Abstract
Non-traumatic intracerebral hemorrhage is a highly destructive intracranial disease with high mortality and morbidity rates. The main risk factors for cerebral hemorrhage include hypertension, amyloidosis, vasculitis, drug abuse, coagulation dysfunction, and genetic factors. Clinically, surviving patients with intracerebral hemorrhage exhibit different degrees of neurological deficits after discharge. In recent years, with the development of regenerative medicine, an increasing number of researchers have begun to pay attention to stem cell and exosome therapy as a new method for the treatment of intracerebral hemorrhage, owing to their intrinsic potential in neuroprotection and neurorestoration. Many animal studies have shown that stem cells can directly or indirectly participate in the treatment of intracerebral hemorrhage through regeneration, differentiation, or secretion. However, considering the uncertainty of its safety and efficacy, clinical studies are still lacking. This article reviews the treatment of intracerebral hemorrhage using stem cells and exosomes from both preclinical and clinical studies and summarizes the possible mechanisms of stem cell therapy. This review aims to provide a reference for future research and new strategies for clinical treatment.
Similar content being viewed by others
Introduction
Non-traumatic intracerebral hemorrhage (ICH), which accounts for almost 15% of strokes, is a highly destructive intracranial disease associated with high mortality and morbidity1, half the deaths occur in the first two days post-stroke, and the mortality rate is as high as 40% within the 1st month after the onset of a stroke2. ICH can significantly reduce patients’ quality of life and place a huge economic burden on the family and society, as most survivors experience severe neurological deficits. The main risk factors associated with ICH include hypertension, amyloidosis, vasculitis, drug abuse, coagulation dysfunction, and genetic factors 3–6. Genetically, gene polymorphisms, such as endogenous tissue inhibitors of metalloproteinases; tumor necrosis factor-α (TNF-α); interleukin (IL), angiotensin-converting enzyme, coagulation factor XIII, and plasminogen activator inhibitor-1 genes; and point mutations, including Ras homolog family member A (RhoA), apoproteins H (apoH), and platelet-activating factor acetylhydrolase, may be associated with ICH [7,8,9,10,11,12,13,14].
Brain injury after cerebral hemorrhage can be primary or secondary. In primary brain injury, blood vessels rupture to form a hematoma, causing direct mechanical damage owing to local compression. Hematoma expansion further compresses the surrounding normal brain tissue, resulting in loss of nerve function [15]. Hematomas not only cause mechanical compression but also induce toxicity, inflammation, oxidative stress, and apoptosis-of-hematoma metabolites, which can further damage the nervous system and cause secondary brain injury [16,17,18]. Surgical removal of a compressing hematoma, when indicated, may relieve intracranial pressure, prevent brain herniation, improve cerebral blood perfusion, remove toxic substances, reduce cerebral edema severity, and improve survival [19]. Hematoma evacuation techniques range from traditional craniotomy to minimally invasive techniques, such as neuroendoscopic hematoma removal and stereotactic hematoma aspiration. However, these treatments have a moderate effect on outcomes, and despite surgical evacuation or optimal medical treatment, the prognosis of ICH remains poor [20, 21]. Experimental results have been promising but have not yet been translated into therapeutic benefits. Recently, with the growing popularity of regenerative medicine, research has focused on stem cell and/or exosome therapy for ICH. Stem cells have been widely used in the study of ICH owing to their strong reproductive ability, low differentiation degree, multi-lineage differentiation potential, anti-inflammatory properties, low immunogenicity, immunomodulatory ability, and secretory ability. Exosomes derived from stem cells are also sought after, because they possess some of the characteristics and transport capabilities of primary cells [22].
In addition, stem cells and exosomes can be harvested from a wide range of sources, including embryos, bone marrow, umbilical cord, adipose tissue, nerve tissue, and induced somatic cells [23,24,25,26]. Numerous animal studies have shown that stem cells can directly or indirectly participate in ICH treatment through regeneration, differentiation, or secretion. However, considering the uncertainty of its safety and efficacy, clinical studies are still lacking. This article reviews preclinical and clinical studies of ICH treatment using stem cells and exosomes and summarizes the possible mechanisms of stem cell therapy. Simultaneously, it provides a reference for future research and “point-to-point therapy.”
Stem cells sources for ICH treatment
Stem cells have unlimited reproductive capacity and can differentiate into cells with specific functions. Depending on their differentiation potential, they can be divided into totipotent, pluripotent, and multipotent stem cells. In a broad sense, totipotent stem cells can produce all extraembryonic tissues and can differentiate into any type of cell in the body, such as blastomeres. Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can only differentiate into certain cells in the three germ layers. Multipotent stem cells, including mesenchymal stem cells (MSCs) and neural stem cells (NSCs), can produce all cell types within a particular lineage [27,28,29].
In order to simulate a human ICH, two mouse models of cerebral hemorrhage have been mainly used: the collagenase model, where injection of a bacterial enzyme digests the collagen present in the basal lamina of the blood vessels to produce spontaneous brain hemorrhage [30], and the blood infusion model, where a fixed amount of blood (autologous or donor) is injected into the brain parenchyma to mimic a primary accumulation, similar to human ICH [31]. In the former, the bleeding process mimics the continuous process in patients with ICH, and mice show more typical and obvious neurological dysfunction. Owing to its rapid emergence, the latter model can better mimic brain tissue damage from hematoma pressure and blood outside the blood vessels. The blood infusion model best mimics the nerve recovery process of patients with ICH [32, 33]. Therefore, different model establishment methods can be chosen for the intended research purpose. After establishing a good ICH animal model, researchers can inject stem cells or exosomes through a variety of routes for treatment, as shown in Fig. 1
Stem cells and stem cell-derived exosomes are used to treat ICH mice. Phase 1: Cell extraction: stem cell extraction from various sources followed by cultivation and extraction of stem cells or exosomes as required (③, ④). Phase 2: ICH animal model establishment: cerebral hemorrhage model through autologous blood or collagenase stereotactic injection (①, ②). Phase 3: cell culture and extraction of stem cells or exosomes. Phase 4: Treat ICH animals with different administration methods. IPSCs: induced pluripotent stem cells; NSCs: neural stem cells; AD-MSCs: adipose-derived mesenchymal stem cells; hUC-MSCs: human umbilical cord mesenchymal stem cells; BM-MSCs: bone marrow mesenchymal stem cells; ESCs: embryonic stem cells; ICH: intracerebral hemorrhage; ABI: autologous blood injection; Col: collagenase; ICI: intracerebral injection; TVI: tail vein injection; IND: intranasal deliver; Exos: exosomes; and SCs: stem cells
Pluripotent stem cells
Pluripotent stem cells can be found in blastocysts and early embryonic developmental stages and can differentiate into multiple cell lineages [34]. These stem cells include mesenchymal stem cells (MSCs) and iPSCs.
Embryonic stem cells
In 1981, pluripotent ESCs were first extracted from in vitro mouse blastocysts [23]. These cells are highly undifferentiated and can self-renew, reproduce indefinitely, mature, and differentiate into any cell, including nerve cells [35, 36]. In 1995, ESCs were extracted from primates [37] and 3 years later from human cell lines. ESCs exist only during the early stages of embryonic development [38]. Because ESCs are capable of multidirectional differentiation, they have been used for in vitro and in vivo regeneration studies. Nonaka et al. used collagenase to establish an ICH rat model and utilized all-trans retinoic acid (ATRA) to induce nestin-positive neural stem cells in vitro. After 7 days of model establishment, ATRA-treated ESCs were infused into the healthy hemispheres. Ultimately, researchers have found neurons and astrocytes originating from ESCs in the area around the hematoma, highlighting the therapeutic potential of ESCs [39]. Owing to its low migratory ability, cell therapy for ICH remains a challenge. To solve this problem, Kang et al. selected safe and efficient ferromagnetic iron oxide nanocubes to label spherical neural masses derived from human embryonic stem cells and used magnetically embedded helmets for targeted delivery under an external magnetic field. In the experimental group, the hematoma volume and edema area were reduced, the infiltration capacity of inflammatory cells was low, and the inflammation cascade was weakened [40].
To date, we have identified very few publications regarding ESCs in cerebral hemorrhage, with even fewer human clinical trials. This is primarily due to two reasons. Although human ESCs (hESCs) can theoretically differentiate into more than 200 types of human cells, in reality, the majority of cell types are difficult to generate, and only 10 types of cells are truly functionally equivalent to normal human cells. Moreover, ESCs are difficult to separate and acquire. Moreover, ESCs may have oncogenic potential, raising serious concerns regarding their safety in clinical studies [41].
