Abstract
M6A methyltransferases, acting as a writer in N6-methyladenosine, have attracted wide attention due to their dynamic regulation of life processes. In this review, we first briefly introduce the individual components of m6A methyltransferases and explain their close connections to each other. Then, we concentrate on the extensive biological functions of m6A methyltransferases, which include cell growth, nerve development, osteogenic differentiation, metabolism, cardiovascular system homeostasis, infection and immunity, and tumour progression. We summarize the currently unresolved problems in this research field and propose expectations for m6A methyltransferases as novel targets for preventive and curative strategies for disease treatment in the future.
Similar content being viewed by others
Background
N6-methyladenosine (m6A) is acknowledged as one of the most ubiquitous and abundant mRNA methylation modifications that occurs in eukaryotes. It was discovered in 1974, when Desrosiers and colleagues studied the methylation status of hepatoma cells mRNA using the polyadenosinic acid (PolyA) structure in eukaryotes [1]. M6A is an epigenetic mark, a heritable change driven by chemical modifications that alters gene expression without changing the nucleotide sequence [2]. M6A methylation modification is a reversible modification, which is methylated by m6A methyltransferases (writers), demethylated by m6A demethylases (erasers) and also recognized by m6A binding proteins (readers), participating in a series of biological processes [3, 4]. This review focuses on the writers, i.e. m6A methyltransferases.
M6A methyltransferases form a stable complex catalysing the methylation of RNA [5]. This complex consists of two core components, methyltransferase-like 3 protein (METTL3) and methyltransferase-like 14 protein (METTL14), and other accessory regulatory subunits, such as Wilm’s tumour-1-associated protein (WTAP)/(Fl(2)d) [6], KIAA1429 (Virilizer), Hakai, RBM15, METTL16, which need to be further explored. METTL3 (initially called MT-A70) was first discovered in 1997 as a major component in an ~ 200-kDa complex isolated from a mammalian cell nuclear extract that exhibited methyltransferase activity, marking a significant breakthrough in m6A methyltransferase research [7, 8]. Although METTL14 shares 43% homology with METTL3 [9], it does not have catalytic activity, as indicated through crystal structure studies [10]. METTL14 provides an RNA-binding scaffold, facilitates allosteric activation and promotes the catalytic activity of METTL3 [3]. These two proteins form a stable heterodimer core complex with a 1:1 stoichiometry and function synergistically both in vitro and in vivo [8, 11]. WTAP localizes the m6A methyltransferase complex to nuclear speckle targets enriched with pre-mRNA and increases its catalytic activity [6, 12]. In mammalian cells, KIAA1429, Hakai and RBM15 are components associated with WTAP that recognize candidate methylation sites and perform precise post-transcriptional regulation [13], while ZC3H13 acts as a bridge between RBM15 and WTAP [14]. METTL16 is an effective component that has recently been discovered, with only partial cellular localization abilities and RNA-binding preferences worthy of further exploration [15]. Moreover, these m6A methyltransferases in mammals have homologues in Saccharomyces cerevisiae, Drosophila, zebrafish, Arabidopsis thaliana and others (described in more detail below), which play similar roles in life processes.
Growth and development
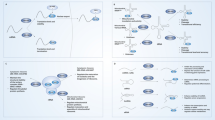
M6A methyltransferases participate in germ cell maturation and preimplantation embryonic development in animals or plants and even in the reproduction of microorganisms [16]. Although mutations in METTL3 are lethal to embryos, limited research has been conducted on METTL3 in germ cells and more is needed [17, 18], with scientists developing special methods to perform experiments. In female animal germ cells, m6A methyltransferases promoted oocyte development and meiosis. In murine and zebrafish oocytes, METTL3 mutations led to arrest in early developmental stage, suppressed maturation and caused defects in the maternal-to-zygotic transition [19, 20] (Fig. 1a). Another study found that KIAA1429 specific deficiency in oocytes led to failure of germinal vesicle breakdown (GVBD), and consequently, the ability to resume meiosis was lost [21] (Fig. 1a). Studying male animal germ cells, Xu K et al. also found that the ablation of METTL3 inhibited the differentiation of spermatogonia and blocked the initiation of meiosis [22] (Fig. 1a); however, Lin et al. suggested that only the combined deletion of METTL3 and METTL14 produced this effect [23, 24] (Fig. 1a). Interestingly, while METTL3 exerted its role in maintaining sperm motility in zebrafish, it caused asthenozoospermia in humans [20, 25] (Fig. 1a). In addition, inactivity of the core RNA methyltransferase (MIS) complex in yeast comprising Ime4 (an orthologue of METTL3), Mum2 (an orthologue of WTAP), and a third ancillary factor, Slz1, inhibited meiosis and sporulation [26, 27] (Fig. 1a). From a micro level, knocking down METTL3 or RBM15 was proven to impair XIST-mediated transcriptional silencing of genes on the X chromosome [28] (Fig. 1a). In addition, m6A methyltransferases directly influence sex determination through selective splicing of specific genes in germ cells. Corresponding to METTL3, METTL4, WTAP, KIAA1429, Rbm15/15B and Zc3h13 in mammals, Ime4, dMETTL14, Virm, Fl(2)d, Nito and Flacc orthologues, respectively, together with a newly discovered unique conservative component, xio, form a functional methyltransferase complex that facilitates Sxl pre-mRNA splicing in Drosophila, suggesting that this complex has a role in sex determination [29, 30] (Fig. 1a). Mutants show a sex bias towards maleness because knocking down these methyltransferases suppresses male-specific lethal 2 (msl-2), preventing female dosage compensation [14, 31] (Fig. 1a). These Drosophila also show flight defects and held out wings. However, fl(2)d, vir, and nito mutants die during larval stages, preventing the analysis of their adult phenotypes [30] (Fig. 1a).
A large body of evidence shows that methyltransferase mutations are embryonic lethal in many species. In mice, knocking out either METTL3 or METTL14 in embryonic stem cells (ESCs) causes inadequate termination of their naive cell state and resistance to differentiation, which accounts for early embryonic lethality [17, 32] (Fig. 1a). METTL16 also exerts its role in the early development of blastocysts [33] (Fig. 1a). In plants, inactivation of Arabidopsis MTA and FIP37, which are orthologues of METTL3 and WTAP in mammals, causes defects in shoot meristems, emergence of lateral roots, and eventually the death of the plant [18, 34, 35] (Fig. 1a). In addition, destabilized METTL3 and METTL14 mRNA encoding developmental regulators in embryonic stem cells help sustain their self-renewal capability [36, 37] (Fig. 1a). Other scientists studied this function of METTL3 to control pluripotency by targeting the SOCS3/JAK2/STAT3 pathway [38] (Fig. 1a). Similarly, depletion of other regulatory subunits, such as WTAP, Virilizer, Hakai and Zc3h13, also impairs ESC self-renewal and induced premature ESC differentiation [39] (Fig. 1a). Notably, increasing the expression of methyltransferase can promote SC reprogramming [40] (Fig. 1a).
Nerve development and regulation
Memory and learning processes are indispensable to m6A methyltransferase. A series of studies found that METTL3 enhances hippocampal long-term memory by promoting the translation efficacy of activity-induced immediate early genes (IEGs), which are DNA binding proteins (e.g., c-Fos, Egr1 and Npas4) that can activate downstream neurotrophic factors to modulate synaptic plasticity and are thus closely related to impaired learning ability and memory formation [41]. Zhang et al. [41] speculated that medicines enhancing METTL3 expression or m6A formation may improve learning ability and slow ageing- and/or disease-related memory loss (Fig. 1b). Another study found that METTL14 is essential for the transcriptional regulation of striatal function and learning epitopes. Conditional deletion of METTL14 in striatonigral and striatopallidal neurons increased neuronal excitability, decreased spike frequency adaptation, altered dopamine signalling and seriously disrupted striatal-mediated performance and learning [42] (Fig. 1b). In addition, METTL3 knockdown in adult neurons not only increased fear memory but also altered the transcriptomic response to fear conditioning stress via the regulation of several genes crucial for neuronal systems, such as neurotransmitter receptors, transporters and transcription factors [43] (Fig. 1b). The expression of METTL3 in the cortex and the hippocampus of mice models of Alzheimer’s disease (AD) was significantly higher, which suggests that methylases may be potential targets for the treatment of AD [44].
