashi PY, Dana E, Bowers CY, Veldhuis JD. Effects of toremifene, a selective estrogen receptor modulator, on spontaneous and stimulated GH secretion, IGF-I, and IGF-binding proteins in healthy elderly subjects. J Endocr Soc. 2018;2:154–65.
Furue M, Hashimoto-Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20215424.
Pohjanvirta R. The AH receptor in biology and toxicology. Hoboken: John Wiley & Sons; 2011.
Bazzi R, Bradshaw TD, Rowlands JC, Stevens MF, Bell DR. 2-(4-Amino-3-methylphenyl)-5-fluorobenzothiazole is a ligand and shows species-specific partial agonism of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol. 2009;237(1):102–10.
Choi YJ, Seelbach MJ, Pu H, et al. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environ Health Perspect. 2010;118(7):976–81.
Phillips MC, Dheer R, Santaolalla R, et al. Intestinal exposure to PCB 153 induces inflammation via the ATM/NEMO pathway. Toxicol Appl Pharmacol. 2018;339:24–33.
Wei Y, Zhao L, He W, et al. Benzo [a] pyrene promotes gastric cancer cell proliferation and metastasis likely through the Aryl hydrocarbon receptor and ERK-dependent induction of MMP9 and c-myc. Int J Oncol. 2016;49(5):2055–63.
Abbas S, Alam S, Singh KP, Kumar M, Gupta SK, Ansari KM. Aryl hydrocarbon receptor activation contributes to benzanthrone-induced hyperpigmentation via modulation of melanogenic signaling pathways. Chem Res Toxicol. 2017;30(2):625–34.
Baba N, Rubio M, Kenins L, et al. The aryl hydrocarbon receptor (AhR) ligand VAF347 selectively acts on monocytes and naïve CD4+ Th cells to promote the development of IL-22-secreting Th cells. Hum Immunol. 2012;73(8):795–800. https://doi.org/10.1016/j.humimm.2012.05.002.
Lawrence BP, Denison MS, Novak H, et al. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112(4):1158–65.
Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111(16):1877–82.
Bjeldanes LF, Kim J-Y, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci. 1991;88(21):9543–7.
Perez-Vizcaino F, Fraga CG. Research trends in flavonoids and health. Arch Biochem Biophys. 2018;646:107–12. https://doi.org/10.1016/j.abb.2018.03.022.
Rannug A, Rannug U. The tryptophan derivative 6-formylindolo [3, 2-b] carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit Rev Toxicol. 2018;48(7):555–74.
Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340:715–22.
Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem Res Toxicol. 2012;25(9):1878–84.
Xue Y, Shui X, Su W, et al. Baicalin inhibits inflammation and attenuates myocardial ischaemic injury by aryl hydrocarbon receptor. J Pharm Pharmacol. 2015;67(12):1756–64.
Dvorak Z, Vrzal R, Henklova P, et al. JNK inhibitor SP600125 is a partial agonist of human aryl hydrocarbon receptor and induces CYP1A1 and CYP1A2 genes in primary human hepatocytes. Biochem Pharmacol. 2008;75(2):580–8.
Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev. 2018;31(1):85–97. https://doi.org/10.1017/s095442241700021x.
Xun W, Fu Q, Shi L, Cao T, Jiang H, Ma Z. Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. Int Immunopharmacol. 2021;99:107989.
Stejskalova L, Dvorak Z, Pavek P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab. 2011;12(2):198–212.
Fukuda I, Sakane I, Yabushita Y, et al. Pigments in green tea leaves (Camellia sinensis) suppress transformation of the aryl hydrocarbon receptor induced by dioxin. J Agric Food Chem. 2004;52(9):2499–506.
Ashida H, Nishiumi S, Fukuda I. An update on the dietary ligands of the AhR. Expert Opin Drug Metab Toxicol. 2008;4(11):1429–47.
Nishiumi S, Yoshida K-i, Ashida H. Curcumin suppresses the transformation of an aryl hydrocarbon receptor through its phosphorylation. Arch Biochem Biophys. 2007;466(2):267–73.
Xue Z, Li D, Yu W, et al. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017;8(4):1414–37.
Choi H, Chun YS, Shin YJ, Ye SK, Kim MS, Park JW. Curcumin attenuates cytochrome P450 induction in response to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008;99(12):2518–24.
Vrzal R, Zdařilová A, Ulrichová J, Bláha L, Giesy JP, Dvořák Z. Activation of the aryl hydrocarbon receptor by berberine in HepG2 and H4IIE cells: Biphasic effect on CYP1A1. Biochem Pharmacol. 2005;70(6):925–36.
Gao J, Xu K, Liu H, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol. 2018;8:13. https://doi.org/10.3389/fcimb.2018.00013.
Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med. 2017;95(9):915–25.
Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52(4):590–9.
Schaldach C, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38(23):7594–600.
Bian Y, Li Y, Shrestha G, et al. ITE, an endogenous aryl hydrocarbon receptor ligand, suppresses endometrial cancer cell proliferation and migration. Toxicology. 2019;421:1–8.
Keshavarzi M, Khoshnoud MJ, Ghaffarian Bahraman A, Mohammadi-Bardbori A. An endogenous ligand of aryl hydrocarbon receptor 6-formylindolo [3, 2-b] carbazole (FICZ) is a signaling molecule in neurogenesis of adult hippocampal neurons. J Mol Neurosci. 2020;70:806–17.
Maitre M, Klein C, Patte-Mensah C, Mensah-Nyagan A-G. Tryptophan metabolites modify brain Aβ peptide degradation: a role in Alzheimer’s disease? Prog Neurobiol. 2020;190:101800.
Abron JD, Singh NP, Mishra MK, et al. An endogenous aryl hydrocarbon receptor ligand, ITE, induces regulatory T cells and ameliorates experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2018;315(2):G220–30. https://doi.org/10.1152/ajpgi.00413.2017.