Induced pluripotent stem cells
In 2006, Takahashi et al. transported four exogenous genes (OCT4, KLF4, SOX2, and c-MYC) into somatic cells and successfully induced iPSCs, a new pluripotent stem cell line [25]. These newborn stem cells express marker genes, growth characteristics, and embryonic stem cell morphology. Subsequently, the distinctive features of these cells were revealed: iPSCs can replace injured or diseased tissue, resist inflammation, secrete biologically active substances, and exhibit low immunogenicity [42]. Eight years later, iPSCs were first implanted in patients with ophthalmopathy [43]. As pluripotent stem cells, iPSCs can differentiate into nerve cells and neuroepithelium-like/neuroepithelioid stem cells with secretory function. Qin et al. successfully transfected all the above-mentioned transcription factors into the fibroblasts of patients with ICH using viral vectors and eventually obtained iPSCs. In vitro, these cells were cultured in a fibroblast medium to induce differentiation of iPSCs into neural cells and neuroepithelial-like stem cells (NESCs). After injecting the differentiated cells into rats, nerve function was significantly improved, and NESCs appeared in the lesion area. Differentiated nerve cells can play a role in replacing damaged cells [44]. In addition to the site of differentiation and injury, subsequent experiments by Qin et al. found that iPSCs secreted brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) cytokines and inflammation-related factors (IL-1β, IL-6, TNF-α, and IL-10) during treatment [45, 46].
Although iPSCs have been available for less than 20 years, much research has been conducted [25]. However, few clinical and animal studies on iPSCs have been conducted. This seems related to the inefficiency of reprogramming, which represents a persistent problem in both laboratory and clinical studies. More importantly, in many experimental studies, the uncontrolled reproduction of iPSCs ultimately led to the formation of teratomas [47,48,49,50].
Multipotent stem cells
As an integral part of the stem cell family, multipotent cells differentiate into all cell types within a particular lineage. Mesenchymal stem cells and neural stem cells, as their representatives, are often used in preclinical and clinical studies [29].
Mesenchymal stem cells
In addition to the general differentiation and proliferation potential, MSCs proposed by the International Society for Cell Therapy must meet three minimum requirements. First, MSCs must adhere to plastic when maintained in standard culture conditions. Secondly, MSCs-surface markers must positively express CD73, CD90, and CD105 and negatively express CD11b or CD14, CD19 or CD79a, CD34, and CD45. Third, in vitro, they should be able to differentiate into osteoblasts, adipocytes, and chondroblasts [51] (Table 1). MSCs can be divided into bone marrow mesenchymal stem cells (BM-MSCs), human umbilical cord mesenchymal stem cells (hUC-MSCs), and adipose-derived mesenchymal stem cells (AD-MSCs) [26].
Bone marrow mesenchymal stem cells
BM-MSCs are stem cells derived from bone marrow. They possess multiple functions such as secretion, self-renewal, and differentiation [52, 53]. These cells can differentiate into chondroblasts, adipocytes, osteoblasts, and neural cells. BM-MSCs have the advantages of a simple extraction process, rapid expansion in vitro, low antigenicity, sufficient donor sources [54, 55], and the ability to pass through the blood–brain barrier (BBB) [56]. Hence, BM-MSCs have become a popular source of stem cells.
Researchers have found that bone marrow mesenchymal stem cells can not only differentiate into target cells and promote endogenous neurogenesis but may also reverse the ICH-induced destruction of the blood–brain barrier [57, 58]. In the ICH mouse model, exogenous BM-MSCs were injected into the brain parenchyma of ICH mice. The implanted cells differentiated into neurons and astrocytes, which promoted endogenous neurogenesis [59]. Meanwhile, intranasal injection of hypoxia-pretreated bone marrow mesenchymal stem cells into ICH mice promoted endogenous neurogenesis, induced the expression of neurotrophic factors, and improved functional defects [60] (Table 2). Additionally, BM-MSCs can improve the prognosis of ICH by genetic modification. Taking advantage of adenoviruses, BM-MSCs were eventually transfected into genetically engineered cells. Glial cell-derived neurotrophic factor (GDNF)-transduced rat bone marrow MCSs were implanted into ICH rats. Compared with BM-MSCs, modified cells can preferentially transform into astrocytes, which improves the outcome of sensory, motor, balance, and reflex tests, reduces lesions, and inhibits apoptosis [71]. Liang reported that the limb function of rats with cerebral hemorrhage recovered well after injection of BM-MSCs. The proportion of brain reorganization increased post-treatment, but the hematoma volume size of the experimental group was not significantly different after treatment, inconsistent with previous studies. Although BM-MSCs may not be able to prevent ICH-induced pontine atrophy, they can enhance corticospinal tract nerve regeneration and effectively improve nerve function recovery [69].
Interestingly, BM-MSCs can repair the BBB while repairing nerve injury [57, 58]. Chen et al. [58] reported that after intravenous injection of BM-MSCs, the neuromotor function of ICH mice improved by inhibiting inflammation, reducing cell apoptosis, and ultimately reversing the destruction of the BBB. The repair effect of BM-MSCs may be due to endothelial progenitor cells (EPC), which can repair damaged endothelial cells, form new neurons, and promote the recovery of new blood vessels and endothelial function [64, 91, 92]. Pías et al. reported that ICH can cause an increase in EPCs in vivo. The mechanism by which EPC affects cerebral hemorrhage may involve multiple processes, including re-endothelialization, angiogenesis, paracrine mechanisms, and protection of the BBB [93]. Chemokine ligand 12 (CXCL12), also called stromal derived factor-1, is significantly higher in injured areas and has the ability to activate various stem cells [94]. Li et al. utilized endothelial cell (EC) marker tyrosine kinase receptor-2, von Willebrand factor, and VE-cadherin to detect the degree of EPC differentiation and found that by the C-X-C chemokine receptor type 4 pathway, CXCL12 can stimulate EPC differentiation into EC and promote formation of new blood vessels. Of course, there is a dose-dependent relationship between CXCL12 and EPC [92]. EC is also important for nerve repair after ICH injury. Matta and colleagues found that EC could increase the expression of VEGF receptor 3 in NSCs by secreting VEGF-C and improve the survival rate of NCSs. This finding provides hope for the promotion of endogenous nerve cells to treat ICH in the future [95].
In addition to rodent models, studies using primate models have shown promising results. Feng et al. transfected BM-human mesenchymal stem cells into Macaca fascicularis monkeys and found that not only did the clinical test results significantly improve but also that a series of 18F-FDG PET and neurological function scores showed improvement compared to the control group [74].
Human umbilical cord mesenchymal stem cells
hUC-MSCs are pluripotent stem cells obtained using a painless procedure. In 2003, Archers et al. isolated and obtained hUC-MSCs from Wharton's jelly (WJ) [96]. Currently, hUC-MSCs are commonly acquired from the umbilical cord or fetal cord blood [97, 98]. Owing to advantages such as abundance, accessibility, low immunogenicity, strong reproductive ability, multi-lineage differentiation potential, and ease of harvesting, hUC-MSCs have been frequently utilized in ICH research [89, 98,99,100,101,102,103,104]. Unlike other stem cells, however, they do not seem able to directly replace defective neurons through differentiation into astrocytes or neurons [105,106,107,108,109]. In a rat model, Nan et al. found that after intravenous administration of hUC-MSCs, neurological functional recovery was significantly improved, although rather surprisingly, very few MSC were found in the brain parenchyma [103]. These results suggest that hUC-MSCs do not need to penetrate the brain parenchyma to induce therapeutic effects. To achieve better results, Zhang et al. combined the application of hUC-MSCs with minimally invasive hematoma removal. They reported that this dual treatment was superior to any single treatment and reduced nerve cell death [77]. Acute hematoma removal may reduce hematoma compression, whereas hUC-MSCs directly or indirectly exert neuroprotective effects with the help of soluble substances [77]. A recently published study that combined umbilical cord-derived MSCs with hyperbaric oxygen to treat ICH in mice reported that combined treatment was significantly better than a single treatment [77]. Although they believe that this is inseparable from the low immunogenicity of hUC-MSCs and the effects of hyperbaric oxygen therapy on improving MSCs levels, the specific molecular mechanism remains to be elucidated [110, 111]. WJ-derived mesenchymal stromal/stem cells (WJ-MSCs) are hUC-MSCs with primitive stemness properties. Fasudil is a Rho kinase (ROCK) inhibitor that inhibits the ROCK pathway, which is involved in neuronal functions, including synapse outgrowth and retraction. The microenvironment of ICH is hypoxic compared with that of normal brain tissue [112]. Lee et al. reported that under hypoxic microenvironmental conditions that mimic ICH, fasudil activates WJ-MSCs, which can significantly promote the transformation of WJ-MSCs to neuron-like cells and accelerate the secretion of GDNF to drive endogenous neurogenesis. In addition, neurological deficits in ICH rats were reversed by transferring these primed cells. Interestingly, primed MSCs mediated by fasudil significantly enhanced the number of double-positive cells (microtubule-associated protein 2 (MAP2) + /HuMit +), although the specific molecular mechanism requires further research [113]. In a similar experiment, Liu et al. found that under hypoxic conditions, miR-326 could target the PTBP1/PI3K signaling pathway to reduce the aging of olfactory mucosa MSCs (OM-MSCs), thereby augmenting the therapeutic efficacy of MSCs in ICH. They believe that the reduction in OM-MSC senescence is closely related to an increase in autophagy in OM-MSCs [114]. Placenta-derived mesenchymal stem cells (PSCs) can also be obtained when hUC-MSCs are acquired. In the acute phase after ICH, transfer of PSCs into mice can decrease intracranial pressure and reduce the size of the hematoma, which may be because PSCs increase the number of tight junction proteins, which are related to vascular integrity [115]. Choi et al. [115] also noted that compared with the PSC group, there was a significantly higher level of coagulation factor VII mRNA expression in the control group, indicating that the increased consumption of coagulation factors was the cause of this phenomenon. Interestingly, although PSCs enhanced the motor performance of ICH mice, no evidence of PSC differentiation into astrocytes or neurons was found in lesions surrounding the hematoma [116].
