Abstract
Fowl cholera caused by Pasteurella multocida exerts a massive economic burden on the poultry industry. Lipopolysaccharide (LPS) is essential for the growth of P. multocida genotype L1 strains in chickens and specific truncations to the full length LPS structure can attenuate bacterial virulence. Here we further dissected the roles of the outer core transferase genes pcgD and hptE in bacterial resistance to duck serum, outer membrane permeability and virulence in ducks. Two P. multocida mutants, ΔpcgD and ΔhptE, were constructed, and silver staining confirmed that they all produced truncated LPS profiles. Inactivation of pcgD or hptE did not affect bacterial susceptibility to duck serum and outer membrane permeability but resulted in attenuated virulence in ducks to some extent. After high-dose inoculation, ΔpcgD showed remarkably reduced colonization levels in the blood and spleen but not in the lung and liver and caused decreased injuries in the spleen and liver compared with the wild-type strain. In contrast, the ΔhptE loads declined only in the blood, and ΔhptE infection caused decreased splenic lesions but also induced severe hepatic lesions. Furthermore, compared with the wild-type strain, ΔpcgD was significantly attenuated upon oral or intramuscular challenge, whereas ΔhptE exhibited reduced virulence only upon oral infection. Therefore, the pcgD deletion caused greater virulence attenuation in ducks, indicating the critical role of pcgD in P. multocida infection establishment and survival.
Similar content being viewed by others
Introduction
Pasteurella multocida (P. multocida), an encapsulated gram-negative bacterium, can cause endemic and epizootic diseases in a wide range of animal species, including fowl cholera (FC) in domestic and wild birds. Human infections by P. multocida also occasionally occur after cat or dog bites [1, 2]. P. multocida isolates are classified into five capsular types (A, B, D, E and F) based on capsular polysaccharide and 16 Heddleston serovars based on lipopolysaccharide (LPS) antigens. Capsular type A and Heddleston serovars 1 and 3 are most commonly associated with FC outbreaks [3,4,5]. P. multocida strains now can also be differentiated into 8 different LPS genotypes (L1–L8) based on distinct LPS outer core biosynthesis loci [6]. This new classification is achieved by PCR and thus easy to operate and more accurate for typing. FC manifests as acute or peracute systemic disease or chronic localized infection, leading to a high rate of morbidity and mortality in birds and posing a massive economic burden on the poultry industry worldwide. The clinical course of the disease usually ranges from a few hours to several days with a virulent strain, and death occurs suddenly in peracute cases [7]. Despite the economic importance of the bacterium, P. multocida is still an enigmatic pathogen, and the exact molecular mechanisms responsible for pathogenesis in pasteurellosis remain largely unknown. To date, only a few virulence factors, such as capsule and LPS [8, 9], and several regulators, including Hfq, Fis, Crp, and PhoP [10,11,12,13], have been shown to have roles in P. multocida virulence.
As a major cell wall component of gram-negative bacteria, LPS plays an essential role in the host-bacteria interaction and is referred to as endotoxin due to its ability to induce sepsis [14]. During infection with gram-negative bacteria, LPS is recognized by pathogen pattern recognition receptors (PRRs) in both extracellular and intracellular sites, which activates signalling pathways of innate immunity that stimulate the secretion of proinflammatory cytokines and interferons to defend against invasive bacteria [15]. However, when such responses become uncontrolled, overwhelming production of proinflammatory cytokines may lead to the development of immunopathology, manifesting as septic shock, tissue damage and even death. P. multocida LPS has been shown to be endotoxic. Stimulation with LPS from the B:2, A:1 and A:3 serotypes induces cell death in bovine leukocytes [16], and intravenous inoculation with LPS isolated from a serotype B:2 strain induces clinical signs of haemorrhagic septicaemia in buffalo [17].
Pasteurella multocida LPS lacks an O-antigen and is composed of three distinct regions; a hydrophobic lipid A domain, an inner core oligosaccharide and an outer core oligosaccharide. While the lipid A structure is still unknown, the nucleotide sequences of the biosynthesis genes required for assembly of the inner and outer core polysaccharides and the structures of those polysaccharides for all 16 Heddleston serovars have been determined by mass spectrometry compositional analysis over the last two decades [18]. The inner core is highly conserved and usually exists simultaneously with two different structures, termed glycoform A and glycoform B [19]. Transferase genes required for the assembly of the inner core are located at different sites in the P. multocida genome. In contrast, the outer core structures vary extraordinarily between the type strains representing different Heddleston serovars, except that Heddleston serovars 2 and 5 possess an identical outer core structure but show differences in the inner core with or without a phosphoethanolamine (PEtn) residue on heptose (Hep) II [20]. The transferase genes required for assembly of the outer core are diverse and located together in the chromosome between the two conserved genes priA and fpg [6].