The regulation of structure and function also includes cerebellar development and axonal regeneration. Some studies have shown precise spatiotemporal expression of the m6A methyltransferase METTL3 and a decreased apoptosis rate of new cerebellar granule cells (CGCs) [45] (Fig. 1b), thus maintaining normal Purkinje cell numbers, laminal structure, and the function of glial cell fibres to regulate the development and related behaviours of the mouse cerebellum [46] (Fig. 1b). Moreover, METTL14 is required for promoting injury-induced protein synthesis, functional axon regeneration in the peripheral nervous system and retinal ganglion neurons in the central nervous system of adult mammals [47] (Fig. 1b).
From the perspective of neural stem cell research, some studies have revealed key roles for m6A methyltransferases in neural stem cells (NSCs). Lack of METTL14 conspicuously decreased the proliferation and induced premature differentiation of NSCs in vitro, suggesting that METTL14 enhances NSC self-renewal, ensuring the reserves of the neural stem cell bank, while analysis in vivo during cortical neurogenesis showed that a decrease in NSCs in the ventricular zone (radial glial cells, RGCs) was accompanied by fewer cortical neurons [36] (Fig. 1b). Silencing METTL3 induced more NSCs differentiated into the glial lineage, and inhibited morphological maturation of new neurons [48] (Fig. 1b). METTL3 and METTL14 regulate the cell cycle and maintain neural stem cells to enhance the transcriptional coordination of mammalian cortical neurogenesis [49].
Osteogenic differentiation
METTL3 efficiently and specifically regulates dynamic equilibrium to advance the differentiation of adipocytes and osteoblasts in bone marrow stem cells (BMSCs) [50]. On the one hand, METTL3 expression positively correlates with BMSC-driven osteogenesis. Conditional deletion of METTL3 in BMSCs resulted in incompetent osteogenic differentiation potential, reduced bone mass and impaired bone formation [51, 52] (Fig. 1c). METTL3 knockdown decreased the expression of bone formation-related genes (such as Runx2 and Osterix), precursor (pre-) miR-320 and the PI3K-Akt pathway. Furthermore, the activity of alkaline phosphatase (ALP), the formation of mineralized nodules, the expression of Vegfa and its splice variants vegfa-164 and vegfa-188 were also influenced [52, 53] (Fig. 1c). On the other hand, METTL3 expression is negatively related to BMSC-driven adipogenesis. Loss of METTL3 in BMSCs increased adipogenic differentiation and led to high marrow adiposity [51] (Fig. 1c). Another study explained that METTL3 negatively regulates BMSC adipogenic differentiation. Knocking down METTL3 increased JAK1 protein expression in the JAK1/STAT5/C/EBPb pathway in an m6A-YTHDF2-dependent manner, subsequently mediating adipogenic differentiation [54] (Fig. 1c). These pathological changes led to pathological features of osteoporosis in mice. In contrast, the overexpression of METTL3 reduced the probability of oestrogen deficiency-induced osteoporosis [51] (Fig. 1c). Nevertheless, Yu J et al. came to the opposite conclusion. They revealed that METTL3 played an inhibitory role in osteogenesis by inhibiting the calcium deposition and alkaline phosphatase activity of BMSCs and by attenuating the activation of NF-κB, which is universally regarded as a repressor of osteogenesis [55] (Fig. 1c).
To maintain bone homeostasis and preserve skeletal integrity, in addition to osteoblast-mediated bone formation, osteoclast-mediated bone resorption is necessary. METTL3 knockdown inhibited osteoclastic differentiation and bone resorption through an integrated mechanism, including decreasing the expression levels of transcription factors (such as c-Fos and Nfatc1) involved in osteoclast differentiation and factors in bone-resorbing activity (Acp5 and Ctsk), upregulating the expression of the cellular fusion-specific gene Atp6v0d2, entrapping the Traf6 transcript in the nucleus, and subsequently suppressing the activation of MAPK, NF-κB and PI3K-AKT signalling pathways [56] (Fig. 1c). When referring to muscle attached to the bone, METTL3 facilitated mRNA expression of myogenic transcription factor MyoD in proliferative myoblasts to maintain their differentiation potential in skeletal muscle [57] (Fig. 1c).
Metabolism
M6A methyltransferases exert their important roles in nutritional physiology and metabolism [58] Among these roles, their ability to regulate circadian rhythm-related metabolism is particularly interesting. Fustin JM et al. found that knocking down METTL3 elicited circadian period elongation by decreasing RNA processing efficiency [59] (Fig. 2a). However, genetic perturbation involving disruption of circadian rhythms can lead to metabolic diseases, especially lipid-related diseases, such as hyperleptinaemia, hypertriglyceridaemia, hepatic steatosis, diabetes, and obesity [60] (Fig. 2a). The absence of liver Bmal1, an essential component of the mammalian circadian rhythm regulatory network, leads to ROS accumulation and disruption of lipid metabolism, but via knockdown of m6A methyltransferase METTL3, lipid accumulation can be reduced because of a decrease in peroxisome proliferator-activator a (PPaRa), m6A abundance, extended mRNA lifetimes and increased expression [60] (Fig. 2a). Furthermore, m6A methyltransferases also act on lipid metabolism independently. Some studies have suggested that m6A methyltransferases negatively correlate with adipogenesis [61, 62] (Fig. 2a). WTAP, together with METTL3 and METTL14, impaired adipogenesis by inducing cell cycle arrest during the mitotic clonal expansion (MCE) of adipocytes [62] (Fig. 2a). Mechanistically similar to its depletion in osteoclasts, depletion of METTL3 entrapped Traf6 transcripts in the nucleus and suppressed the NF-kB and MAPK signalling pathways of inflammation, promoting the absorption of long-chain fatty acids (LCFAs) [63] (Fig. 2a). A similar effect was found in the metabolism of fungi. IME4 (an orthologue of METTL3) deletion induced dysfunction of peroxisomes, which are the sole sites of fatty acid β-oxidation in yeast [64] (Fig. 2a). In addition to its direct effect on regulating triacylglycerol (TAG) metabolism in haploid cells, IME4 deletion caused mitochondrial fragmentation and dysfunction, which indirectly influenced TAG metabolism [65] (Fig. 2a).
In addition, carbohydrate metabolism is involved. The relationship between m6A methyltransferase and glucose metabolism was mentioned above. Because diabetes is a prevalent metabolic disease, many studies on methyltransferases in diabetes have been performed in recent years. Reduced METTL3 in mice increased insulin sensitivity, decreased fatty acid synthesis and protected the mice from diet-induced obesity [66] (Fig. 2a). A similar conclusion on lipid and glucose metabolism had been reached in other studies of WTAP and METTL3 [62, 67] (Fig. 2a). In addition, by changing the stability of ICAM-1 mRNA, METTL3 knockdown repressed the apoptosis of human lens epithelial cells (HLECs) in diabetic cataracts caused by high levels of glucose [68] (Fig. 2a). Compared to these methyltransferase-disease interactions, the relationship of METTL14 with pancreatic β cells seems to be the closest. METTL14 deficiency destroys pancreatic β-cell homeostasis, specifically shown as decreased β-cell mass and increased cell death, as well as glucose intolerance, enhanced insulin sensitivity, and reduced body weight despite increased adipose tissue mass [69]. Other studies that reached similar conclusions explained that METTL14 regulates the functions of β cells through the insulin/IGF1-AKT-PDX1 or IRE1a/sXBP-1 pathway [70, 71] (Fig. 2a).
Knockdown of METTL3/14 inhibited the expression levels and activities of the drug metabolizing enzyme cytochrome P450 in HepaRG and Huh-7 cells [72].
Cardiovascular system homeostasis
The cardiovascular system consists of the heart and blood vessels. Recently, some studies have tried to explain the relationship between m6A methyltransferases and the growth of cardiomyocytes. However, interestingly, because of differences in study designs and modelling methods, these studies came to opposite conclusions. Dorn et al. reported that METTL3 maintained cardiac homeostasis and the heart response to pressure-overload stress. Increasing the expression of the m6A methyltransferase METTL3 in the heart drove spontaneous, compensated hypertrophy but did not affect cardiac function, whereas METTL3 knockdown led to morphological and functional signs of heart failure, which demonstrated that METTL3 may have the ability to block maladaptive eccentric remodelling [73] (Fig. 2b). In contrast, Kmietczyk V et al. demonstrated that METTL3 conspicuously decreased the expression of hypertrophic markers Nppa and Nppb to prevent pathological growth in cardiac myocytes [74] (Fig. 2b). METTL3 downregulated the expression levels of transcription factor EB (TFEB), which is directly involved with lysosomal biogenesis and autophagy, subsequently inhibiting autophagy and increasing the apoptosis rate of H/R-treated cardiomyocytes [75] (Fig. 2b).
