Abstract
Purpose
Recruitment of lung volume is often cited as the reason for using positive end-expiratory pressure (PEEP) in acute respiratory distress syndrome (ARDS) patients. We performed a systematic review on PEEP-induced recruited lung volume measured from inspiratory volume-pressure (VP) curves in ARDS patients to assess the prevalence of patients with PEEP-induced recruited lung volume and the mortality in recruiters and non-recruiters.
Methods
We conducted a systematic search of PubMed to identify studies including ARDS patients in which the intervention of an increase in PEEP was accompanied by measurement of the recruited volume (Vrec increase versus no increase) using the VP curve in order to assess the relation between Vrec and mortality at ICU discharge.
We first analysed the pooled data from the papers identified and then analysed individual patient level data received from the authors via personal contact. The risk of bias of the included papers was assessed using the quality in prognosis studies tool and the certainty of the evidence regarding the relationship of mortality to Vrec by the GRADE approach. Recruiters were defined as patients with a Vrec > 150 ml. A random effects model was used for the pooled data. Multivariable logistic regression analysis was used for individual patient data.
Results
We identified 16 papers with a total of 308 patients for the pooled data meta-analysis and 14 papers with a total of 384 patients for the individual data analysis. The quality of the articles was moderate. In the pooled data, the prevalence of recruiters was 74% and the mortality was not significantly different between recruiters and non-recruiters (relative risk 1.20 [95% confidence intervals 0.88–1.63]). The certainty of the evidence regarding this association was very low and publication bias evident. In the individual data, the prevalence of recruiters was 70%. In the multivariable logistic regression, Vrec was not associated with mortality but Simplified Acute Physiology Score II and driving pressure at PEEP of 5 cmH2O were.
Conclusion
After a PEEP increment, most patients are recruiters. Vrec was not associated with ICU mortality. The presence of similar findings in the individual patient level analysis and the driving pressure at PEEP of 5 cmH2O was associated with mortality as previously reported validate our findings. Publication bias and the lack of prospective studies suggest more research is required.
Similar content being viewed by others
We conducted systematic literature search in PubMed of studies on ARDS patients where recruited volume (Vrec) elicited by a PEEP increase was measured by inspiratory VP curve. Our analysis showed that, after a PEEP increment, most of the patients had a Vrec measured with VP curve greater than 150 ml. Vrec was not associated with ICU mortality but this may be affected by publication bias. |
Introduction
Lung protective mechanical ventilation is the cornerstone feature of ARDS management. It includes setting low tidal volumes (VT) and maintaining plateau pressure (Pplat) ≤ 30 cmH2O [1], to prevent much of the damage incurred to the injured ARDS lung by alveolar over-distension during mechanical ventilation. Positive end-expiratory pressure (PEEP) is the second main ventilator setting that aims at preventing lung derecruitment during expiration, thereby improving oxygenation and distribution of ventilation. These concepts, together with haemodynamic preservation, currently underlie the use of PEEP in ARDS.
In daily ICU practice, recruited lung volume is often not measured. Surrogates such as the changes in lung compliance and gas exchange are used instead. However, recruited lung volume can be measured at the bedside by using the volume-pressure (VP) curves available in almost every ICU ventilator. To the best of our knowledge, there has been no study of the relation between the recruited lung volume (Vrec) measured from the VP curves used to set PEEP and mortality in ARDS patients. We therefore performed a systematic review of the literature describing PEEP-induced recruited lung volume as measured from inspiratory VP curves in ARDS patients, with the aim of estimating lung recruitability rate in association with mortality.
Two methods for bedside measurement of Vrec from VP curves were studied in the present systematic review, namely the occlusion technique and the low flow inflation technique. A description of these methods can be found in the ESM and also in Fig. 1.