Adipose-derived mesenchymal stem cells
AD-MSCs, a type of mesenchymal stem cell with tri-potential differentiation ability, are easily separated from adipose tissue [102]. These cells are not only rich in content and sources but can also induce targeted differentiation [117]. Compared to other stem cells, AD-MSCs have several advantages, such as large numbers, easy accessibility and isolation, stable reproduction, and minimal damage to donors [40, 118, 119]. Therefore, AD-MSCs have attracted significant attention.
In a mouse model of ICH, human AD-MSCs can be used not only to enhance neural function by inhibiting CD11b + CD45 + subpopulation inflammation [120], but also to improve behavioral testing capabilities by differentiating into endothelial lineages [65]. Through in vivo and in vitro experiments, Chen et al. found that the neurological function of mice with cerebral hemorrhage was significantly improved after treatment with rat AD-MSCs (rAD-MSCs). However, no differentiation into endothelial cells was observed. In contrast, these cells transform into neuron-like and glial-like cells, and the expression of neuronal nuclei or glial fibrillary acidic protein is positive. These two discrepancies may be due to differences in cell culture and composition. Therefore, the loss of neuron-like and glial-like cells, secretion of the neuroprotective substance VEGF, and reduction of apoptosis may induce these beneficial effects [62]. Li et al. reported that although stem cell therapy may offer certain advantages, treatment may be delayed owing to the long cell preparation time. To overcome these limitations, utilizing rAD-MSCs with high expression of CX3CR1 can improve the migration ability of transplanted cells in vitro and in vivo and achieve better prognosis [121].
Neural stem cells
In 1992, Reynolds and Weiss obtained a self-renewing and multi-differentiation cell from the striatum of the adult mouse brain called neural stem cells (NSC) [24]. This discovery has changed the long-standing notion that nerves cannot be regenerated. Subsequently, an increasing number of NSCs were confirmed to exist in the subgranular zone of the dentate gyrus and the subventricular zone [122]. NSCs are multipotent cells that can differentiate into astrocytes, oligodendrocytes, and neurons [123]. These characteristics have driven scientific research [124]. When ICH occurs, the brain tissue around the hematoma is ischemic and hypoxic, and the cells change from aerobic phosphorylation to anaerobic glycolysis [125]. Owing to the low energy production of anaerobic glucose metabolism, it is particularly important to use limited energy to maintain cell life. In in vitro studies, Lehane et al. [126] found that carbimazole could maintain adenosine triphosphate content by inhibiting the translation process of energy-consuming proteins under hypoxic conditions to protect neurons from hypoxic injury. This undoubtedly reduces nerve damage caused by secondary hypoxia after hematoma. In addition, transplanted NSCs can improve neurological deficits in rodents, but the survival rate of exogenous nerve cells in the host has still plagued researchers. In in vitro experiments using human NSCs (hNSCs), Santilli et al. found that different oxygen concentrations have an important effect on the proliferation and differentiation of nerve cells. They further concluded that mild hypoxic (2.5–5% oxygen) conditions in vitro could promote the proliferation and differentiation of human neural stem cells, which raises hopes for ICH in vivo research [127]. Wakai et al. reported that reactive oxygen species can induce apoptosis in rNSCs transplanted into ICH mice [79]. However, subsequent experiments found that after rNSCs pretreated for 24 h under 5% hypoxia were implanted into ICH rats, hypoxia preconditioning promoted the proliferation of rNSCs. Hypoxic preconditioning accelerated the functional recovery of ICH mice and increased the expression of VEGF in the perihematoma region. The above results may be because hypoxia-inducible factor-1 alpha (HIF-1α) upregulates phosphorylated serine threonine kinase/Akt, enhances NSC resistance to hemoglobin cytotoxicity, and improves the proliferation of NSCs [128, 129]. Additionally, Lee et al. confirmed that genetic modification caused hNSCs to overexpress BDNF, GDNF, Akt1, and VEGF and significantly improved the recovery of neurological function in ICH mice [81,82,83].
In addition to transplanting exogenous stem cells, promoting the proliferation and differentiation of endogenous NSCs is promising. After repetitive transcranial magnetic stimulation in rats with ICH, NSCs directly differentiate into neurons, inhibit their differentiation into glial-like cells, and restore neural function [130]. In addition, transplantation of hESC-derived NSCs into mice with stroke increased endogenous neurogenesis [131]. Although NSCs have broad prospects in the treatment of cerebral hemorrhage, there are understandable ethical issues with NSCs obtained from fetal brains, which hinder their further clinical application [132, 133].
Mechanism of stem cell treatment in ICH
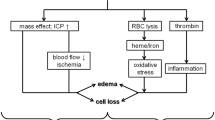
Although the mechanism of stem cell treatment in ICH remains unclear, researchers have discovered that it may involve anti-inflammatory mechanisms, neuroprotection, reduction of brain edema, and reversal of BBB damage [58, 60, 63, 78, 82, 134,135,136,137] (Fig. 2).
The mechanisms of mesenchymal stem cell therapy for ICH. A After ICH occurs, it can cause brain edema through the MAPK signaling pathway. Macrophages can also cause brain tissue edema by secreting cytokines such as TNF-α, IL-1β, and IL-6. In addition to directly inhibiting the above two methods, MSCs can also secrete BDNF to relieve cerebral edema. TNF-α secreted by macrophages can act on MSCs with the help of TNFR-1 and finally increase the level of Prostaglandin E2 (PGE2) through the NF-κB signaling pathway. PGE2 binds to EP2/EP4 on the surface of macrophages to promote IL-10 secretion, thereby inhibiting inflammation. MSCs can directly increase the expression of growth-associated protein-43 (GAP-43) through ERK1/2, thereby exerting neuroprotective effects. B Damaged tissue stimulates inflammatory cells (macrophages, astrocytes) to increase peroxynitrite (ONOO–), a strong oxidant, levels through signal pathways. Increased ONOO– can directly damage tight junction proteins and can also promote the production of Matrix metalloproteinase-9 (MMP-9) and damage tight junction proteins, ultimately leading to the blood–brain barrier BBB damage. TIMP-1 as a MMP inhibitor can inhibit MMP activation, but increasing ONOO– can also inhibit the biological effects of TIMP-1. ONOO– can also inhibit sodium potassium pump and cell metabolism to damage BBB. MSCs can secrete TSG-6, which inhibits the NF-κB signaling pathway through CD44, thereby inhibiting subsequent biological processes to improve damaged BBB
Relation to inflammation and low immunogenicity
Although hUC-MSCs cannot be directly differentiated into target cells to participate in damage repair, Liao et al. [138] observed that hUC-MSCs can treat ICH by increasing pro-angiogenic activity and suppressing the immune response. The upregulation of a variety of soluble bioactive substances, such as intracellular adhesion molecule-5, BDNF, oncostatin M, macrophage colony-stimulating factor, interleukin-1 receptor antagonist, and ciliary neurotrophic factor, contributes to the immune suppression response [57, 139,140,141,142,143]. Moreover, migrating cells can concurrently weaken leukocyte infiltration and microglial activation [134]. Interestingly, NSCs can not only directly alleviate the functional defects of cerebral hemorrhage by replacing damaged tissue but can also regulate immune cells and some inflammatory factors to play a neuroprotective role. Gao et al. [78] injected NSCs into mouse brain parenchyma and found a neuroprotective effect via modulation of T cell subpopulations and anti-inflammatory (IL-4, IL-10, and transforming growth factor beta), pro-inflammatory [IL-6, interferon gamma (IFN-γ)], and γδ-T cell cytokines. Gao et al. [144] also reported in vivo animal studies and in vitro cellular experiments showing that IL-17 can impede NSC differentiation into astrocytes and neurons and reverse their differentiation by neutralizing anti-IL-17 antibodies. In an ICH mouse model, Chen et al. [110] found that iPSCs-MSC therapy not only improved the recovery of nerve function but also significantly reduced inflammatory factors, including I-κB, NF-κB, TNF-α, IL-β, and inducible nitric oxide synthase (iNOS). iPSCs perform better than BM-MSCs in terms of immunogenic and immunomodulatory properties [145]. Human iPSCs do not express major histocompatibility complex (MHC)-II or costimulatory molecules but only show low levels of MHC-I [42].
