Abstract
Background
Indigo Naturalis (IN) is used as a traditional herbal medicine for ulcerative colitis (UC). However, the mechanisms of action of IN have not been clarified. We aimed to evaluate the efficacy of IN for ameliorating colonic inflammation. We further investigated the mechanisms of action of IN.
Methods
Colitis severity was assessed in dextran sodium sulfate-induced colitis and trinitrobenzene sulfonic acid-induced colitis models with or without the oral administration of IN or indigo, which is a known major component of IN. Colonic lamina propria (LP) mononuclear cells isolated from IN-treated mice were analyzed with quantitative reverse transcription polymerase chain reaction (qRT-PCR) and flow cytometry. LP and splenic mononuclear cells cultured in vitro with IN or indigo were also analyzed. The role of the candidate receptor for indigo, the aryl hydrocarbon receptor (AhR), was analyzed using Ahr-deficient mice.
Results
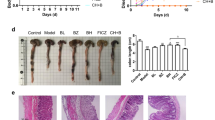
Colitis severity was significantly ameliorated in the IN and indigo treatment groups compared with the control group. The mRNA expression levels of interleukin (Il)-10 and Il-22 in the LP lymphocytes were increased by IN treatment. The treatment of splenocytes with IN or indigo increased the expression of anti-inflammatory cytokines and resulted in the expansion of IL-10-producing CD4+ T cells and IL-22-producing CD3−RORγt+ cells, but not CD4+Foxp3+ regulatory T cells. The amelioration of colitis by IN or indigo was abrogated in Ahr-deficient mice, in association with diminished regulatory cytokine production.
Conclusions
IN and indigo ameliorated murine colitis through AhR signaling activation, suggesting that AhR could be a promising therapeutic target for UC.









Similar content being viewed by others
Abbreviations
- AhR:
-
Aryl hydrocarbon receptor
- CD:
-
Crohn’s disease
- CECs:
-
Colonic epithelial cells
- DMSO:
-
Dimethyl sulfoxide
- DSS:
-
Dextran sodium sulfate
- Foxp3:
-
Forkhead box p3
- IBD:
-
Inflammatory bowel disease
- ILC:
-
Innate lymphoid cells
- IN:
-
Indigo Naturalis
- LP:
-
Lamina propria
- MLN:
-
Mesenteric lymph node
- RORγ:
-
RAR-related orphan receptor gamma
- TCDD:
-
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- TNBS:
-
Trinitrobenzene sulfonic acid
- Tr1 cells:
-
Type 1 regulatory T cells
- UC:
-
Ulcerative colitis
References
Ochsenkühn T, D’Haens G. Current misunderstandings in the management of ulcerative colitis. Gut. 2011;60:1294–9.
Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–15.
Ng SC, Lam YT, Tsoi KK, et al. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854–63.
Triantafyllidi A, Xanthos T, Papalois A, et al. Herbal and plant therapy in patients with inflammatory bowel disease. Ann Gastroenterol. 2015;28:210–20.
Plitzko I, Mohn T, Sedlacek N, et al. Composition of Indigo naturalis. Planta Med. 2009;75:860–3.
Deng S, May BH, Zhang AL, et al. Plant extracts for the topical management of psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:769–82.
Suzuki H, Kaneko T, Mizokami Y, et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol. 2013;19:2718–22.
Fukunaga K, Ohda Y, Hida N, et al. Placebo controlled evaluation of Xilei San, a herbal preparation in patients with intractable ulcerative proctitis. J Gastroenterol Hepatol. 2012;27:1808–15.
Zhang F, Li Y, Xu F, et al. Comparison of Xilei-san, a Chinese herbal medicine, and dexamethasone in mild/moderate ulcerative proctitis: a double-blind randomized clinical trial. J Altern Complement Med. 2013;19:838–42.
Sugimoto S, Naganuma M, Kiyohara H, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93:193–201.
Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–61.
Stockinger B, Di Meglio P, Gialitakis M, et al. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32.
Sugihara K, Okayama T, Kitamura S, et al. Comparative study of aryl hydrocarbon receptor ligand activities of six chemicals in vitro and in vivo. Arch Toxicol. 2008;82:5–11.
Adachi J, Mori Y, Matsui S, et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276:31475–8.
Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–48, 248.e1.
Nguyen NT, Nakahama T, Le DH, et al. Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front Immunol. 2014;5:551.
Shiraishi E, Iijima H, Shinzaki S, et al. Vitamin K deficiency leads to exacerbation of murine dextran sulfate sodium-induced colitis. J Gastroenterol. 2016;51:346–56.
Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–40.
Shinzaki S, Ishii M, Fujii H, et al. N-acetylglucosaminyltransferase V exacerbates murine colitis with macrophage dysfunction and enhances colitic tumorigenesis. J Gastroenterol. 2016;51:357–69.
Furumatsu K, Nishiumi S, Kawano Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56:2532–44.
Aicher WK, Fujihashi K, Yamamoto M, et al. Effects of the lpr/lpr mutation on T and B cell populations in the lamina propria of the small intestine, a mucosal effector site. Int Immunol. 1992;4:959–68.