The two methods to measure recruited volume (Vrec) from volume-pressure curve of the respiratory system that were investigated in present study. a Occlusion technique at different tidal volume (VT). Airway pressure (Paw) is recorded at different VT during an end-inspiratory occlusion. Each dot is the plateau pressure at a given VT. The procedure is done at PEEP 0 (red dots) and PEEP14 cmH2O (blue dots). Both curves are referred to the relaxation volume of the respiratory system at PEEP 0 (Vr). Vrec is the change in lung volume at a given pressure, i.e. 20 cmH2O, between the 2 PEEP (broken horizontal black lines). b Low flow inflation technique. The respiratory system is insufflated from PEEP 5 (blue line) or PEEP10 (grey line) at constant low flow (7 l/min). Volumes are referred to Vr at PEEP 0. Vrec can be obtained as the difference in lung volume at same 20 cmH2O Paw (broken horizontal green lines) or from PEEP10 down to PEEP5 (broken horizontal red lines)
Methods
Following registration in PROSPERO (CRD 420 2017 5380) the current systematic review was performed in order to study adult patients with ARDS as defined by the authors, in whom the effect of PEEP on recruited lung volume as assessed by bedside VP curves was investigated. We extracted Vrec, oxygenation, compliance and mortality at ICU discharge or at last assessment from the included studies. We defined patients as recruiters when Vrec was > 150 ml and as non-recruiters when Vrec was ≤ 150 ml. The primary end-point was ICU mortality in recruiters and non-recruiters. This threshold was selected because it has been previously used in the literature [2].
Literature search
Two collaborators (ET and CG) performed a comprehensive search of the Pubmed database from inception to 19/07/2019 for relevant papers. The search was the search was updated on 20/05/2020 to ensure full capture of the available literature. The keywords used were: "acute respiratory distress syndrome", "acute lung injury", “positive end expiratory pressure”, “continuous positive airway pressure”, "Positive-Pressure Respiration/methods", “lung recruitment”, “alveolar recruitment”,”recruited lung volume” and”PEEP-induced recruitment”. We also used a ‘snowballing’ method, using study references to find additional studies to be included.
Study selection
The citations identified in the preliminary search were screened in duplicate by the same investigators to assess fulfilment of inclusion criteria in three stages: first by title, then by abstract review and finally in full text review. Only articles reporting Vrec elicited by PEEP and measured with either the VP curve or nitrogen wash out in ARDS patients were selected. Disagreements regarding fulfilment of inclusion criteria were resolved by discussion between the two researchers performing screening. Animal studies, studies in which recruitment was performed by other means than PEEP (e.g. recruitment manoeuvers or high frequency oscillation ventilation), pediatric studies, case reports, bench studies, reviews, abstracts and articles not in the English language were excluded.
Data extraction
The relevant data were extracted independently and in duplicate to a predefined form (ET and CE). Disagreements were resolved by discussion. Extracted data included patient characteristics (e.g. age, gender, height, weight, predicted body weight [PBW]), disease details (cause and number of days since onset of ARDS, simplified acute physiology score [SAPS] II), ventilation parameters (oxygen arterial partial pressure [PaO2] to inspired fraction [FiO2] ratios, FiO2, PEEP, compliance, Pplat, driving pressure, total respiratory rate [RR] and VT), Vrec and mortality at ICU discharge or at last assessment. If in the original papers Vrec was available only as graphs, the GetData Gaph Digitizer software (getdata-graph-digitizer.com) was used to transform it to numeric values.
The corresponding authors of the original papers were also contacted directly to obtain individual patient-level data regarding the above listed variables as well as the respiratory mechanics and ventilator settings at two specific levels of PEEPs: 5 and 15 cmH2O.
Quality assessment
The Quality In Prognosis Studies (QUIPS) tool was used to assess the risk of bias in the included papers [3]. The QUIPS checklist examines the risk of bias across six domains: study participation, study attrition, prognostic factor measurement, outcome measurement, adjustment for other prognostic factors, and statistical analysis and reporting. Two authors (ET and CG) independently assessed the risk of bias of each article included in the meta-analysis, using the QUIPS tool. For each domain we considered the full list of potential issues suggested by the tool. These were then taken together to inform an overall judgment of potential bias for each domain and to classify the risk in this domain as either high, moderate or low. This process was repeated for each of the 6 domains in each of the papers included (see Supplemental material 2). We also considered the items 1, 2 and 6 of the QUIPS tool for rating the prevalence of recruiters. The same two authors then assigned an overall judgment regarding the quality of the study; this too was classified as either high, moderate or low. The overall risk of bias was assigned the quality of the domain with the highest risk of bias assessment (conservative approach).
Statistical analysis
The statistical analysis was divided into two parts. In the first part, we analyzed the data obtained directly from previously published papers (pooled data analysis). In the second part, we analysed the individual patient-level data.