Blood vessel physiology is complex and involves multiple molecules in multiple cells. METTL14 selectively hypermethylates the transcript of Klotho, a vascular system-protecting protein, promoting its degradation and attenuating the harmful expression of this protein induced by indoxyl sulfate in vascular calcification, thereby decreasing the vascular repair function. Interestingly, the forced expression of METTL14 in stressed human artery smooth muscle cells (HASMCs) has the opposite effect, which may predict the therapeutic potential of METTL14 in vascular calcification-involved diseases [76] (Fig. 2b). However, METTL14 seems to be associated with inflammatory infiltrates and neovascularization to lead to a greater risk of human abdominal aortic aneurysm (AAA) rupture [77] (Fig. 2b). One study suggested that silencing METTLE3 not only reduced the expression of VSMC-specific markers, including α-SMA, SM22α, calponin, and SM-MHC but also decreased the expression of paracrine factors, including VEGF, HGF, TGF-β, GM-CSF, bFGF, and SDF-1, which revealed a positive role for METTL3 in vascular smooth muscle differentiation [78] (Fig. 2b). Another study reported that WTAP, a component of m6A methyltransferases, exerted a negative role by impairing the viability, proliferation, and migration potential of vascular smooth muscle cells (VSMCs) by mechanistically regulating p16 via m6A modification, thereby preventing arterial restenosis induced by intimal hyperplasia [79] (Fig. 2b).
Moreover, m6A methyltransferases exert their role in the generation and differentiation of blood cells. METTL3 was reported to maintain balanced self-renewal and differentiation in the fate determination of haematopoietic stem/progenitor cells (HSPCs) [80] (Fig. 2b). It can repress arterial-endothelial Notch activity, thereby promoting HSPC generation through the endothelial-to-haematopoietic transition (EHT) [80, 81] (Fig. 2b). In vitro knockdown of METTL3 or METTL14 in HSPCs led to myeloid differentiation [82, 83] (Fig. 2b), whereas in vitro deletion blocked HSC differentiation to cause an accumulation of HSCs in the bone marrow and a reduction in reconstitution potential [84] (Fig. 2b), which can be interpreted as regulating the expression of the asymmetric or symmetric cell division marker MYC in HSPCs [85]. Moreover, the m6A methyltransferase complex promoted the translation of genes required for human erythropoiesis, including those encoding SETD histone methyltransferases, ribosomal components, and poly(A) RNA-binding proteins [86].
Infection and immunity
Recent studies suggest that m6A methyltransferases play diverse roles in either restricting or modulating the lifecycles of viruses. We first focus on RNA viruses, which are classified into positive-sense, single-stranded RNA viruses, such as Flaviviridae. Zika virus (ZIKV) replication efficiency was enhanced after METTL3 or METTL14 knockdown, modifying its host mRNA landscape [87] (Fig. 3), whereas the hepatitis C virus (HCV) infection rate was increased, not through viral RNA replication but through increased production of infectious viral particles [88] (Fig. 3). However, in another single-stranded RNA virus that similarly replicates in the cytoplasm, enterovirus 71 (EV71) of the Picornaviridae family displayed the opposite pattern. METTL3 increased SUMOylation and ubiquitination of the viral RNA polymerase 3D to boost viral replication, and through interaction, 3D recruits METTL3 to sites of viral RNA replication [89] (Fig. 3). In influenza A virus (IAV) and respiratory syncytial virus (RSV), for which infection by either is characterized by respiratory symptoms, inactivation of METTL3 inhibited virus replication and pathogenesis [90, 91] (Fig. 3). In addition to suppressing viral replication as explained above, by reducing Rev protein, which preferentially interacts with methylated RRE, the export of viral RNA is constrained [92] (Fig. 3), and silencing of METTL3 or METTL14 decreased HIV-1 Gag expression, which is crucial in the assembly of virus particles [93, 94] (Fig. 3). In regard to DNA viruses, METTL14 not only maintains the expression of latent Epstein-Barr virus (EBV) transcripts but also drives EBV-mediated tumorigenesis via direct interaction with the viral-encoded latent oncoprotein EBNA3C [95] (Fig. 3). METTL14 also plays a positive role in the growth cycle of human cytomegalovirus (HCMV), without which interferon β accumulates to reduce virus protein expression and reproduction [96] (Fig. 3). Consistent with the role of m6A methyltransferases in the DNA virus infection, in Kaposi’s sarcoma-associated herpesvirus (KSHV) and simian virus 40 (SV40), METTL3 reduced the post-transcriptional accumulation of the major viral lytic transactivator ORF50 [97,98,99] (Fig. 3) and enhanced the translation of late SV40 transcripts, respectively [100] (Fig. 3). Another DNA virus, hepatitis B virus (HBV), is an exception. METTL3 and METTL14 depletion led to increased expression of the HBc and HBs proteins, consequently promoting the progression of infection [101] (Fig. 3). This regulatory effect of methyltransferases in pathogenic infections is found not only in humans but also in animals and plants. Silencing of METTL3 or METTL14 decreased the Bombyx mori nucleopolyhedrovirus (BmNPV) structural protein VP39 [102] (Fig. 3). Moreover, Pyricularia oryzae, a filamentous phytopathogenic fungus that causes unfavourable declines in rice production, showed decreased density at lesion areas and lesion numbers after deletion of PoIME (an m6A methyltransferase in P. oryzae) [103] (Fig. 3).
In addition to acting directly on viruses, m6A methyltransferases can regulate the immune response to infection [104] (Fig. 3). In antiviral innate immunity, METTL3 depletion results in the modular and highly specific induction of hundreds of interferon-stimulated genes after viral infection, constituting one of the first lines of pathogen defence [105] (Fig. 3). However, in macrophage polarization, METTL3 plays a dual regulatory role, and knocking down METTL3 significantly inhibits M1 macrophage polarization, which has high bactericidal and proinflammatory activities, but enhances M2 macrophage polarization, which has anti-inflammatory properties [106] (Fig. 3). METTL3 also enhanced the translation of CD40, CD80 and cytokine IL-12 transcripts in dendritic cells, strengthening the cytokine production induced by TLR4/NF-κB signalling and dendritic cell (DC) activation, further eliciting the proliferation and differentiation naive T cells, which are involved adaptive immunity [107] (Fig. 3). METTL3 induced the decay of SOCS family genes that encode STAT signalling inhibitory proteins, consequently promoting IL-7-mediated STAT5 activation and T cell homeostatic proliferation and differentiation [108] (Fig. 3). Consistent with these observations, in CD4+ regulatory T cells (Tregs), SOCS targets the IL-2-STAT5 signalling pathway to sustain the suppressive functions of Tregs [109] (Fig. 3).
Recent work on inflammation has yielded some intriguing mechanistic insights into how it might be affected by m6A methyltransferases. METTL3 fostered pri-miR-65-3p processing in a microprocessor protein DiGeorge critical region 8-dependent manner to induce inflammatory pain and neuronal sensitization [110]. It also suppressed the expression of MyD88S, which inhibited inflammatory cytokine production, and then promoted the expression of inflammatory cytokines in LPS-induced human dental pulp cells (HDPCs), as well as related markers in the NF-κB and MAPK signalling pathways [111]. Corresponding with this signalling pathway, NF-κB signalling, together with extracellular matrix ECM synthesis, is involved in mediating progression of METTL3 in osteoarthritis [112], whereas another study demonstrated that, in contrast, METTL3 knockdown facilitates LPS-induced inflammation by regulating MAPK signalling [113]. In autoimmune diseases, although the overexpression of METTL3 has been shown to attenuate the inflammatory response induced by LPS in macrophages dependent on NF-κB to influence rheumatoid arthritis (RA) progression [114], the effect of METTL3 on systemic lupus erythaematosus (SLE) remains a matter of speculation [115].
Tumour progression
Recently, the function of m6A methyltransferases in oncology has become a focus, and a variety of studies have led to breakthroughs in understanding and treatment.