Dolciami D, Gargaro M, Cerra B, et al. Binding mode and structure-activity relationships of ITE as an Aryl Hydrocarbon Receptor (AhR) agonist. ChemMedChem. 2018;13(3):270–9. https://doi.org/10.1002/cmdc.201700669.
Yan J, Tung HC, Li S, et al. Aryl hydrocarbon receptor signaling prevents activation of hepatic stellate cells and liver fibrogenesis in mice. Gastroenterology. 2019;157(3):793–806. https://doi.org/10.1053/j.gastro.2019.05.066.
Kiyomatsu-Oda M, Uchi H, Morino-Koga S, Furue M. Protective role of 6-formylindolo [3, 2-b] carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J Dermatol Sci. 2018;90(3):284–94.
Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. Metabolic fate of the Ah receptor ligand 6-formylindolo [3, 2-b] carbazole. Chem Biol Interact. 2004;149(2–3):151–64.
Nebert DW. MINIREVIEW: proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 1991;5(9):1203–14.
Romani L, Zelante T, Luca AD, et al. Microbiota control of a tryptophan–AhR pathway in disease tolerance to fungi. Eur J Immunol. 2014;44(11):3192–200.
Alexeev EE, Lanis JM, Kao DJ, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188(5):1183–94.
Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science. 2017;357(6353):806–10. https://doi.org/10.1126/science.aah5825.
Cheng Y, Jin U-H, Allred CD, Jayaraman A, Chapkin RS, Safe S. Aryl hydrocarbon receptor activity of tryptophan metabolites in young adult mouse colonocytes. Drug Metab Dispos. 2015;43(10):1536–43.
Hubbard TD, Murray IA, Bisson WH, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5(1):1–13.
Cervantes-Barragan L, Chai JN, Tianero MD, et al. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science. 2017;357(6353):806–10.
Islam J, Sato S, Watanabe K, et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J Nutr Biochem. 2017;42:43–50.
Nishiumi S, Yamamoto N, Kodoi R, Fukuda I, Yoshida K-i, Ashida H. Antagonistic and agonistic effects of indigoids on the transformation of an aryl hydrocarbon receptor. Arch Biochem Biophys. 2008;470(2):187–99.
Guengerich FP, Martin MV, McCormick WA, Nguyen LP, Glover E, Bradfield CA. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch Biochem Biophys. 2004;423(2):309–16.
Adachi J, Mori Y, Matsui S, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276(34):31475–8.
Rosser EC, Piper CJM, Matei DE, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31(4):837–51. https://doi.org/10.1016/j.cmet.2020.03.003.
Di Stadio A, Costantini C, Renga G, Pariano M, Ricci G, Romani L. The microbiota/host immune system interaction in the nose to protect from COVID-19. Life (Basel, Switzerland). 2020. https://doi.org/10.3390/life10120345.
Ren F, Huang Y, Tao Y, et al. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol Appl Pharmacol. 2020;398:115029. https://doi.org/10.1016/j.taap.2020.115029.
Martey CA, Pollock SJ, Turner CK, et al. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L981–91. https://doi.org/10.1152/ajplung.00239.2003.
Tang J, Hu B, Zheng H, et al. 2, 2′, 4, 4′-Tetrabromodiphenyl ether (BDE-47) activates Aryl hydrocarbon receptor (AhR) mediated ROS and NLRP3 inflammasome/p38 MAPK pathway inducing necrosis in cochlear hair cells. Ecotoxicol Environ Saf. 2021;221:112423.
Brinchmann BC, Skuland T, Rambøl MH, et al. Lipophilic components of diesel exhaust particles induce pro-inflammatory responses in human endothelial cells through AhR dependent pathway (s). Part Fibre Toxicol. 2018;15(1):1–17.
Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem Pharmacol. 2013;86(5):561–70.
Hahn ME, Poland A, Glover E, Stegeman JJ. Photoaffinity labeling of the Ah receptor: phylogenetic survey of diverse vertebrate and invertebrate species. Arch Biochem Biophys. 1994;310(1):218–28.
Veiga-Parga T, Suryawanshi A, Rouse BT. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 2011;7(12):e1002427.
Hanieh H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends. BioMed Res Int. 2014;2014:520763. https://doi.org/10.1155/2014/520763.
Kerkvliet NI, Shepherd DM, Baecher-Steppan L. T lymphocytes are direct, aryl hydrocarbon receptor (AhR)-dependent targets of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD): AhR expression in both CD4+ and CD8+ T cells is necessary for full suppression of a cytotoxic T lymphocyte response by TCDD. Toxicol Appl Pharmacol. 2002;185(2):146–52.
Julliard W, Owens L, O’Driscoll C, Fechner J, Mezrich J. Environmental exposures—the missing link in immune responses after transplantation. Am J Transplant. 2016;16(5):1358–64.
Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14(12):801–14.
Trombino AF, Near RI, Matulka RA, et al. Expression of the aryl hydrocarbon receptor/transcription factor (AhR) and AhR-regulated CYP1. Breast Cancer Res Treat. 2000;63:117–31.
Pohjanvirta R. Short-term toxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549.
Angata T. Possible influences of endogenous and exogenous ligands on the evolution of human siglecs. Front Immunol. 2018;9:2885. https://doi.org/10.3389/fimmu.2018.02885.
Walczak K, Wnorowski A, Turski WA, Plech T. Kynurenic acid and cancer: facts and controversies. Cell Mol Life Sci. 2020;77(8):1531–50. https://doi.org/10.1007/s00018-019-03332-w.
Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51(9):853–61. https://doi.org/10.1007/s00535-016-1220-2.
Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013;65(4):1148–61.