Pooled data analysis
The data extracted for the pooled data analysis were mean and standard deviation (SDs) or counts (± 95% confidence intervals [CI]s). Data provided in the included papers as median and quartiles were converted to mean (± SDs) [4]. Forest plots were constructed using the random effects model since heterogeneity was expected. For the same reason the Cochran’s Q test and ɪ2 were used to quantify data heterogeneity.
The prevalence of recruiters was presented as percent among the total number of patients. To compare continuous and dichotomous outcome variables in recruiters and non-recruiters, their mean difference and relative risks were used, respectively. The mortality in recruiters and non-recruiters was expressed as relative risk (95% CI).
Funnel plots were created for the articles retrieved in order to identify potential publication bias. Finally, several subgroup analyses were performed to assess the stability of our model when taking into account potential sources of confounding and heterogeneity. These included the PEEP levels used in the VP curve to measure Vrec (5 cmH2O versus 15 cmH2O), the PaO2/FiO2 ratio, the cause of ARDS, the timing of paper publication in relation to the ARMA trial (before or after) [1] and the overall risk of bias assessment (low versus moderate versus high).
Following GRADE methodology the certainty in the relationship between Vrec and mortality in the pooled data was rated [5]. Data from observational studies were initially designated as low certainty evidence. The degree of certainty was downgraded for concerns related to individual study risk of bias, inconsistency, indirectness, imprecision or publication bias and additional concerns. Disagreements were resolved by discussion.
Individual data analysis
In the individual data analysis variables were presented as median (1st and 3rd quartiles). The extracted variables were compared between recruiters and non-recruiters, and between survivors and non- survivors at ICU discharge by using non parametric tests. The association between Vrec and patient outcome was assessed using the area under curve (AUC) of the receiving operating characteristics (ROC) analysis.
We performed a multivariable logistic regression analysis of survival to ICU discharge as the dependent variable (enter method). Variables were selected as follows: Variables with a P value < 0.20 in the univariate comparisons between groups were eligible as independent covariates [6] and were required to reach this P value in each univariate analysis to be included in the multivariable logistic regression analysis. Some of the variables that met this criterion had obvious co-linearity and mathematical coupling (e.g. driving pressure, compliance, Pplat, PEEP) and were expected to have a high rate of missing values. Therefore, we prioritized variables based on prior clinical knowledge, our first choice being the driving pressure because it has been shown to be most strongly predictive of mortality in ARDS [7]. We forced Vrec as a covariate in the model regardless of whether it was significant in the univariate comparisons. To handle missing data as recommended for a rate was greater than 50% [8], the missing driving pressure values (where this occurred) were replaced once with a low value and once with a high value for both PEEP 5 and PEEP 15. The low value was the first quartile and the high value was the third quartile of the existing data. The results are presented with the final ORs and 95% CIs for each covariate.
Statistical analyses were performed using R [R version 3.5.2 (2018-12-20)] with the meta, pROC and epi2 packages [9].
Results
Study selection and individual data
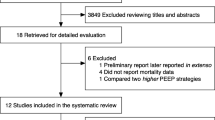
We identified 837 papers in the Pubmed search (Fig. 2). Among these, 803 articles were excluded based on title and abstract and another 15 of the 34 remaining papers were excluded following full text review. After inclusion of two papers retrieved from the references of previously selected articles [10, 11] a total of twenty-one papers fulfilled inclusion criteria [10,11,12,13,14,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] (Table 1). The 21 retrieved articles were all observational studies. Five of the 21 papers provided the data in a format that did not enable pooled data extraction [10–13, 24]. As these five articles could not be included in the pooled analysis, we contacted the authors and they provided us with their individual patient data, which were included in the individual data analysis. For the pooled data meta-analysis we used sixteen papers for a total of 308 patients (Table 1). The median number of patients per paper was 18 (10–23). Individual patient–level data were available in 6 of the published articles [22, 24, 26–28, 30] and were provided directly by the authors of 8 papers [10,11,12,13, 16,17,18, 20] [12, 13, 16,17,18, 20] (Table 1). Therefore, individual data were available for 384 patients included in 14 papers (Table 1). As shown in Table 1 ESM (Supplemental material 1) the rate of missing values ranged between 2% (Vrec) to 61% (driving pressure and compliance) for the papers included in the individual data meta-analysis. Eighteen papers used the Berlin definition [31] or the previous American-European Consensus Conference (AECC) [32] criteria for ARDS (Table 1). Three papers published before the AECC definition, used the authors’ definition of ARDS (Table 1).