FC disease is frequently caused by P. multocida LPS genotype L1 strains. The full-length outer core of the well-studied LPS genotype L1 strain VP161 consists of Hep IV linked to galactose (Gal) I and Gal II at the 4 and 6 positions, respectively, and one phosphocholine (PCho) molecule attached to each Gal residue [21]. The synthesis of the outer core is achieved by the transferases HptE (adding Hep IV to the inner core), GatA (transferring both Gal I and Gal II to Hep IV) and PcgD (transferring PCho to both Gal residues) (Figure 1). Interestingly, inactivation of the phosphocholine pathway in VP161 also leads to the loss of a galactose from the end of the LPS structure so that the corresponding LPS lacks PCho and one galactose residue [22]. Moreover, decoration with PEtn attached to both Gal residues via the transferase PetG also exists in another LPS genotype L1 strain X73 [23]. The absence of PEtn decoration on Gal residues in VP161 is due to a single base deletion in the petG gene, which is located outside the outer core gene locus [23]. Previous studies have shown that the complete LPS molecule is important for LPS genotype L1 strains to display full virulence in chickens. Mutations in the genes required for decoration of LPS with PCho or PEtn, including pcgC, petL or petK rather than petG, significantly increase bacterial susceptibility to chicken antimicrobial peptide but have limited effects on bacterial virulence [22, 23]. Nevertheless, mutation of LPS biosynthesis genes for the main oligosaccharide extension of the inner core (gctB, hptD and hptC) or outer core (hptE and gatA) not only gives rise to reduced defence against the antimicrobial peptide but also results in bacterial growth defects in vivo and virulence attenuation to various extents [19, 21, 24]. These mutants, except ΔhptC, are only partially attenuated in virulence and can still induce FC symptoms in chickens. The mutants, including ΔhptE, ΔgatA, ΔhptD and ΔgctB, were shown to transform into wild-type (WT) revertants in vivo post-infection, which might eventually contribute to animal death [21]. The revertants emerged from the instability of the single-crossover insertional mutagenesis or the signature-tagged mutagenesis method used for mutant constructions [21, 24]. Thus, it is of interest and importance to further measure the virulence of more stable double crossover mutants within each of these genes. Moreover, the animal species is an important factor influencing the roles of LPS synthesis genes in the virulence of P. multocida [25]. Therefore, it is also necessary to evaluate the function of P. multocida LPS in other avian species.
Schematic representation of the LPS genotype L1 outer core gene locus (A) and LPS structures (B) expressed by the P. multocida WT and mutant strains. The LPS structures were predicted through previous studies that uncovered the outer core structures and related synthesis genes belonging to genotype L1 of P. multocida [21, 22].
In this study, two LPS outer core transferase genes, pcgD and hptE, were deleted in the WT P. multocida strain PM0818 via double-crossover homologous recombination, and then the abilities of the mutants were investigated and compared with the WT strain in response to duck serum complement and a variety of hydrophobic antibiotics in vitro; to colonize tissues, and to cause pathological lesions and death in ducks.
Materials and methods
Bacterial strains and growth conditions
The bacterial strains and plasmids used are shown in Table 1. All the mutant strains were derived from the virulent WT P. multocida A:1 strain PM0818 with a muscular 50% lethal dose (LD50) of < 100 CFU and an oral LD50 of approximately 106 CFU in ducklings [10, 13]. P. multocida strains were grown at 37 °C in brain heart infusion (BHI) broth or on BHI agar (BD Bioscience, USA), and Escherichia coli (E. coli) strains were grown in Luria–Bertani (LB) broth or on LB agar. Tryptic soybean agar (TSA, Difco Laboratories, USA) was generally used for colony counts of P. multocida strains. When required, antibiotics were added to the medium at the following concentrations: kanamycin, 50 μg/mL; chloramphenicol, 25 μg/mL.
Plasmid and mutant strain construction
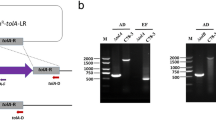
The primers used in this study are listed in Additional file 1. The P. multocida mutant strains were constructed by allelic exchange using the suicide T-vector pRE112 [26] as previously described [13]. The primers used for the mutant construction and characterization are depicted in Figure 2. To delete the pcgD gene, the primer pairs DpcgD-1F/1R, DpcgD-2F/2R and kanR-pcgD-F/R were used to amplify the upstream segment (440 bp) and downstream segment (335 bp) of the pcgD gene from the PM0818 genome and the kanamycin resistance (kanR) gene (837 bp) from the pET28a plasmid, respectively. The three gene fragments were then linked together by overlap PCR using primers DpcgD-1F and DpcgD-2R. The resulting PCR product was digested with KpnI-HF and XmaI and ligated into pRE112 to generate the plasmid pCZ51, which carries a deletion of the entire pcgD gene sequence. This plasmid was subsequently introduced into P. multocida PM0818 from E. coli SM10 λ pir via conjugation, and the ΔpcgD mutant strain designated PMZ1 was selected on BHI agar containing kanamycin. The pcgD mutation was confirmed by PCR with three primer pairs (P1-F/R, P2-F/R and P3-F/R) for the deleted region and flanking DNA, and the PCR products from P1-F/R and P2-F/R were subjected to DNA sequencing (BGI-Shenzhen, China) (data not shown). The same method was applied to construct the mutant strain PMZ3 (ΔhptE).
Schematic strategy used for deletion of the target pcgD (A) or hptE (B) gene in P. multocida. The target gene was replaced with the kanR gene via homologous recombination. The primer pairs DpcgD-1F/1R, DpcgD-2F/2R, and kanR-pcgD-F/R were designed for the construction of PMZ1 (ΔpcgD) and DhptE-1F/1R, DhptE-2F/2R, and kanR-hptE-F/R for the construction of PMZ3 (ΔhptE). The primer pairs P1-F/R, P2-F/R, and P3-F/R were designed for the characterization of PMZ1 (ΔpcgD) and P4-F/P1-R, P2-F/P5-R, and P6-F/R for the characterization of PMZ3 (ΔhptE).
