Abstract
Bone marrow mesenchymal stromal cells (BM-MSCs) have anti-inflammatory and pro-survival properties. Naturally, they do not express human leukocyte antigen class II surface antigens and have immunosuppressive capabilities. Together with their relatively easy accessibility and expansion, they are an attractive tool for organ support in transplantation and regenerative therapy. Autologous BM-MSC transplantation alone or together with transplanted islets improves β-cell function, graft survival, and glycemic control in diabetes. Albeit MSCs’ capacity to transdifferentiate into β-cell is limited, their protective effects are mediated mainly by paracrine mechanisms through BM-MSCs circulating through the body. Direct cell–cell contact and spontaneous fusion of BM-MSCs with injured cells, although at a very low rate, are further mechanisms of their supportive effect and for tissue regeneration. Diabetes is a disease of long-term chronic inflammation and cell therapy requires stable, highly functional cells. Several tools and protocols have been developed by mimicking natural fusion events to induce and accelerate fusion in vitro to promote β-cell-specific gene expression in fused cells. BM-MSC-islet fusion before transplantation may be a strategy for long-term islet survival and improved function. This review discusses the cell-protective and anti-inflammatory characteristics of BM-MSCs to boost highly functional insulin-producing cells in vitro and in vivo, and the efficacy of their fusion with β-cells as a path to promote β-cell regeneration.
Similar content being viewed by others
Background
For the first time in 1966, Friedenstein et al. introduced and characterized bone marrow-derived cells from mice and called a group of fibroblast–shaped cells in culture with the potential to differentiate into multilineages in vitro “bone marrow mesenchymal stromal cells (BM-MSCs)” [1]. Later studies found these mesenchymal stromal/stem cells (MSCs) in many other tissues such as blood, umbilical cord blood, amniotic fluid, skin, foreskin, heart muscle, lung, pancreas, adipose tissue, dental pulp [2,3,4], and also in human islets [5]. BM-MSCs are multipotent cells that can be isolated from bone marrow aspiration and easily expand in culture without loss of function. Besides their capacity to self–renew in vivo [6, 7], they can differentiate into several cell types of mesenchymal, endodermal, and ectodermal origins [8]. In 2006, the international society for cellular therapy introduced minimal characterization criteria for MSCs, i.e., the expression of surface antigens CD73 (identified by the MAb SH3 and SH4), CD90 and CD105 (identified by the MAb SH2) and the negativity for CD34 (primitive hematopoietic progenitors marker), CD45 (pan–leukocyte marker), CD19 and CD79a (B cell marker), CD14 and CD11b (monocyte and macrophage marker) and HLA class II as well as their adherence to plastic dishes in culture and their differentiation capacity into chondrogenic, osteogenic, and adipogenic lineages [9]. The bone marrow only consists of approximately 0.01–0.001% MSCs [10]. However, BM-MSCs rapidly proliferate in culture, and their proliferation rate accelerates in platelet lysate instead of fetal bovine serum (FBS) [11] and at lower oxygen tension as mimicry of their native microenvironment [12]. Injected BM-MSCs into blastocyst can proliferate and differentiate into all organs in response to tissue-specific signals [8]. MSCs, despite their high proliferative potential, are negative for OCT4, preventing teratoma formation [13].
MSCs circulate through the bloodstream, migrate, and home in on injured tissues. They have not only the potential to transdifferentiate into different lineages [14, 15] but can also fuse with somatic cells in vitro as well as in vivo [16,17,18]. Transplantation of MSCs into injured tissue improves repair mechanisms [19,20,21] through modulation of the immune response [22], transdifferentiation [23, 24], fusion with target cells in injured tissues [21] and increased proliferation [23].
The lack of donor antigens, low level of HLA class I, and absence of HLA class II make allogeneic MSCs a suitable source for transplantation [10, 25]. Therefore, BM-MSCs are in trials for the therapy of autoimmune diseases, e.g., graft versus host disease (GVHD) [26], Crohn’s disease [27], multiple sclerosis [28], and type 1 diabetes [29,30,31].
In summary, multiple cell regeneration supportive properties make MSCs a suitable tool for clinical studies through their
-
Easy accessibility
-
Immunomodulatory, anti-inflammatory, and anti-apoptotic effects
-
Angiogenic potential
-
Capability to differentiate into multilineages like adipocytes, neurons, and pancreatic β-cells
-
Lack of teratoma formation.
Many studies show MSCs derived from adipose tissue and umbilical cord as promising sources for therapy (please see previous comprehensive overviews [32,33,34,35,36,37]). This review focuses specifically on multipotent bone marrow mesenchymal stromal cells (BM-MSCs), underlying mechanisms how they can protect pancreatic β-cells, their potential use for β-cell regeneration and as source for cell-based diabetes therapy. Furthermore, studies of spontaneous and induced BM-MSCs-target cell fusion as method for cellular differentiation are discussed. Fusion of MSCs with β-cells to make β-MSCs have been performed exclusively using BM-MSCs. We hypothesize that fusion of MSCs with target cells shares similar mechanisms independently of the source of MSCs.