Assessment of risk of bias (Quality in Prognostic Studies [QUIPS] tool): All papers were rated as having a moderate to low risk of bias in the domains of study participation, study attrition, prognostic factor measurement and statistical analysis and reporting (Table 2 and Supplemental material 2). The risk of bias was rated as low to moderate in 12/21 papers for the domain of outcome measurement and in 17/21 papers for the domain of study confounding (Table 2). The overall risk of bias was rated as low in 3 papers, moderate in 9 and high in 9 papers (Table 2).
Baseline data
The baseline data of patients in each paper are displayed in Table 3 ESM (Supplemental material 1) and summarized in each study in Table 4 ESM (Supplemental material 1) for the pooled analysis and in Table 5 ESM (Supplemental material 1) for the individual data analysis. The distribution of most of the values used in converted form for the pooled and individual analyses approximated normal (Tables 4 ESM and 5 ESM Supplemental material 1).
Pooled analysis of recruiters versus non-recruiters
Among the 308 patients included in the 16 papers used for the pooled analysis, the overall prevalence of recruiters was 74% (95% CI 64–84%) (Fig. 3). The overall between-study heterogeneity was 85% (Fig. 3). The mean difference in compliance at baseline between recruiters and non-recruiters was 13.83 ml/cmH2O (95% CI 0.14–27.52) (P = 0.048) (Table 3), i.e. compliance was significantly higher in recruiters than in non-recruiters. Otherwise the two groups did not differ in any of the independent variables studied including mortality and days in ARDS before the investigation (Table 3). Funnel plots showed bias in favor of publication supporting an association between recruitability and mortality; nine articles were within and seven were outside the CIs (Fig. 1 ESM Supplemental material 1).
Subgroup analyses on pooled data
The prevalence of recruiters was 80% and 89% when Vrec was measured between PEEP 5 and 15 cmH2O vs. between PEEP 0 and 10 cmH2O, respectively (Table 4 and Fig. 2 ESM Supplemental material 1). The prevalence of recruiters was 94% and 65% in studies where PaO2/FiO2 was greater than vs. lower than 150 mmHg, respectively (Table 4 and Fig. 4). The prevalence of recruiters was 75% and 66% in articles where primary ARDS accounted for by more vs. less than 50% of the cases, respectively (Table 4 and Fig. 4 ESM Supplemental material 1). The prevalence of recruiters was 61% and 77% for those papers published before vs. after the ARMA trial release, respectively (Table 4 and Fig. 5 ESM Supplemental material 1). The prevalence of recruiters was 70% in articles with an overall low risk of bias vs. 75% in those with a moderate risk of bias and 72% in those with a high risk of bias according the QUIPS tool (Table 4 and Fig. 6 ESM Supplemental material 1). The relative risk of ICU death for recruiters vs. non-recruiters was 1.20 (95% CI 0.88–1.63) (Fig. 5).
GRADE assessment of certainty of evidence
We assessed the certainty of the evidence with regards to the relation of Vrec with mortality at ICU discharge to be overall very low (Table 5).
Individual data meta-analysis
Prevalence of recruiters versus non-recruiters
Among the 384 patients included in the 14 papers used for the individual data meta-analysis the prevalence of recruiters was 70% (266/380 patients with data available for Vrec).
Comparison of recruiters to non-recruiters
At the threshold of P < 0.20, age, baseline PaO2/FiO2, compliance at PEEP15, and VT (ml/kg/PBW) were higher in recruiters whilst days in ARDS, Pplat at PEEP5 and at PEEP15, driving pressure at PEEP5, baseline respiratory rate and SAPSII were lower in recruiters than in non-recruiters (Table 6).
Overall mortality
Fourteen studies contributed to the individual data analysis on mortality at ICU discharge (Tables 6ESM and 4ESM Supplemental material 1). ICU mortality was 44% (152/344 patients with data on mortality available).