Nerve protection and improvement of neurological symptoms
To study the potential ICH treatment mechanisms, Cui et al. established an accurate ICH model using rat autologous arterial blood. BM-MSCs were injected into rats via the retroorbital vein. At 3 and 7 days after administration, the neurological deficit had improved, and the expression of neurite extension marker growth-associated protein-43 (GAP-43) had increased. When mice were injected with the p-ERK1/2 inhibitor (PD98059) and/or PI3K inhibitor (LY294002), GAP-43 expression was reduced, especially when the two drugs were injected simultaneously. This is the first study to report that PD98059 and LY294002 impede the neuroprotective effect of BM-MSCs. The neuroprotective role of BM-MSC may be achieved by regulating the expression of GAP-43, which can be activated by the ERK1/2 and PI3K/Akt signaling pathways [63]. Cui et al. [146] reported a similar study on a BM-MSC conditioned medium. Chen et al. used IV collagen to establish an ICH mouse model and infused BM-MSCs into the lesions. They found that BM-MSCs can accelerate cell proliferation via the MST1/YAP/Hippo signaling pathway, ensure mesenchymal phenotype switching, and relieve neurological symptoms [147].
Reduce brain edema
Secondary brain edema after ICH is another cause of brain damage. Zhang et al. confirmed that rAD-MSCs could reduce brain edema, improve motor symptoms, and achieve this goal by limiting inflammation and Aquaporin4 protein expression. Aquaporin4 is an important factor related to cerebral edema, which can be reduced by downregulating the c-Jun N-terminal kinase pathway (JNK) pathway and phosphorylation of p38/MAPK [135].
Promoting angiogenesis
As a selective interface between the central nervous system and periphery [148], the blood–brain barrier (BBB) is localized at the level of the brain microvascular endothelial cells (BMVECs) that are interconnected together via intercellular junction proteins. Brain pericytes, astrocytes, and neurons communicate with the BMVECs to form the neurovascular unit (NVU). Junction proteins are composed of multiple transmembrane proteins and junctional adhesion molecules. The former includes claudins and occludins, while the latter includes zonula occludens (ZO) and cytoskeleton-related proteins [149, 150]. When ICH occurs, the BBB structure is damaged, which can be improved by EPCs and their secreted external vesicles [64, 91, 92, 151]. Zeng et al. [152] found that EVs secreted by mouse EPCs could promote proliferation, migration, and tube formation of BMECs. In another study on human cells, Loiola Azevedo et al. [151] found that human EPC-secretome decreased the permeability of confluent monolayers of endothelial cells by increasing the expression of occludin, VE-cadherin, and ZO-1. The number of EPCs was positively correlated with the prognosis. This finding has laid the foundation for future cell-free therapies. Annexin A1 (ANXA1), an anti-inflammatory agent, is expressed on microglia, BMVECs, and EVs and plays a role via formyl peptide receptors (FPRs) [153,154,155,156,157]. It has been reported that BBB permeability increases due to endothelial tight junction protein (TJ) degradation and actin microfilament instability when ANXA1 is knocked out [158]. However, ANXA1-FPR2 receptors interact with TJ formation by inactivating the small GTPase, RhoA [158, 159]. Therefore, this is a potential target for future research. In addition, MSCs can promote the maturation of astrocytes and BMVECs, which may be related to the role of VEGF; however, the specific molecular mechanism remains to be studied.
Protect the integrity of the BBB
The protective effect of BM-MSCs on the BBB can be achieved by increasing the levels of tumor necrosis factor-stimulated gene 6 (TSG-6). Specifically, TNF-α stimulates TSG-6 secretion by BM-MSCs. Increased TSG-6 expression can regulate astrocytes by inhibiting the NF-κB signaling pathway. Elevated TSG-6 levels inhibit iNOS, thereby inhibiting peroxynitrite (ONOO¯) production. Finally, this increase prevents BBB leakage and increases the content of tight proteins such as ZO-1 and occludin, thereby protecting the integrity of the BBB [58]. In addition, BM-MSCs may inhibit NF-κB signaling. When the NF-κB signal is weakened, the activation of MMP-9, which destroys vascular integrity, is hindered [137]. MMP-9 can also be hydrolyzed by endogenous tissue inhibitors of metalloproteinases. It has been shown to improve BBB in rats with stroke by TIMP-1 [160, 161]. Reuter et al. also reported that TIMP-2 gene polymorphism was related to ICH [12]. This may be a potential therapeutic target in the future.
BM-MSCs are very effective in improving endothelial function, avoiding BBB leakage, preventing ICH, and reducing intracerebral hematoma volume by selectively blocking IL-1 and TNF-α [136]. IL-1 and TNF-α gene polymorphisms reportedly increase the probability of rupture and bleeding in cerebral arteriovenous malformations [13, 14]. Future research may bring new hope for the treatment of ICH.
Exosomes
In the past decade, cell-free therapy has attracted widespread attention. As a safe cell-free therapy, exosomes derived from SCs have been tested in many diseases, such as spinal cord injury, nerve injury, Alzheimer's disease, pancreatic ductal adenocarcinoma, inflammatory bowel disease, and ocular disease [162].
Source and structure of exosomes
Heterogeneous vesicles derived from cells are called extracellular vesicles, which are mainly distinguished based on their size, such as exosomes (50–150 nm), microvesicles (100–1000 nm), oncosomes (1000–10,000 nm), and apoptotic bodies (100–5000 nm) [163, 164]. These vesicles may be difficult to distinguish from high-density lipoproteins, low-density lipoproteins, chylous particles, protein aggregates, and cell debris. First, the plasma membrane continuously sags inward to form multivesicular bodies, which then fuse with the plasma membrane. Finally, intraluminal vesicles are released into the extracellular cavity as exosomes [165,166,167]. Exosomes are microvesicles secreted by stem cells and other cells with a diameter of approximately 40–160 nm and have a lipid bilayer similar to that of a cell membrane. Exosomes from different cell sources contain different components, such as proteins, nucleic acids, lipids, amino acids, and metabolites. Meanwhile, exosomes have marker proteins (CD9, CD81, CD63, flotillin, TSG101, neuroide, and Alix) and some proteins related to exosome biogenesis (Rab GTPases and ESCRT proteins) [163, 164, 166, 168, 169]. Additionally, exosomes also transport RNA, antisense oligonucleotides, and drugs to their intended destinations [167] (Fig. 3). With the increasing attention paid to EVs, an increasing number of molecular components have been found in EVs. Therefore, three different databases have been developed to regroup the molecular data collected during studies on EVs: (i) Vesiclepedia (http://www.microvesicles.org/), (ii) ExoCarta (http://www.exocarta.org/), listing the identified contents of EXOs from multiple organisms, and (iii) EV-TRACK (http://evtrack.org/) using EV-METRIC not only designed as a tool to validate EV studies, but also to generate a wide public EV-TRACK knowledgebase from submitted and previously published experiments [163, 164, 170].
Exosomes carry substances for the treatment of ICH
Recently, with the in-depth study of exosomes, researchers have begun to experiment with their use in the treatment of ICH. Zhang et al. reported that BM-MSCs can secrete exosomes that highly express microRNA-21. Researchers have found that the therapeutic effect of high-expression microRNA-21 BM-MSCs is to deliver microRNA-21 through exosomes to regulate the transient receptor potential melastatin 7 and the NF-κB pathway, thereby protecting nerve cells [158]. In an autologous arterial blood ICH rat model, Shen et al. injected miR-133b-rich exosomes from BM-MSCs and found that the miR-133b content in the brain tissue increased. This is contrary to the report that ICH in the phosphate-buffered saline (PBS) group reduced the content of miR-133b. Therefore, it is speculated that the improved motor performance by increasing miR-133b after ICH is mediated by targeting blocked RhoA and activating the ERK1/2-CREB pathway [159]. After ICH, the levels of interleukin receptor-associated kinase 1 (IRAK1) and nuclear factor of activated T cells 5 (NFAT5) increased. The former activates the NF-κB signaling pathway to induce a variety of inflammatory responses. The latter can be activated by inflammation and participates in the neuroinflammatory response, promoting the polarization of microglia M1. Duan et al. injected exosomes derived from BM-MSCs loaded with miR-146a-5p into ICH mice via the tail vein. They found that in the BMSCs-miR-146a-5p-exosome group, neuronal apoptosis and inflammation were reduced, microglial polarization was inhibited, and damaged nerve function was significantly improved. They believe that this result is because exosomes transport miR-146a-5p to target cells, which in turn inhibits IRAK1 and NFAT5, thereby reducing ICH damage [171]. Ding et al. used extracellular vesicles derived from BM-MSCs to transfer miR-183-5p into the brains of mice with diabetic ICH. They observed that the inflammatory response was suppressed and behavioral function was improved. They speculated that extracellular vesicles carry miR-183-5p into target cells, and in turn, miR-183-5p targets and reduces programmed cell death 4, thereby inhibiting the expression of nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3. Ultimately, this inhibits neuroinflammation [172].
