Abstract
Natural killer (NK) cells play a crucial role in host immunity by detecting cells that downregulate MHC class I presentation and upregulate stress ligands, as commonly seen in cancers. Current NK therapies using primary NK cells are prone to manufacturing issues related to expansion and storage. Alternative cell sources utilizing immortalized NK cell lines require irradiation and are dependent on systemic IL-2 administration, which has been associated with adverse effects. In contrast, NK cells differentiated from induced pluripotent stem cells (iPSC-NK cells) offer an off-the-shelf alternative that may overcome these bottlenecks. The development of a serum-free and feeder-free differentiation protocol allows for the manufacturing of clinically adaptable iPSC-NK cells that are equally as effective as primary NK cells and the NK-92 cell line for many indications. Moreover, genetic modifications targeting NK-mediated antibody-dependent cellular cytotoxicity capabilities, cytotoxicity, and checkpoint inhibitors may increase the therapeutic potential of iPSC-NK products. This review will highlight the current sources for NK therapies and their respective constraints, discuss recent developments in the manufacturing and genetic engineering of iPSC-NK cells, and provide an overview of ongoing clinical trials using NK cells.
Similar content being viewed by others
Introduction
Despite recent advances in standard of care, cancers still account for more than 600,000 deaths annually in the USA alone [1], causing researchers to investigate alternative therapies. Immunotherapy, which seeks to harness and augment natural functions of the patient’s immune system to treat tumors, has now generated numerous approaches: antibodies, cytokines, dendritic cell vaccines, checkpoint inhibitors, adoptive cell transfer with tumor infiltrating lymphocytes (TIL) or genetically modified T cells, or some combination of these treatments [2]. While many of these are promising options (reviewed elsewhere [2,3,4]), this review will focus on natural killer (NK) cell-based approaches and their potential for off-the-shelf therapeutics.
Progress with T cell therapies over the last decade has provided guidance for the development of NK cell therapies. One approach to T cell therapies is to use autologous or human leukocyte antigen (HLA)-matched allogeneic T cells with an engineered chimeric antigen receptor (CAR). These cells are genetically modified to express antibody fragments fused to T cell signaling domains, providing antigen-specific cytotoxicity independent of the HLA [5].
There are currently two Food and Drug Administration (FDA)-approved CAR T cell therapies, tisagenlecleucel [6,7,8] and axicabtagene ciloleucel [9, 10]. Both therapies employ a virally inserted CD19 CAR that allows T cells to recognize and kill malignant B cells expressing the CD19 antigen, including B cell acute lymphoblastic leukemia and non-Hodgkin lymphomas [6,7,8,9,10]. Patients undergoing CAR T cell therapies can experience remission of their cancer after receiving lymphodepletion chemotherapy followed by CAR T cell infusion, but can also be susceptible to on-target/off-tumor B cell aplasia, due in part to the formation of memory CAR T cells [6,7,8,9,10]. Patients may also develop cytokine release syndrome (CRS) and/or immune effector cell-associated neurotoxicity syndrome (ICANS), which require further treatment in an intensive care unit (ICU) due to high likelihood of progression into other life-threatening complications [6,7,8,9,10]. Although more effective than traditional chemotherapy, T cell therapies require long production times, are expensive (~$373–475,000 USD per dose) [11], and are also partly dependent on the quality of the leukapheresis product from the patient, making them harder to implement in large-scale treatments [12]. Allogeneic T cell therapies are also prone to these issues, in theory, and also require HLA matching or knockout of the T cell receptor (TCR) to prevent graft-versus-host disease (GvHD) [5, 13].
NK cells are another important component of the immune system which have proven to be effective at killing malignant cells [14,15,16,17]. In contrast to T cells, NK cells do not require antigen priming and are HLA agnostic—thus avoiding GvHD—making them an excellent candidate for off-the-shelf therapies [17,18,19]. Clinical trials using NK cells have been available for the last 20 years and currently involve treatment for hematologic malignancies and solid tumors (see Supplementary Table 1, Additional file 1) [19,20,21,22,23,24,25,26,27]. Most of these trials examine the maximum tolerated dose of ex vivo activated and expanded primary peripheral blood NK cells (PB-NK), primary cord blood NK cells (CB-NK), or immortalized NK cell lines (e.g., NK-92 cells), while some trials focus on genetically modified NK cells, including CAR NK cells. Several trials using PB-NK cells implement lymphodepleting chemotherapy, and some trials administer the NK cells in combination with interleukin-2 (IL-2) or FDA-approved monoclonal antibodies to promote antibody-dependent cellular cytotoxicity (ADCC). However, the use of autologous and allogeneic NK cells in nearly all of these trials leads to the same long production times of weeks as observed with T cells. Developing NK cell-based immunotherapies from induced pluripotent stem cells (iPSCs) circumvents these issues while providing off-the-shelf capabilities. Additionally, genetic modifications—similar to those in T cells—can further improve the specificity, strength, and efficacy of iPSC-NK cell therapies. In this review, we will discuss the current sources for NK cells and recent developments in gene-modified iPSC-NK cells for universal therapies applicable to a global population.