BM-MSCs in clinical studies for diabetes therapy
Over 50 clinical trials for MSC applications, reaching from tackling diabetes-related vascular damage and impaired wound healing to treating new-onset type 1 diabetes (T1D) and type 2 diabetes (T2D), have been registered in the last decade [30, 38]. The first trial of autologous BM-MSCs transplantation for T1D has started in 2010 at Uppsala University Hospital (NCT01068951); 20 adult patients with newly diagnosed T1D received combined BM-MSCs/insulin or insulin only, which resulted in a preserved or even increased C-peptide response in BM-MSC-treated patients without any discernible side effects during the 1-year study period. However, there was no difference in HbA1c levels or insulin doses [29]. In T2D, several studies show improved C-peptide and lowered insulin requirements by BM-MSCs therapy [39, 40]. A recent meta-analysis which included bone marrow-, umbilical cord-, adipose-, and Wharton’s jelly-derived MSC therapy in patients with T1D and T2D confirmed improved C-peptide levels, lower HbA1c and blood glucose levels, and lesser insulin requirement without serious or chronic adverse responses [38] and concludes a more beneficial effect of MSCs in the treatment of T1D than in T2D. In contrast, another recent meta-analysis [41] presented no difference in C-peptide levels in T1D but improvement in T2D and comes to the conclusion of MSC therapy being more effective in improving β-cell function in T2D.
Inclusion of several underpowered studies, different study designs, trial durations, heterogenous patient groups (e.g., age and time of diagnosis), non-transparent data reports from several trials and the use of MSCs of various origins raise some doubts on reproducibility of various studies and their conclusions. Therefore, well-designed standardized randomized studies with larger numbers of patients and longer observation periods are needed to finally prove a long-term effective MSC therapy for patients with T1D and T2D [30, 38].
MSCs in diabetes: mechanisms of action
The clinical application of MSCs is motivated from in vitro cell culture and in vivo studies in animal models of numerous diseases with promising results [28, 30, 42, 43]. BM-MSCs can differentiate into functional β-cells [3, 44]; (Tables 1, 2), but also only little if any transdifferentiation capacity of MSCs-to-β-cells [45,46,47] and no spontaneous cell–cell fusion [24, 48] have been observed.
MSCs regulate both innate and adaptive immune responses by coordinating cell-to-cell contacts and the secretion of soluble humoral factors with immunosuppressive effects on target cells, such as immune, vascular, and endocrine cells [22]. Such factors show supportive anti-inflammatory, anti-apoptotic, and pro-survival effects on pancreatic β-cells [31, 49] (Fig. 1, Table 1).
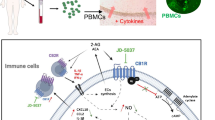
Protective mechanisms of MSCs in vivo. Both sole transplantation and co-transplantation of MSCs with islets or dissociated islet cells into the portal vein or the kidney capsule affect β-cell mass replenishment and transplantation outcome through indirect (immune and endothelial cells) and direct effects between MSCs and the β-cell. Danger signals sent out from the β-cell are detected by MSCs and tissue repair mechanisms are in place, e.g., via CXCL12/SDF signals, growth factor signals, N-cadherin-mediated direct cell–cell contacts, and secreted annexin, or by the formation of tunneling nanotubes which enable the exchange of mitochondria. In concert, these mechanisms promote β-cell survival/apoptosis protection, proliferation, and improved β-cell function
BM-MSCs reduce activation, survival, and proliferation of CD4+ or CD8+ cytotoxic T cells; they were shown to diminish activation of dendritic and natural killer (NK) cells, increasing Tregs and reducing cytokine secretion [22, 50, 51], thus balancing the immune response. Thereby, the lesser T cell proliferation and the induction of regulatory Tregs can simultaneously occur in a monocyte-dependent manner [52] with the outcome of decreased inflammation [53]. All these effects would be of great importance toward inhibiting autoimmunity and T1D progression. An inflammatory environment propels MSCs to respond with anti-inflammatory effects to promote rapid tissue regeneration. Activated MSCs diminish pro-inflammatory signals mainly by releasing soluble factors: (1) secreting Prostaglandin E2 (PGE2) and Indolamine-2,3-dioxygenase (IDO) in response to pro-inflammatory insults, such as lipopolysaccharide, TNF-α and nitric oxide (NO) secreted from active macrophages and injured tissues, (2) producing TNF-stimulated gene-6 protein (TSG-6) that interacts with pro-inflammatory macrophages to reduce NF-κB signaling cascades and (3) promoting expression and secretion of anti-inflammatory Interleukin-1 receptor antagonist (IL-1Ra) [54] with highly protective effects on β-cell function and survival [55, 56]. MSCs also secrete several other factors with immunomodulatory properties on β-cells, e.g., transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), interleukin-10 (IL-10) [57], heme oxygenase-1 (HO-1) [14] and matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9) [15, 29] (Table 1).