The effects of m6A methyltransferases on the proliferation, invasion and metastasis of different tumours vary tremendously. In the majority of tumours of comparatively high incidence, such as digestive system tumours, including gastric cancer (GC) [116,117,118], colorectal cancer (CRC) [119,120,121], hepatocellular carcinoma (HCC) [122, 123] and pancreatic cancer (PAAD) [124], as well as lung cancer (LCA) [125, 126], endometrial cancer [127], thyroid carcinoma [128], prostate cancer [129], osteosarcoma [130], melanoma [131], ovarian carcinoma [132] and more (Fig. 4), knocking down METTL3 was verified to inhibit the proliferation, invasion and migration of cancer cells in vitro by regulating the expression of relevant genes and pathways. Some studies even showed METTL3 roles in tumorigenesis- and metastasis-promoting effects in vivo, including in CRC [121], GC [133], HCC [123, 134] and prostate cancer [129] (Fig. 4). Of particular interest, in GC [116, 117], LCA [135] and ovarian carcinoma [132] the epithelial-mesenchymal transition (EMT) control of METTL3 seems to be particularly important. In addition, in acute myeloid leukaemia (AML), METTL3 depletion caused a favourable outcome by delaying the occurrence of disease through the promotion of the terminal myeloid differentiation of HSPCs and impairment of AML cell survival [82] (Fig. 4). In addition to METTL3, other m6A methyltransferase components, namely, WTAP and KIAA1429, also play adverse roles in GC [130, 136] and HCC [137, 138] progression, respectively. METTL14 is also expressed in CRC [139], HCC [134], AML [83], breast cancer [140] and endometrial cancer [127] (Fig. 4). Although the effects of METTL3 are mechanistically similar to those in GC and LCA in terms of the EMT and PI3K-Akt-mTOR pathways, in renal cell carcinoma (RCC), knocking down METTL3 significantly promoted cell proliferation, migration and invasion [141] (Fig. 4). Thus, opposite functions of METTL3 are not unique and are similar to those of its counterpart, METTL14, in GC and CRC [142, 143]. Interestingly, in glioma and breast cancer, the role of m6A methyltransferases remains controversial. Some studies have suggested that overexpression of METTL3 profoundly inhibited the proliferation, tumorigenicity and migration ability of glioma [144, 145], breast cancer [146] or bladder cancer cells [147] by altering the mRNA expression of genes or proteins. Paradoxically, other studies have shown that by silencing METTL3 [148,149,150,151,152,153] or WTAP (only in glioma) [154], similar cell growth and aggressiveness inhibition was achieved (Fig. 4).
Because m6A methyltransferase plays a vital role in the proliferation, invasion and migration of cancer cells, its level can be used to judge the stage and prognosis of tumours. Survival analysis showed that METTL3 serves as a prognostic factor for poor outcomes for CRC [121, 143], GC [117, 133], pancreatic cancer [124], HCC [122, 123] and thyroid carcinoma [128] patients. Specifically, Hua W et al. pointed out that a high level of METTL3 was significantly associated with tumour TMN grade and FIGO stage [132] (Fig. 4). In contrast, corresponding to the aforementioned functions, RCC patients with high METTL3 expression had an obviously longer survival time [141, 155] (Fig. 4). Moreover, WTAP expression served as an independent predictor for the survival of patients with HCC [137], GC [136], RCC [156] and high-grade serous ovarian carcinoma [157]. A high WTAP level is also closely correlated with increased postoperative recurrence risk of bladder cancer [158] and glioma grade [159] (Fig. 4). However, METTL14 acted in the opposite manner in HCC [134], CRC [143] and GC [142]. In other words, METTL14 downregulation demonstrated adverse clinical outcomes (Fig. 4).
In terms of treatment, while METTL3-silenced GSCs and pancreatic cancer cells showed enhanced sensitivity to irradiation [160, 161], METTL3-depleted cells induced NSCLC and pancreatic cancer chemotherapeutic drug resistance to gemcitabine, 5-fluorouracil, cisplatin, etc. [161, 162] (Fig. 4).
Conclusions
M6A is acknowledged as one of the most ubiquitous and abundant mRNA methylation modifications in eukaryotes. Therefore, m6A methyltransferases have attracted increasing attention due to their various functions in mediating growth and development, metabolism, behavioural activity and even disease development. In addition to these major aspects, their effects in other areas have been discovered, including drug toxicity [163] and cytoplasmic turnover [164]. In this emerging and hot research direction, many gratifying results have been revealed in recent years, among which some difficult miscellaneous diseases have been effectively resolved with current medical level advancements. These achievements may provide new approaches for delaying memory decline in AD, interrupting metastasis and recurrence of tumours, and controlling autoimmune disease progression. Nevertheless, there remains many functions in related fields that deserve further exploration.
According to the current research foundation, the following points need to be addressed. First, as far as m6A methyltransferases are concerned, existing research on several major components (METTL3/METTL14/WTAP) has been relatively intensive, but studies on the later-discovered regulatory subunits, such as METTL16 [15], METTL5, RBM15, VIRMA, and ZCCHC4 [165], are few and cursory. Secondly, there is also room for exploration into the interactions between components including m6A methyltransferases, demethylases and binding proteins. With the increasing number of discovered methyltransferases and binding proteins, it still unclear if any special m6A modification sites mediated by different methyltransferases; if any special m6A modification sites recognized by different binding proteins; if any special interactions among methyltransferases, demethylases and binding proteins. For instance, In addition to the basic structure and functional relationships mentioned in the introduction, Sorci M et al. illustrated multiple dimensions of mutual regulation in mRNA translation and stability, by which either knockdown or overexpression of METTL3 upregulated WTAP protein and influenced its homeostasis. In addition, WTAP upregulation can have a carcinogenic effect only in the presence of a functional m6A methylation complex [166]; that is, its function is m6A-dependent. Most m6A methyltransferases act as indispensable writers of N6-methyladenosine to play roles in biology. However, Qian JY et al. reported that KIAA1429 can change CDK1 transcript stability and extend its half-life to induce breast cancer [167]. Lin S et al. revealed that METTL3 facilitated the translation of the mRNAs of epidermal growth factor receptor (EGFR) and the Hippo pathway effector TAZ to promote the progression of human lung cancer [125], which suggests that some aspects of m6A methyltransferase can regulate life processes in an m6A-independent manner. This possibility has attracted little attention but may eventually lead to improvements in this field. Thirdly, the bio-function of m6A methyltransferase remains contradictory. For instance, in osteogenesis, while most scientists have proved that METTL3 expression in BMScs is positively correlated with osteogenesis and negatively correlated with adipogenesis [51, 52], Yu J et al. revealed that METTL3 inhibits the calcium deposition and alkaline phosphatase activity of BMSCs and attenuates the activation of NF-κB to impair osteogenesis [55]. In another example, METTL3 exhibited an inhibitory role in the proliferation, tumorigenicity and migration ability in glioma cells [144], which had a paradoxical effect [149]. Similar but different types of cells used in experiments, dynamic impacts at different stages of the same life process, and horizontal staggering of sequencing-based methodologies used in each study may contribute to the contradictory results obtained. This outcome is a reminder that more accurate detection methods and more extensive cooperation and communication are needed at the international level. Last, but most significant, more exploration is needed for the quantification of modifications on a transcriptome-wide level, identification of precise sites and discovery of upstream and downstream regulation mechanisms of m6A methyltransferases. Currently, very few drugs are based on m6A methyltransferase function, and some related ideas are at the speculative stage. We anticipate an increased transition from mature in vitro cell experiments to in vivo studies and expansion of the development of some targeted clinical drugs. All these findings may reveal insights for developing novel preventive and curative strategies for related diseases.