Activation and inhibition of NK cells
NK cells are large granular lymphocytes present in the innate immune system that provide defense against virally infected cells and malignant tumors. In humans, NK cells are often defined by the presence of CD56, a marker also detected on other lymphocytes including CD8+ T cells, dendritic cells, and γδ T cells, and by their lack of CD3 [28,29,30,31]. NK cells also express the low-affinity Fcγ receptor CD16 [32, 33] to mediate ADCC and can generally be classified into two major subsets: CD56brightCD16+ and CD56dimCD16+ [31]. Although there is evidence of a small population of CD56negCD16+ NK cells in healthy individuals that undergoes expansion during HIV-1 infection [34], the CD56bright cells are abundant in lymphoid and nonlymphoid tissues and exhibit high cytokine secretion [35,36,37]. CD56dim cells are prevalent in peripheral blood and exhibit high cytotoxicity through secretion of perforin and granzyme B to kill target cells [35].
NK cell activation is dependent on the integration of signals received by the multitude of membrane bound activating and inhibiting receptors (Fig. 1). This unique regulation allows NK cells to mount an immune response that is independent of antigen presentation. NK cells distinguish healthy cells from abnormal cells through detection of “self-ligands” such as major histocompatibility complex (MHC) molecules [38]. The detection of MHC molecules from the MHC class I recognizing receptors, such as killer cell immunoglobulin-like receptors (KIR) and lectin-like NKG2A heterodimers, results in inhibitory signals (Fig. 2a) [38]. Malignant tumors have been shown to downregulate MHC molecules [39, 40] and upregulate stress ligands such as MICA and MICB [41,42,43], rending them susceptible to NK lysis (Fig. 2c). In cases when malignant cells escape immune surveillance through normal MHC expression, NK cytotoxicity can still be induced through sufficient stimulation of activating receptors (Fig. 2b), including the stress ligand receptor NKG2D [44], and natural cytotoxic receptors (NCR) NKp30 [45], NKp44 [46], and NKp46 [47]. In instances when target cells are opsonized with antibodies, the CD16 receptor on NK cells can bind to the Fc portion of the immunoglobulin G (IgG) and trigger granule secretion (Fig. 2d). Though NK cells are part of the innate immune response, there is evidence indicating that they are capable of forming a memory-like phenotype in acute myeloid leukemia (AML) [48]. Thus, NK cells provide additional levels of immune surveillance by targeting tumors that evade T cell-mediated killing.
Major NK cell receptors and ligands. NK cells express a repertoire of activating (“+”; NKG2C, NCRs, CD16, NKG2D) and inhibitory (“−”; NKG2A, KIRs) receptors on their cell surface. The activation of NK cells is dependent on the integration of the signals received by these receptors. The ligands responsible for NK activation and inhibition are shown above their corresponding receptors. Additionally, NK cells also express cytokine receptors that regulate cell functions such as proliferation and persistence and, in certain disease contexts, a memory-like phenotype
Schematic of NK cell functions. a In normal cells, MHC class I molecules can engage inhibitory receptors on NK cells that can outnumber and override activating receptors, preventing NK-mediated cytotoxicity. b In malignant cells, downregulation of MHC class I molecules can result in the absence of NK inhibitor receptor engagement, resulting in NK-mediated cytotoxicity. c In cases where tumor cells retain expression of MHC class I molecules, NK cytotoxicity can still be induced through the upregulation of stress-induced activation ligands that can bind activation receptors on NK cells. d In instances when target cells are opsonized by antibodies, NK cells can exhibit ADCC by detecting antibodies on the target cell through the low-affinity Fcγ CD16 receptor. The outcome of the NK cell response is determined by the amount of activating versus inhibitory receptors on the NK cell surface
Current sources of NK cells
Current immunotherapies employ NK cells from various sources: primary PB-NK cells (Fig. 3a, e), primary CB-NK cells (Fig. 3b), immortalized NK cell lines (Fig. 3c), and recently, iPSC-derived NK cells (Fig. 3d), each with its own benefits and disadvantages. Studies in the late 1990s to early 2000s focused on improving anti-tumor activity of NK cells using cytokine activated, autologous primary NK cells [49,50,51]. Isolating large numbers of primary PB-NK cells, however, has proven to be difficult as only 5–15% of circulating blood lymphocytes are NK cells, and quantities derived from leukapheresis products are highly donor dependent [52,53,54]. Additionally, isolation from peripheral blood lymphocytes often results in a heterogenous population of lymphocytes with only a 10–20% yield of NK cells [55]. The use of primary NK cells is further complicated by their decrease in cytotoxicity following cryopreservation [56, 57]. While the low yield and loss in cytotoxicity from cryopreservation can be partially overcome with cytokine support and feeder cell expansion, this approach makes PB-NK cells suboptimal for an “off-the-shelf” therapy [58,59,60]. In contrast, CB-NK cells recover well after cryopreservation and may be a potential candidate for “off-the-shelf” therapy using primary NK cells [61]. Though there is a greater percentage of NK cells in CB (23%) than in PB (11%), one disadvantage is the limited number of NK cells per cord blood unit, potentially requiring expansion of multiple units per dose [62]. When compared to PB-NK cells, CB-NK cells express lower levels of KIRs and granzyme B and higher levels of the NKG2A inhibitory receptor, suggesting that CB-NK cells have an immature phenotype [62, 63]. While this particular phenotype results in less potent cytotoxicity, CB-NK cell effector function can be improved to that of PB-NK cells with cytokine stimulation [63].