Comparison of survivors to non-survivors
Several variables met the threshold of P < 0.20 in the comparison between survivors and non-survivors. These included the proportion of primary ARDS proportion, compliance at PEEP15 and tidal volume (ml) (all higher in survivors) and baseline FiO2, baseline PEEP, Pplat at PEEP5 and PEEP15, driving pressure at PEEP5 and PEEP15, respiratory rate and SAPSII (all lower in survivors) (Table 7).
The relative risk of mortality in recruiters was 1.05 (0.86–1.30). The performance of Vrec for prediction of ICU mortality was poor (AUC ROC 0.52, 95% CI 0.46–0.58). The ROC curve did not suggest any other specific threshold to be tested (Fig. 6).
Multivariable logistic regression on ICU mortality
Three variables were selected as covariates for the multivariable logistic regression analysis on ICU mortality because they had reached a P value < 0.20 in the univariate comparison between both survivors and non-survivors and recruiters versus non-recruiters. These variables were baseline respiratory rate, driving pressure at PEEP5 and SAPSII. In addition, as planned, we forced Vrec (as a continuous variable) into the model. In accordance with our preplanned analysis strategy driving pressure PEEP 15 was also entered into specific models. We did it too because we felt it may be wrong to impute only the driving pressure at a low PEEP because of the literature. The low and high cut-off values of 8 and 13 cmH2O for the driving pressure at PEEP5 and 9 and 14 cmH2O for the driving pressure at PEEP15 were used to replace the missing values. Vrec was not significantly associated with ICU mortality in any model (Table 8 and Table 6 ESM in Supplemental material 1). The single variables significantly associated with ICU mortality were SAPSII and driving pressure at PEEP 5 (Table 8). However, in models that included both driving pressures at PEEP5 and at PEEP15 the driving pressure at PEEP 15 was consistently significantly associated with ICU mortality (Table 6 ESM Supplemental material 1).
Discussion
This is the first systematic review and meta-analysis on PEEP-induced recruited lung volume in ARDS patients. Our main finding was that at the threshold tested Vrec was very prevalent but was not associated with mortality. However, the certainty of the evidence with regards to this association was very low. We also identified significant publication bias in studies reporting in favor of significant associations between Vrec and mortality using proportions and found that the number of studies reporting risk ratios was too low to enable assessment of publication bias. These findings suggest that the existing literature does not really suffice to determine the relation of Vrec with patient outcomes.
Most papers assessing Vrec used a cut-off of 150 ml to differentiate between "recruiters" and "non-recruiters. While this recruited lung volume appears clinically meaningful, such dichotomization makes little biological sense. We used recruited lung volume as a continuous variable in the individual data analysis. Although both analyses indicated that recruitment of lung volume as a result of increased PEEP does not seem to modify important effect measures in any meaningful way, the uncertainly regarding these conclusions remains significant due to the small amount of aggregated data available. This issue, concomitant with the existence of clear publication bias, suggests that the prognostic value of Vrec may have been unjustifiably inflated.
The combination of negative results of large trials investigating higher versus lower PEEP [33] and studies showing an increased risk with the use of very high PEEP [34] highlights the need for a bedside tool for assessing individual alveolar recruitment potential. Esophageal pressure has been put forward as one such tool [35] but to date there is no evidence of clinical benefit of its use [36]. The measurement of Vrec with the VP curve is easy to carry out at the bedside. However, our findings raise important questions regarding the justification for using "recruitability" versus "non-recruitablility" as a criterion for enrolment to clinical trials or for post-hoc stratification. Recent advances to assess lung recruitability at the bedside [11, 17] share the same approach, and hence have the same implication as the VP curve explored in present study.
Physiological meaning of Vrec
Vrec as measured from the VP curve of the respiratory system reflects the amount of air that enters previously non-aerated lung regions as a result of PEEP increase [29]. It may also include some overdistension of previously normally aerated lung. By contrast, lung CT scan measures the amount of non-aerated tissue that becomes aerated as a result of the increase in PEEP. Comparison of these methods showed they do not correlate [14]. Contrary to Chiumello et al. [14], Lu et al. [21] found a tight correlation between Vrec and recruited lung volume measured by CT. Part of this discrepancy could be explained by the fact that Lu et al. defined recruited lung volume on CT by the amount of gas penetrating not only non-aerated lung area but also poorly ventilated lung regions [21]. They also pointed out that the morphology of lung involvement (focal vs. diffuse) may affect the degree of correlation of the two techniques [21].