To complement each gene mutation in the P. multocida mutants, the region (12 bp) immediately upstream of the start codon and the complete coding sequences of pcgD and hptE were amplified from the PM0818 genome with primer pairs CpcgD-F/R and ChptE-F/R, respectively. The resulting pcgD or hptE segment was inserted into the KpnI and NotI sites of the shuttle plasmid pMC-Express [27] to generate the complementation plasmid pCZ54 or pCZ56. Then, the recombinant plasmids were transformed into the corresponding mutant strains, generating two complemented strains, PMZ1 (pCZ54) and PMZ3 (pCZ56). Moreover, the empty plasmid pMC-Express was also transformed into the mutant strains, generating the control strain, PMZ1 (pMC-Express) or PMZ3 (pMC-Express).
Phenotype detection
To determine the relative growth rate of each P. multocida strain grown under normal in vitro growth conditions, overnight bacterial cultures were diluted to an optical density (OD) = 0.05 in fresh BHI medium, and then the bacterial growth was determined by measuring the OD600 value every 0.5 h during the first 0 h-2 h and every 2 h from 3 to 15 h at 37 °C. To detect the LPS phenotype, whole-cell lysates of the P. multocida WT strain or mutants were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) on 15% (w/v) acrylamide gels using a Tricine-SDS buffer system (Bio-Rad Laboratories, California, USA) followed by silver staining as previously described [28].
Susceptibility to duck serum
The bactericidal activity of duck serum complement against the P. multocida strains was measured as previously described [10]. In brief, the P. multocida WT and mutant strains were grown to the mid-log growth phase (OD = 0.6) in BHI broth and re-suspended in phosphate-buffered saline (PBS) at a concentration of 104 CFU/mL. The bacterial suspensions were then treated with 90% normal duck serum or heat-inactivated duck serum for 3 h at 37 °C with shaking. After incubation, serial dilutions of the samples in PBS were cultured on TSA plates at 37 °C overnight. The survival rate of each strain was calculated as the CFU of normal serum group divided by the CFU of the inactivated serum group.
NPN assay
Outer membrane (OM) permeability was determined by utilizing N-phenyl-1-naphthylamine (NPN) (Sigma-Aldrich, USA), which is a hydrophobic fluorescent probe, as described previously, with minor modification [29]. In brief, the P. multocida WT and mutant strains were grown to the mid-log phase and then harvested by centrifugation and resuspended in PBS to an OD600 of 0.5. The NPN dissolved in acetone was added to the suspension at a final concentration of 80 µM. Control samples without added cells were also treated with NPN. Then, the fluorescence of the mixture was measured immediately using a spectrofluorometer (Thermo Scientific Varioskan Flash). The excitation wavelength, emission wavelength and slit width used were 350 nm, 420 nm, and 5 nm, respectively.
The minimum inhibitory concentrations (MICs) of antibiotics
The MICs of several hydrophobic antibiotics (Dalian Meilun Biotechnology, China) and SDS (Sigma-Aldrich), including azithromycin, novobiocin, spiramycin, ciprofloxacin, rifampicin, and nalidixic acid, against the P. multocida WT and mutant strains were measured using the standard microscale broth dilution method performed according to the Clinical and Laboratory Standard Institute criteria as previously described [30]. The E. coli ATCC25922 strain was included as quality control.
Colonization and virulence of P. multocida strains and resultant histopathology changes in ducks
One-day-old partridge Sichuan ducklings were purchased from Grimaud Breeding Co., Ltd. (Chengdu, China) and acclimated for several days after arrival. To measure the colonization of the mutants, 7-day-old ducks were orally inoculated with approximately 109 CFU of the P. multocida WT strain or each mutant strain (6 ducks/group). Then, blood and tissues, including the lung, spleen and liver, were collected from animals 12 h and 24 h after infection. Each tissue sample was weighed and homogenized in 1 mL of PBS. Next, the suspension or the collected blood was serially diluted, and the appropriate dilution was plated onto TSA to determine the number of viable bacteria. The colonization level is represented as CFU per gram of tissue (CFU/g) or CFU per mL of blood (CFU/mL).
Moreover, to observe histopathological changes, the spleens and livers randomly collected from 4 ducks within each group in the colonization analysis at 24 h were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, sectioned into 4-μm-thick sections, placed on microscope slides, and stained with haematoxylin and eosin (HE) using standard procedures. Four ducks inoculated with the PBS were also included as a negative control. Scoring with light microscopy evaluation was based on the severity of pathological changes in terms of tissue structure, congestion, inflammatory infiltration, haemorrhage, degeneration and necrosis. Scores ranging from 0 to 4 represent different levels of significant pathological lesions and were assessed by three diagnostic pathologists. Furthermore, to determine bacterial virulence, 7-day-old ducks were challenged with approximately 109 CFU of each P. multocida strain orally or 100 CFU intramuscularly (10 ducks/group). Then, the survival of ducks in each infected group was observed and recorded in a 7-day period.
Statistical analysis
The data are shown as the means ± SDs and were analysed by one-way ANOVA followed by Dunnett’s multiple comparison test in GraphPad Prism (GraphPad Software, California, USA). The survival curves in the virulence assay were analysed by the Log-rank test in GraphPad Prism. A probability value of p < 0.05 was considered statistically significant. The animal experiments were performed twice, and the in vitro experiments were conducted in triplicate independently at least three times.