Sources of NK cells. Current NK therapies use NK cells from the following sources: primary PB-NK cells, primary CB-NK cells, NK cell lines, and recently, iPSC-NK cells, each with its own benefits and limitations. See Supplemental Tables 1 and 2 (Additional files 1 and 2) for a list of products in clinical trials
Alternative approaches have been considered using immortalized NK cell lines. Eight malignant NK cell lines have been established: NK-92, YT, NKL, HANK-1, KHYG-1, NK-YS, NKG, and NK101 [64, 65]. Of the eight cell lines, NK-92 is the only established cell line that is FDA approved for patient testing in phase 1 and 2 clinical trials [27, 66,67,68,69]. The NK-92 cell line was established from the blood of 50-year-old male patient with rapidly progressive non-Hodgkin lymphoma [70]. The NK-92 cell line can easily be maintained in vitro and expanded to large numbers through good manufacturing practices (GMP) with the addition of recombinant IL-2 [71]. Unlike primary NK cells, the NK-92 cell line can be cryopreserved with high recovery and function post-thaw [72]. NK-92 cells lack most of the KIRs while expressing several activating receptors making them suitable for anti-tumor therapies [73]. However, one disadvantage of NK-92 cells is that they do not express the low-affinity Fcγ CD16 receptor, making them incapable of an ADCC response [70]. To address this, NantKwest has developed an NK product engineered with a high-affinity CD16 receptor using their proprietary NK-92 platform [74]. The product, haNK, has been tested in a phase I clinical trial for solid tumors and is undergoing phase II clinical trials for Merkel cell carcinoma (see Supplementary Table 1, Additional file 1, ClinicalTrials.gov: NCT03027128, NCT03853317). Additionally, the malignant origin of the cell line requires them to be irradiated before adoptive transfer into patients [27, 66,67,68,69], inhibiting their proliferative capacity and persistence in vivo from a range of 48 h to 1 week, depending on modifications such as CARs [68, 69]. It has been shown previously using PB-NK cells that increased in vivo persistence (> 7 days) correlates with greater anti-tumor activity [75], weakening the rationale for using NK-92 therapies unless serial dosing is utilized. While multi-dose treatments would circumvent this issue, it would also require systemic administration of IL-2 to support NK growth, which may trigger additional side effects such as vascular leak syndrome and upregulation of regulatory T cells, which suppress NK cells [76,77,78].
Recently, there has been increasing interest in iPSC-NK therapies due to their ability to address the supply-chain bottlenecks associated with primary and cell line NK therapies. Advantages include that iPSCs can be generated from easily accessible sources such as fibroblasts or peripheral blood, retain pluripotency during expansion, and be banked for long-term storage [79]. NK cells derived from iPSCs have proven to be equally as effective as primary NK cells and NK-92 cells. One study showed that iPSC-NK cytotoxicity is comparable to activated and expanded PB-NK in MA148 and A1847 ovarian tumor xenograft models [80]. Donor peripheral blood-iPSC-NK cells have also been shown to have greater cytotoxicity against SKOV3, SW480, HCT-8, MCF7, and SCC-25 cancer cell lines compared to donor PB-NK cells, and similar efficacy against K562s as donor PB-NK cells [81]. iPSC-NK cells also prove advantageous over the established NK-92 cell line in that iPSC-NK cells do not need to be irradiated [80,81,82]. Another benefit of iPSC-NK cells is that they express the CD16 receptor and are therefore capable of ADCC [81, 82]. Zeng et al. demonstrated that PB-iPSC-NK cells are capable of ADCC by showing successful killing of Raji cells opsonized with an anti-CD20-hIgG1 antibody [81]. Typical degranulation of NK cells is achieved through phosphorylation of the Syk-PLCγ-DAG/inositol triphosphate or Syk-Vav-RAC-PAK-MEK-ERK pathways, while the secretion of inflammatory cytokines results from phosphorylation of the NF-κB pathway [83, 84]. However, it is not yet entirely clear if iPSC-NK cells require the same activation pathways to induce granule and cytokine secretion. Future studies need to mechanistically interrogate the signaling pathways activated upon iPSC-NK stimulation. Still, these data suggest that iPSC-NK-based strategies combine the most attractive qualities of primary NK cells and the NK-92 cell line, namely high potential for cytotoxicity, including ADCC function, and potential for expansion and persistence in vivo, even after cryopreservation.
Manufacturing NK cells from iPSCs
Serum-/feeder-free iPSC-NK derivation methods have already been established, in contrast to iPSC-T cell differentiations, which largely require xenogeneic Notch signaling from feeder cells [85,86,87]. A serum-, stromal, and feeder-free method for differentiation has recently been shown to repeatably produce clinical-scale mature NK cells in just 5 weeks (Fig. 4) [88] as opposed to a previously published longer protocol [89]. This new method eliminates the need for feeder-dependent single-cell adaptation, a non-uniform process that typically takes 2–3 months, and updates it to include mTeSR and Matrigel for quicker and more controlled single-cell iPSC generation [88].