Exosomes are directly involved in the treatment of ICH
MSCs exosomes have a high proliferation rate and exhibit immunosuppressive activity. With increasing maturity levels, the capacity of MSCs to secrete exosomes is reduced [173]. Exosomes may not only serve as carriers for drug delivery [173, 174] but also play a therapeutic role. Otero et al. administered rAD-MSC-derived exosomes into mice via tail vein injection and found recovery of motor function, elevated levels of oligodendrocyte-associated markers, restoration of nerve fiber integrity, and promotion of axonal sprouting [175]. Han et al. injected bone marrow-derived exosomes into ICH mice and discovered that the number of new blood vessels and mature neurons around the hematoma increased and the neurological function of ICH mice improved. Angiopoietin is postulated to increase the density of blood vessels and promote the formation and growth of synapses in the brain. Additionally, by testing the three markers, doublecortin, MAP2, and β-tubulin-III, it was found that compared with the PBS control group, the content of subventricular zone precursor cells, which are important for the recovery of nerve function, increased in the exosome-treated group. It was also proposed that white matter increased in the exosome treatment group by increasing the remyelination of newborn microglia [176]. In addition, hUC-MSC-derived exosomes can potentially inhibit apoptosis and accelerate cell reproduction [177]. However, studies regarding hUC-MSC-derived exosomes are only experimental at the level of small animal studies [178], lacking large animal and clinical studies (Table 3).
Clinical aspects
With the progress in stroke and stem cell research, many clinical breakthroughs have been achieved. Some clinical and preclinical studies have demonstrated that BM-MSCs may eventually provide a novel, safe, reliable, and effective therapy for ICH, which can enhance nerve function and promote nerve recovery [179,180,181,182]. According to a case report published in 2016, researchers injected autologous BMSCs into the ventricles of two patients who were in a minimally conscious state after cerebral hemorrhage. After 12 months, the National Institutes of Health Stroke Scale scores of both patients had improved [183]. In a double-blind, phase I/II randomized controlled trial, autologous BM-MSCs were injected intravenously into patients with ICH. Patients were randomized to receive two intravenous injections of autologous MSCs or placebo 4 weeks apart. Stem cell treatment was concluded to be safe with improved neurological function compared with that of a placebo group [184]. In addition to hemorrhagic stroke, bone marrow mesenchymal stem cells play a pivotal role in clinical studies on ischemic stroke [185,186,187].
Recently, clinical trials of NSCs for the treatment of non-traumatic cerebral hemorrhage have also achieved exciting results, including transplantation of NSCs into the subarachnoid space of patients with ICH via lumbar puncture, demonstrating that exogenous cells repaired function deficiencies and reduced ICH volume [188]. A clinical follow-up study also confirmed that EPC levels in patients with cerebral hemorrhage are related to prognosis. Seven days after ICH, circulating EPC levels were positively related to prognosis and negatively related to the residual amount of hematoma [189]. From a clinical perspective, this finding may also reveal an appropriate time window for EPC administration, namely within 1 week after cerebral hemorrhage.
Although these results provide a theoretical basis for future applications, the clinical application of BM-MSCs is still progressing. Extracting bone marrow mesenchymal stem cells may be difficult for patients. With age, the normal bone marrow cavity is inevitably replaced by fat cells, making it more difficult to obtain BM-MSCs [190]. Furthermore, with increasing age, the proliferation and differentiation abilities of hBM-MSCs also decrease [191]. On the other hand, and more importantly, these transplanted cells may eventually develop into malignant tumors [192]. Zhu et al. [193] proposed that hMSCs secrete soluble substances, including VFGF, which are of great significance in promoting tumor growth.
Conclusion and future perspectives
Stem cell therapy has become an attractive potential treatment option for ICH. Stem cells may improve neurological deficits, enhance motor performance, and protect neural cells from damage. Nevertheless, research and application of stem cell therapy for ICH remain immature. At present, the mechanisms that regulate the therapeutic action between the host and migrated cells remain unclear and may involve filling and replacing damaged tissues, accelerating tissue regeneration, improving inflammation, regulating immune responses, and secreting soluble factors and exosomes. In vivo, only hUC-MSCs failed to differentiate into the target cell, but they seemed to be able to indirectly exert therapeutic effects through biologically active substances. At the molecular level, the underlying mechanisms are unclear. Currently, we believe that there are many deficiencies in the research on stem cell therapy for ICH. First, neither of the two animal models completely reproduced the actual pathological process of cerebral hemorrhage. Therefore, an appropriate modeling method should be selected according to the purpose of the study, and better modeling methods are warranted to address this issue. Second, the model animals were mostly selected from healthy adult animals, and there were deviations from ICH patients with other diseases. Third, after ICH, the extracellular microenvironment may impact the transplanted cells, and simple in vitro experiments may not fully simulate the real situation in vivo. More clinical trials should be conducted under suitable conditions. Moreover, although various administration methods have been utilized, the optimal route of administration remains to be determined. Furthermore, issues such as safety, differentiation uncertainty, immune rejection, low number of stem cells reaching the lesion, and tumorigenicity should be considered. Despite these uncertainties, stem cell and exosome therapy remains a promising but challenging treatment option for ICH.
Availability of data and materials
Not applicable.
Abbreviations
- ABI:
-
Autologous blood injection
- AD-MSCs:
-
Adipose-derived mesenchymal stem cells
- ANXA1:
-
Annexin A1
- apoH:
-
Apoproteins H
- ATRA:
-
All-trans retinoic acid
- BBB:
-
Blood–brain barrier
- BDNF:
-
Brain-derived neurotrophic factor
- BM-MSCs:
-
Bone marrow mesenchymal stem cells
- BMVECs:
-
Brain microvascular endothelial cells
- Col:
-
Collagenase
- CXCL12:
-
Chemokine ligand 12
- EC:
-
Endothelial cell
- EPC:
-
Endothelial progenitor cell
- ESCs:
-
Embryonic stem cells
- GAP-43:
-
Growth-associated protein-43
- GDNF:
-
Glial cell-derived neurotrophic factor
- hAD-MSCs:
-
Human AD-MSCs
- hESCs:
-
Human ESCs
- HIF-1α:
-
Hypoxia-inducible factor-1 alpha
- hUC-MSCs:
-
Human umbilical cord mesenchymal stem cells
- ICH:
-
Intracerebral hemorrhage
- ICI:
-
Intracranial injection
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- IND:
-
Intranasal deliver
- iNOS:
-
Inducible nitric oxide synthase
- iPSCs:
-
Induced pluripotent stem cells
- IRAK1:
-
Interleukin receptor-associated kinase 1
- JNK:
-
C-Jun N-terminal kinase pathway
- MAP2:
-
Microtubule-associated protein 2
- MHC:
-
Major histocompatibility complex
- MMP-9:
-
Matrix metalloproteinase 9
- MSCs:
-
Mesenchymal stem cells
- MVB:
-
Multivesicular bodies
- NESCs:
-
Neuroepithelial-like stem cells
- NFAT5:
-
Nuclear factor of activated T cells 5
- NSCs:
-
Neural stem cells
- NVU:
-
Neurovascular unit
- OM-MSCs:
-
Olfactory mucosa MSCs
- ONOO¯:
-
Peroxynitrite
- PBS:
-
Phosphate-buffered saline
- PGE2:
-
Prostaglandin E2
- PSCs:
-
Placenta-derived mesenchymal stem cells
- rAD-MSCs:
-
Rat AD-MSCs
- RhoA:
-
Ras homolog family member A
- rNSCs:
-
Rat NSCs
- ROCK:
-
Rho kinase
- SCs:
-
Stem cells
- TJ:
-
Tight junction protein
- TNF-α:
-
Tumor necrosis factor-α
- TSG-6:
-
TNF-α-stimulated gene/protein 6
- TVI:
-
Tail vein injection
- VEGF:
-
Vascular endothelial growth factor
- WJ:
-
Wharton’s jelly
- WJ-MSCs:
-
Wharton’s jelly-derived MSCs
- ZO:
-
Zonula occludens
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–60.
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–76.
Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934–43.
Arima H, Tzourio C, Anderson C, Woodward M, Bousser MG, MacMahon S, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the progress trial. Stroke. 2010;41(2):394–6.