Availability of data and materials
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALP:
-
Alkaline phosphatase
- BMSCs:
-
Bone marrow stem cells
- BmNPV:
-
Bombyx mori nucleopolyhedrovirus
- CGSs:
-
Cerebellar granule cells
- CNS:
-
Central nervous system
- EBV:
-
Epstein-Barr virus
- ESC:
-
Embryonic stem cells
- EV71:
-
Enterovirus 71
- GVBD:
-
Germinal vesicle breakdown
- HASMC:
-
Human artery smooth muscle cell
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HCMV:
-
Human cytomegalovirus
- HIV:
-
Human immunodeficiency virus
- HSPCs:
-
Haematopoietic stem/progenitor cells
- IAV:
-
Influenza A virus
- IEGs:
-
Immediate early genes
- KSHV:
-
Kaposi’s sarcoma-associated herpesvirus
- LCFA:
-
Long-chain fatty acids
- M6A:
-
N6-methyladenosine
- METTL3:
-
Methyltransferase-like 3 protein
- METTL14:
-
Methyltransferase-like 14 protein
- NSCs:
-
Neural stem cells
- PNS:
-
Peripheral nervous system
- PolyA:
-
Polyadenosinic acid
- RBC:
-
Red blood cell
- SV40:
-
Simian virus 40
- TAG:
-
Triacylglycerol
- Tregs:
-
CD4 + regulatory T cells
- VSMCs:
-
Vascular smooth muscle cell
- WTAP:
-
Wilm’s tumour-1-associated protein
- ZIKV:
-
Zika virus
References
Desrosiers RFK, Rottman F. Identification of methylated nucleosides in messenger-RNA from Novikoff hepatoma-cells. Proc Natl Acad Sci USA. 1974;71:3971–5.
Yue YLJ, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–55.
Bi Z, Liu Y, Zhao Y, Yao Y, Wu R, Liu Q, Wang Y, Wang X. A dynamic reversible RNA N(6)-methyladenosine modification: current status and perspectives. J Cell Physiol. 2019;234:7948–56.
Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–24.
Wu B, Li L, Huang Y, Ma J, Min J. Readers, writers and erasers of N(6)-methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89.
Bokar JASM, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–47.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5.
Bujnicki JMFM, Radlinska M, Blumenthal RM. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA: m(6)A methyltransferase. J Mol Evol. 2002;55:431–44.
Wang P, Doxtader KA, Nam Y. Structural basis for cooperative function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016;63:306–17.
Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–8.
Schöller EWF, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, Meister G. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. RNA. 2018;24:499–512.
Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, Hamakubo T. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–302.
Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villasenor R, Hess D, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–29.
Nance DJ, Satterwhite ER, Bhaskar B, Misra S, Carraway KR, Mansfield KD. Characterization of METTL16 as a cytoplasmic RNA binding protein. PLoS ONE. 2020;15:e0227647.
Wang YK, Yu XX, Liu YH, Li X, Liu XM, Wang PC, Liu S, Miao JK, Du ZQ, Yang CX. Reduced nucleic acid methylation impairs meiotic maturation and developmental potency of pig oocytes. Theriogenology. 2018;121:160–7.
Geula SM-MS, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, Ben-Haim MS, Eyal E, Yunger S, Pinto Y, Jaitin DA, Viukov S, Rais Y, Krupalnik V, Chomsky E, Zerbib M, Maza I, Rechavi Y, Massarwa R, Hanna S, Amit I, Levanon EY, Amariglio N, Stern-Ginossar N, Novershtern N, Rechavi G, Hanna JH. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–6.
Shen L, Liang Z, Gu X, Chen Y, Teo ZW, Hou X, Cai WM, Dedon PC, Liu L, Yu H. N(6)-Methyladenosine RNA modification regulates shoot stem cell fate in arabidopsis. Dev Cell. 2016;38:186–200.
Sui X, Hu Y, Ren C, Cao Q, Zhou S, Cao Y, Li M, Shu W, Huo R. METTL3-mediated m(6)A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle. 2020;19:391–404.
Xia H, Zhong C, Wu X, Chen J, Tao B, Xia X, Shi M, Zhu Z, Trudeau VL, Hu W. Mettl3 mutation disrupts gamete maturation and reduces fertility in Zebrafish. Genetics. 2018;208:729–43.
Hu Y, Ouyang Z, Sui X, Qi M, Li M, He Y, Cao Y, Cao Q, Lu Q, Zhou S, et al. Oocyte competence is maintained by m(6)A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ. 2020;27(8):2468–83.
Xu K, Yang Y, Feng GH, Sun BF, Chen JQ, Li YF, Chen YS, Zhang XX, Wang CX, Jiang LY, et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–14.
Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou Q, Zhang KJ, Zhang X, Zhou Y, Zhang T, et al. Mettl3-/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Res. 2017;27:1216–30.
Lin Z, Tong MH. m(6)A mRNA modification regulates mammalian spermatogenesis. Biochim Biophys Acta Gene Regul Mech. 2019;1862:403–11.
Yang Y, Huang W, Huang JT, Shen F, Xiong J, Yuan EF, Qin SS, Zhang M, Feng YQ, Yuan BF, Liu SM. Increased N6-methyladenosine in Human Sperm RNA as a Risk Factor for Asthenozoospermia. Sci Rep. 2016;6:24345.
Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155:1409–21.
Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 2012;8:e1002732.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73.
Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M, Roignant JY. m(6)A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–7.
Guo JTH, Li J, Perrimon N, Yan D. Xio is a component of the Drosophila sex determination pathway and RNA N(6)-methyladenosine methyltransferase complex. Proc Natl Acad Sci USA. 2018;115:3674–9.
Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, Soller M. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–4.
Meng TG, Lu X, Guo L, Hou GM, Ma XS, Li QN, Huang L, Fan LH, Zhao ZH, Ou XH, et al. Mettl14 is required for mouse postimplantation development by facilitating epiblast maturation. FASEB J. 2019;33:1179–87.
Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, McCarthy AA, Pillai RS. Methylation of Structured RNA by the m(6)A writer METTL16 Is essential for mouse embryonic development. Mol Cell. 2018;71:986–1000.
Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–88.
Bhat SS, Bielewicz D, Jarmolowski A, Szweykowska-Kulinska Z. N(6)-methyladenosine (m(6)A): Revisiting the Old with Focus on New, an Arabidopsis thaliana Centered Review. Genes (Basel). 2018;9(12):596.
Wang Y, Li Y, Yue M, Wang J, Kumar S, Wechsler-Reya RJ, Zhang Z, Ogawa Y, Kellis M, Duester G, Zhao JC. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci. 2018;21:195–206.
Lin S, Gregory RI. Methyltransferases modulate RNA stability in embryonic stem cells. Nat Cell Biol. 2014;16:129–31.
Wu R, Liu Y, Zhao Y, Bi Z, Yao Y, Liu Q, Wang F, Wang Y, Wang X. m(6)A methylation controls pluripotency of porcine induced pluripotent stem cells by targeting SOCS3/JAK2/STAT3 pathway in a YTHDF1/YTHDF2-orchestrated manner. Cell Death Dis. 2019;10:171.
Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell. 2018;69:1028–38.
Guo M, Liu X, Zheng X, Huang Y, Chen X. m(6)A RNA modification determines cell fate by regulating mrna degradation. Cell Reprogram. 2017;19:225–31.
Zhang Z, Wang M, Xie D, Huang Z, Zhang L, Yang Y, Ma D, Li W, Zhou Q, Yang YG, Wang XJ. METTL3-mediated N(6)-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Res. 2018;28:1050–61.
Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, Rao MN, Chen K, Lu Z, Yi Y, Chi W, et al. Mettl14 Is essential for epitranscriptomic regulation of striatal function and learning. Neuron. 2018;99:283–92.
Engel M, Eggert C, Kaplick PM, Eder M, Roh S, Tietze L, Namendorf C, Arloth J, Weber P, Rex-Haffner M, et al. The role of m(6)A/m-RNA methylation in stress response regulation. Neuron. 2018;99:389–403.
Han M, Liu Z, Xu Y, Liu X, Wang D, Li F, Wang Y, Bi J. Abnormality of m6A mRNA Methylation Is Involved in Alzheimer’s Disease. Front Neurosci. 2020;14:98.
Wang CX, Cui GS, Liu X, Xu K, Wang M, Zhang XX, Jiang LY, Li A, Yang Y, Lai WY, et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018;16:e2004880.
Ma C, Chang M, Lv H, Zhang ZW, Zhang W, He X, Wu G, Zhao S, Zhang Y, Wang D, et al. RNA m(6)A methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biol. 2018;19:68.
Weng YL, Wang X, An R, Cassin J, Vissers C, Liu Y, Liu Y, Xu T, Wang X, Wong SZH, et al. Epitranscriptomic m(6)a regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–25.
Chen J, Zhang YC, Huang C, Shen H, Sun B, Cheng X, Zhang YJ, Yang YG, Shu Q, Yang Y, Li X. m(6)A regulates neurogenesis and neuronal development by modulating histone methyltransferase Ezh2. Genom Proteomics Bioinformat. 2019;17:154–68.
Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. Temporal control of mammalian cortical neurogenesis by m(6)A Methylation. Cell. 2017;171:877–89.
Chen X, Hua W, Huang X, Chen Y, Zhang J, Li G. Regulatory Role of RNA N(6)-Methyladenosine Modification in Bone Biology and Osteoporosis. Front Endocrinol. 2019;10:911.
Wu Y, Xie L, Wang M, Xiong Q, Guo Y, Liang Y, Li J, Sheng R, Deng P, Wang Y, et al. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun. 2018;9:4772.
Yan G, Yuan Y, He M, Gong R, Lei H, Zhou H, Wang W, Du W, Ma T, Liu S, et al. m(6)A methylation of precursor-miR-320/RUNX2 controls osteogenic potential of bone marrow-derived mesenchymal stem cells. Mol Ther Nucleic Acids. 2020;19:421–36.
Tian C, Huang Y, Li Q, Feng Z, Xu Q. Mettl3 regulates osteogenic differentiation and alternative splicing of vegfa in bone marrow mesenchymal stem cells. Int J Mol Sci. 2019;20(3):551.
Yao Y, Bi Z, Wu R, Zhao Y, Liu Y, Liu Q, Wang Y, Wang X. METTL3 inhibits BMSC adipogenic differentiation by targeting the JAK1/STAT5/C/EBPbeta pathway via an m(6)A-YTHDF2-dependent manner. FASEB J. 2019;33:7529–44.
Yu J, Shen L, Liu Y, Ming H, Zhu X, Chu M, Lin J. The m6A methyltransferase METTL3 cooperates with demethylase ALKBH5 to regulate osteogenic differentiation through NF-kappaB signaling. Mol Cell Biochem. 2020;463:203–10.
Li D, Cai L, Meng R, Feng Z, Xu Q. METTL3 modulates osteoclast differentiation and function by controlling RNA stability and nuclear export. Int J Mol Sci. 2020;21(5):1660.
Kudou KKT, Nogami J, Maehara K, Harada A, Saeki H, Oki E, Maehara Y, Ohkawa Y. The requirement of Mettl3-promoted MyoD mRNA maintenance in proliferative myoblasts for skeletal muscle differentiation. Open Biol. 2017;7(9):170119.
Wu J, Frazier K, Zhang J, Gan Z, Wang T, Zhong X. Emerging role of m(6) A RNA methylation in nutritional physiology and metabolism. Obes Rev. 2020;21:e12942.
Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806.
Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM, Dolan K, Zhu X, Hubert N, Tao Y, et al. Circadian clock regulation of hepatic lipid metabolism by modulation of m(6)A mRNA Methylation. Cell Rep. 2018;25:1816–28.
Wang X, Zhu L, Chen J, Wang Y. mRNA m(6)A methylation downregulates adipogenesis in porcine adipocytes. Biochem Biophys Res Commun. 2015;459:201–7.
Kobayashi MOM, Sasako T, Awazawa M, Umehara T, Iwane A, Kobayashi N, Okazaki Y, Kubota N, Suzuki R, Waki H, Horiuchi K, Hamakubo T, Kodama T, Aoe S, Tobe K, Kadowaki T, Ueki K. The RNA methyltransferase complex of WTAP, METTL3, and METTL14 regulates mitotic clonal expansion in adipogenesis. Mol Cell Biol. 2018;38(16):e00116-e118.
Zong X, Zhao J, Wang H, Lu Z, Wang F, Du H, Wang Y. Mettl3 deficiency sustains long-chain fatty acid absorption through suppressing Traf6-dependent inflammation response. J Immunol. 2019;202:567–78.
Yadav PK, Rajvanshi PK, Rajasekharan R. The role of yeast m(6)A methyltransferase in peroxisomal fatty acid oxidation. Curr Genet. 2018;64:417–22.
Yadav PK, Rajasekharan R. The m(6)A methyltransferase Ime4 and mitochondrial functions in yeast. Curr Genet. 2018;64:353–7.
Xie W, Ma LL, Xu YQ, Wang BH, Li SM. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem Biophys Res Commun. 2019;518:120–6.
Li Y, Zhang Q, Cui G, Zhao F, Tian X, Sun B-F, Yang Y, Li W. m6A Regulates Liver Metabolic Disorders and Hepatogenous Diabetes. 2020;S1672–0229(20)30129–7.
Yang J, Liu J, Zhao S, Tian F. N(6)-Methyladenosine METTL3 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract. Mol Ther Nucleic Acids. 2020;20:111–6.
Liu J, Luo G, Sun J, Men L, Ye H, He C, Ren D. METTL14 is essential for beta-cell survival and insulin secretion. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2138–48.
De Jesus DF, Zhang Z, Kahraman S, Brown NK, Chen M, Hu J, Gupta MK, He C, Kulkarni RN. m(6)A mRNA Methylation regulates human beta-cell biology in physiological states and in type 2 diabetes. Nat Metab. 2019;1:765–74.
Men L, Sun J, Luo G, Ren D. Acute Deletion of METTL14 in beta-cells of adult mice results in glucose intolerance. Endocrinology. 2019;160:2388–94.
Nakano M, Ondo K, Takemoto S, Fukami T, Nakajima M. Methylation of adenosine at the N(6) position post-transcriptionally regulates hepatic P450s expression. Biochem Pharmacol. 2020;171:113697.
Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, Hanna JH, van Berlo JH, Accornero F. The N(6)-Methyladenosine mRNA Methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139:533–45.
Kmietczyk V, Riechert E, Kalinski L, Boileau E, Malovrh E, Malone B, Gorska A, Hofmann C, Varma E, Jurgensen L, et al. m(6)A-mRNA methylation regulates cardiac gene expression and cellular growth. Life Sci Alliance. 2019;2(2):e201800233.
Song H, Feng X, Zhang H, Luo Y, Huang J, Lin M, Jin J, Ding X, Wu S, Huang H, et al. METTL3 and ALKBH5 oppositely regulate m(6)A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15:1419–37.
Chen J, Ning Y, Zhang H, Song N, Gu Y, Shi Y, Cai J, Ding X, Zhang X. METTL14-dependent m6A regulates vascular calcification induced by indoxyl sulfate. Life Sci. 2019;239:117034.
He Y, Xing J, Wang S, Xin S, Han Y, Zhang J. Increased m6A methylation level is associated with the progression of human abdominal aortic aneurysm. Ann Transl Med. 2019;7:797.
Lin J, Zhu Q, Huang J, Cai R, Kuang Y. Hypoxia promotes vascular smooth muscle cell (VSMC) differentiation of adipose-derived stem cell (ADSC) by regulating Mettl3 and paracrine factors. Stem Cells Int. 2020;2020:2830565.
Zhu B, Gong Y, Shen L, Li J, Han J, Song B, Hu L, Wang Q, Wang Z. Total Panax notoginseng saponin inhibits vascular smooth muscle cell proliferation and migration and intimal hyperplasia by regulating WTAP/p16 signals via m(6)A modulation. Biomed Pharmacother. 2020;124:109935.
Zhang C, Chen Y, Sun B, Wang L, Yang Y, Ma D, Lv J, Heng J, Ding Y, Xue Y, et al. m(6)A modulates haematopoietic stem and progenitor cell specification. Nature. 2017;549:273–6.
Lv J, Zhang Y, Gao S, Zhang C, Chen Y, Li W, Yang YG, Zhou Q, Liu F. Endothelial-specific m(6)A modulates mouse hematopoietic stem and progenitor cell development via Notch signaling. Cell Res. 2018;28:249–52.
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–76.
Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes Leukemogenesis via mRNA m(6)A Modification. Cell Stem Cell. 2018;22:191–205.
Lee H, Bao S, Qian Y, Geula S, Leslie J, Zhang C, Hanna JH, Ding L. Stage-specific requirement for Mettl3-dependent m(6)A mRNA methylation during haematopoietic stem cell differentiation. Nat Cell Biol. 2019;21:700–9.
Cheng Y, Luo H, Izzo F, Pickering BF, Nguyen D, Myers R, Schurer A, Gourkanti S, Bruning JC, Vu LP, et al. m(6)A RNA Methylation maintains hematopoietic stem cell identity and symmetric commitment. Cell Rep. 2019;28:1703–16.