A potential pipeline to manufacture iPSC-NK cells. At the iPSC stage, the cells can be modified and cryopreserved as a master cell bank for later use, or directly differentiated into NK cells. Spin-embryoid bodies can be used to induce CD34+ progenitors and self-supporting stromal cells without the use of feeder cells. The CD34+ progenitors can be differentiated into NK cells before expansion with cytokines and irradiated artificial antigen presenting cells (aAPCs). At this stage, the iPSC-NK cells can be cryopreserved as a working cell bank until infused into the patient. It may also be useful to enrich the CD34+ progenitor population (indicated by gray arrows) for cells with potent degranulation profiles before differentiating into NK cells
Another advantage to using iPSC-NK products, compared to iPSC-T cell therapies, is that iPSC-NK products have the capacity to be truly off-the-shelf as they would not need to be HLA matched between donors and patients. To create a universal therapy using iPSC-T products, cell banks would still require several different HLA diverse iPSC lines to cater to a large population. Okita et al. found that 50 unique HLA iPSC lines are required to treat over 73% of the Japanese population [90]. Taylor et al. found that 150 homozygous HLA iPSC lines are required to match 93% of the UK population [91]. One strategy to overcome the high number of required cell lines regarding iPSC-T therapies would be to genetically delete the HLA genes, but this particular modification is not necessary in iPSC-NK cells. However, the biomanufacturing time of 5 weeks for iPSC-NK cells could be a limitation to establishing and maintaining iPSC-NK cell banks, especially if genetic modifications such as CARs are necessary. Strategies to shorten and streamline the manufacturing process would prove useful.
Groups have successfully differentiated pluripotent stem cells (PSCs) into NK cells that are similar in phenotype and effector function when compared to primary NK cells [80, 81, 89, 92,93,94,95,96,97,98]. The differentiated cells express several NK-associated receptors such as CD56, KIRs, CD16, NKp44, NKp46, NKG2D, and TRAIL [80, 82, 92,93,94,95]. However, it is unclear if the derived cells resemble the bright or dim subset of the CD56 population, suggesting that further investigation into the expression profile of differentiated NK cells may be necessary. A recent study by Dege et al. further probed the ontogeny of hPSC-derived NK cells and found two distinct CD34+ populations that dictate final effector function [97]. The HOXAneg/low/CD34+ progenitors gave rise to a population that exhibits more potent degranulation while the HOXA+/CD34+ progenitors resulted in an NK population with robust inflammatory cytokine secretion [97]. These findings suggest that the origin of differentiated NK cells should be considered when adapting for immunotherapy applications and that it may be beneficial to enrich progenitor populations for markers of a more potent degranulation profile during differentiation.
Given the therapeutic potential of iPSC-NK products, the FDA has already approved a phase I clinical trial to investigate Fate Therapeutics’ off-the-shelf iPSC-NK product, FT500 [99]. FT500 is the first FDA-approved clinical investigation of an iPSC-derived cell product in the USA [99]. The NK cells are developed from a clonal master iPSC line cell bank, therefore making it possible to mass produce iPSC-NK cells that are relatively homogenous, quality controlled, and able to be cryopreserved for long-term storage [100]. The trial seeks to assess the safety and tolerability of multiple doses of FT500 in combination with checkpoint blockade therapy to treat adults with advanced solid tumors (see Supplementary Table 2, Additional file 2, ClinicalTrials.gov: NCT03841110). A separate observational study has been initiated to assess the long-term safety and efficacy of patients who receive FT500 treatment (see Supplementary Table 2, Additional file 2, ClinicalTrials.gov: NCT04106167).
Genetic modification of iPSC-NK cells
Despite the promising functionality of NK cells for treating cancer, the cells can become less effective over time due to a decrease in NK persistence and cytotoxicity. Therefore, genetic modifications to overcome these issues would prove useful to enhance antigen specificity and cytotoxicity (Fig. 5). Though groups have successfully modified primary NK cells and NK-92 cells using retroviral [101,102,103,104,105,106], lentiviral [107,108,109,110,111,112,113], and mRNA transfection [107, 114,115,116,117] approaches, there is wide variability in the efficiencies of transduction/transfection across these studies. The range of efficiencies span from 13 to 69% for retroviral transductions [101,102,103,104,105,106], 4 to 90% for lentiviral [107,108,109,110,111], and < 10 to > 60% for mRNA transfections [107, 114,115,116,117]. It is feasible to modify iPSCs using similar approaches including viral vectors, transposons, and CRISPR-Cas9 [85, 118,119,120,121,122,123,124]. Moreover, editing at the pluripotent stage would result in fully edited clonal lines that can be differentiated towards a NK lineage.
A summary of genetically modified iPSC-NK therapies. iPSC-NK cells have been genetically engineered to overcome the various limitations associated with NK therapies. Attempted modifications include mutated versions of the CD16 receptor to inhibit cleavage by the ADAM17 protease, fused cytokine receptor-ligand receptors to improve in vivo persistence, CARs to improve anti-tumor cytotoxicity, receptor knockout to prevent fratricide from combinatorial antibody treatments, and recombinant receptors to increase NK cytotoxicity outside the context of cancer. Another approach has been to target checkpoint inhibitors through deletion of the CISH gene
One of the issues associated with loss of cytotoxicity is the cleavage and shedding of the CD16 receptor. Upon NK activation, CD16 undergoes cleavage by the ADAM17 protease and is shed from the membrane, causing the NK cell to lose its ability to perform ADCC [121]. To circumvent this issue, Jing et al. transduced a mutated version of the CD16a receptor (CD16a/S197P) into iPSCs using a sleeping beauty transposon [121]. A site-directed mutagenesis of the CD16a receptor (S197P) prevented cleavage by ADAM17 and resulted in stable expression of CD16 even upon activation by the K562 cell line. In a separate abstract, Blum et al. reported that the CD16a/S197P-transduced iPSC-NK cells were 97.5% CD16+ before stimulation and 95.2% CD16+ after stimulation [122]. They also reported that CD16a/S197P-transduced iPSC-NK also showed improved degranulation and better killing of the SKOV3 ovarian cancer cell line when compared to unmodified iPSC-NK and PB-NK [122]. Studies incorporating CRISPR/Cas9 are also underway to determine the larger effects of deleting the ADAM17 gene [122]. Another successful modification to prevent CD16 cleavage has been to transduce iPSCs with a recombinant Fcγ receptor with the extracellular region of CD64, and the intracellular and transmembrane region of CD16a (CD64/16A) using a sleeping beauty transposon [124]. The CD64/16A receptor lacks the ADAM17 cleavage site, preventing CD16 downregulation upon NK activation. In an in vitro cytotoxicity assay, the transduced iPSC-NK cells exhibited greater ADCC against SKOV3 ovarian cancer cells when compared to untransduced iPSC-NK cells.