Relationship between Vrec and mortality
We found no association between Vrec and mortality at the predefined threshold we used. This threshold was selected from the literature and had a large prevalence in our study. The lack of a significant relationship between Vrec and mortality may be explained by a lack of power of present study, prevalence of focal and diffuse ARDS, and the threshold definition for Vrec. This threshold was selected because it has been previously used by other investigators. Moreover, the ROC curve did not suggest any other value to be tested for assessing mortality further. Interestingly, the amount of 150 ml was the lowest value of Vrec observed in patients used by Chiumello et al. to compare Vrec to CT-based recruited lung volume [14]. Furthermore, at this value of 150 ml of Vrec the amount of recruited lung volume can range between 0 and 350 g [14] and with this latter value patients would be defined as higher recruiters [37]. Mortality is increased in patients with higher versus lower percentage of potentially recruitable lung, i.e. 41 vs. 15% (P < 0.05), respectively [37]. The lack of prognostic role of Vrec may also reflect that the included studies were not designed and did not aim to assess mortality. However, that driving pressure at PEEP 15 was a significant independent predictor of ICU mortality in present study adds reliability to our analysis.
Prevalence of recruiters
The prevalence of recruiters was large in our study, at the threshold used. We found only a small difference in the prevalence of recruiters in the pooled versus the individual data. This difference could be due to the inclusion of different papers into the two analyses.
Subgroup analysis of the pooled data revealed some of the sources of the significant heterogeneity observed between studies. Some of the heterogeneity stemmed from differences in the level of PEEP applied. The prevalence of recruiters was also clearly related to the initial patient PaO2/FiO2 and to the risk of bias as assessed by the QUIPS tool. Our findings differ from those of several previous studies. Our subgroup analysis showed that the subgroup of articles with a mean PaO2/FiO2 > 150 mmHg had a higher percentage of recruiters. This could be due to the fact that the high Vrec found in patients with a high PaO2/FiO2 does not reflect recruitment alone, but also includes some component of over-distension. This discrepancy could also be a chance finding, as is often the case when multiple subgroup analyses are conducted.
There is no threshold to define recruiters based on CT scan. In the studies that investigated the CT-based lung recruitment the authors used the median value of the observed data to define recruiters and non-recruiters. Across studies the percentage of potentially recruitable lung varies between 13% [37] and 42% [38] depending on definition of lung recruitment, range of pressure investigated and whether ARDS was early or late. When lung recruitment was defined as a ≥ 10% decrease of non-aerated lung tissue between PEEP and zero PEEP 62% of the patients studied were recruiters [39].
Limitations
Our study has several limitations. It was registered in PROSPERO 9 months after the first search due to an excessive time lag in the PROSPERO answer. Some discrepancies are now present between the version submitted to PROSPERO and the current version. These discrepancies partly come from the request of the reviewers to use the QUIPS tool to assess the risk of bias and be clearer for the time of assessment of mortality (ICU discharge in the current version and not ICU discharge or at the last assessment). We added in the current version a subgroup analysis based on risk of bias in the studies. Lacking RCTs, our analysis is based on observational data. Such data is by nature prone to bias by uncontrolled confounders. Some of the papers we included are dated before widespread use of several important therapeutic mechanical ventilation strategies. This could explain why, as shown in Fig. 3, older articles had a lower percentage of recruiters when compared to the more recent ones. The data in the present study did not enable investigation of airway opening pressure (AOP) which may also affect the determination of Vrec [40]. However, since we extracted data on Vrec at a pressure of 20 cmH2O, it is likely that this level would be greater than AOP on average [40]. Lung recruitability has been suggested to decrease over time. As temporal data was not provided by the authors we were therefore unable to assess the effect of this variable on Vrec. Based on previously published data we felt that driving pressure is a variable than cannot be ignored in this analysis. Despite the consistent finding in our sensitivity analysis, our result regarding this variable should be interpreted with caution due to the amount of missing data imputed. An analysis with this amount of missing data is concerning and may be of limited value (and potentially misleading). Finally selection of covariates was performed based on a P value of 0.20. While this method has the advantage of relatively broad inclusion as benefits an exploratory study such as ours and a threshold of 0.157 (corresponding to selection based on the Akaike information criterion) is often used, variable selection bias remains a possibility. The primary reason for this is a limitation due to the risk of chance findings in the screening process which may inflate the risk of random errors. This is the reason that statistical variable selection is generally recommended against [41].