Results
Construction and characterization of P. multocida mutants
The LPS genotype L1 outer core biosynthesis locus of P. multocida contains seven genes, rpL31_2, pcgD, pcgA, pcgB, pcgC, gatA and hptE, among which rpL31_2 is unrelated to LPS biosynthesis (Figure 1A). Genome sequencing and bioinformatics analyses showed that the outer core locus of the WT strain PM0818 (GenBank accession number MT542700) was intact and highly conserved compared with the well-studied LPS genotype L1 strains VP161 and X73. Additionally, a homologue of petG (responsible for the addition of PEtn to the Gal residues) was present in the PM0818 genome but was a pseudogene because of a one-base deletion at nucleotide 1076 compared with the functional petG gene of the X73 strain (Additional file 2), similar to previously reported observations for the nonfunctional petG gene of the VP161 strain [23]. Thus, we predicted that PM0818 possessed an outer core structure identical to the VP161 strain, without PEtn attached to the Gal residues (Figure 1B).
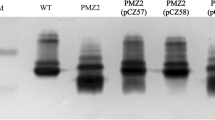
The outer core transferase genes, pcgD and hptE, were deleted in WT strain PM0818 by suicide plasmid-based homologous recombination, as depicted in Figures 2A and B, generating two mutants termed PMZ1 (ΔpcgD) and PMZ3 (ΔhptE), respectively. The genotypes of the mutants were verified via PCR using several pairs of primers. The DNA segment containing the upstream sequence of pcgD or hptE and a partial kanR cassette (P1-F/R or P4-F/P1-R) and the segment containing the downstream sequence of pcgD or hptE and a partial kanR cassette (P2-F/R or P2-F/P5-R) were present in the mutant strain but not in the WT strain, whereas the partial sequence of pcgD or hptE (P3-F/R or P6-F/R) was only amplified from the WT strain (Additional files 3A and 3B). Next, two complementation plasmids, pCZ54 encoding pcgD and pCZ56 encoding hptE, were constructed and transformed into the corresponding mutant strain, generating two complemented strains, PMZ1 (pCZ54) and PMZ3 (pCZ56). Moreover, each mutant harbouring the empty plasmid pMC-Express was also constructed as a control. The outer core structures of the two mutants were predicted according to previous studies whereby mass spectrometry and nuclear magnetic resonance were performed to determine exact LPS core structures (Figure 1B) [21]. To confirm the prediction, LPS phenotypes of the P. multocida strains were detected by silver staining. As expected, genetic inactivation of pcgD or hptE led to the production of truncated LPS, as demonstrated by the observation that the LPS from the two mutants migrated further within the gel than WT LPS. The LPS of PMZ3 (ΔhptE), with the shorter oligosaccharide extension, moved further than that of ΔpcgD (Figure 3). Moreover, the mutants carrying the empty plasmid also produced truncated LPS phenotypes, while complementation of each mutation with the appropriate gene provided in trans resulted in the production of a longer LPS molecule compared with the LPS produced by the corresponding mutant (Figure 3), suggesting the production of functional PcgD or HptE protein and formation of the full-length LPS outer core in the complemented strain. Additionally, the growth curves of the mutants in vitro were similar to those of the WT strain at 37 ℃ (Additional file 4).
LPS phenotypes. LPS extracted from the P. multocida WT strain, two mutant strains, PMZ1 (ΔpcgD) and PMZ3 (ΔhptE), and two complemented strains, PMZ1 (pCZ54) and PMZ3 (pCZ56), and two control strains, PMZ1 (pMC-Express) and PMZ3 (pMC-Express), were subjected to SDS-PAGE followed by silver staining. M refers to the protein marker.
Susceptibility to duck serum and outer membrane permeability of the P. multocida mutants
Loss of the full-length LPS outer or inner core in P. multocida was previously shown to increase susceptibility to the antimicrobial peptide fowlicidin-1 rather than chicken serum complement [21]. Here we evaluated the role of the LPS outer core genes pcgD and hptE in bacterial resistance to duck serum complement and OM permeability using the probe NPN. The mutants PMZ1 (ΔpcgD) and PMZ3 (ΔhptE) showed similar resistances to duck serum complement (Additional file 5) and comparable NPN fluorescence signals to the WT strain (Additional file 6). Additionally, compared with the WT strain, the two mutants had similar susceptibilities to SDS and a subset of hydrophobic antibiotics, including azithromycin, novobiocin, spiramycin, ciprofloxacin, rifampicin, nalidixic acid and bacitracin (Additional file 7). These results indicated that the bacterial resistance to duck serum and OM integrity was not affected by the truncation of the LPS structure due to the inactivation of the outer core transferase genes pcgD or hptE.
Colonization of P. multocida mutants and resultant pathological lesions in ducks
To detect the effects of pcgD or hptE mutation on bacterial colonization, approximately 109 CFU of each mutant strain or the WT strain was inoculated orally into seven-day-old ducks, and the bacterial loads in the blood, spleen, liver and lung were measured 12 h and 24 h post-infection. Compared with the WT strain, the colonization levels of the two mutants were not changed in the blood or all detected tissues at 12 h post-infection (Figures 4A–D); however, at 24 h post-infection, both mutants colonized the blood at a significantly lower level than the WT strain (Figure 4A). Additionally, the bacterial counts of PMZ1 (ΔpcgD) decreased significantly in the spleen rather than the liver and lung, while the counts of PMZ3 (ΔhptE) were similar to those of the WT strain in all three tissues (Figures 4B–D). This result indicated that truncation of the LPS due to deletion of the pcgD or hptE gene had a significantly negative effect on the colonization ability of P. multocida in the blood, and the pcgD mutation further attenuated bacterial colonization in the spleen of ducks.
Colonization in ducks. Groups of ducklings (n = 6/group) were inoculated orally with approximately 109 CFU of the P. multocida WT strain or each mutant strain. The blood (A), spleen (B), lung (C) and liver (D) were collected at 12 h or 24 h post-infection, and the bacterial loads of each strain were calculated as CFU/mL in the blood and CFU/g in the three tissues. The asterisk above the error bar indicates significance compared with the WT group. **, p < 0.01; ***, p < 0.001.