Fate Therapeutics is conducting an analogous line of research with their iPSC-NK products, FT516 and FT538 (see Supplementary Table 2, Additional file 2). FT516 is an iPSC-NK product that has been engineered with a high-affinity, non-cleavable CD16 (hnCD16) receptor at the iPSC stage to enhance ADCC and anti-tumor capabilities, and is undergoing phase I clinical trials in adults with hematologic malignancies (see Supplementary Table 2, Additional file 2, ClinicalTrials.gov: NCT04023071) [125]. A preclinical study reported that hnCD16-iPSC-NK cells displayed greater ADCC capabilities, CD107a expression, and IFN-gamma production compared to peripheral and unmodified iPSC-NK cells against various antibody-treated cancer cell lines [126]. While treatment with hnCD16-iPSC-NK, iPSC-NK, or PB-NK cells alone did not show a change in tumor burden, a combinatorial treatment of anti-CD20 and hnCD16-iPSC-NK showed a decrease in tumor burden 10 days after treatment in an in vivo B cell lymphoma mouse xenograft model. However, relapse occurred in most treated groups, but was rescued by multiple doses of treatment extending the mean survival from 52 to 76 days. The FT538 product also addresses issues with NK cytotoxicity, specifically in cases of multiple myeloma. Myeloma cells strongly express CD38 and are often treated with daratumumab, an anti-CD38 monoclonal antibody [127]. However, administration with daratumumab has been shown to demonstrate reductions in NK cell counts and activation due to fratricide from NK expression of CD38 [128]. To circumvent this, FT538, derived from a clonal master iPSC line, has been modified with a knockout of the CD38 receptor and knock-in of the hnCD16 receptor to increase ADCC and prevent exhaustion when used with anti-CD38 antibody treatments [129].
Though useful strategies, completely preventing cleavage of CD16, could also be problematic since CD16 shedding has been established as a regulatory mechanism that sustains NK survival by assisting with detachment of NK cells from target cells [130]. Once a target cell is lysed, the NK cell detaches and continues its surveillance for other malignant cells and can normally kill up to seven target cells in a 12-h period [131]. However, Srpan et al. observed a 67% decrease in detachment of NK cells and found that NK cells stayed in contact with their target cells even after 8 h when modified with the mutant CD16 receptor (S197P) from the Jing et al. study [130]. While the cytotoxicity of the NK cells was not affected, there was less target cell lysis overall. The authors also noted an increase in NK cell death from 23 to 38% in the presence of an ADAM17 inhibitor due to the inability of the NK cell to detach itself [130]. These results suggest that a fully non-cleavable CD16 receptor could be advantageous initially but may be a limiting factor for “serial killing.” Moreover, constant engagement of the NK cells may also lead to a hyperinflammatory environment due to increased cytokine secretion, causing deleterious side effects such as CRS that could contribute to morbidity or mortality. Strategies to induce CD16 re-expression or increase the rate of CD16 receptor turnover after shedding may provide more effective alternatives.
iPSC-NK cells have also been investigated in the context of human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) due to the ability of NK cells to kill virally infected cells. Ni et al. found that iPSC-NK cells were capable of inhibiting replication of HIV-infected CD4+ T cells through degranulation, cytokine secretion, and ADCC within in vitro co-culture assays [94]. In another study, Ni et al. engineered embryonic stem cells (ESCs) and iPSCs with a receptor composed of the extracellular region of CD4 and the intracellular signaling chain of CD3ζ before differentiating to NK cells to target HIV-infected cells [98]. The engineered ESC/iPSC-NK cells showed better suppression of HIV-infected T cells in in vitro assays when compared to unmodified NK cells. In a humanized mouse model, the authors found inhibition of HIV replication in mice treated with modified and unmodified ESC/iPSC-NK cells, but noted that the difference was not significant between the two groups. These findings suggest that iPSC-NK therapies, modified or not, may provide potential treatments outside the context of cancer.
CAR NK and iPSC-CAR-NK cells
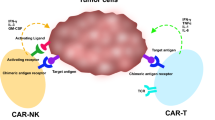
Another proposed approach to increasing the anti-tumor activity of NK cells has been to engineer them with CARs. Clinical trials have been initiated for CAR NK treatments using donor derived primary NK cells (see Supplementary Table 1, Additional file 1, ClinicalTrials.gov: NCT03056339, NCT00995137) and completed but not yet published (NCT00995137). A phase I/II clinical trial administering cord blood CAR NK cells for relapsed or refractory CD19-positive cancers showed encouraging outcomes with seven of eleven patients experiencing complete remission, with no development of CRS or ICANS noted (ClinicalTrials.gov: NCT03056339) [132]. The CAR NK treatment, coined “TAK-007,” is currently being developed by Takeda Pharmaceuticals, alongside three other CAR NK treatments, in anticipation of a multicenter phase II clinical trial in 2021 [133]. Other immunotherapy companies, such as Nantkwest and Nkarta, are also conducting preclinical research for CAR NK products for B cell malignancies and solid tumors. NantKwest is developing a CAR NK product (taNK) using its uniquely engineered NK-92 platform for HER2/NEU expressing cancers [74]. Nkarta Therapeutics is working to create NKX101, a unique CAR NK therapy that targets NKG2D stress ligands in place of cancer-specific antigens, and an anti-CD19 CAR NK product [134].