Kuppers DA, Arora S, Lim Y, Lim AR, Carter LM, Corrin PD, Plaisier CL, Basom R, Delrow JJ, Wang S, et al. N(6)-methyladenosine mRNA marking promotes selective translation of regulons required for human erythropoiesis. Nat Commun. 2019;10:4596.
Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, Rana TM. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe. 2016;20:666–73.
Gokhale NS, McIntyre ABR, McFadden MJ, Roder AE, Kennedy EM, Gandara JA, Hopcraft SE, Quicke KM, Vazquez C, Willer J, et al. N6-Methyladenosine in Flaviviridae viral RNA genomes regulates infection. Cell Host Microbe. 2016;20:654–65.
Hao H, Hao S, Chen H, Chen Z, Zhang Y, Wang J, Wang H, Zhang B, Qiu J, Deng F, Guan W. N6-methyladenosine modification and METTL3 modulate enterovirus 71 replication. Nucleic Acids Res. 2019;47:362–74.
Xue M, Zhao BS, Zhang Z, Lu M, Harder O, Chen P, Lu Z, Li A, Ma Y, Xu Y, et al. Viral N(6)-methyladenosine upregulates replication and pathogenesis of human respiratory syncytial virus. Nat Commun. 2019;10:4595.
Courtney DG, Kennedy EM, Dumm RE, Bogerd HP, Tsai K, Heaton NS, Cullen BR. Epitranscriptomic enhancement of influenza a virus gene expression and replication. Cell Host Microbe. 2017;22:377–86.
Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, Mason CE, Rana TM. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011.
Tirumuru NZB, Lu W, Lu Z, He C, Wu L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 2016;5:e15528.
Riquelme-Barrios S, Pereira-Montecinos C, Valiente-Echeverria F, Soto-Rifo R. Emerging roles of N(6)-methyladenosine on HIV-1 RNA metabolism and viral replication. Front Microbiol. 2018;9:576.
Lang F, Singh RK, Pei Y, Zhang S, Sun K, Robertson ES. EBV epitranscriptome reprogramming by METTL14 is critical for viral-associated tumorigenesis. PLoS Pathog. 2019;15:e1007796.
Rubio RM, Depledge DP, Bianco C, Thompson L, Mohr I. RNA m(6) A modification enzymes shape innate responses to DNA by regulating interferon beta. Genes Dev. 2018;32:1472–84.
Ye FCE, Nilsen TW. Kaposi’s sarcoma-associated herpesvirus utilizes and manipulates RNA N(6)-Adenosine methylation to promote lytic replication. J Virol. 2017;91(16):e00466-e517.
Hesser CR, Karijolich J, Dominissini D, He C, Glaunsinger BA. N6-methyladenosine modification and the YTHDF2 reader protein play cell type specific roles in lytic viral gene expression during Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog. 2018;14:e1006995.
Tan B, Gao SJ. RNA epitranscriptomics: Regulation of infection of RNA and DNA viruses by N(6)-methyladenosine (m(6) A). Rev Med Virol. 2018;28:e1983.
Tsai K, Courtney DG, Cullen BR. Addition of m6A to SV40 late mRNAs enhances viral structural gene expression and replication. PLoS Pathog. 2018;14:e1006919.
Imam H, Khan M, Gokhale NS, McIntyre ABR, Kim GW, Jang JY, Kim SJ, Mason CE, Horner SM, Siddiqui A. N6-methyladenosine modification of hepatitis B virus RNA differentially regulates the viral life cycle. Proc Natl Acad Sci USA. 2018;115:8829–34.
Zhang X, Zhang Y, Dai K, Liang Z, Zhu M, Pan J, Zhang M, Yan B, Zhu H, Zhang Z, et al. N (6)-Methyladenosine level in silkworm midgut/ovary cell line is associated with Bombyx mori Nucleopolyhedrovirus Infection. Front Microbiol. 2019;10:2988.
Shi Y, Wang H, Wang J, Liu X, Lin F, Lu J. N6-methyladenosine RNA methylation is involved in virulence of the rice blast fungus Pyricularia oryzae (syn. Magnaporthe oryzae). FEMS Microbiol Lett. 2019;366(1):fny286.
Williams GDGN, Horner SM. Regulation of Viral Infection by the RNA Modification N6-Methyladenosine. Annu Rev Virol. 2019;6:235–53.
Winkler R, Gillis E, Lasman L, Safra M, Geula S, Soyris C, Nachshon A, Tai-Schmiedel J, Friedman N, Le-Trilling VTK, et al. m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat Immunol. 2019;20:173–82.
Liu YLZ, Tang H, Shen Y, Gong Z, Xie N, Zhang X, Wang W, Kong W, Zhou Y, Fu Y. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am J Physiol Cell Physiol. 2019;317(4):C762–75.
Wang H, Hu X, Huang M, Liu J, Gu Y, Ma L, Zhou Q, Cao X. Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun. 2019;10:1898.
Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, Bailis W, Cao G, Kroehling L, Chen Y, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–42.
Tong J, Cao G, Zhang T, Sefik E, Amezcua Vesely MC, Broughton JP, Zhu S, Li H, Li B, Chen L, et al. m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res. 2018;28:253–6.
Zhang C, Wang Y, Peng Y, Xu H, Zhou X. METTL3 regulates inflammatory pain by modulating m(6)A-dependent pri-miR-365-3p processing. FASEB J. 2020;34:122–32.
Feng Z, Li Q, Meng R, Yi B, Xu Q. METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide-induced inflammatory response in human dental pulp cells. J Cell Mol Med. 2018;22:2558–68.
Liu Q, Li M, Jiang L, Jiang R, Fu B. METTL3 promotes experimental osteoarthritis development by regulating inflammatory response and apoptosis in chondrocyte. Biochem Biophys Res Commun. 2019;516:22–7.
Zhang Y, Gu X, Li D, Cai L, Xu Q. METTL3 regulates osteoblast differentiation and inflammatory response via smad signaling and MAPK signaling. Int J Mol Sci. 2019;21(1):199.
Wang J, Yan S, Lu H, Wang S, Xu D. METTL3 Attenuates LPS-induced inflammatory response in macrophages via NF-kappaB signaling pathway. Mediators Inflamm. 2019;2019:3120391.
Li LJ, Fan YG, Leng RX, Pan HF, Ye DQ. Potential link between m(6)A modification and systemic lupus erythematosus. Mol Immunol. 2018;93:55–63.
Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, Zhao G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142.
Liu T, Yang S, Sui J, Xu SY, Cheng YP, Shen B, Zhang Y, Zhang XM, Yin LH, Pu YP, Liang GY. Dysregulated N6-methyladenosine methylation writer METTL3 contributes to the proliferation and migration of gastric cancer. J Cell Physiol. 2020;235:548–62.
Lin S, Liu J, Jiang W, Wang P, Sun C, Wang X, Chen Y, Wang H. METTL3 promotes the proliferation and mobility of gastric cancer cells. Open Med (Wars). 2019;14:25–31.
Peng W, Li J, Chen R, Gu Q, Yang P, Qian W, Ji D, Wang Q, Zhang Z, Tang J, Sun Y. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res. 2019;38:393.
Deng R, Cheng Y, Ye S, Zhang J, Huang R, Li P, Liu H, Deng Q, Wu X, Lan P, Deng Y. m(6)A methyltransferase METTL3 suppresses colorectal cancer proliferation and migration through p38/ERK pathways. Onco Targets Ther. 2019;12:4391–402.
Li T, Hu PS, Zuo Z, Lin JF, Li X, Wu QN, Chen ZH, Zeng ZL, Wang F, Zheng J, et al. METTL3 facilitates tumor progression via an m(6)A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112.
Lin Y, Wei X, Jian Z, Zhang X. METTL3 expression is associated with glycolysis metabolism and sensitivity to glycolytic stress in hepatocellular carcinoma. Cancer Med. 2020;9:2859–67.
Chen MWL, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, Wong CC, Ng IO, Wong CM. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–70.
Xia T, Wu X, Cao M, Zhang P, Shi G, Zhang J, Lu Z, Wu P, Cai B, Miao Y, Jiang K. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215:152666.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A Methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–45.
Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–60.
Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–83.
Wang K, Jiang L, Zhang Y, Chen C. Progression of thyroid carcinoma is promoted by the m6A Methyltransferase METTL3 through regulating m(6)A Methylation on TCF1. Onco Targets Ther. 2020;13:1605–12.