Pathological changes in the spleen and liver were also detected by HE staining at 24 h after infection. The ducks in the PBS control group showed normal tissue structures without histopathological lesions, while the WT strain-infected ducks displayed severe focal necrosis with abundant fragmented nuclei remaining and infiltration of a number of heterophilic granulocytes and structural disorder in the spleen and the liver (Figure 5). The pcgD or hptE mutation alleviated infection-induced tissue lesions to some extent. Neither PMZ1 (ΔpcgD) nor PMZ3 (ΔhptE) infection induced necrosis and merely resulted in significant granulocyte infiltration and slight congestion in the spleen; the former induced only hepatocyte degeneration, while the latter induced moderate granulocyte infiltration and inflammatory exudation in the liver (Figure 5). Of note, the changes in histopathological scores were highly coincident; deletion of pcgD or hptE significantly reduced splenic lesions, and inactivation of pcgD but not hptE weakened the pathological liver lesions (Table 2).
Pathological changes in ducks. Groups of ducklings (n = 4/group) were infected orally with approximately 109 CFU of the P. multocida WT strain or each mutant strain or with control PBS. The histopathological lesions in the spleen and liver caused by each bacterial strain were analysed by HE staining.
Virulence of the P. multocida mutants in ducks
The virulence of the P. multocida strains was determined by inoculation of ducks with approximately 109 CFU of each bacterial strain orally or 100 CFU intramuscularly. The ducks infected with the WT strain showed 100% death following oral inoculation and 20% survival following intramuscular inoculation, whereas the PMZ1 (ΔpcgD) and PMZ3 (ΔhptE) challenges resulted in 50% and 30% survival following oral inoculation (Figure 6A) and 80% and 30% survival following intramuscular inoculation, respectively (Figure 6B). The log-rank test showed that compared with the WT strain, PMZ1 (ΔpcgD) was significantly attenuated in virulence by both the oral and intramuscular routes, while the virulence of PMZ3 (ΔhptE) was reduced only in the oral infection route. Moreover, PMZ1 (ΔpcgD) was less virulent than PMZ3 (ΔhptE) during intramuscular infection (Figure 6B).
Virulence trial. Groups of ducklings (n = 10/group) were challenged with approximately 109 CFU of the P. multocida WT strain or each mutant strain orally (A) or with 100 CFU of each strain intramuscularly (B). Then, the animal survival in each challenge group was monitored. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Discussion
FC is one of the most important diseases, leading to massive economic losses to the poultry industry worldwide; however, the underlying mechanisms of the high mortality and severe tissue damage caused by the causative agent, P. multocida, are still poorly understood [7]. Revealing such mechanisms is essential for developing effective live attenuated vaccines against pasteurellosis, such as the P. multocida auxotrophic aroA mutant and the aroA mutant carrying a deletion of a certain LPS synthesis gene [31, 32]. The polysaccharide components of LPS have been shown to be closely associated with the pathogenicity of P. multocida in chickens [18]; nevertheless, their roles in virulence have not been studied in other avian species. Here we constructed two defined P. multocida mutants with deletions of the outer core glycotransferase genes, pcgD or hptE, via double-crossover homologous recombination, and we evaluated their resistance to duck serum, outer membrane permeability, and their colonization and virulence in ducks, another natural host. The gatA is another transferase gene involved in the synthesis of the LPS outer core and must be studied, unfortunately, however, we did not successfully construct the gatA mutant strain in the WT strain.
It was found that the LPS outer core was irrelevant for the stress response to duck serum, which is in agreement with previous studies showing that P. multocida mutants with incomplete LPS inner cores or without PEtn on lipid A show the same level of serum resistance as the WT parent strain [23, 24]. It also further supports a previous finding showing that the resistance of P. multocida to serum complement is dominantly mediated by the capsule rather than by LPS [33]. The OM of gram-negative bacteria is composed of the outer LPS layer and a phospholipid inner leaflet that confers a semipermeable barrier function against toxic molecules [34]. The loss of lipid A synthesis or destruction of the LPS core region or porin proteins has been previously shown to increase membrane permeability and a range of antibiotics in several gram-negative bacteria [35,36,37,38]. Our results demonstrated that the two outer core mutants maintained normal OM permeability. The irrelevance of the LPS outer core and OM integrity might be a consequence of the outer core being the most distal and exposed region of the LPS molecule. It would be of interest to investigate whether the LPS inner core or lipid A is closely associated with the OM permeability of P. multocida.
The capacities of the two P. multocida mutants to colonize tissues and induce histopathological changes and animal mortality were assessed in ducks. Both strains showed defective growth in the blood, decreased pathological splenic lesion induction and increased animal survival in comparison to those of the WT strain after high-dose oral infection. Nevertheless and interestingly, the virulence attenuation exhibited by PMZ1 (ΔpcgD) was greater than that exhibited by PMZ3 (ΔhptE), as demonstrated by the finding that deletion of pcgD but not hptE led to reduced bacterial colonization in the spleen and alleviated liver injury, and attenuated virulence upon intramuscular infection. The defective in vivo growth of the ΔpcgD and ΔhptE was similar to previous virulence studies on P. multocida in chickens to some extent [21, 22]; however, the virulence attenuation by intramuscular injection exhibited by the ΔpcgD was somewhat contradictory to a previous study in which all the chickens injected with 60 CFU of a PCho mutant (pcgC mutant) showed symptoms of FC but did not succumb to disease, indicating that the addition of PCho to A:1 LPS was not essential for the virulence of P. multocida in chickens [22]. As the four genes of the pcgDCBA locus work together for assembly of PCho on the outer core [18], the discrepancy triggered by the two mutants might be due to differences in the inherent nature of chickens and ducks, or the possibility that pcgD has functions beyond PCho synthesis.