Given the accessibility of iPSCs [79] and efficacy of iPSC-NK cells [80,81,82], it can be beneficial to engineer CARs in iPSCs. For instance, the Kaufman group has had success in engineering iPSCs with an optimized NK-CAR construct constructed with an anti-mesothelin scFv region, NKG2D transmembrane domain, 2B4 co-stimulatory domain, and a CD3ζ cytotoxicity domain using a PiggyBac transposon vector, prior to differentiation into functional CAR-iPSC-NK cells [120]. The derivation efficiency of NK cells from CAR bearing iPSCs was similar to the efficiency from unmodified iPSCs, and the phenotype of the CAR-iPSC-NK cells was comparable to that of the PB-NK cells. In an ovarian cancer xenograft model, NK-CAR-iPSC-NK cells displayed better anti-tumor activity when compared to PB-NK, iPSC-NK, and T-CAR-expressing-iPSC-NK cells and had greater circulation at day 10. When compared to third-generation CAR T cells, NK-CAR-iPSC-NK cells demonstrated similar anti-tumor effects but with less overall toxicity in vivo. Through a mechanistic study with site-specific mutations in each CAR signaling domain, the authors also demonstrated that the cytotoxicity of the NK-CAR-iPSC-NK cells resulted from activation of established NK signaling pathways, Syk-vav1-Erk and NF-κB. Despite the positive results, the NK-CAR-iPSC-NK cells had limited in vivo persistence as they returned to baseline levels similar to those of PB-NK cells by day 21 and were barely detectable at day 28. The Rezvani group reported that the inclusion of an IL-15 domain in an anti-CD19 CAR resulted in detection of cord blood CAR NK cells up to 68 days after infusion [106]. Though the microenvironment of solid tumors adds an extra layer of complexity, the Rezvani group’s CAR could provide a blueprint for improving the in vivo lifespan of CAR NK cells as in vivo persistence has been correlated with the efficacy in CAR T cell treatment for solid tumors [135].
Along a similar line of research, Fate Therapeutics has also been conducting preclinical investigations regarding their iPSC-NK product FT596 (see Supplementary Table 2, Additional file 2), which has been modified to include an anti-CD19 CAR, a hnCD16 receptor, and a novel IL-15/IL-15r fusion receptor to target B cell malignancies [136]. The CD19-CAR enhances anti-tumor activity against CD19 expressing B cells, while the CD16 receptor increases ADCC, and the novel IL-15/IL-15r fusion receptor promotes in vivo persistence and expansion. FT596 is being developed for use as a monotherapy or in combination with monoclonal antibodies. It has been shown to kill both CD19+ and CD19− B cells in a co-culture assay when used in conjunction with rituximab, a monoclonal antibody for CD20 expressing B cells [136]. It has also been shown to prevent tumor progression and promote survival in a B cell leukemia xenograft model [136].
Limitations of NK cell therapies
Despite the encouraging results of preclinical and clinical studies of NK therapies, there are still significant barriers to overcome with current approaches. Though efforts have been made to improve in vivo persistence through cytokine support, the short lifespan of NK cells in vivo may require multiple infusions. Investigations into serial dosing of cryopreserved stocks, combinatorial approaches with other immunotherapies such as CAR T cells, and using cytokines such as IL-15/IL-15r or IL-21 for additional support will be required.
An alternative approach to enhance NK effector functions has been to target checkpoint inhibitors of NK cells, such as the downregulation of IL-15 signaling. IL-15 is a critical chemokine involved in maintaining NK homeostasis [137]. However, it has been shown that the CIS regulatory element, encoded by the CISH gene, negatively regulates IL-15 signaling and thus acts as a major inhibitory checkpoint [138]. Using a murine model, Delconte et al. showed that CISH−/− mice were immune to the formation of melanoma, breast, and prostate cancer in vivo due to an upregulation of IL-15 signaling in NK cells [138]. To overcome this inhibitory checkpoint, the Kaufman group used the CRISPR/Cas9 system to delete CISH at the iPSC stage prior to NK differentiation. When compared to unmodified iPSC-NK cells, the CISH−/− iPSC-NK cells demonstrated better cytotoxicity against AML cell lines and greater in vivo persistence in an AML xenograft model [123]. Biallelic deletion of CISH also increased sensitivity to IL-15 and resulted in greater expansion when compared to unedited iPSC-NK cells, even at low concentrations of IL-15. In addition, groups are also examining the combination of adoptive NK cell infusions with checkpoint blockade antibodies such as anti-PD1, anti-TIM3, and anti-TIGIT to overcome NK exhaustion [139, 140], although there is some debate on whether PD-1 is a relevant checkpoint on NK cells [141]. Zhang et al. showed that blockade of TIGIT, an immunosuppressive factor, improved murine NK cell anti-tumor activity and prevented exhaustion in vivo of tumor bearing mice, suggesting that blocking inhibiting receptors may be a compelling strategy to improve anti-tumor responses [140].