Di Sciascio G, Patti G, Pasceri V, Gatto L, Colonna G, Montinaro A, et al. Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention: results of the ARMYDA-5 PRELOAD (antiplatelet therapy for reduction of myocardial damage during angioplasty) randomized trial. J Am Coll Cardiol. 2010;56(7):550–7.
Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33(5):1190–5.
Loehrer E, Ikram MA, Akoudad S, Vrooman HA, van der Lugt A, Niessen WJ, et al. Apolipoprotein E genotype influences spatial distribution of cerebral microbleeds. Neurobiol Aging. 2014;35(4):899–905.
Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc Drugs Ther. 2009;23(1):73–83.
Fu Z, Chen Y, Qin F, Yang S, Deng X, Ding R, et al. Increased activity of Rho kinase contributes to hemoglobin-induced early disruption of the blood-brain barrier in vivo after the occurrence of intracerebral hemorrhage. Int J Clin Exp Pathol. 2014;7(11):7844–53.
Cao Y, Chen W, Qian Y, Zeng Y, Liu W. Plasminogen activator inhibitor-1 4G/5G polymorphism and ischemic stroke risk: a meta-analysis in Chinese population. Int J Neurosci. 2014;124(12):874–81.
Yamada M. Brain hemorrhages in cerebral amyloid angiopathy. Semin Thromb Hemost. 2013;39(8):955–62.
Reuter B, Bugert P, Stroick M, Bukow S, Griebe M, Hennerici MG, et al. TIMP-2 gene polymorphism is associated with intracerebral hemorrhage. Cerebrovasc Dis. 2009;28(6):558–63.
Fontanella M, Rubino E, Crobeddu E, Gallone S, Gentile S, Garbossa D, et al. Brain arteriovenous malformations are associated with interleukin-1 cluster gene polymorphisms. Neurosurgery. 2012;70(1):12–7.
Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37(1):231–4.
Brouwers HB, Goldstein JN. Therapeutic strategies in acute intracerebral hemorrhage. Neurotherapeutics. 2012;9(1):87–98.
Wang S, Yu L, Sun G, Liu Y, Hu W, Liu Y, et al. Danhong injection protects hemorrhagic brain by increasing peroxiredoxin 1 in aged rats. Front Pharmacol. 2020;11:346.
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019;178: 101610.
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48(4):1033–43.
Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. The Lancet. 2013;382(9890):397–408.
Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–31.
Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27(3):268–79.
Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, et al. Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transplant. 2018;27(12):1723–30.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Turbil E, Terzi N, Cour M, Argaud L, Einav S, Guerin C. Positive end-expiratory pressure-induced recruited lung volume measured by volume-pressure curves in acute respiratory distress syndrome: a physiologic systematic review and meta-analysis. Intensive Care Med. 2020;46(12):2212–25.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Bedini G, Bersano A, Zanier ER, Pischiutta F, Parati EA. Mesenchymal stem cell therapy in intracerebral haemorrhagic stroke. Curr Med Chem. 2018;25(19):2176–97.
Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8.
Baker CL, Pera MF. Capturing totipotent stem cells. Cell Stem Cell. 2018;22(1):25–34.
Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132(4):567–82.
Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21(5):801–7.
Cordeiro MF, Horn AP. Stem cell therapy in intracerebral hemorrhage rat model. World J Stem Cells. 2015;7(3):618–29.
MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28(3):516–25.
MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke. 2010;41(10 Suppl):S95–8.
Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100(1):12–27.
Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–75.
Barut GA, Tunc M, Sahin S, Ulus F, Sazak H. Effects of epidural morphine and levobupivacaine combination before incision and after incision and in the postoperative period on thoracotomy pain and stress response. Turk J Med Sci. 2018;48(4):716–23.
Ouyang Y, Tang Y, Fu L, Peng S, Wu W, Tan D, et al. Exosomes secreted by chronic hepatitis B patients with PNALT and liver inflammation grade >/= A2 promoted the progression of liver cancer by transferring miR-25-3p to inhibit the co-expression of TCF21 and HHIP. Cell Prolif. 2020;53(7):e12833.
Bax M, McKenna J, Do-Ha D, Stevens CH, Higginbottom S, Balez R, et al. The ubiquitin proteasome system is a key regulator of pluripotent stem cell survival and motor neuron differentiation. Cells. 2019;8(6):581.
Parakrama R, Fogel E, Chandy C, Augustine T, Coffey M, Tesfa L, et al. Immune characterization of metastatic colorectal cancer patients post reovirus administration. BMC Cancer. 2020;20(1):569.
Kang MK, Kim TJ, Kim YJ, Kang L, Kim J, Lee N, et al. Targeted delivery of iron oxide nanoparticle-loaded human embryonic stem cell-derived spherical neural masses for treating intracerebral hemorrhage. Int J Mol Sci. 2020;21(10):3658.
Ishii T, Eto K. Fetal stem cell transplantation: past, present, and future. World J Stem Cells. 2014;6(4):404–20.
Lu Q, Yu M, Shen C, Chen X, Feng T, Yao Y, et al. Negligible immunogenicity of induced pluripotent stem cells derived from human skin fibroblasts. PLoS ONE. 2014;9(12):e114949.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous Induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–46.
Qin J, Song B, Zhang H, Wang Y, Wang N, Ji Y, et al. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci Lett. 2013;548:95–100.
Qin J, Gong G, Sun S, Qi J, Zhang H, Wang Y, et al. Functional recovery after transplantation of induced pluripotent stem cells in a rat hemorrhagic stroke model. Neurosci Lett. 2013;554:70–5.
Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, et al. Transplantation of induced pluripotent stem cells alleviates cerebral inflammation and neural damage in hemorrhagic stroke. PLoS ONE. 2015;10(6):e0129881.
Simerman AA, Perone MJ, Gimeno ML, Dumesic DA, Chazenbalk GD. A mystery unraveled: nontumorigenic pluripotent stem cells in human adult tissues. Expert Opin Biol Ther. 2014;14(7):917–29.
Nishimori M, Yakushiji H, Mori M, Miyamoto T, Yaguchi T, Ohno S, et al. Tumorigenesis in cells derived from induced pluripotent stem cells. Hum Cell. 2014;27(1):29–35.
Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27(8):743–5.
Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30(8):1487–93.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3(1):63–70.
Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841–8.
Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16(1):12–20.
Matsiko A, Levingstone TJ, O’Brien FJ. Advanced strategies for articular cartilage defect repair. Materials. 2013;6(2):637–68.
Imam YZ, D’Souza A, Malik RA, Shuaib A. Secondary stroke prevention: improving diagnosis and management with newer technologies. Transl Stroke Res. 2016;7(6):458–77.
Wang C, Cao J, Duan S, Xu R, Yu H, Huo X, et al. Effect of microRNA-126a-3p on bone marrow mesenchymal stem cells repairing blood-brain barrier and nerve injury after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(5):104748.
Chen M, Li X, Zhang X, He X, Lai L, Liu Y, et al. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: contribution of TSG-6. J Neuroinflammation. 2015;12:61.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–8.
Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, et al. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015;272:78–87.
Nonaka M, Yoshikawa M, Nishimura F, Yokota H, Kimura H, Hirabayashi H, et al. Intraventricular transplantation of embryonic stem cell-derived neural stem cells in intracerebral hemorrhage rats. Neurol Res. 2004;26(3):265–72.
Chen J, Tang YX, Liu YM, Chen J, Hu XQ, Liu N, et al. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci Ther. 2012;18(10):847–54.
Cui J, Cui C, Cui Y, Li R, Sheng H, Jiang X, et al. Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem. 2017;42(1):137–44.
Ribeiro F, Ribeiro IP, Goncalves AC, Alves AJ, Melo E, Fernandes R, et al. Effects of resistance exercise on endothelial progenitor cell mobilization in women. Sci Rep. 2017;7(1):17880.
Kim JM, Lee ST, Chu K, Jung KH, Song EC, Kim SJ, et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50.
Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34(9):2258–63.
Vaquero J, Otero L, Bonilla C, Aguayo C, Rico MA, Rodriguez A, et al. Cell therapy with bone marrow stromal cells after intracerebral hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy. 2013;15(1):33–43.
Bao XJ, Liu FY, Lu S, Han Q, Feng M, Wei JJ, et al. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and anti-inflammatory and angiogenesis effects in an intracerebral hemorrhage rat model. Int J Mol Med. 2013;31(5):1087–96.
Liang H, Yin Y, Lin T, Guan D, Ma B, Li C, et al. Transplantation of bone marrow stromal cells enhances nerve regeneration of the corticospinal tract and improves recovery of neurological functions in a collagenase-induced rat model of intracerebral hemorrhage. Mol Cells. 2013;36(1):17–24.