Lee J, Park EJ, Yuki Y, et al. Profiles of microRNA networks in intestinal epithelial cells in a mouse model of colitis. Sci Rep. 2015;5:18174.
Dohi T, Ejima C, Kato R, et al. Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology. 2005;128:411–23.
Iijima H, Neurath MF, Nagaishi T, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–82.
Gandhi R, Kumar D, Burns EJ, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–53.
Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–32.
Spits H, Artis D, Colonna M, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9.
Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34.
Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–85.
Chiang YR, Li A, Leu YL, et al. An in vitro study of the antimicrobial effects of indigo naturalis prepared from Strobilanthes formosanus Moore. Molecules. 2013;18:14381–96.
Xiao HT, Peng J, Hu DD, et al. Qing-dai powder promotes recovery of colitis by inhibiting inflammatory responses of colonic macrophages in dextran sulfate sodium-treated mice. Chin Med. 2015;10:29.
Li Y, Ligr M, McCarron JP, et al. Natura-alpha targets forkhead box m1 and inhibits androgen-dependent and -independent prostate cancer growth and invasion. Clin Cancer Res. 2011;17:4414–24.
Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549.
Takamura T, Harama D, Matsuoka S, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol. 2010;88:685–9.
Kociba RJ, Schwetz BA. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Drug Metab Rev. 1982;13:387–406.
Pot C. Aryl hydrocarbon receptor controls regulatory CD4+ T cell function. Swiss Med Wkly. 2012;142:w13592.
Roncarolo MG, Gregori S, Battaglia M, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50.
Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42.
Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–61.
Mascanfroni ID, Takenaka MC, Yeste A, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med. 2015;21:638–46.
Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104.
Wagage S, Harms Pritchard G, Dawson L, et al. The group 3 innate lymphoid cell defect in aryl hydrocarbon receptor deficient mice is associated with T cell hyperactivation during intestinal infection. PLoS One. 2015;10:e0128335.
Nikoopour E, Bellemore SM, Singh B. IL-22, cell regeneration and autoimmunity. Cytokine. 2015;74:35–42.
Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–44.
Ji T, Xu C, Sun L, et al. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci. 2015;60:1958–66.
Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495–507.
Zhao Y, Ma T, Chen W, et al. MicroRNA-124 promotes intestinal inflammation by targeting aryl hydrocarbon receptor in Crohn’s disease. J Crohns Colitis. 2016;10:703–12.
Acknowledgements
Supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (Grant No. 26460969).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2016_1292_MOESM3_ESM.tif
Fig. S1. Flow cytometric analysis of the LP mononuclear cells of mice treated with IN. Similar populations of CD4-IL-10+cells (A) and CD3+IL-22+cells (B) were observed in IN-treated and control mice. A representative figure for flow cytometric analysis is shown on the left side (A). Shaded area isotype control, solid line IN, dotted line control. The data are expressed as the mean + standard error of 3 mice per group (TIFF 785 kb)
535_2016_1292_MOESM4_ESM.tif
Fig. S2. Cytokine expression profiles of the MLN mononuclear cells of mice treated with IN. Cytokine expression levels in the MLN cells isolated from mice with DSS-induced colitis were not significantly different between the high-dose IN treatment (IN) and control groups. The expression of Il-22 mRNA was undetectable in both groups. The measurements are expressed as the mean + standard error of 5 mice. *p < 0.05 (TIFF 985 kb)
535_2016_1292_MOESM5_ESM.tif
Fig. S3. Cytokine expression profile of the splenic mononuclear cells in mice treated with IN. Cytokine expression levels in the splenocytes isolated from mice with DSS-induced colitis were not significantly different between the high-dose IN treatment (IN) and control groups. The expression of Il-22 was undetectable in both groups. The measurements are expressed as the mean + standard error of 5 mice. *p < 0.05 (TIFF 990 kb)
535_2016_1292_MOESM6_ESM.tif
Fig. S4. Cytokine expression profile in LP mononuclear cells in Ahr-deficient mice treated with or without IN. There were no significant differences between the IN and control groups. The data are expressed as the mean + standard error of 4 mice per group (TIFF 838 kb)
535_2016_1292_MOESM7_ESM.tif
Fig. S5. AHR expression was elevated in the inflamed mucosa of UC patients. The expression levels of AHR mRNA in uninflamed mucosa (Mayo endoscopic score of 0) and inflamed mucosa (Mayo endoscopic score: 1–3) were compared in 10 UC patients. *p < 0.05 (TIFF 712 kb)
535_2016_1292_MOESM8_ESM.tif
Fig. S6. Expressions of STAT3 and phosphorylated (p)-STAT3 in CECs of mice treated with IN or vehicle control. Mice had DSS-induced colitis that was treated with IN or vehicle control. CECs were isolated from the colon by gentle stirring in the medium followed by Percoll gradient. Western blotting of the cellular lysates was performed by using anti-STAT3, anti-phospho-STAT3, and anti-β-actin antibodies. There was no apparent difference in the expression of p-STAT3 between the IN-treated and control groups. The data are representative of three independent experiments (TIFF 909 kb)
Rights and permissions
About this article
Cite this article
Kawai, S., Iijima, H., Shinzaki, S. et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol 52, 904–919 (2017). https://doi.org/10.1007/s00535-016-1292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1292-z