BM-MSC-released SDF1/CXCL12 bound to CXCR4 on β-cells improve their survival via activation of AKT [58]. Both in vitro and in vivo studies showed that BM-MSCs activate important pro-survival AKT, ERK, and FoxO1, all leading to the expansion of the β-cell population [59,60,61,62]. Additionally, activation of the major downstream executor of the apoptotic pathway, caspase 3, is reduced in β-cells when islets are co-transplanted with MSCs [63]. MSCs can also reduce endoplasmic reticulum (ER) stress-induced apoptosis in islets through downregulation of ER chaperone (BIP) and apoptosis-inducing ER stress protein (CHOP) and enhanced c-Myc expression (64). The functional improvement in MSC-islet co-cultures partially comes from annexin A1 which is secreted and highly expressed in MSCs. The direct exposure of high concentrations of secreted ANXA1 during MSC-islet co-culture adds to the paracrine mediated supportive effects [65].
Also, direct cell–cell contacts of MSCs and islet cells after co-transplantation contribute to the β-cell-protective effect, shown through N-cadherin interactions which improves insulin secretion efficiently [66]. Mitochondria from MSCs can transfer through tunneling nanotube-like structures to β-cells, a response probably induced through “danger signals” sent from damaged islets during transplantation stress to MSCs, which then support survival and insulin secretory function [67] (Fig. 1).
MSCs express a set of chemokine receptors such as CX3C chemokine receptor 1 (CX3CR1) and CXC chemokine receptor 12 (CXCR12), which are attracted by their specific ligands CX3CL1 and CXCL12 expressed in pancreatic islets [68]. Hence, MSCs could also serve as vehicle for effective drug, gene, or protein delivery to targeted cells, i.e., to islet cells in vivo [69, 70]. Such gene transfers could promote insulin production and vascularization and prevent apoptosis in islets [71]. For example, co-transplantation of MSCs overexpressing hepatocyte growth factor (HGF) and interleukin-1 receptor agonist (IL-1Ra) (HGF+IL-IRa+MSCs) in islets improves the outcome of islet transplantation [70]. Also, PDX1-expressing MSCs can transform into β-like cells which produce insulin after transplantation in vivo, display glucose-stimulated insulin secretion, and reduce hyperglycemia in diabetic mice [72].
BM-MSCs have been widely used for autologous, allogeneic transplantation or co-transplantation, to reduce the severity of disease and injuries [73]. Initially, autologous bone marrow-derived stromal progenitor cells were infused to facilitate engraftment and contribute to the recovery of hematopoiesis after bone erosion and bone marrow transplantation for cancer treatment [74]. BM-MSCs have been progressively explored as adjuvants to improve the outcome of therapies and avoid relapse. For instance, BM-MSCs increase body weight in weak and severely diabetic STZ (streptozotocin)-induced mice [73], together with the restoration of normoglycemia and β-cell function. Co-transplantation of MSCs improves and prolongs transplantation efficiency and outcome [68], again through paracrine effects which suppress immune response and inflammation [63, 75,76,77] (Fig. 1).
BM-MSCs for β-cell regeneration
BM-MSCs promote physiological tissue repair by migrating into injury sites, such as in cardiac infarction, where systemically as well as locally transplanted MSCs home in on injured tissue. As diabetes is a chronic disease with subclinical levels of inflammation rather than an acute tissue injury, spontaneous migration of endogenous MSCs may be limited. However, GFP-labeled infused BM-MSCs localize near pancreatic ducts, possibly to accelerate differentiation of pancreatic progenitor cells in addition to promote immune regulation and functional improvement in β-cells within islets [59]. BM-MSC-promoted β-cell regeneration has been observed indirectly by proliferation induction of pancreatic ductal cells and islet cell clusters, which led to increased pancreatic progenitor cell numbers and insulin production [23]. Successful induction of cell fusion, in vivo or in vitro, maybe a strategy to combine favorable characteristics of BM-MSC with the powerful glucose metabolism regulation by highly functional β-cells (Fig. 3) [78].
Cell fusion as tool for cell regeneration
Life begins with cell fusion. During fertilization, sperm fuses with the egg. Later, fusion repeatedly happens during organ development and in adults, e.g., macrophage fusion with target cells during infection or MSC fusion with damaged cells for tissue repair [79].