Cai J, Yang F, Zhan H, Situ J, Li W, Mao Y, Luo Y. RNA m(6)A Methyltransferase METTL3 promotes the growth of prostate cancer by regulating hedgehog pathway. Onco Targets Ther. 2019;12:9143–52.
Miao W, Chen J, Jia L, Ma J, Song D. The m6A methyltransferase METTL3 promotes osteosarcoma progression by regulating the m6A level of LEF1. Biochem Biophys Res Commun. 2019;516:719–25.
Dahal U, Le K, Gupta M. RNA m6A methyltransferase METTL3 regulates invasiveness of melanoma cells by matrix metallopeptidase 2. Melanoma Res. 2019;29:382–9.
Hua W, Zhao Y, Jin X, Yu D, He J, Xie D, Duan P. METTL3 promotes ovarian carcinoma growth and invasion through the regulation of AXL translation and epithelial to mesenchymal transition. Gynecol Oncol. 2018;151:356–65.
Wang QCC, Ding Q, Zhao Y, Wang Z, Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, Zhou J, Sun B, Zou X, Wang S. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2019;69(7):1193–205.
Ma JZYF, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, Sun SH. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–43.
Wanna-Udom S, Terashima M, Lyu H, Ishimura A, Takino T, Sakari M, Tsukahara T, Suzuki T. The m6A methyltransferase METTL3 contributes to Transforming Growth Factor-beta-induced epithelial-mesenchymal transition of lung cancer cells through the regulation of JUNB. Biochem Biophys Res Commun. 2020;524:150–5.
Li H, Su Q, Li B, Lan L, Wang C, Li W, Wang G, Chen W, He Y, Zhang C. High expression of WTAP leads to poor prognosis of gastric cancer by influencing tumour-associated T lymphocyte infiltration. J Cell Mol Med. 2020;24:4452–65.
Chen Y, Peng C, Chen J, Chen D, Yang B, He B, Hu W, Zhang Y, Liu H, Dai L, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18(1):127.
Cheng X, Li M, Rao X, Zhang W, Li X, Wang L, Huang G. KIAA1429 regulates the migration and invasion of hepatocellular carcinoma by altering m6A modification of ID2 mRNA. Onco Targets Ther. 2019;12:3421–8.
Yang X, Zhang S, He C, Xue P, Zhang L, He Z, Zang L, Feng B, Sun J, Zheng M. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46.
Yi D, Wang R, Shi X, Xu L, Yilihamu Y, Sang J. METTL14 promotes the migration and invasion of breast cancer cells by modulating N6methyladenosine and hsamiR146a5p expression. Oncol Rep. 2020;43(5):1375–86.
Li XTJ, Huang W, Wang F, Li P, Qin C, Qin Z, Zou Q, Wei J, Hua L, Yang H, Wang Z. The M6A methyltransferase METTL3: acting as a tumor suppressor in renal cell carcinoma. Oncotarget. 2017;8:96103–16.
Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J, Gong J, Shen L. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8:4766–81.
Liu XLL, Dong Z, Li J, Yu Y, Chen X, Ren F, Cui G, Sun R. Expression patterns and prognostic value of m(6)A-related genes in colorectal cancer. Am J Transl Res. 2019;11:3972–91.
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, et al. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–34.
Li F, Zhang C, Zhang G. m6A RNA methylation controls proliferation of human glioma cells by influencing cell apoptosis. Cytogenet Genome Res. 2019;159:119–25.
Wu L, Wu D, Ning J, Liu W, Zhang D. Changes of N6-methyladenosine modulators promote breast cancer progression. BMC Cancer. 2019;19:326.
Zhao S, Liu J, Nanga P, Liu Y, Cicek AE, Knoblauch N, He C, Stephens M, He X. Detailed modeling of positive selection improves detection of cancer driver genes. Nat Commun. 2019;10:3399.
Li F, Yi Y, Miao Y, Long W, Long T, Chen S, Cheng W, Zou C, Zheng Y, Wu X, et al. N(6)-Methyladenosine modulates nonsense-mediated mRNA decay in human glioblastoma. Cancer Res. 2019;79:5785–98.
Visvanathan A, Patil V, Abdulla S, Hoheisel JD, Somasundaram K. N(6)-Methyladenosine landscape of glioma stem-like cells: METTL3 is essential for the expression of actively transcribed genes and sustenance of the oncogenic signaling. Genes. 2019;10:141.
Wang H, Xu B, Shi J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene. 2020;722:144076.
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, Liu Y, Zhang X, Zhang W, Ye L. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–9.
Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110.
Cheng M, Sheng L, Gao Q, Xiong Q, Zhang H, Wu M, Liang Y, Zhu F, Zhang Y, Zhang X, et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-kappaB/MYC signaling network. Oncogene. 2019;38:3667–80.
Jin DI, Lee SW, Han ME, Kim HJ, Seo SA, Hur GY, Jung S, Kim BS, Oh SO. Expression and roles of Wilms’ tumor 1-associating protein in glioblastoma. Cancer Sci. 2012;103:2102–9.
Zhou JWJ, Hong B, et al. Gene signatures and prognostic values of m6A regulators in clear cell renal cell carcinoma–a retrospective study using TCGA database. Aging. 2019;11:1633–47.
Tang J, Wang F, Cheng G, Si S, Sun X, Han J, Yu H, Zhang W, Lv Q, Wei JF, Yang H. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res. 2018;37:40.
Yu HL, Ma XD, Tong JF, Li JQ, Guan XJ, Yang JH. WTAP is a prognostic marker of high-grade serous ovarian cancer and regulates the progression of ovarian cancer cells. Onco Targets Ther. 2019;12:6191–201.
Chen L, Wang X. Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis. Oncol Lett. 2018;16:6966–70.
Xi Z, Xue Y, Zheng J, Liu X, Ma J, Liu Y. WTAP expression predicts poor prognosis in malignant glioma patients. J Mol Neurosci. 2016;60:131–6.
Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, Somasundaram K. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–33.
Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T, Doki Y, Mori M, Ishii H, Ogawa K. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52:621–9.
Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, Di W, Hu B, An J, Kong L, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:135.
Wang J, Ishfaq M, Xu L, Xia C, Chen C, Li J. METTL3/m(6)A/miRNA-873-5p Attenuated oxidative stress and apoptosis in colistin-induced kidney injury by modulating Keap1/Nrf2 Pathway. Front Pharmacol. 2019;10:517.
Ke SP-JA, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE Jr, Darnell RB. m(6)A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 2017;31:990–1006.
Liu S, Zhuo L, Wang J, Zhang Q, Li Q, Li G, Yan L, Jin T, Pan T, Sui X, et al. METTL3 plays multiple functions in biological processes. Am J Cancer Res. 2020;10:1631–46.
Sorci M, Ianniello Z, Cruciani S, Larivera S, Ginistrelli LC, Capuano E, Marchioni M, Fazi F, Fatica A. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9:796.
Qian JY, Gao J, Sun X, Cao MD, Shi L, Xia TS, Zhou WB, Wang S, Ding Q, Wei JF. KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner. Oncogene. 2019;38:6123–41.
Funding
This study was supported by the National Natural Science Foundation of China (81802371, 81730108, 81973805); Zhejiang Provincial Natural Science Foundation (LGD20H290001); Key Project of Hangzhou Ministry of Science and Technology (20162013A07); Zhejiang Province Medical Science and Technology Project (2018KY108, 2021RC117), Hangzhou Agricultural and Social Development Scientific Research Independent Application Project (20191203B22); and Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Chinese Traditional Medicine), Zhejiang Chinese Medical University (ZYX2018005).
Author information
Authors and Affiliations
Contributions
JZG, SPL, YZ and LJZ wrote the manuscript and created the figures. SPL, TX and XBS provided direction and guidance throughout the preparation of this manuscript. SPL, QZ, YYS, YG and JZG reviewed and made significant revisions to the manuscript. QL, GHL, QJL, SSQ and JYZ collected and prepared the related papers. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All of the authors are aware of and agree to the content of the paper and their being listed as a co-author of the paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gu, J., Zhan, Y., Zhuo, L. et al. Biological functions of m6A methyltransferases. Cell Biosci 11, 15 (2021). https://doi.org/10.1186/s13578-020-00513-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-020-00513-0