The virulence assay with the hptE mutant in chickens resulted in a delayed onset of fowl cholera symptoms in the presence of wild-type revertants post-intramuscular infection [21]. Our study further confirmed the comparable virulence of the stable ΔhptE with the WT strain upon intramuscular challenge. Inactivation of hptE results in the loss of the full outer core structure and was also found to produce a novel outer core extension comprising β-Gal-(1–4)-β-GlcNAc-(1–3)-β-Gal-(1–3)-β-GlcNAc-(1–4) that is present in approximately 15% of the LPS molecules [21], which might compensate for the loss of the original outer core and contribute to high virulence in ducks. LPS is a strong stimulus for the production of innate immunity by binding to PRRs, while large amounts of LPS can also cause excessive proinflammatory responses that can lead to sepsis and even death [15]. Although lipid A is the main ligand for TLR4/MD-2, parts of the LPS polysaccharide backbone are also involved in the binding of the TLR4 ectodomain [39, 40], and the core oligosaccharide of the Pseudomonas aeruginosa LPS has been proven to be a kind of pathogen-associated molecular pattern recognized by the innate immune system, leading to host bactericidal defences [41]. The different LPS structures produced by ΔpcgD and ΔhptE might stimulate dissimilar inflammatory responses that partially led to the distinct consequences in virulence phenotypes displayed by the two mutants. To determine whether the innate immunity was altered, the cytokine profiles induced by purified LPS from the WT strain, the pcgD mutant, and the hptE mutant need to be evaluated both in vitro and in vivo. Furthermore, the colonization of both mutant strains was markedly decreased in the blood from 12 to 24 h post-infection, suggesting a profound clearance of the bacteria by the innate immunity in this period. As the pcgD or hptE mutation did not affect bacterial tolerance to the duck serum, it is worth detecting the susceptibility of both mutants to phagocyte ingestion and intracellular killing in the future, which are major components of innate immunity [42].
In conclusion, our study systematically investigated the role of two outer core transferase genes, pcgD and hptE, in susceptibility to duck serum, OM permeability and the virulence of P. multocida in ducks. The two gene mutations had no adverse effect on bacterial resistance to duck serum complement and OM permeability. Mutations in pcgD or hptE reduced bacterial virulence to a different extent compared with the WT strain. PMZ3 (ΔhptE) showed compromised colonization in only the blood, moderate splenic lesions, and severe liver injuries as well as reduced virulence via the oral route rather than the intramuscular route, whereas PMZ1 (ΔpcgD) displayed decreased colonization in the blood and spleen, moderate splenic and liver lesions and further reduced virulence via both the oral and intramuscular route. Therefore, deletion of the pcgD gene led to greater P. multocida virulence reduction in ducks.
Availability of data and materials
All datasets are presented in the paper or additional files supporting the manuscript.
Abbreviations
- P. multocida :
-
Pasteurella multocida
- FC:
-
Fowl cholera
- LPS:
-
Lipopolysaccharide
- PRR:
-
Pathogen pattern recognition receptor
- PEtn:
-
Phosphoethanolamine
- Hep:
-
Heptose
- Gal:
-
Galactose
- PCho:
-
Phosphocholine
- WT:
-
Wild-type
- BHI:
-
Brain heart infusion
- E. coli :
-
Escherichia coli
- LB:
-
Luria–Bertani
- TSA:
-
Tryptic soybean agar
- OD:
-
Optical density
- PBS:
-
Phosphate buffered saline
- Cm:
-
Chloramphenicol
- Amp:
-
Ampicillin
- Kan:
-
Kanamycin
- CFU:
-
Colony forming unit
- SDS-PAGE:
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- OM:
-
Outer membrane
- NPN:
-
N-phenyl-1-naphthylamine
- MIC:
-
Minimum inhibitory concentration
References
Wilson BA, Ho M (2013) Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev 26:631–655
Abreu F, Rodriguez-Lucas C, Rodicio MR, Vela AI, Fernandez-Garayzabal JF, Leiva PS, Cuesta F, Cid D, Fernandez J (2018) Human Pasteurella multocida infection with likely zoonotic transmission from a pet dog, Spain. Emerg Infect Dis 24:1145–1146
Blackall PJ, Pahoff JL, Marks D, Fegan N, Morrow CJ (1995) Characterisation of Pasteurella multocida isolated from fowl cholera outbreaks on turkey farms. Aust Vet J 72:135–138
Kardos G, Kiss I (2005) Molecular epidemiology investigation of outbreaks of fowl cholera in geographically related poultry flocks. J Clin Microbiol 43:2959–2961
Mohamed MA, Mohamed MW, Ahmed AI, Ibrahim AA, Ahmed MS (2012) Pasteurella multocida in backyard chickens in Upper Egypt: incidence with polymerase chain reaction analysis for capsule type, virulence in chicken embryos and antimicrobial resistance. Vet Ital 48:77–86
Harper M, John M, Turni C, Edmunds M, St Michael F, Adler B, Blackall PJ, Cox AD, Boyce JD (2015) Development of a rapid multiplex PCR assay to genotype Pasteurella multocida strains by use of the lipopolysaccharide outer core biosynthesis locus. J Clin Microbiol 53:477–485
Wilkie IW, Harper M, Boyce JD, Adler B (2012) Pasteurella multocida: diseases and pathogenesis. Curr Top Microbiol 361:1–22
Harper M, Boyce JD, Adler B (2012) The key surface components of Pasteurella multocida: capsule and lipopolysaccharide. Curr Top Microbiol 361:39–51