For the first time, spontaneous cell fusion resulting in multinucleated cells in vitro was seen in 1927 by W. H. Lewis [80]. Later in 1960, fusion of two heteroploid mouse cells resulted in the generation of hybrid cell lines from two different sarcomas [81]. Then, Harris and Ringertz successfully fused various cell types from different species and discovered that UV-inactivated Sendai virus induces fusion in cell culture. By fusing mammalian Hela cells with chicken erythrocytes, they observed condensed and inactive chromosomes in birds’ erythrocytes, but interspecies heterokaryons express chicken-specific RNA, indicating that DNA and RNA synthesis had been re-activated in the chicken nuclei after fusion [82]. Constant co-culture of two different mouse cell lines results in approximately 10% hybrid cells after three months, according to chromosome variations due to spontaneous fusion [81]. Fusion of mouse BM-MSCs with other cell types in culture showed that fused cells adopt the phenotype of the recipient cells, such as beating cardiac myocytes [83].
That BM-MSCs can transdifferentiate both directly and indirectly after fusion with injured cells in vivo is supported by a previous study: While a sub-population of BM-MSCs within a mixed population of injured epithelial cells transdifferentiate into epithelial cells, fusion is another phenomenon in up to 1% of the cells identified as epithelial+polyploid cells [57].
Cell fusion is a powerful attempt toward tissue regeneration. Three fundamental methods to induce it in culture have been developed using (1) inactivated viruses, (2) chemical agents including PEG, and (3) electric pulses [82, 84, 85]. Fusion occurs between the same or different cell types and results in transient homo- or heterokaryons with three distinct outcomes: homokaryons (> 1 of the same nuclei in a polyploid cell), heterokaryons (> 1 of different nuclei in a polyploid cell), and synkaryons (nuclei have fused in a polyploid cell) (Fig. 2). While homo- or heterokaryons are non-dividing cells and frequently transient, the nucleus of a synkaryon has a combined chromosome pool of all nuclei; they can become proliferative and eventually make hybrids. During cell fusion, the epigenetic and genetic information of the different cell types is combined. This leads to new cellular expression patterns, starting within few hours of the heterokaryon state by remodeling the chromatin and switching-on transacting regulators at key loci [79, 86]. Fusion between interspecies cells, like rat and human cells, often leads to the formation of stable interspecies heterokaryons, where nuclei are not combined. This phenomenon brings a unique opportunity to trace the variation of the chromosome pool in an intact nucleus after the fusion event and to study the first phases and epigenetic changes in lineage reprogramming [87], in iPSC generations [86, 88] and differentiation [89]. The cell fate could be bidirectional after fusion; but is later fixed to only one of the parental cell fates, depending on the relative nuclear dosage [90]. Fusion of a pluripotent with a somatic cell gives rise to a dominant pluripotent cell fate [91]. The dominant fate after fusion of two different somatic cells depends on their cell type, e.g., heterokaryons from human keratinocytes and mouse myotubes display a more keratinocyte fate [90]; from mouse melanoma and rat hepatoma results in both melanin and albumin production, but not at the same time [92]. By increasing the ratio of somatic to pluripotent stem cells, one can activate the expression of all cell lineage determinants sequentially [93]. The new cell fate in heterokaryons is determined by the structural information in the chromosomes, such as DNA methylation/demethylation patterns, transcription factors, and non-coding RNAs leading to repression or activation of specific genes; dominant and recessive fates are changeable after fusion depending on the activity of chromatin-modifying enzymes [94].
Outcome of cell fusion in culture. Fusion is a natural phenomenon that can occur between cells of the same or of different types. In vitro cell fusion can be achieved through three major methods, i.e., chemicals including polyethylene glycol (PEG), inactivated viruses, and electric pulse (electrofusion). At the beginning, a cell is formed in which the nuclei do not merge (heterokaryon). This state could be transient in case both cells are from the same species which ends up in a synkaryon, or they remain separated in interspecies heterokaryons. Chromatin remodeling starts within few hours after heterokaryon formation and its combination with the genetic reprograms will define the fate of the resultant synkaryon cell up to the development of hybrid cells
BM-MSCs and cell fusion in vivo
BM-MSCs can turn into a new phenotype via fusion with target cells in vivo [17, 57, 79, 95, 96]. BM-MSCs traced by the Cre-loxP system in mice after transplantation show transdifferentiation into cardiac, lymphatic and kidney tissues. Surprisingly, cell fusion is the ubiquitous phenomenon. Monitoring these cells over 5 months did not reveal any signs of cancer development [96]. The ratio of spontaneous fusion increases with age and time after transplantation [97] and the frequency of fusion is higher with larger cells with larger cytoplasmic volume, e.g., Purkinje neurons, skeletal myotubes, cardiomyocytes, and hepatocytes [79]. One must still accept that spontaneous cell fusion in vivo without the presence of pro-inflammatory conditions or intense tissue injury is a rare event [57, 79, 98].