Otero L, Zurita M, Bonilla C, Aguayo C, Rico MA, Rodriguez A, et al. Allogeneic bone marrow stromal cell transplantation after cerebral hemorrhage achieves cell transdifferentiation and modulates endogenous neurogenesis. Cytotherapy. 2012;14(1):34–44.
Yang C, Zhou L, Gao X, Chen B, Tu J, Sun H, et al. Neuroprotective effects of bone marrow stem cells overexpressing glial cell line-derived neurotrophic factor on rats with intracerebral hemorrhage and neurons exposed to hypoxia/reoxygenation. Neurosurgery. 2011;68(3):691–704.
Otero L, Bonilla C, Aguayo C, Zurita M, Vaquero J. Intralesional administration of allogeneic bone marrow stromal cells reduces functional deficits after intracerebral hemorrhage. Histol Histopathol. 2010;25(4):453–61.
Otero L, Zurita M, Bonilla C, Aguayo C, Vela A, Rico MA, et al. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy. 2011;13(5):562–71.
Feng M, Zhu H, Zhu Z, Wei J, Lu S, Li Q, et al. Serial 18F-FDG PET demonstrates benefit of human mesenchymal stem cells in treatment of intracerebral hematoma: a translational study in a primate model. J Nucl Med. 2011;52(1):90–7.
Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS ONE. 2007;2(12):e1272.
Seyfried D, Ding J, Han Y, Li Y, Chen J, Chopp M. Effects of intravenous administration of human bone marrow stromal cells after intracerebral hemorrhage in rats. J Neurosurg. 2006;104(2):313–8.
Zhang Q, Shang X, Hao M, Zheng M, Li Y, Liang Z, et al. Effects of human umbilical cord mesenchymal stem cell transplantation combined with minimally invasive hematoma aspiration on intracerebral hemorrhage in rats. Am J Transl Res. 2015;7(11):2176–86.
Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ, Huang WL, et al. Transplanted neural stem cells modulate regulatory T, gammadelta T cells and corresponding cytokines after intracerebral hemorrhage in rats. Int J Mol Sci. 2014;15(3):4431–41.
Wakai T, Sakata H, Narasimhan P, Yoshioka H, Kinouchi H, Chan PH. Transplantation of neural stem cells that overexpress SOD1 enhances amelioration of intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2014;34(3):441–9.
Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, et al. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15(12):2624–33.
Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010;88(15):3282–94.
Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS ONE. 2009;4(5):e5586.
Lee HJ, Park IH, Kim HJ, Kim SU. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16(9):1066–76.
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25(5):1204–12.
Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2(1):e156.
Seyfried DM, Han Y, Yang D, Ding J, Savant-Bhonsale S, Shukairy MS, et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–9.
Li F, Liu Y, Zhu S, Wang X, Yang H, Liu C, et al. Therapeutic time window and effect of intracarotid neural stem cells transplantation for intracerebral hemorrhage. NeuroReport. 2007;18(10):1019–23.
Zhang H, Huang Z, Xu Y, Zhang S. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006;28(1):104–12.
Xie J, Wang B, Wang L, Dong F, Bai G, Liu Y. Intracerebral and intravenous transplantation represents a favorable approach for application of human umbilical cord mesenchymal stromal cells in intracerebral hemorrhage rats. Med Sci Monit. 2016;22:3552–61.
Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(Pt 3):616–29.
Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–53.
Li B, Bai W, Sun P, Zhou B, Hu B, Ying J. The effect of CXCL12 on endothelial progenitor cells: potential target for angiogenesis in intracerebral hemorrhage. J Interferon Cytokine Res. 2015;35(1):23–31.
Pias-Peleteiro J, Campos F, Castillo J, Sobrino T. Endothelial progenitor cells as a therapeutic option in intracerebral hemorrhage. Neural Regen Res. 2017;12(4):558–61.
Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–7.
Matta R, Feng Y, Sansing LH, Gonzalez AL. Endothelial cell secreted VEGF-C enhances NSC VEGFR3 expression and promotes NSC survival. Stem Cell Res. 2021;53:102318.
Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–10.
Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2(2):34–8.
Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202.
Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126(2):220–32.
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8–15.
Ilancheran S, Moodley Y, Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30(1):2–10.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301.
Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96.
Hao S, Wang B. Editorial: review on intracerebral haemorrhage: multidisciplinary approaches to the injury mechanism analysis and therapeutic strategies. Curr Pharm Des. 2017;23(15):2159–60.
Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87(3):350–9.
Visco DB, Toscano AE, Juarez PAR, Gouveia H, Guzman-Quevedo O, Torner L, et al. A systematic review of neurogenesis in animal models of early brain damage: Implications for cerebral palsy. Exp Neurol. 2021;340:113643.
Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25(1):98–106.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19(1):112.
Chen KH, Lin KC, Wallace CG, Li YC, Shao PL, Chiang JY, et al. Human induced pluripotent stem cell-derived mesenchymal stem cell therapy effectively reduced brain infarct volume and preserved neurological function in rat after acute intracranial hemorrhage. Am J Transl Res. 2019;11(9):6232–48.
Yip HK, Lin KC, Sung PH, Chiang JY, Yin TC, Wu RW, et al. Umbilical cord-derived MSC and hyperbaric oxygen therapy effectively protected the brain in rat after acute intracerebral haemorrhage. J Cell Mol Med. 2021;25(12):5640–54.
McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676.
Lee HS, Kim KS, Lim HS, Choi M, Kim HK, Ahn HY, et al. Priming wharton’s jelly-derived mesenchymal stromal/stem cells with ROCK inhibitor improves recovery in an intracerebral hemorrhage model. J Cell Biochem. 2015;116(2):310–9.
Liu J, He J, Ge L, Xiao H, Huang Y, Zeng L, et al. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res Ther. 2021;12(1):413.
Choi BY, Kim OJ, Min SH, Jeong JH, Suh SW, Chung TN. Human placenta-derived mesenchymal stem cells reduce mortality and hematoma size in a rat intracerebral hemorrhage model in an acute phase. Stem Cells Int. 2018;2018:1658195.
Zhou H, Zhang H, Yan Z, Xu R. Transplantation of human amniotic mesenchymal stem cells promotes neurological recovery in an intracerebral hemorrhage rat model. Biochem Biophys Res Commun. 2016;475(2):202–8.
Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26(5):485–9.
Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3(4):25–33.
Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6(3):256–65.
Kuramoto Y, Takagi T, Tatebayashi K, Beppu M, Doe N, Fujita M, et al. Intravenous administration of human adipose-derived stem cells ameliorates motor and cognitive function for intracerebral hemorrhage mouse model. Brain Res. 2019;1711:58–67.
Li G, Yu H, Liu N, Zhang P, Tang Y, Hu Y, et al. Overexpression of CX3CR1 in adipose-derived stem cells promotes cell migration and functional recovery after experimental intracerebral hemorrhage. Front Neurosci. 2019;13:462.
Martinez-Galdamez M, Biondi A, Kalousek V, Pereira VM, Ianucci G, Gentric JC, et al. Periprocedural safety and technical outcomes of the new Silk Vista Baby flow diverter for the treatment of intracranial aneurysms: results from a multicenter experience. J Neurointerv Surg. 2019;11(7):723–7.
Ai Z, Cheng C, Zhou L, Yin S, Wang L, Liu Y. Bone marrow mesenchymal stem cells-derived extracellular vesicles carrying microRNA-221-3p protect against ischemic stroke via ATF3. Brain Res Bull. 2021;172:220–8.
Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80(3):588–601.
Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. J Cell Physiol. 2009;219(2):271–5.
Lehane C, Guelzow T, Zenker S, Erxleben A, Schwer CI, Heimrich B, et al. Carbimazole is an inhibitor of protein synthesis and protects from neuronal hypoxic damage in vitro. J Pharmacol Exp Ther. 2013;347(3):781–93.
Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS ONE. 2010;5(1):e8575.
Wakai T, Narasimhan P, Sakata H, Wang E, Yoshioka H, Kinouchi H, et al. Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2016;36(12):2134–45.
Yu Z, Chen LF, Tang L, Hu CL. Effects of recombinant adenovirus-mediated hypoxia-inducible factor-1alpha gene on proliferation and differentiation of endogenous neural stem cells in rats following intracerebral hemorrhage. Asian Pac J Trop Med. 2013;6(10):762–7.
Cui M, Ge H, Zeng H, Yan H, Zhang L, Feng H, et al. Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation and differentiation after intracerebral hemorrhage in mice. Cell Transpl. 2019;28(5):568–84.
Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;49:68–78.
Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31(3):146–53.
Pullicino PM, Burke WJ. Cell-based interventions for neurologic conditions: ethical challenges for early human trials. Neurology. 2009;72(19):1709.
Pasi M, Sugita L, Xiong L, Charidimou A, Boulouis G, Pongpitakmetha T, et al. Association of cerebral small vessel disease and cognitive decline after intracerebral hemorrhage. Neurology. 2021;96(2):e182–92.