Brawner KM, Yeramilli VA, Duck LW, et al. Depletion of dietary aryl hydrocarbon receptor ligands alters microbiota composition and function. Sci Rep. 2019;9(1):14724. https://doi.org/10.1038/s41598-019-51194-w.
Geusau A, Tschachler E, Meixner M, et al. Olestra increases faecal excretion of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Lancet. 1999;354(9186):1266–7.
Shen H, Robertson LW, Ludewig G. Regulation of paraoxonase 1 (PON1) in PCB 126-exposed male Sprague Dawley rats. Toxicol Lett. 2012;209(3):291–8.
Bradshaw TD, Bell DR. Relevance of the aryl hydrocarbon receptor (AhR) for clinical toxicology. Clin Toxicol. 2009;47(7):632–42.
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–47.
Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B. 2007;853(1–2):183–9.
Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82.
Reed GA, Sunega JM, Sullivan DK, et al. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3, 3′-diindolylmethane in healthy subjects. Cancer Epidemiol Prev Biomarkers. 2008;17(10):2619–24.
Tan Y-Q, Wang Y-N, Feng H-Y, et al. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic Biol Med. 2022;184:30–41. https://doi.org/10.1016/j.freeradbiomed.2022.03.025.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24. https://doi.org/10.1016/j.chom.2018.05.003.
Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discovery. 2013;12(1):64–82.
Ehrlich AK, Kerkvliet NI. Is chronic AhR activation by rapidly metabolized ligands safe for the treatment of immune-mediated diseases? Curr Opin Toxicol. 2017;2:72–8.
Henry E, Bemis J, Henry O, Kende A, Gasiewicz T. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450(1):67–77.
Wincent E, Kubota A, Timme-Laragy A, Jönsson ME, Hahn ME, Stegeman JJ. Biological effects of 6-formylindolo [3, 2-b] carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem Pharmacol. 2016;110:117–29.
Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14(12):801–14. https://doi.org/10.1038/nrc3846.
Ikuta T, Tachibana T, Watanabe J, Yoshida M, Yoneda Y, Kawajiri K. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J Biochem. 2000;127(3):503–9.
Ciolino HP, MacDonald CJ, Memon OS, Bass SE, Yeh GC. Sulindac regulates the aryl hydrocarbon receptor-mediated expression of Phase 1 metabolic enzymes in vivo and in vitro. Carcinogenesis. 2006;27(8):1586–92.
Backlund M, Ingelman-Sundberg M. Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high-and low-affinity ligand binding and receptor activation. Mol Pharmacol. 2004;65(2):416–25.
Lemaire G, Delescluse C, Pralavorio M, Ledirac N, Lesca P, Rahmani R. The role of protein tyrosine kinases in CYP1A1 induction by omeprazole and thiabendazole in rat hepatocytes. Life Sci. 2004;74(18):2265–78.
Maayah ZH, El Gendy MA, El-Kadi AO, Korashy HM. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome P450 1A1 gene in human breast cancer MCF7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch Toxicol. 2013;87(5):847–56.
MacPherson L, Ahmed S, Tamblyn L, et al. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. Int J Mol Sci. 2014;15(5):7939–57.
Xiao W, Son J, Vorrink SU, Domann FE, Goswami PC. Ligand-independent activation of aryl hydrocarbon receptor signaling in PCB3-quinone treated HaCaT human keratinocytes. Toxicol Lett. 2015;233(3):258–66.
Piper CJ, Rosser EC, Oleinika K, et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. 2019;29(7):1878–92.
Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–48.
Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104.
Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605.
Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242–5.
Moura-Alves P, Puyskens A, Stinn A, et al. Host monitoring of quorum sensing during Pseudomonas aeruginosa infection. Science. 2019;366(6472):1629.
Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32(6):256–64.
Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11(4):1024–38. https://doi.org/10.1038/s41385-018-0019-2.
Gasaly N, De Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. 2021;12:658354.
Stockinger B, Shah K, Wincent E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat Rev Gastroenterol Hepatol. 2021;18(8):559–70.
Prokipcak RD, Okey AB. Downregulation of the Ah receptor in mouse hepatoma cells treated in culture with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Can J Physiol Pharmacol. 1991;69(8):1204–10.
Reick M, Robertson RW, Pasco DS, Fagan JB. Down-regulation of nuclear aryl hydrocarbon receptor DNA-binding and transactivation functions: requirement for a labile or inducible factor. Mol Cell Biol. 1994;14(9):5653–60.
Pollenz RS. The aryl-hydrocarbon receptor, but not the aryl-hydrocarbon receptor nuclear translocator protein, is rapidly depleted in hepatic and nonhepatic culture cells exposed to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1996;49(3):391–8.
Leclerc D, Pires ACS, Guillemin GJ, Gilot D. Detrimental activation of AhR pathway in cancer: an overview of therapeutic strategies. Curr Opin Immunol. 2021;70:15–26.
Dantsuka A, Ichii O, Hanberg A, et al. Histopathological features of the proper gastric glands in FVB/N-background mice carrying constitutively-active aryl-hydrocarbon receptor. BMC Gastroenterol. 2019;19(1):102–102. https://doi.org/10.1186/s12876-019-1009-x.
Takamura T, Harama D, Matsuoka S, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88(6):685–9. https://doi.org/10.1038/icb.2010.35.
Exon J, South E, Magnuson B, Hendrix K. Effects of indole-3-carbinol on immune responses, aberrant crypt foci, and colonic crypt cell proliferation in rats. J Toxicol Environ Health A. 2001;62(7):561–73.
Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40. https://doi.org/10.1016/j.cell.2011.09.025.
Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334(6062):1561–5.
Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch [Article]. Nat Immunol. 2011;13:144. https://doi.org/10.1038/ni.2187.
Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242–5. https://doi.org/10.1038/nature21080.
Ji T, Xu C, Sun L, et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci. 2015;60(7):1958–66. https://doi.org/10.1007/s10620-015-3632-x.