First evidence of in vivo reprogramming after fusion was based on changes in the chromatin as sign of a fused cell: BM-MSCs injection into mice leads to BM-MSCs-purkinje-neuron-binucleated-heterokaryons in the mouse brain [97]. First time reported in humans in a cerebellar tissue autopsy from a female recipient of male MSCs shows 0.1% of her Purkinje neurons tetraploid (XXXX) or containing both X and Y chromosomes (XXXY) as a sign of cell fusion of male BM-MSCs and female neurons [97]. Similar results come from pancreas autopsies from opposite-gender cord blood MSCs recipients, which detected nuclear fusion based on two sets of opposite sex chromosomes in one nucleus. 1.5% of opposite-sex insulin-expressing cells were seen and 0.76% of the insulin-producing cells are polyploid with three or even more sex chromosomes and enlarged nuclei, assuming that at least half of the differentiation events resulted from cell fusion in the pancreas, even in a non-diabetic environment. From other studies, there is lesser evidence of transdifferentiation via cell fusion to β-cells in vivo in humans [99] or mice [24]. As functional heterokaryons do exist in vivo [97, 100, 101], it remains to be assessed whether these polyploid cells seen after BM-MSC transplantation will progress to fully functional cells.
In line with the hypothesis that tissue damage is a prerequisite for MSC trafficking and fusion, other studies observed an increase in heterokaryon cells after apoptosis induction [18] or chronic inflammation [98]. Therefore, various methods were established to improve fusion efficiency in vitro [82, 84, 85], e.g., a microfluidic device which improves fusion efficiency in suspension culture [102].
BM-MSCs-β-cell fusion: evidence for highly functional β-MSCs
Compared to other differentiation methods, e.g., gene transfer, in vitro cell fusion imitates nature as a phenomenon that occurs to rescue cells in danger and has a well-documented safety profile in numerous clinical studies. Improving cell fusion in vitro can be a strategy for transplantation to restore survival of cells in damaged tissue. As a proof of concept, Flatt et al. established a functional human β-cell line (1.1B4) through electrofusion of the epithelial-like human pancreatic carcinoma cell line (PANC–1) with human pancreatic islet cells. 1.1B4 hybrid cells have stable characteristics in culture and secrete insulin upon glucose stimulation. The implantation of 1.1B4 cells into Streptozotocin (STZ)-diabetic mice decreases glucose levels and improves diabetes [89]. However, recently, several 1.1B4 clones have been found inhomogeneous as they contain a mixture of rodent and human cells and do not fully retain the human β-cell phenotype [103].
Given the protective nature of BM-MSC-islet co-transplantation and the feasibility of cell fusion, Yanai et al. produced β-MSCs from rat BM-MSCs and mouse dispersed islet cells by electrofusion. They showed slightly higher insulin secretion after one day in β-MSCs which dramatically increases compared to co-cultured BM-MSCs/dispersed islets after 20 days, with higher proliferation rates and lower caspase 3 expression levels [104].
Later, we developed and improved a fusion protocol of human BM-MSCs with rat INS1E β-cells, which led to 5% to up to 30% successfully fused cells seen as β-MSC heterokaryons. Fusion showed an increase in β-cell functionality and identity genes, compared to INS1E control cells, such as insulin, NKX6.1, Nkx2.2, Neurod1, MafA, and Pdx1. Such induction of functional genes after fusion was also confirmed upon fusion of BM-MSCs with human-dispersed islet cells from organ donors. Importantly, fused cells show improved functionality over co-cultured MSC-dispersed islets [78] (Fig. 3). In vivo β-MSC-islets also gradually normalized blood glucose levels after transplantation [104].
MSCs and β-cells and fusion to β-MSCs. β-MSC transdifferentiation after fusion may initially result in immature polyhormonal cells and later process to the expression of mature β-cell markers. Epigenetic modifiers and transcription factors are major contributors to this process inside the shared cytoplasm. Further differentiation may be achieved after transplantation
Despite promising results, cell fusion is still in its experimental stage and many questions on the characteristics of newly fused cells and how to steer the fusion process remain unanswered. None of the previous studies compared numbers of heterokaryons and hybrids that were formed in culture. Long-term stability and functionality of newly formed islets in vivo and to what extend MSCs remain mature in β-MSC-islets has never been investigated.
When somatic cells fuse with pluripotent cells, most of the resulting hybrids maintain their pluripotent character, and only few hybrids switch to somatic types. Our studies showed higher fusion rates by increasing the number of β-cells over BM-MSCs. Whether higher fusion rates correlate with the number of insulin positive β-MSCs and how fused cells choose to transdifferentiate into their desirable fate has not been addressed. In-depth investigation and characterization of interspecies heterokaryon stages will help answering these questions and evaluate quantity and quality of fusion and the genetic profile and stability of fused cells before they are ready to enter clinical trials.
Conclusion
BM-MSCs are a natural physiological source for β-cell protection after injury and can be fused with β-cells to obtain reprogrammed functional β-MSCs with improved survival. In vivo studies confirm their stability and glucose-normalizing efficacy over weeks. However, long-term in vivo studies and the characterization of genomic and epigenetic patterns of insulin-producing β-MSCs are needed to prove safety, stability, and potential for diabetes therapy. Nature’s imitation of spontaneous in vivo fusion gives hope in regenerative medicine as another strategy for cell differentiation, including β-cells.