Conclusion
We found that after a PEEP increment most patients had a Vrec > 150 ml measured with VP curve, and that Vrec was not associated with ICU mortality. In the pooled data the certainty of evidence with regards to this association was overall very low as all of the studies identified were observational and had quality limitations. Two of our results suggest this association is valid nonetheless: the fact that the findings were present in the individual patient level analysis and our finding that driving pressure at PEEP of 5 cmH2O was associated with mortality as previously reported. Conversely, the presence of significant publication bias towards studies reporting significant associations between Vrec and mortality and using proportions rather than risk ratios and, as well as the paucity of studies reporting risk ratios, suggest that the existing literature still does not suffice to determine the relation of Vrec to outcome.
References
ARDSnet, (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Marco Ranieri V (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Hayden JA, van der Windt DA, Cartwright JL, Coté P, Bombardier C (2013) Assessing bias in studies of prognostic factors. Ann Intern Med 158:280–286
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G (2015) Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 350:h870
Hosmer DW, Lemeshow SL (2000) Applied logistic regression. Wiley-Interscience, Hoboken
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
Vesin A, Azoulay E, Ruckly S, Vignoud L, Rusinova K, Benoit D, Soares M, Azeivedo-Maia P, Abroug F, Benbenishty J, Timsit JF (2013) Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med 39:1396–1404
R Core Team (2013) R: A language and environment for statistical computing. In: Book R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Guerin C, Terzi N, Galerneau LM, Mezidi M, Yonis H, Baboi L, Kreitmann L, Turbil E, Cour M, Argaud L, Louis B (2020) Lung and chest wall mechanics in patients with acute respiratory distress syndrome, expiratory flow limitation and airway closure. J Appl Physiol 128:1594–1603
Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, Sklar MC, Rauseo M, Ferguson ND, Fan E, Richard JM, Brochard L (2020) Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care Med 201:178–187
Aguirre-Bermeo H, Turella M, Bitondo M, Grandjean R, Italiano S, Festa O, Moran I, Mancebo J (2018) Lung volumes and lung volume recruitment in ARDS: a comparison between supine and prone position. Ann Intensive Care 8:25
Yonis H, Mortaza S, Baboi L, Mercat A, Guerin C (2018) Expiratory flow limitation assessment in patients with acute respiratory distress syndrome. A reappraisal. Am J Respir Crit Care Med 198:131–134
Chiumello D, Marino A, Brioni M, Cigada I, Menga F, Colombo A, Crimella F, Algieri I, Cressoni M, Carlesso E, Gattinoni L (2016) Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome. What is the relationship? Am J Respir Crit Care Med 193:1254–1263
Stahl CA, Möller K, Steinmann D, Henzler D, Lundin S, Stenqvist O (2015) Determination of “recruited volume” following a PEEP step is not a measure of lung recruitability. Acta Anaesthesiol Scand 59:35–46
Wallet F, Delannoy B, Haquin A, Debord S, Leray V, Bourdin G, Bayle F, Richard JC, Boussel L, Guerin C (2013) Evaluation of recruited lung volume at inspiratory plateau pressure with PEEP using bedside digital chest X-ray in patients with acute lung injury/ARDS. Respir Care 58:416–423
Dellamonica J, Lerolle N, Sargentini C, Beduneau G, Di Marco F, Mercat A, Richard JC, Diehl JL, Mancebo J, Rouby JJ, Lu Q, Bernardin G, Brochard L (2011) PEEP-induced changes in lung volume in acute respiratory distress syndrome. Two methods to estimate alveolar recruitment. Intensive Care Med 37:1595–1604
Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ (2011) Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med 183:341–347
Patroniti N, Bellani G, Cortinovis B, Foti G, Maggioni E, Manfio A, Pesenti A (2010) Role of absolute lung volume to assess alveolar recruitment in acute respiratory distress syndrome patients. Crit Care Med 38:1300–1307
Thille AW, Richard JC, Maggiore SM, Ranieri VM, Brochard L (2007) Alveolar recruitment in pulmonary and extrapulmonary acute respiratory distress syndrome: comparison using pressure-volume curve or static compliance. Anesthesiology 106:212–217