Chinen I, Nakahama T, Kimura A, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015;27(8):405–15.
Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. https://doi.org/10.1038/nm.4102.
Wang Q, Yang K, Han B, et al. Aryl hydrocarbon receptor inhibits inflammation in DSS-induced colitis via the MK2/p-MK2/TTP pathway. Int J Mol Med. 2018;41(2):868–76. https://doi.org/10.3892/ijmm.2017.3262.
Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2011;13(2):144–51. https://doi.org/10.1038/ni.2187.
Collins JW, Keeney KM, Crepin VF, et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol. 2014;12(9):612–23. https://doi.org/10.1038/nrmicro3315.
Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity. 2015;42(4):731–43. https://doi.org/10.1016/j.immuni.2015.03.012.
Han B, Sheng B, Zhang Z, et al. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK-MLC phosphorylation pathway. Shock (Augusta, Ga). 2016;46(3):319–28.
Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci. 2010;107(1):228–33.
Akedo I, Ishikawa H, Ioka T, et al. Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Prev Biomarkers. 2001;10(9):925–30.
Metidji A, Omenetti S, Crotta S, et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2019;50(6):1542. https://doi.org/10.1016/j.immuni.2019.05.024.
Yin J, Yang K, Zhou C, Xu P, Xiao W, Yang H. Aryl hydrocarbon receptor activation alleviates dextran sodium sulfate-induced colitis through enhancing the differentiation of goblet cells. Biochem Biophys Res Commun. 2019;514(1):180–6. https://doi.org/10.1016/j.bbrc.2019.04.136.
Han B, Sheng B, Zhang Z, et al. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK-MLC phosphorylation pathway. Shock (Augusta, Ga). 2016;46(3):319–28. https://doi.org/10.1097/shk.0000000000000594.
Metidji A, Omenetti S, Crotta S, et al. The environmental sensor ahr protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity. 2018;49(2):353–62. https://doi.org/10.1016/j.immuni.2018.07.010.
Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32. https://doi.org/10.1146/annurev-immunol-032713-120245.
Chinen I, Nakahama T, Kimura A, et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int Immunol. 2015;27(8):405–15. https://doi.org/10.1093/intimm/dxv015.
Zhu J, Luo L, Tian L, et al. Aryl hydrocarbon receptor promotes IL-10 expression in inflammatory macrophages through Src-STAT3 signaling pathway. Front Immunol. 2018;9:2033.
Goudot C, Coillard A, Villani A-C, et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity. 2017;47(3):582–96.
Chng SH, Kundu P, Dominguez-Brauer C, et al. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci Rep. 2016;6(1):1–14.
Piper CJM, Rosser EC, Oleinika K, et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. 2019;29(7):1878–92. https://doi.org/10.1016/j.celrep.2019.10.018.
Bock KW. Human AHR functions in vascular tissue: Pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem Pharmacol. 2019;159:116–20. https://doi.org/10.1016/j.bcp.2018.11.021.
Nohara K, Pan X, Tsukumo S-i, et al. Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization-induced increase in splenocytes. J Immunol. 2005;174(5):2770–7.
von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161(10):5673–80.
Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–48. https://doi.org/10.1053/j.gastro.2011.04.007.
Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–40.
Hanieh H. Toward understanding the role of aryl hydrocarbon receptor in the immune system: current progress and future trends. BioMed Res Int. 2014. https://doi.org/10.1155/2014/520763.
Mjösberg J, Bernink J, Peters C, Spits H. Transcriptional control of innate lymphoid cells. Eur J Immunol. 2012;42(8):1916–23.
Kiss EA, Diefenbach A. Role of the aryl hydrocarbon receptor in controlling maintenance and functional programs of RORγt+ innate lymphoid cells and intraepithelial lymphocytes. Front Immunol. 2012;3:124.
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53(4):465–74. https://doi.org/10.1007/s00535-017-1401-7.
Rannug A. How the AHR became important in intestinal homeostasis-a diurnal FICZ/AHR/CYP1A1 feedback controls both immunity and immunopathology. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21165681.
Parks OB, Pociask DA, Hodzic Z, Kolls JK, Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front Cell Dev Biol. 2015;3:85. https://doi.org/10.3389/fcell.2015.00085.
Yeste A, Mascanfroni ID, Nadeau M, et al. IL-21 induces IL-22 production in CD4+ T cells. Nat Commun. 2014;5:3753. https://doi.org/10.1038/ncomms4753.
Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Investig. 2008;118(2):534–44.
Liu Z, Fan H, Jiang S. CD4+ T-cell subsets in transplantation. Immunol Rev. 2013;252(1):183–91.
Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206(1):43–9.
Quintana FJ, Basso AS, Iglesias AH, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71.
Singh NP, Singh UP, Rouse M, et al. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of microRNA. J Immunol. 2016;196(3):1108–22.
Mascanfroni ID, Takenaka MC, Yeste A, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21(6):638–46.
Gagliani N, Vesely MCA, Iseppon A, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–5.
Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE. 2011;6(8):e23522.
Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Investig. 2014;124(11):4678–80.
Liu C, Li Y, Chen Y, et al. Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediators Inflamm. 2020. https://doi.org/10.1155/2020/5918587.
Goettel J, Gandhi R, Kenison J, et al. AHR activation is protective against colitis driven by T cells in humanized mice. Cell Rep. 2016;17(5):1318–29.
Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Investig. 1995;95(6):2945–53.
Hara T, Shitara S, Imai K, et al. Identification of IL-7–producing cells in primary and secondary lymphoid organs using IL-7–GFP knock-in mice. J Immunol. 2012;189(4):1577–84.
Shalapour S, Deiser K, Kühl AA, et al. Interleukin-7 links T lymphocyte and intestinal epithelial cell homeostasis. PLoS ONE. 2012;7(2):e31939.