Availability of data and materials
Not applicable. No specific data have been generated for this review.
References
Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90.
Bongso AaEHL. Stem cells: from bench to bedside2005.
Pavathuparambil Abdul Manaph N, Sivanathan KN, Nitschke J, Zhou XF, Coates PT, Drogemuller CJ. An overview on small molecule-induced differentiation of mesenchymal stem cells into beta cells for diabetic therapy. Stem Cell Res Ther. 2019;10(1):293.
Cheung TS, Bertolino GM, Giacomini C, Bornhauser M, Dazzi F, Galleu A. Mesenchymal stromal cells for graft versus host disease: mechanism-based biomarkers. Front Immunol. 2020;11:1338.
Carlotti F, Zaldumbide A, Loomans CJ, van Rossenberg E, Engelse M, de Koning EJ, et al. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets. 2010;2(3):164–73.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34.
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–7.
Verbeek R. Generation of mesenchymal stem cells as a medicinal product in organ transplantation. Curr Opin Organ Transplant. 2013;18(1):65–70.
Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213(1):18–26.
Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207(2):331–9.
Li HL, Wei JF, Fan LY, Wang SH, Zhu L, Li TP, et al. miR-302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4-dependent manner. Cell Death Dis. 2016;7: e2078.
Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82(1):66–76.
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–6.
Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422(6934):901–4.
Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10(5):575–83.
Yang WJ, Li SH, Weisel RD, Liu SM, Li RK. Cell fusion contributes to the rescue of apoptotic cardiomyocytes by bone marrow cells. J Cell Mol Med. 2012;16(12):3085–95.
Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–19.
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–94.
Rustad KC, Gurtner GC. Mesenchymal stem cells home to sites of injury and inflammation. Adv Wound Care (New Rochelle). 2012;1(4):147–52.
Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7: e2062.
Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21(7):763–70.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111(6):843–50.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–6.
Yin F, Battiwalla M, Ito S, Feng X, Chinian F, Melenhorst JJ, et al. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem cells (Dayton, Ohio). 2014;32(5):1278–88.
Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis. 2019;14(1):64–70.
Iacobaeus E, Kadri N, Lefsihane K, Boberg E, Gavin C, Törnqvist Andrén A, et al. Short and long term clinical and immunologic follow up after bone marrow mesenchymal stromal cell therapy in progressive multiple sclerosis—a phase I study. J Clin Med. 2019;8(12):2102.
Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64(2):587–92.
de Klerk E, Hebrok M. Stem cell-based clinical trials for diabetes mellitus. Front Endocrinol (Lausanne). 2021;12: 631463.
Path G, Perakakis N, Mantzoros CS, Seufert J. Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism. 2019;90:1–15.
Camara BOS, Bertassoli BM, Ocarino NM, Serakides R. Differentiation of mesenchymal stem cells from humans and animals into insulin-producing cells: an overview in vitro induction forms. Curr Stem Cell Res Ther. 2021;16(6):695–709.
Refaie AF, Elbassiouny BL, Kloc M, Sabek OM, Khater SM, Ismail AM, et al. From mesenchymal stromal/stem cells to insulin-producing cells: immunological considerations. Front Immunol. 2021;12: 690623.
Ghoneim MA, Refaie AF, Elbassiouny BL, Gabr MM, Zakaria MM. From mesenchymal stromal/stem cells to insulin-producing cells: progress and challenges. Stem Cell Rev Rep. 2020;16(6):1156–72.
Qi Y, Ma J, Li S, Liu W. Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res Ther. 2019;10(1):274.
Takahashi H, Sakata N, Yoshimatsu G, Hasegawa S, Kodama S. Regenerative and transplantation medicine: cellular therapy using adipose tissue-derived mesenchymal stromal cells for type 1 diabetes mellitus. J Clin Med. 2019;8(2).
Stiner R, Alexander M, Liu G, Liao W, Liu Y, Yu J, et al. Transplantation of stem cells from umbilical cord blood as therapy for type I diabetes. Cell Tissue Res. 2019;378(2):155–62.
Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, et al. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. 2021;12(1):273.
Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23(9):1075–85.
Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, et al. Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: a randomized. Placebo-Controlled Compar Study Stem Cells Dev. 2017;26(7):471–81.
Hwang G, Jeong H, Yang HK, Kim HS, Hong H, Kim NJ, et al. Efficacies of stem cell therapies for functional improvement of the beta cell in patients with diabetes: a systematic review of controlled clinical trials. Int J Stem Cells. 2019;12(2):195–205.
Fu J, Wang Y, Jiang Y, Du J, Xu J, Liu Y. Systemic therapy of MSCs in bone regeneration: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12(1):377.
Henriksen JL, Sorensen NB, Fink T, Zachar V, Porsborg SR. Systematic review of stem-cell-based therapy of burn wounds: lessons learned from animal and clinical studies. Cells. 2020;9(12).