Lu Q, Constantin JM, Nieszkowska A, Elman M, Vieira S, Rouby JJ (2006) Measurement of alveolar derecruitment in patients with acute lung injury: computerized tomography versus pressure-volume curve. Crit Care 10:R95
Demoule A, Decailliot F, Jonson B, Christov C, Maitre B, Touqui L, Brochard L, Delclaux C (2006) Relationship between pressure-volume curve and markers for collagen turn-over in early acute respiratory distress syndrome. Intensive Care Med 32:413–420
Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T (2005) Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med 171:1002–1008
Koutsoukou A, Bekos B, Sotiropoulou C, Koulouris NG, Roussos C, Milic-Emili J (2002) Effects of positive end-expiratory pressure on gas exchange and expiratory flow limitation in adult respiratory distress syndrome. Crit Care Med 30:1941–1949
Maggiore SM, Jonson B, Richard JC, Jaber S, Lemaire F, Brochard L (2001) Alveolar derecruitment at decremental positive end-expiratory pressure levels in acute lung injury: comparison with the lower inflection point, oxygenation, and compliance. Am J Respir Crit Care Med 164:795–801
Chelucci GL, Dall'Ava-Santucci J, Dhainaut JF, Chelucci A, Allegra A, Lockhart A, Zin WA, Milic-Emili J (2000) Association of PEEP with two different inflation volumes in ARDS patients: effects on passive lung deflation and alveolar recruitment. Intensive Care Med 26:870–877
Ranieri VM, Mascia L, Fiore T, Bruno F, Brienza A, Giuliani R (1995) Cardiorespiratory effects of positive end-expiratory pressure during progressive tidal volume reduction (permissive hypercapnia) in patients with acute respiratory distress syndrome. Anesthesiology 83:710–720
Ranieri VM, Giuliani R, Fiore T, Dambrosio M, Milic-Emili J (1994) Volume-pressure curve of the respiratory system predicts effects of PEEP in ARDS: "occlusion" versus "constant flow" technique. Am J Respir Crit Care Med 149:19–27
Ranieri VM, Eissa NT, Corbeil C, Chasse M, Braidy J, Matar N, Milic-Emili J (1991) Effects of positive end-expiratory pressure on alveolar recruitment and gas exchange in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 144:544–551
Valta P, Takala J, Eissa NT, Milic-Emili J (1993) Does alveolar recruitment occur with positive end-expiratory pressure in adult respiratory distress syndrome patients? J Crit Care 8:34–42
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873
Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, Romano ER, Regenga MM, Taniguchi LNT, Teixeira C, Pinheiro de Oliveira R, Machado FR, Diaz-Quijano FA, Filho MSA, Maia IS, Caser EB, Filho WO, Borges MC, Martins PA, Matsui M, Ospina-Tascon GA, Giancursi TS, Giraldo-Ramirez ND, Vieira SRR, Assef M, Hasan MS, Szczeklik W, Rios F, Amato MBP, Berwanger O, Ribeiro de Carvalho CR (2017) Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 318:1335–1345
Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH (2008) Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359:2095–2104
Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D (2019) JAMA 321:846–857
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Borges JB, Carvalho CR, Amato MB (2006) Lung recruitment in patients with ARDS. N Engl J Med 355: 319–320
Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ (1998) A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 158:1644–1655
Chen L, Del Sorbo L, Grieco LG, Shklar B, Junhasavasdikul D, Telias I, Fan E, Brochard L (2018) Airway closure in acute respiratory distress syndrome: an underestimated and misinterpreted phenomenon. Am J Respir Crit Care Med 197:132–135
Kattan MW, Gerds TA (2020) A framework for the evaluation of statistical prediction models. Chest 158:S29–S38
Acknowledgements
The authors would like to thank H. Aguirre-Bermeo, L. Chen, J. Dellamonica, A. Koutsoukou, J.J. Rouby, and A. Thille for sharing their data. The authors are grateful to the ESICM systematic review group for assistance received in data analysis and presentation.
Funding
This study has received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Turbil, E., Terzi, N., Cour, M. et al. Positive end-expiratory pressure-induced recruited lung volume measured by volume-pressure curves in acute respiratory distress syndrome: a physiologic systematic review and meta-analysis. Intensive Care Med 46, 2212–2225 (2020). https://doi.org/10.1007/s00134-020-06226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06226-9