Zhang Y, Deng H, Hu Y, Pan C, Wu G, Li Q, et al. Adipose-derived mesenchymal stem cells stereotactic transplantation alleviate brain edema from intracerebral hemorrhage. J Cell Biochem. 2019;120(9):14372–82.
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002–7.
Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20.
Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, et al. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24(3–4):307–16.
Jiang Y, Wei N, Zhu J, Lu T, Chen Z, Xu G, et al. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm. 2010;2010:372423.
Tian L, Lappalainen J, Autero M, Hanninen S, Rauvala H, Gahmberg CG. Shedded neuronal ICAM-5 suppresses T-cell activation. Blood. 2008;111(7):3615–25.
Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech. 2012;5(6):823–33.
Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2020;91(7):720–9.
Kim K, Park HW, Moon HE, Kim JW, Bae S, Chang JW, et al. The effect of human umbilical cord blood-derived mesenchymal stem cells in a collagenase-induced intracerebral hemorrhage rat model. Exp Neurobiol. 2015;24(2):146–55.
Gao L, Li PP, Shao TY, Mao X, Qi H, Wu BS, et al. Neurotoxic role of interleukin-17 in neural stem cell differentiation after intracerebral hemorrhage. Neural Regen Res. 2020;15(7):1350–9.
Schnabel LV, Abratte CM, Schimenti JC, Felippe MJ, Cassano JM, Southard TL, et al. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen Med. 2014;9(5):621–35.
Cui C, Cui Y, Gao J, Li R, Jiang X, Tian Y, et al. Intraparenchymal treatment with bone marrow mesenchymal stem cell-conditioned medium exerts neuroprotection following intracerebral hemorrhage. Mol Med Rep. 2017;15(4):2374–82.
Chen X, Xu CX, Liang H, Xi Z, Pan J, Yang Y, et al. Bone marrow mesenchymal stem cells transplantation alleviates brain injury after intracerebral hemorrhage in mice through the Hippo signaling pathway. Aging. 2020;12(7):6306–23.
Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201.
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol. 2015;38:16–25.
Loiola RA, Garcia-Gabilondo M, Grayston A, Bugno P, Kowalska A, Duban-Deweer S, et al. Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res Ther. 2021;12(1):552.
Zeng W, Lei Q, Ma J, Gao S, Ju R. Endothelial progenitor cell-derived microvesicles promote angiogenesis in rat brain microvascular endothelial cells in vitro. Front Cell Neurosci. 2021;15:638351.
McArthur S, Loiola RA, Maggioli E, Errede M, Virgintino D, Solito E. The restorative role of annexin A1 at the blood-brain barrier. Fluids Barriers CNS. 2016;13(1):17.
Luo ZZ, Gao Y, Sun N, Zhao Y, Wang J, Tian B, et al. Enhancing the interaction between annexin-1 and formyl peptide receptors regulates microglial activation to protect neurons from ischemia-like injury. J Neuroimmunol. 2014;276(1–2):24–36.
Chen K, Bao Z, Gong W, Tang P, Yoshimura T, Wang JM. Regulation of inflammation by members of the formyl-peptide receptor family. J Autoimmun. 2017;85:64–77.
He HQ, Ye RD. The formyl peptide receptors: diversity of ligands and mechanism for recognition. Molecules. 2017;22(3):455.
Gussenhoven R, Klein L, Ophelders D, Habets DHJ, Giebel B, Kramer BW, et al. Annexin A1 as neuroprotective determinant for blood-brain barrier integrity in neonatal hypoxic-ischemic encephalopathy. J Clin Med. 2019;8(2):137.
Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z. MicroRNA-21 overexpression promotes the neuroprotective efficacy of mesenchymal stem cells for treatment of intracerebral hemorrhage. Front Neurol. 2018;9:931.
Shen H, Yao X, Li H, Li X, Zhang T, Sun Q, et al. Role of exosomes derived from miR-133b modified MSCs in an experimental rat model of intracerebral hemorrhage. J Mol Neurosci. 2018;64(3):421–30.
Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, et al. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(10):1674–85.
Tejima E, Guo S, Murata Y, Arai K, Lok J, van Leyen K, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26(11):1935–41.
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733.
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–9.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–92.
Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:6478.
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585–606.
Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727.
Hong SB, Yang H, Manaenko A, Lu J, Mei Q, Hu Q. Potential of exosomes for the treatment of stroke. Cell Transplant. 2019;28(6):662–70.
Consortium ET, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228–32.
Duan S, Wang F, Cao J, Wang C. Exosomes derived from microRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. 2020;14:3143–58.
Ding H, Jia Y, Lv H, Chang W, Liu F, Wang D. Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neuroinflammation after diabetic intracerebral hemorrhage via the miR-183-5p/PDCD4/NLRP3 pathway. J Endocrinol Invest. 2021;44(12):2685–98.
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–41.
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–46.
Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, et al. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2018;38(5):767–79.
Han Y, Seyfried D, Meng Y, Yang D, Schultz L, Chopp M, et al. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2018;131(1):290–300.
Zhang B, Shen L, Shi H, Pan Z, Wu L, Yan Y, et al. Exosomes from human umbilical cord mesenchymal stem cells: identification, purification, and biological characteristics. Stem Cells Int. 2016;2016:1929536.
Mello TG, Rosado-de-Castro PH, Campos RMP, Vasques JF, Rangel-Junior WS, Mattos R, et al. Intravenous human umbilical cord-derived mesenchymal stromal cell administration in models of moderate and severe intracerebral hemorrhage. Stem Cells Dev. 2020;29(9):586–98.
Chang Z, Mao G, Sun L, Ao Q, Gu Y, Liu Y. Cell therapy for cerebral hemorrhage: five year follow-up report. Exp Ther Med. 2016;12(6):3535–40.
Zhu J, Xiao Y, Li Z, Han F, Xiao T, Zhang Z, et al. Efficacy of surgery combined with autologous bone marrow stromal cell transplantation for treatment of intracerebral hemorrhage. Stem Cells Int. 2015;2015:318269.
Li ZM, Zhang ZT, Guo CJ, Geng FY, Qiang F, Wang LX. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-a prospective clinical observation. Clin Neurol Neurosurg. 2013;115(1):72–6.
Turnbull MT, Zubair AC, Meschia JF, Freeman WD. Mesenchymal stem cells for hemorrhagic stroke: status of preclinical and clinical research. NPJ Regen Med. 2019;4:10.
Fauzi AA, Suroto NS, Bajamal AH, Machfoed MH. Intraventricular transplantation of autologous bone marrow mesenchymal stem cells via ommaya reservoir in persistent vegetative state patients after haemorrhagic stroke: report of two cases & review of the literature. J Stem Cells Regen Med. 2016;12(2):100–4.
Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, et al. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9(8):133–43.
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–106.
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–807.
Doeppner TR, Hermann DM. Stem cell-based treatments against stroke: observations from human proof-of-concept studies and considerations regarding clinical applicability. Front Cell Neurosci. 2014;8:357.
Xue YZ, Li XX, Li L, Pang SL, Yao JG, Hao PL. Curative effect and safety of intrathecal transplantation of neural stem cells for the treatment of cerebral hemorrhage. Genet Mol Res. 2014;13(4):8294–300.
Pias-Peleteiro J, Perez-Mato M, Lopez-Arias E, Rodriguez-Yanez M, Blanco M, Campos F, et al. Increased endothelial progenitor cell levels are associated with good outcome in intracerebral hemorrhage. Sci Rep. 2016;6:28724.
Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–73.
Tomecka E, Lech W, Zychowicz M, Sarnowska A, Murzyn M, Oldak T, et al. Assessment of the neuroprotective and stemness properties of human Wharton’s Jelly-derived mesenchymal stem cells under variable (5% vs. 21%) Aerobic conditions. Cells. 2021;10(4):717.
Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, et al. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24(4):1095–103.
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, et al. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10(18):3198–207.
Acknowledgements
We are grateful to the Science and Technology Bureau of Quanzhou (Grant Number 2020CT003).
Funding
This work was supported by Science and Technology Bureau of Quanzhou (Grant Number 2020CT003) and Doctoral Startup Fund of the Second Affiliated Hospital of Fujian Medical University (Grant Number BS202205).
Author information
Authors and Affiliations
Contributions
All the authors have contributed to the concept and main ideas of the work. XK, ZP, and QZ search for relevant literature. ZJ drafted a manuscript. JZ, YX, RG, LS, SL, WH, FZ, and PS modified the manuscript. All the authors read and approved the final version of the forthcoming works.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Jf., Xiong, Y., Kang, X. et al. Application of stem cells and exosomes in the treatment of intracerebral hemorrhage: an update. Stem Cell Res Ther 13, 281 (2022). https://doi.org/10.1186/s13287-022-02965-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02965-2