Yang H, Madison B, Gumucio DL, Teitelbaum DH. Specific overexpression of IL-7 in the intestinal mucosa: the role in intestinal intraepithelial lymphocyte development. Am J Physiol Gastrointest Liver Physiol. 2008;294(6):G1421–30.
Singh ND, Paul AK, Paul RK. Selecting appropriate nonlinear growth models using Bootstrap technique. Indian J Animal Sci. 2015;85(8):920–2.
Wagage S, John B, Krock BL, et al. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol. 2014;192(4):1661–70.
Brockmann L, Soukou S, Steglich B, et al. Molecular and functional heterogeneity of IL-10-producing CD4(+) T cells. Nat Commun. 2018;9(1):5457. https://doi.org/10.1038/s41467-018-07581-4.
Rojas OL, Pröbstel AK, Porfilio EA, et al. Recirculating intestinal IgA-producing cells regulate neuroinflammation via IL-10. Cell. 2019;176(3):610–24. https://doi.org/10.1016/j.cell.2018.11.035.
Zhu J, Luo L, Tian L, et al. Aryl hydrocarbon receptor promotes IL-10 expression in inflammatory macrophages through Src-STAT3 signaling pathway [original research]. Front Immunol. 2018. https://doi.org/10.3389/fimmu.2018.02033.
Lanis JM, Alexeev EE, Curtis VF, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10(5):1133–44. https://doi.org/10.1038/mi.2016.133.
Lanis JM, Alexeev EE, Curtis VF, et al. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10(5):1133–44.
Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci. 2008;105(28):9721–6.
Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48(1):19–33.
Li S, Bostick JW, Ye J, et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity. 2018;49(5):915–28.
Han H, Safe S, Jayaraman A, Chapkin RS. Diet–Host–microbiota interactions shape aryl hydrocarbon receptor ligand production to modulate intestinal homeostasis. Annu Rev Nutr. 2021;41:455–78.
Mar JS, Ota N, Pokorzynski ND, et al. IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome. 2023;11(1):47. https://doi.org/10.1186/s40168-023-01486-1.
Lee J-H, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34(4):426–44.
Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23(6):705–15. https://doi.org/10.1016/j.chom.2018.05.012.
Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50.
Kaisar MM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, Everts B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein-coupled receptor 109A signaling. Front Immunol. 2017;8:1429.
Ribière C, Peyret P, Parisot N, et al. Oral exposure to environmental pollutant benzo [a] pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci Rep. 2016;6(1):1–11.
Petriello MC, Brandon JA, Hoffman J, et al. Dioxin-like PCB 126 increases systemic inflammation and accelerates atherosclerosis in lean LDL receptor-deficient mice. Toxicol Sci. 2018;162(2):548–58.
Murray IA, Perdew GH. Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis. Curr Opin Toxicol. 2017;2:15–23.
Vogel CF, Haarmann-Stemmann T. The aryl hydrocarbon receptor repressor–more than a simple feedback inhibitor of AhR signaling: clues for its role in inflammation and cancer. Curr Opin Toxicol. 2017;2:109–19.
Minton K. Negative regulation of AHR essential for intestinal homeostasis. Nat Rev Immunol. 2023;23(4):202. https://doi.org/10.1038/s41577-023-00852-2.
Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 2011;120(1):68–78.
Qi L, Kai W, Si-Miao Q, Yue D, Zhi-Feng W. Norisoboldine, a natural aryl hydrocarbon receptor agonist, alleviates TNBS-induced colitis in mice, by inhibiting the activation of NLRP3 inflammasome. Chin J Nat Med. 2018;16(3):161–74.
Liu X, Li X, Tao Y, et al. TCDD inhibited the osteogenic differentiation of human fetal palatal mesenchymal cells through AhR and BMP-2/TGF-β/Smad signaling. Toxicology. 2020;431:152353. https://doi.org/10.1016/j.tox.2019.152353.
Murai M, Yamamura K, Hashimoto-Hachiya A, Tsuji G, Furue M, Mitoma C. Tryptophan photo-product FICZ upregulates AHR/MEK/ERK-mediated MMP1 expression: implications in anti-fibrotic phototherapy. J Dermatol Sci. 2018;91(1):97–103. https://doi.org/10.1016/j.jdermsci.2018.04.010.
Lv Q, Wang K, Qiao S, et al. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 2018;9(3):258. https://doi.org/10.1038/s41419-018-0297-3.
Hubbard TD, Murray IA, Nichols RG, et al. Dietary broccoli impacts microbial community structure and attenuates chemically induced colitis in mice in an Ah receptor dependent manner. J Funct Foods. 2017;37:685–98.
Xiao H-T, Peng J, Hu D-D, et al. Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice. Chin Med. 2015;10(1):1–13.
Furumatsu K, Nishiumi S, Kawano Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56(9):2532–44.
Lippmann D, Lehmann C, Florian S, et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014;5(6):1073–81.
Xu X, Dong Q, Zhong Q, et al. The flavonoid kurarinone regulates macrophage functions via aryl hydrocarbon receptor and alleviates intestinal inflammation in irritable bowel syndrome. J Inflamm Res. 2021;14:4347.
Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65(11):1906–15.
Silva MJB, Carneiro MBH, dos Anjos Pultz B, Pereira Silva D, de Lopes MEM, dos Santos LM. The multifaceted role of commensal microbiota in homeostasis and gastrointestinal diseases. J Immunol Res. 2015. https://doi.org/10.1155/2015/321241.
Takamura T, Harama D, Fukumoto S, et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89(7):817–22.
Fukumoto S, Toshimitsu T, Matsuoka S, et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol Cell Biol. 2014;92(5):460–5.