Petruzzi B, Briggs RE, Tatum FM, Swords WE, De Castro C, Molinaro A, Inzana TJ (2017) Capsular polysaccharide interferes with biofilm formation by Pasteurella multocida serogroup A. Bio 8:e01843-17
Zhao X, Liu Q, Xiao K, Hu Y, Liu X, Li Y, Kong Q (2016) Identification of the crp gene in avian Pasteurella multocida and evaluation of the effects of crp deletion on its phenotype, virulence and immunogenicity. BMC Microbiol 16:125
Steen JA, Steen JA, Harrison P, Seemann T, Wilkie I, Harper M, Adler B, Boyce JD (2010) Fis is essential for capsule production in Pasteurella multocida and regulates expression of other important virulence factors. PLoS Pathog 6:e1000750
Megroz M, Kleifeld O, Wright A, Powell D, Harrison P, Adler B, Harper M, Boyce JD (2016) The RNA-binding chaperone Hfq is an important global regulator of gene expression in Pasteurella multocida and plays a crucial role in production of a number of virulence factors, including hyaluronic acid capsule. Infect Immun 84:1361–1370
Xiao K, Liu Q, Liu X, Hu Y, Zhao X, Kong Q (2015) Identification of the Avian Pasteurella multocida phoP gene and evaluation of the effects of phoP deletion on virulence and immunogenicity. Int J Mol Sci 17:12
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420:885–891
Tan Y, Kagan JC (2014) A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol cell 54:212–223
Periasamy S, Praveena PE, Singh N (2018) Effects of Pasteurella multocida lipopolysaccharides on bovine leukocytes. Microb Pathog 119:225–232
Horadagoda NU, Hodgson JC, Moon GM, Wijewardana TG, Eckersall PD (2002) Development of a clinical syndrome resembling haemorrhagic septicaemia in the buffalo following intravenous inoculation of Pasteurella multocida serotype B:2 endotoxin and the role of tumour necrosis factor-alpha. Res Vet Sci 72:194–200
Harper M, Boyce JD (2017) The myriad properties of Pasteurella multocida lipopolysaccharide. Toxins 9:254
Harper M, Boyce JD, Cox AD, St Michael F, Wilkie IW, Blackall PJ, Adler B (2007) Pasteurella multocida expresses two lipopolysaccharide glycoforms simultaneously, but only a single form is required for virulence: identification of two acceptor-specific heptosyl I transferases. Infect Immun 75:3885–3893
St Michael F, Harper M, Parnas H, John M, Stupak J, Vinogradov E, Adler B, Boyce JD, Cox AD (2009) Structural and genetic basis for the serological differentiation of Pasteurella multocida Heddleston serotypes 2 and 5. J Bacteriol 191:6950–6959
Boyce JD, Harper M, St Michael F, John M, Aubry A, Parnas H, Logan SM, Wilkie IW, Ford M, Cox AD, Adler B (2009) Identification of novel glycosyltransferases required for assembly of the Pasteurella multocida A:1 lipopolysaccharide and their involvement in virulence. Infect Immun 77:1532–1542
Harper M, Cox A, St Michael F, Parnas H, Wilkie I, Blackall PJ, Adler B, Boyce JD (2007) Decoration of Pasteurella multocida lipopolysaccharide with phosphocholine is important for virulence. J Bacteriol 189:7384–7391
Harper M, Wright A, St Michael F, Li J, Deveson Lucas D, Ford M, Adler B, Cox AD, Boyce JD (2017) Characterization of two novel lipopolysaccharide phosphoethanolamine transferases in Pasteurella multocida and their role in resistance to cathelicidin-2. Infect Immun 85:e00557-e617
Harper M, Cox AD, St Michael F, Wilkie IW, Boyce JD, Adler B (2004) A heptosyltransferase mutant of Pasteurella multocida produces a truncated lipopolysaccharide structure and is attenuated in virulence. Infect Immun 72:3436–3443
Harper M, Boyce JD, Wilkie IW, Adler B (2003) Signature-tagged mutagenesis of Pasteurella multocida identifies mutants displaying differential virulence characteristics in mice and chickens. Infect Immun 71:5440–5446
Edwards RA, Keller LH, Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157
Bosse JT, Durham AL, Rycroft AN, Kroll JS, Langford PR (2009) New plasmid tools for genetic analysis of Actinobacillus pleuropneumoniae and other pasteurellaceae. Appl Environ Microbiol 75:6124–6131
Kittelberger R, Hilbink F (1993) Sensitive silver-staining detection of bacterial lipopolysaccharides in polyacrylamide gels. J Biochem Biophys Methods 26:81–86
Loh B, Grant C, Hancock RE (1984) Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551
Luo HY, Liu MF, Wang MS, Zhao XX, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Biville F, Zou YF, Jing B, Cheng AC, Zhu DK (2018) A novel resistance gene, lnu(H), conferring resistance to lincosamides in Riemerella anatipestifer CH-2. Int J Antimicrob Agents 51:136–139
Scott PC, Markham JF, Whithear KG (1999) Safety and efficacy of two live Pasteurella multocida aro-A mutant vaccines in chickens. Avian Dis 43:83–88
Harper M, John M, Edmunds M, Wright A, Ford M, Turni C, Blackall PJ, Cox A, Adler B, Boyce JD (2016) Protective efficacy afforded by live Pasteurella multocida vaccines in chickens is independent of lipopolysaccharide outer core structure. Vaccine 34:1696–1703
Chung JY, Wilkie I, Boyce JD, Townsend KM, Frost AJ, Ghoddusi M, Adler B (2001) Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A. Infect Immun 69:2487–2492
Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D (2016) Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol 14:337–345