Jafarian A, Taghikhani M, Abroun S, Pourpak Z, Allahverdi A, Soleimani M. Generation of high-yield insulin producing cells from human bone marrow mesenchymal stem cells. Mol Biol Rep. 2014;41(7):4783–94.
Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53(3):616–23.
Choi JB, Uchino H, Azuma K, Iwashita N, Tanaka Y, Mochizuki H, et al. Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic beta cells. Diabetologia. 2003;46(10):1366–74.
Ezquer F, Ezquer M, Contador D, Ricca M, Simon V, Conget P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30(8):1664–74.
Dadheech N, Srivastava A, Vakani M, Shrimali P, Bhonde R, Gupta S. Direct lineage tracing reveals Activin-a potential for improved pancreatic homing of bone marrow mesenchymal stem cells and efficient ss-cell regeneration in vivo. Stem Cell Res Ther. 2020;11(1):327.
Hubber EL, Rackham CL, Jones PM. Protecting islet functional viability using mesenchymal stromal cells. Stem Cells Transl Med. 2021;10(5):674–80.
Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7.
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom HJ, et al. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol. 2011;25(1):7–15.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042–6.
Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20.
Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A. 2004;101(21):8138–43.
Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, et al. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci U S A. 2002;99(12):8236–41.
Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100(5):2397–402.
Yano T, Liu Z, Donovan J, Thomas MK, Habener JF, Garcia-Ocaña A. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes. 2007;56:2946–57.
Gao X, Song L, Shen K, Wang H, Qian M, Niu W, et al. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol Cell Endocrinol. 2014;388:41–50.
Khatri R, Mazurek S, Petry SF, Linn T. Mesenchymal stem cells promote pancreatic beta-cell regeneration through downregulation of FoxO1 pathway. Stem Cell Res Ther. 2020;11(1):497.
Liu C, Zhang W, Peradze N, Lang L, Straetener J, Feilen PJ, et al. Mesenchymal stem cell (MSC)-mediated survival of insulin producing pancreatic beta-cells during cellular stress involves signalling via Akt and ERK1/2. Mol Cell Endocrinol. 2018;473:235–44.
Khatri R, Petry SF, Linn T. Intrapancreatic MSC transplantation facilitates pancreatic islet regeneration. Stem Cell Res Ther. 2021;12(1):121.
Borg DJ, Weigelt M, Wilhelm C, Gerlach M, Bickle M, Speier S, et al. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57(3):522–31.
He Y, Zhang D, Zeng Y, Ma J, Wang J, Guo H, et al. Bone marrow-derived mesenchymal stem cells protect islet grafts against endoplasmic reticulum stress-induced apoptosis during the early stage after transplantation. Stem Cells. 2018;36(7):1045–61.
Rackham CL, Vargas AE, Hawkes RG, Amisten S, Persaud SJ, Austin ALF, et al. Annexin A1 is a key modulator of mesenchymal stromal cell-mediated improvements in islet function. Diabetes. 2016;65:129–39.
Montanari E, Meier RPH, Mahou R, Seebach JD, Wandrey C, Gerber-Lemaire S, et al. Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice. Stem Cell Res Ther. 2017;8:199.
Rackham CL, Hubber EL, Czajka A, Malik AN, King AJF, Jones PM. Optimizing beta cell function through mesenchymal stromal cell-mediated mitochondria transfer. Stem Cells. 2020;38(4):574–84.
Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–27.
D’souza N, Rossignoli F, Golinelli G, Grisendi G, Spano C, Candini O, et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015;13:1–15.
Wu H, Lu W, Mahato RI. Mesenchymal stem cells as a gene delivery vehicle for successful islet transplantation. Pharm Res. 2011;28:2098–109.
Chen NKF, Tan SY, Udolph G, Kon OL. Insulin expressed from endogenously active glucose-responsive EGR1 promoter in bone marrow mesenchymal stromal cells as diabetes therapy. Gene Ther. 2010;17:592.
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transpl. 2008;14(6):631–40.
Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–64.
Hayward JA. Co-transplantation of mesenchymal stem cells with islet grafts. 2017.
Wang H, Strange C, Nietert P, Wang J, Turnbull T, Cloud C, et al. Autologous Mesenchymal Stem Cell and Islet Cotransplantation: Safety and Efficacy. STEM CELLS Translational Medicine. 2017;7.
Ben Nasr M, Vergani A, Avruch J, Liu L, Kefaloyianni E, D’Addio F, et al. Co-transplantation of autologous MSCs delays islet allograft rejection and generates a local immunoprivileged site. Acta Diabetol. 2015;52:917–27.
Azizi Z, Lange C, Paroni F, Ardestani A, Meyer A, Wu Y, et al. β-MSCs: successful fusion of MSCs with β-cells results in a β-cell like phenotype. Oncotarget. 2016;7:48963.
Dittmar T, Zänker KS. Cell Fusion in Health and Disease: II: Cell Fusion in Disease. 2011;950.