Popolo A, Pinto A, Daglia M, Nabavi SF, Farooqi AA, Rastrelli L. Two likely targets for the anti-cancer effect of indole derivatives from cruciferous vegetables: PI3K/Akt/mTOR signalling pathway and the aryl hydrocarbon receptor. Semin Cancer Biol. 2017;46:132–7. https://doi.org/10.1016/j.semcancer.2017.06.002.
Busbee PB, Menzel L, Alrafas HR, et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22–dependent manner. JCI Insight. 2020;5(1):e127551. https://doi.org/10.1172/jci.insight.127551.
Paturi G, Mandimika T, Butts CA, et al. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a−/− mice, a model of inflammatory bowel diseases. Nutrition. 2012;28(3):324–30.
Liang H, Dai Z, Kou J, et al. Dietary l-tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: implication of tryptophan-metabolizing microbiota. Int J Mol Sci. 2018;20(1):20.
Dodd D, Spitzer MH, Van Treuren W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–52.
Williams BB, Van Benschoten AH, Cimermancic P, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503.
Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci. 2009;106(10):3698–703.
Whitehead TR, Price NP, Drake HL, Cotta MA. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl Environ Microbiol. 2008;74(6):1950–3.
Russell WR, Duncan SH, Scobbie L, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57(3):523–35.
Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81(3):288–302.
Smith T. A modification of the method for determining the production of indol by bacteria. J Exp Med. 1897;2(5):543.
Kurata K, Kawahara H, Nishimura K, Jisaka M, Yokota K, Shimizu H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem Biophys Res Commun. 2019;510(4):649–55. https://doi.org/10.1016/j.bbrc.2019.01.122.
Wlodarska M, Luo C, Kolde R, et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22(1):25–37. https://doi.org/10.1016/j.chom.2017.06.007.
Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. https://doi.org/10.1038/s41467-018-05470-4.
Ehrlich AM, Pacheco AR, Henrick BM, et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. 2020;20(1):357. https://doi.org/10.1186/s12866-020-02023-y.
Jennis M, Cavanaugh C, Leo G, Mabus J, Lenhard J, Hornby P. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil. 2018;30(2):e13178.
Alexeev EE, Lanis JM, Kao DJ, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188(5):1183–94. https://doi.org/10.1016/j.ajpath.2018.01.011.
Whitfield-Cargile CM, Cohen ND, Chapkin RS, et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut microbes. 2016;7(3):246–61.
Marinelli L, Martin-Gallausiaux C, Bourhis JM, Béguet-Crespel F, Blottière HM, Lapaque N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep. 2019;9(1):643. https://doi.org/10.1038/s41598-018-37019-2.
Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–7. https://doi.org/10.1053/j.gastro.2013.07.050.
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73.
Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5.
Korecka A, Dona A, Lahiri S, et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016;2(1):1–10.
Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci. 2014;111(6):2247–52.
Reigstad CS, Salmonson CE, Rainey JF, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–403.
Manzella C, Singhal M, Alrefai WA, Saksena S, Dudeja PK, Gill RK. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci Rep. 2018;8(1):6103. https://doi.org/10.1038/s41598-018-24213-5.
Pagliai G, Russo E, Niccolai E, et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59(5):2011–24.
Santoro A, Ostan R, Candela M, et al. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci. 2018;75(1):129–48.
Du H, van A DL, Boshuizen HC, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91(2):329–36.
Yang W, Yu T, Huang X, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):1–18.
Mohammadi S, Seyedhoseini FS, Asadi J, Yazdani Y. Effects of berberine on the secretion of cytokines and expression of genes involved in cell cycle regulation in THP-1 monocytic cell line. Iran J Basic Med Sci. 2017;20(5):530.
Kosalec I, Gregurek B, Kremer D, Zovko M, Sanković K, Karlović K. Croatian barberry (Berberis croatica Horvat): a new source of berberine—analysis and antimicrobial activity. World J Microbiol Biotechnol. 2009;25(1):145–50. https://doi.org/10.1007/s11274-008-9860-x.
Jing W, Dong S, Luo X, et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol Res. 2020;164:105358. https://doi.org/10.1016/j.phrs.2020.105358.
Habtemariam S. Berberine and inflammatory bowel disease: a concise review. Pharmacol Res. 2016;113:592–9.
Li H, Fan C, Lu H, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells–intestinal epithelial cells–immune cells interactions. Acta Pharmaceutica Sinica B. 2020;10(3):447–61.
Takahara M, Takaki A, Hiraoka S, et al. Berberine improved experimental chronic colitis by regulating interferon-γ-and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci Rep. 2019;9(1):1–13.
Li Y-h, Xiao H-t, Hu D-d, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses. Pharmacol Res. 2016;110:227–39.
Hou Q, Zhu S, Zhang C, et al. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed Pharmacother. 2019;118:109206.
Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the’French paradox. Eur J Endocrinol. 1998;138(6):619–20.
Tocmo R, Le B, Heun A, van Pijkeren JP, Parkin K, Johnson JJ. Prenylated xanthones from mangosteen (Garcinia mangostana) activate the AhR and Nrf2 pathways and protect intestinal barrier integrity in HT-29 cells. Free Radic Biol Med. 2020;163:102–15. https://doi.org/10.1016/j.freeradbiomed.2020.11.018.
Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–53.
Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751.
Li Y-y, Wang X-j, Su Y-l, et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s. Acta Pharmacologica Sinica. 2022;43(6):1495–1507. https://doi.org/10.1038/s41401-021-00781-7.
Lv Q, Shi C, Qiao S, et al. Alpinetin exerts anti-colitis efficacy by activating AhR, regulating miR-302/DNMT-1/CREB signals, and therefore promoting Treg differentiation. Cell Death Dis. 2018;9(9):1–25.
Hu K, Yang Y, Tu Q, Luo Y, Ma R. Alpinetin inhibits LPS-induced inflammatory mediator response by activating PPAR-γ in THP-1-derived macrophages. Eur J Pharmacol. 2013;721(1–3):96–102.