Chang PC, Wang CJ, You CK, Kao MC (2011) Effects of a HP0859 (rfaD) knockout mutation on lipopolysaccharide structure of Helicobacter pylori 26695 and the bacterial adhesion on AGS cells. Biochem Biophys Res Commun 405:497–502
Dam S, Pages JM, Masi M (2018) Stress responses, outer membrane permeability control and antimicrobial resistance in Enterobacteriaceae. Microbiology 164:260–267
van der Heijden J, Reynolds LA, Deng W, Mills A, Scholz R, Imami K, Foster LJ, Duong F, Finlay BB (2016) Salmonella rapidly regulates membrane permeability to survive oxidative stress. mBio 7:e01238-16
Bojkovic J, Richie DL, Six DA, Rath CM, Sawyer WS, Hu Q, Dean CR (2015) Characterization of an Acinetobacter baumannii lptD deletion strain: permeability defects and response to inhibition of lipopolysaccharide and fatty acid biosynthesis. J Bacteriol 198:731–741
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Conde-Alvarez R, Arce-Gorvel V, Iriarte M, Mancek-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacon-Diaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, Grillo MJ, Jerala R, Brandenburg K, Llobet E, Bengoechea JA, Moreno E, Moriyon I, Gorvel JP (2012) The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog 8:e1002675
Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerceker AA, Golan DE, Pier GB (2002) CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci USA 99:6907–6912
Weiss G, Schaible UE (2015) Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 264:182–203
Rubires X, Saigi F, Pique N, Climent N, Merino S, Alberti S, Tomas JM, Regue M (1997) A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J Bacteriol 179:7581–7586
Acknowledgements
We thank Fuxiang Yang, Huaying Luo, Jin Li, Yanwan Li, Yi Liao and Xiaoli Zeng in our laboratory for generous assistance during the study.
Funding
This research was supported by the National Key Research and Development Program of China (2017YFD0500800), the Applied Basic Research Programs of Science and Technology Department of Sichuan Province (2019YJ0436), the Program Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (SCCXTD-2020–18), the China Agricultural Research System (CARS-42–17) and the Special Fund for Key Laboratory of Animal Disease and Human Health of Sichuan Province (2016JPT0004).
Author information
Authors and Affiliations
Contributions
XZ and AC designed the experiments; XZ and HS analysed the data and drafted the manuscript; XZ, Hui Shen and SL performed most of the experiments; DZ, MW, RJ, and SC constructed the plasmids and mutants; ML, QY, YW, SZ, JH, XO, SM, QG, LZ, YL, YY, LP participated in the animal experiments; AC edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal treatments were approved by the Animal Ethics Committee of Sichuan Agricultural University and the Sichuan Administration Committee of Laboratory Animals under protocol number SYXK2019-187.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2. Alignments of the gene sequences of
petG among three genotype L1 strains, PM0818, VP161 (NZ_CP048792, PmVP161_RS04065) and X73 (NZ_CM001580, X73_RS06225). PM0818 petG shared > 99% identity with the homologs of the VP161 and X73 but was also a pesudogene due to a single base deletion at nucleotide 1076 like the VP161 petG compared with the homolog of the X73 strain. The blue and red indicate the positions of the point mutation and the base deletion, respectively.
Additional file 3. Characterization of the
P. multocida mutant strains via PCR. The WT strain and PMZ1 (ΔpcgD) mutant were identified using primers P1-F/R, P2-F/R, and P3-F/R to confirm the pcgD mutation; similarly, the WT and PMZ3 (ΔhptE) were identified using primers P4-F/P1-R, P2-F/P5-R, and P6-F/R to confirm the hptE mutation. M refers to the DNA marker.

Additional file 4. The growth curves.
The P. multocida strains were grown in BHI medium overnight, and the cultures were diluted to an OD600 of 0.05 in fresh medium. Then the bacterial growth was determined by measuring the OD600 value every 0.5 h during the first 0 h-2 h and every 2 h from 3 to 15 h at 37 °C.
Additional file 5. Serum bactericidal assay.
The P. multocida strains were cultured in media to an OD600 of 0.6 and diluted to a final concentration of 104 CFU/mL in PBS. Then, the bacteria were mixed with 90% normal duck serum or heat-inactivated duck serum and incubated for 3 h at 37 °C. After incubation, serial dilutions of the samples were spread on TSA plates for counting. The survival rate of each strain was calculated as the CFU after active serum treatment divided by the CFU after the heat-inactivated serum treatment.
Additional file 6. NPN assay.
The log phase growth medium of the P. multocida strains was harvested, and the bacteria were resuspended in PBS at an OD600 of 0.5 with 80 µM NPN. PBS without bacteria was used as a control. The fluorescence of the suspension was measured immediately using a spectrofluorometer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, X., Shen, H., Liang, S. et al. The lipopolysaccharide outer core transferase genes pcgD and hptE contribute differently to the virulence of Pasteurella multocida in ducks. Vet Res 52, 37 (2021). https://doi.org/10.1186/s13567-021-00910-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-021-00910-4