Lewis WH. The formation of giant cells in tissue cultures and their similarity to those in tuberculosis lesions. Am Rev Tuberculosis. 1927;15:616.
Barski G, Sorieul S, Cornefert F. “Hybrid” type cells in combined cultures of two different mammalian cell strains. J Natl Cancer Inst. 1961;26:1269–91.
Harris H, Watkins JF. Hybrid cells derived from mouse and man: artificial heterokaryons of mammalian cells from different species. Nature. 1965;205:640.
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542.
Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic cell genetics. 1975;1:397–400.
Teissie J, Knutson VP, Tsong TY, Lane MD. Electric pulse-induced fusion of 3T3 cells in monolayer culture. Science. 1982;216:537–8.
Tsubouchi T, Soza-Ried J, Brown K, Piccolo FM, Cantone I, Landeira D, et al. DNA synthesis is required for reprogramming mediated by stem cell fusion. Cell. 2013;152:873–83.
Brown KE, Fisher AG. Reprogramming lineage identity through cell-cell fusion. Curr Opin Genet Dev. 2021;70:15–23.
Lluis F, Ombrato L, Pedone E, Pepe S, Merrill BJ, Cosma MP. T-cell factor 3 (Tcf3) deletion increases somatic cell reprogramming by inducing epigenome modifications. Proc Natl Acad Sci. 2011;108:11912–7.
McCluskey JT, Hamid M, Guo-Parke H, McClenaghan NH, Gomis R, Flatt PR. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J Biol Chem. 2011;286:21982–92.
Palermo A, Doyonnas R, Bhutani N, Pomerantz J, Alkan O, Blau HM. Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional. FASEB J. 2009;23:1431–40.
Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704.
Fougère C, Weiss MC. Phenotypic exclusion in mouse melanoma-rat hepatoma hybrid cells: pigment and albumin production are not reexpressed simultaneously. Cell. 1978;15:843–54.
Wong WT, Matrone G, Tian X, Tomoiaga SA, Au KF, Meng S, et al. Discovery of novel determinants of endothelial lineage using chimeric heterokaryons. Elife. 2017;6: e23588.
Terranova R, Pereira CF, Du Roure C, Merkenschlager M, Fisher AG. Acquisition and extinction of gene expression programs are separable events in heterokaryon reprogramming. J Cell Sci. 2006;119:2065–72.
Savatier P. Introduction of mouse embryonic fibroblasts into early embryos causes reprogramming and (con)fusion. Cells. 2021;10(4).
Bonde S, Pedram M, Stultz R, Zavazava N. Cell fusion of bone marrow cells and somatic cell reprogramming by embryonic stem cells. FASEB J. 2010;24:364–73.
Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959.
Johansson C, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel S, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–83.
Huang CJ, Butler AE, Moran A, Rao PN, Wagner JE, Blazar BR, et al. A low frequency of pancreatic islet insulin-expressing cells derived from cord blood stem cell allografts in humans. Diabetologia. 2011;54:1066–74.
Kemp K, Gordon D, Wraith DC, Mallam E, Hartfield E, Uney J, et al. Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells. Neuropathol Appl Neurobiol. 2011;37:166–78.
Larsson L-I, Bjerregaard B, Talts JF. Cell fusions in mammals. Histochem Cell Biol. 2008;129:551–61.
Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Microfluidic control of cell pairing and fusion. Nat Methods. 2009;6:147.
Chaffey JR, Young J, Leslie KA, Partridge K, Akhbari P, Dhayal S, et al. Investigation of the utility of the 1.1B4 cell as a model human beta cell line for study of persistent enteroviral infection. Sci Rep. 2021;11(1):15624.
Yanai G, Hayashi T, Zhi Q, Yang KC, Shirouzu Y, Shimabukuro T, et al. Electrofusion of mesenchymal stem cells and islet cells for diabetes therapy: a rat model. PLoS ONE. 2013;8(5): e64499.
Pankajakshan D, Agrawal DK. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J Biomed Technol Res. 2014;1(1).
Acknowledgements
This review was supported by the German Research Foundation (DFG) and the German Academic Exchange Service (DAAD; Re-invitation Programme for Former Scholarship Holders to AN). It has been planned before and finalized during a sabbatical leave at the University of Bremen in September 2021 and extends scientific collaboration between Tehran and Bremen.
Funding
Open Access funding enabled and organized by Projekt DEAL. This review was funded by the German Research Foundation (DFG) and the German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Contributions
ZA wrote the first draft; RA, RS and EM contributed to the ideas; AN helped with figures; KM discussed structure and content, wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Animal or human data or tissue
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azizi, Z., Abbaszadeh, R., Sahebnasagh, R. et al. Bone marrow mesenchymal stromal cells for diabetes therapy: touch, fuse, and fix?. Stem Cell Res Ther 13, 348 (2022). https://doi.org/10.1186/s13287-022-03028-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03028-2