Guan S, Fang B, Song B, Xiong Y, Lu J. Immunosuppressive activity of alpinetin on activation and cytokines secretion of murine T lymphocytes. Immunopharmacol Immunotoxicol. 2014;36(4):290–6.
Riemschneider S, Hoffmann M, Slanina U, Weber K, Hauschildt S, Lehmann J. Indol-3-carbinol and quercetin ameliorate chronic DSS-induced colitis in C57BL/6 mice by AhR-mediated anti-inflammatory mechanisms. Int J Environ Res Public Health. 2021;18(5):2262.
Plitzko I, Mohn T, Sedlacek N, Hamburger M. Composition of Indigo naturalis. Planta Med. 2009;75(08):860–3.
Deng S, May B, Zhang A, Lu C, Xue C. Plant extracts for the topical management of psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169(4):769–82.
Sugihara K, Okayama T, Kitamura S, et al. Comparative study of aryl hydrocarbon receptor ligand activities of six chemicals in vitro and in vivo. Arch Toxicol. 2008;82(1):5–11.
himizu T, Takagi C, Sawano T, et al. Indigo enhances wound healing activity of Caco-2 cells via activation of the aryl hydrocarbon receptor. J Nat Med. 2021;75(4):833–39. https://doi.org/10.1007/s11418-021-01524-y.
Kawai S, Iijima H, Shinzaki S, et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017;52(8):904–19. https://doi.org/10.1007/s00535-016-1292-z.
Yang M, Yan T, Yu M, et al. Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Front. 2020;1(4):398–419.
Xie F, Xiong Q, Li Y, et al. Traditional chinese medicine regulates Th17/treg balance in treating inflammatory bowel disease. Evid Based Complement Alternat Med. 2022. https://doi.org/10.1155/2022/6275136.
Diling C, Xin Y, Chaoqun Z, et al. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget. 2017;8(49):85838.
Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009–2012). Expert Opin Ther Pat. 2013;23(2):215–31.
Chang Y, Zhai L, Peng J, Wu H, Bian Z, Xiao H. Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomed Pharmacother. 2021;141:111931.
Wang K, Lv Q, Miao Y-M, Qiao S-M, Dai Y, Wei Z-F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol. 2018;155:494–509.
Zhao L, Xiao H-t, Mu H-x, et al. Magnolol, a natural polyphenol, attenuates dextran sulfate sodium-induced colitis in mice. Molecules. 2017;22(7):1218.
Lv Q, Shi C, Qiao S, et al. Alpinetin exerts anti-colitis efficacy by activating AhR, regulating miR-302/DNMT-1/CREB signals, and therefore promoting Treg differentiation. Cell Death Dis. 2018;9(9):890.
Wang J, Niu X, Wu C, Wu D. Naringenin modifies the development of lineage-specific effector CD4+ T cells. Front Immunol. 2018;9:2267.
Guo A, He D, Xu H-B, Geng C-A, Zhao J. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci Rep. 2015;5(1):1–12.
Kawai S, Iijima H, Shinzaki S, et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017;52:904–19.
Pierre S, Chevallier A, Teixeira-Clerc F, et al. Aryl hydrocarbon receptor–dependent induction of liver fibrosis by dioxin. Toxicol Sci. 2014;137(1):114–24.
Jönsson ME, Mattsson A, Shaik S, Brunström B. Toxicity and cytochrome P450 1A mRNA induction by 6-formylindolo [3, 2-b] carbazole (FICZ) in chicken and Japanese quail embryos. Comp Biochem Physiol C Toxicol Pharmacol. 2016;179:125–36.
Kerkvliet NI. TCDD: an environmental immunotoxicant reveals a novel pathway of immunoregulation—a 30-year odyssey. Toxicol Pathol. 2012;40(2):138–42.
Park R, Madhavaram S, Ji JD. The role of aryl-hydrocarbon receptor (AhR) in osteoclast differentiation and function. Cells. 2020;9(10):2294.
Großkopf H, Walter K, Karkossa I, Von Bergen M, Schubert K. Non-genomic AhR-signaling modulates the immune response in endotoxin-activated macrophages after activation by the environmental stressor BaP. Front Immunol. 2021;12:620270.
Machala M, Ciganek M, Bláha L, Minksová K, Vondráčk J. Aryl hydrocarbon receptor-mediated and estrogenic activities of oxygenated polycyclic aromatic hydrocarbons and azaarenes originally identified in extracts of river sediments. Environ Toxicol Chem Int J. 2001;20(12):2736–43.
Safe S. PCBs as aryl hydrocarbon receptor agonists. pcbs recent advances in environmental toxicology and health effects. Louisville: University Press of Kentucky; 2001. p. 171–7.
Ibabao CN, Bunaciu RP, Schaefer DM, Yen A. The AhR agonist VAF347 augments retinoic acid-induced differentiation in leukemia cells. FEBS Open Bio. 2015;5:308–18.
Zapadka TE, Lindstrom SI, Batoki JC, et al. Aryl hydrocarbon receptor agonist VAF347 impedes retinal pathogenesis in diabetic mice. Int J Mol Sci. 2021;22(9):4335.
Lin L, Dai Y, Xia Y. An overview of aryl hydrocarbon receptor ligands in the Last two decades (2002–2022): a medicinal chemistry perspective. Eur J Med Chem. 2022;244:114845.
Chen I, Safe S, Bjeldanes L. Indole-3-carbinol and diindolylmethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer cells. Biochem Pharmacol. 1996;51(8):1069–76.
Peng C, Wu C, Xu X, et al. Indole-3-carbinol ameliorates necroptosis and inflammation of intestinal epithelial cells in mice with ulcerative colitis by activating aryl hydrocarbon receptor. Exp Cell Res. 2021;404(2):112638.
Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56(2):197–206.
Nguyen NT, Nakahama T, Nguyen CH, et al. Aryl hydrocarbon receptor antagonism and its role in rheumatoid arthritis. J Exp Pharmacol. 2015;7:29–35. https://doi.org/10.2147/JEP.S63549.
Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43(1):309–34.
Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143(6):1670–80.
Smirnova A, Wincent E, Vikström Bergander L, et al. Evidence for new light-independent pathways for generation of the endogenous aryl hydrocarbon receptor agonist FICZ. Chem Res Toxicol. 2016;29(1):75–86.
Dolciami D, Gargaro M, Cerra B, et al. Binding mode and structure–activity relationships of ITE as an aryl hydrocarbon receptor (AhR) agonist. ChemMedChem. 2018;13(3):270–9.
Gargaro M, Manni G, Scalisi G, Puccetti P, Fallarino F. Tryptophan metabolites at the crossroad of immune-cell interaction via the aryl hydrocarbon receptor: implications for tumor immunotherapy. Int J Mol Sci. 2021;22(9):4644.
Lowe MM, Mold JE, Kanwar B, et al. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS ONE. 2014;9(2):e87877.
Novikov O, Wang Z, Stanford EA, et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER−/PR−/Her2− human breast cancer cells. Mol Pharmacol. 2016;90(5):674–88.
DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115(1):89–97.
Dopkins N, Becker W, Miranda K, Walla M, Nagarkatti P, Nagarkatti M. Tryptamine attenuates experimental multiple sclerosis through activation of aryl hydrocarbon receptor. Front Pharmacol. 2021;11:619265.
Kurata K, Kawahara H, Nishimura K, Jisaka M, Yokota K, Shimizu H. Skatole regulates intestinal epithelial cellular functions through activating aryl hydrocarbon receptors and p38. Biochem Biophys Res Commun. 2019;510(4):649–55.
Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–37.
Zhao H, Chen L, Yang T, et al. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med. 2019;17:1–14.
Wong CB, Tanaka A, Kuhara T, Xiao J-Z. Potential effects of indole-3-lactic acid, a metabolite of human bifidobacteria, on NGF-induced neurite outgrowth in PC12 cells. Microorganisms. 2020;8(3):398.
Puccetti M, Dos Reis LG, Pariano M, et al. Development and in vitro-in vivo performances of an inhalable indole-3-carboxaldehyde dry powder to target pulmonary inflammation and infection. Int J Pharm. 2021;607:121004.
Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-pyruvic acid, an aryl hydrocarbon receptor activator, suppresses experimental colitis in mice. J Immunol. 2018;201(12):3683–93.
Hammerschmidt-Kamper C, Biljes D, Merches K, et al. Indole-3-carbinol, a plant nutrient and AhR-Ligand precursor, supports oral tolerance against OVA and improves peanut allergy symptoms in mice. PLoS ONE. 2017;12(6):e0180321.
Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator–dependent system in gut. J Clin Investig. 2007;117(7):1940–50.
Yin X-F, Chen J, Mao W, Wang Y-H, Chen M-H. A selective aryl hydrocarbon receptor modulator 3, 3’-Diindolylmethane inhibits gastric cancer cell growth. J Exp Clin Cancer Res. 2012;31(1):1–9.
Jellinck PH, Gekforkert P, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45(5):1129–36.
Vyhlídalová B, Krasulová K, Pečinková P, et al. Gut microbial catabolites of tryptophan are ligands and agonists of the aryl hydrocarbon receptor: a detailed characterization. Int J Mol Sci. 2020;21(7):2614.
Jin U-H, Lee S-O, Sridharan G, et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777–88.
Miller CA. Expression of the human aryl hydrocarbon receptor complex in yeast: activation of transcription by indole compounds. J Biol Chem. 1997;272(52):32824–9.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–24.
Meng D, Sommella E, Salviati E, et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res. 2020;88(2):209–17.
Weems JM, Yost GS. 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol. 2010;23(3):696–704.
Marinelli L, Martin-Gallausiaux C, Bourhis J-M, Béguet-Crespel F, Blottière HM, Lapaque N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep. 2019;9(1):1–14.
Jing W, Dong S, Luo X, et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol Res. 2021;164:105358.
Casper RF, Quesne M, Rogers IM, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56(4):784–90.
Tocmo R, Le B, Heun A, van Pijkeren JP, Parkin K, Johnson JJ. Prenylated xanthones from mangosteen (Garcinia mangostana) activate the AhR and Nrf2 pathways and protect intestinal barrier integrity in HT-29 cells. Free Radical Biol Med. 2021;163:102–15.
Acknowledgements
All the figures were created with BioRender.com. The language of this paper has been modified by language editor of American Journal Experts (AJE), and we got Editorial Certificate. This work was supported by the Special funds for the construction of innovative provinces in Hunan (2023JJ20043, 2021NK1009, 2021NK1012 and 2020JJ5635), Laboratory of Lingnan Modern Agriculture Project (NT2021005), the Natural Science Foundation of Guangxi Province (2020JJB130030) and Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2019304).
Funding
This work was supported by the Special funds for the construction of innovative provinces in Hunan (2023JJ20043, 2021NK1009, 2021NK1012 and 2020JJ5635), Laboratory of Lingnan Modern Agriculture Project (NT2021005), the Natural Science Foundation of Guangxi Province (2020JJB130030) and Open Fund of Key Laboratory of Agro-ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2019304).
Author information
Authors and Affiliations
Contributions
KX designed the review framework, YC, KX and YDW wrote and revised the manuscript. YWF and YLY were involved in writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Wang, Y., Fu, Y. et al. Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation. Cell Biosci 13, 85 (2023). https://doi.org/10.1186/s13578-023-01046-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-023-01046-y