As is the case with other immunotherapies [142], NK cells struggle to infiltrate solid tumors. Tumor penetration, in combination with tumor homing, has been strongly correlated with better prognosis [143, 144]. One study’s single-cell RNA-seq analysis showed that murine NK cells represented less than 1–5% of immune cells present in mouse tumor microenvironments, indicating that NK cells do not naturally infiltrate tumors at high percentages [145]. CXCR3 has been identified as a crucial receptor for NK chemotaxis in response to cancer-secreted ligands, CXCL9, CXCL10, and CXCL11 [146]. Therefore, engineering a CXCR3 receptor fused with NK activating signaling domains into an iPSC line may prove beneficial to enhance homing of NK cells to chemokine-secreting tumors. Even if NK cells penetrate the tumor, they encounter an array of immune suppressive factors, such as tumor secreted transforming growth factor-beta (TGF-β), that may downregulate NK activity regardless of genetic modifications [147,148,149]. It has been suggested that a dominant negative TGF-β receptor that inhibits TGF-β signaling or a chimeric receptor that converts TGF-β to an activation signal may provide potential strategies to overcome NK cell exhaustion [150, 151].
Although some of the studies investigating NK cell biology were performed in the context of murine NK cells, there is evidence that regulatory mechanisms may be conserved between human and murine NK cells. For instance, though the human KIR inhibitory receptor is structurally different from its murine analog, Ly49, both receptors contain an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) that phosphorylates similar pathways to inhibit NK activation [152]. Additionally, the localization of receptors and ligands in chromosomes are similar in human and murine NK cells, suggesting that the regulatory mechanisms may be comparable in both populations [152]. However, murine NK cells lack CD56 and are identified through the expression of other markers such as NK1.1 (CD161b, CD161c), DX5 (CD49b), and NKp46 instead. Since the lack of CD56 expression on murine NK cells makes it difficult to identify analogous subsets in human NK populations, there is a possibility that the CD56bright and CD56dim subsets in humans may not have a comparable population in mice, and thus observations from comparable murine NK effector phenotypes may not translate to the clinic [153]. Nevertheless, these differences may be accounted for through appropriate manipulation of mouse models and understanding of their limitations.
Concluding remarks
NK-based immunotherapies already provide a promising approach to treat several types of cancers (see Supplementary Table 1, Additional file 1). These therapies are likely complementary to T cell therapies and may be able to address some of the limitations of T cell therapies (e.g., requiring patient-donor HLA matching, development of CRS/ICANS, and the lack of a clinically adaptable differentiation protocol for iPSC-T products). Importantly, NK-mediated immunotherapy can be applied across all HLA haplotypes without the occurrence of GvHD. Moreover, iPSC technology offers new solutions for obstacles associated with conventional NK therapies regarding manufacturing, banking, and improving anti-tumor activity.
Given the advances made in the development and engineering of iPSC-NK products, it is essential to further examine the therapeutic potential of iPSC-NK immunotherapies in both preclinical and clinical studies, while trying to reduce biomanufacturing time. An “omics”-based approach to resolve tumor-NK cell interactions at the single-cell level may offer new targets to improve persistence in the tumor microenvironment. It would be of interest to examine the transcriptome and secretome of NK cells capable of infiltrating the tumor, fluctuations in NK gene expression induced from cancer cells, and changes, or lack thereof, in the tumor transcriptome in response to NK cell presence. We anticipate that an increase in our understanding of fundamental NK and tumor biology will soon prove useful in selecting appropriate gene modifications and further optimizing the biomanufacturing of NK cell immunotherapies.
Availability of data and materials
Not applicable.
Abbreviations
- aAPC:
-
Artificial antigen presenting cells
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- AIDS:
-
Acquired immunodeficiency syndrome
- AML:
-
Acute myeloid leukemia
- CAR:
-
Chimeric antigen receptor
- CB-NK:
-
Cord blood natural killer
- CRS:
-
Cytokine release syndrome
- ESC:
-
Embryonic stem cells
- FDA:
-
Food and Drug Administration
- GMP:
-
Good manufacturing practices
- GvHD:
-
Graft-versus-host disease
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- ICU:
-
Intensive care unit
- IL-2:
-
Interleukin-2
- IL-15:
-
Interleukin-15
- IgG:
-
Immunoglobulin G
- iPSC:
-
Induced pluripotent stem cells
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
- KIR:
-
Killer cell immunoglobulin-like receptor
- MHC:
-
Major histocompatibility complex
- NCR:
-
Natural cytotoxic receptor
- NK:
-
Natural killer
- PB-NK:
-
Peripheral blood natural killer
- PSC:
-
Pluripotent stem cells
- TCR:
-
T cell receptor
- TGF-β:
-
Transforming growth factor-beta
- TIL:
-
Tumor infiltrating lymphocytes
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 2019;2019:4508794. https://doi.org/10.1155/2019/4508794.
Naran K, Nundalall T, Chetty S, Barth S. Principles of immunotherapy: implications for treatment strategies in cancer and infectious diseases. Front Microbiol. 2018;9. https://doi.org/10.3389/fmicb.2018.03158.
Zhao L, Cao YJ. Engineered T cell therapy for cancer in the clinic. Front Immunol. 2019;10:2250. https://doi.org/10.3389/fimmu.2019.02250.
Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98.
Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Maude SL, Pulsipher MA, Boyer MW, et al. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood. 2016;128:2801.
Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42.
Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Novartis in Society 2018 US Report. Accessed 14 Jan 2019.
Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Therapy Oncolytics. 2016;3:16015. https://doi.org/10.1038/mto.2016.15.
Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pract Res Clin Haematol. 2008;21:205–22.
Cordeau M, Belounis A, Lelaidier M, Cordeiro P, Sartelet H, Herblot S, Duval M. Efficient killing of high risk neuroblastoma using natural killer cells activated by plasmacytoid dendritic cells. PLoS One. 2016;11:e0164401. https://doi.org/10.1371/journal.pone.0164401.
Sun Y, Yao Z, Zhao Z, Xiao H, Xia M, Zhu X, Jiang X, Sun C. Natural killer cells inhibit metastasis of ovarian carcinoma cells and show therapeutic effects in a murine model of ovarian cancer. Exp Ther Med. 2018;16:1071–8.
Pasero C, Gravis G, Granjeaud S, Guerin M, Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A, Olive D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360–73.
Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100.
Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7.
Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9.
Rizzieri DA, Storms R, Chen DF, et al. Natural killer cell-enriched donor lymphocyte infusions from a 3-6/6 HLA matched family member following nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1107–14.
Federico SM, McCarville MB, Shulkin BL, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–9.
Modak S, Le Luduec JB, Cheung IY, et al. Adoptive immunotherapy with haploidentical natural killer cells and anti-GD2 monoclonal antibody m3F8 for resistant neuroblastoma: results of a phase I study. Oncoimmunology. 2018;7. https://doi.org/10.1080/2162402X.2018.1461305.
Shaffer BC, Le Luduec JB, Forlenza C, Jakubowski AA, Perales MA, Young JW, Hsu KC. Phase II study of haploidentical natural killer cell infusion for treatment of relapsed or persistent myeloid malignancies following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:705–9.
Cooley S, He F, Bachanova V, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019;3:1970–80.
Bachanova V, Sarhan D, DeFor TE, Cooley S, Panoskaltsis-Mortari A, Blazar BR, Curtsinger JM, Burns L, Weisdorf DJ, Miller JS. Haploidentical natural killer cells induce remissions in non-Hodgkin lymphoma patients with low levels of immune-suppressor cells. Cancer Immunol Immunother. 2018;67:483–94.
Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: a phase I trial. Biol Blood Marrow Transplant. 2016;22:1290–8.
Boyiadzis M, Agha M, Redner RL, et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy. 2017;19:1225–32.
Pittet MJ, Speiser DE, Valmori D, Cerottini J-C, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52.
Tel J, Smits EL, Anguille S, Joshi RN, Figdor CG, de Vries IJM. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood. 2012;120:3936–44.
Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vγ2Vδ2 T cells. BMC Immunol. 2009;10:50. https://doi.org/10.1186/1471-2172-10-50.
Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16:7–19.
Anegón I, Cuturi MC, Trinchieri G, Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988;167:452–72.
Yeap WH, Wong KL, Shimasaki N, Teo ECY, Quek JKS, Yong HX, Diong CP, Bertoletti A, Linn YC, Wong SC. CD16 is indispensable for antibodydependent cellular cytotoxicity by human monocytes. Sci Rep. 2009;10. https://doi.org/10.1038/srep34310.
Milush JM, López-Vergès S, York VA, Deeks SG, Martin JN, Hecht FM, Lanier LL, Nixon DF. CD56negCD16+NK cells are activated mature NK cells with impaired effector function during HIV-1 infection. Retrovirology. 2013;10. https://doi.org/10.1186/1742-4690-10-158.
Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–6.
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97:3146–51.
Carrega P, Bonaccorsi I, Di Carlo E, et al. CD56brightPerforinlow noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol. 2014;192:3805–15.
Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9.
Garrido F, Cabrera T, Aptsiauri N. “Hard” and “soft” lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–56.
Koopman LA, Corver WE, van der Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med. 2000;191:961–76.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. PNAS USA. 1999;96:6879–84.
Vetter CS, Groh V, Thor Straten P, Spies T, Bröcker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118:600–5.
Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–96.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9.
Pende D, Parolini S, Pessino A, et al. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–16.
Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72.
Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–36.
Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:1–12.
DeMagalhaes-Silverman M, Donnenberg A, Lembersky B, Elder E, Lister J, Rybka W, Whiteside T, Ball E. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother. 2000;23:154–60.
Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, Ohno T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–71.
Escudier B, Farace F, Angevin E, Charpentier F, Nitenberg G, Triebel F, Hercend T. Immunotherapy with interleukin-2 (IL2) and lymphokine-activated natural killer cells: improvement of clinical responses in metastatic renal cell carcinoma patients previously treated with IL2. Eur J Cancer. 1994;30:1078–83.
Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kühne T, Favre G, Gratwohl A. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8.
Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7. https://doi.org/10.3389/fimmu.2016.00091.
Yoon SR, Lee YS, Yang SH, et al. Generation of donor natural killer cells from CD34 progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant. 2010;45:1038–46.
Koepsell SA, Miller JS, McKenna DH Jr. Natural killer cells: a review of manufacturing and clinical utility. Transfusion. 2013;53:404–10.
Mark C, Czerwinski T, Roessner S, et al. Cryopreservation impairs cytotoxicity and migration of NK cells in 3-D tissue: implications for cancer immunotherapy. 2019. Preprint at https://www.biorxiv.org/content/10.1101/812172v1. Accessed 9 May 2020.
Szmania S, Lapteva N, Garg T, et al. Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients. J Immunother. 2015;38:24–36.
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–7.
Lapteva N, Durett AG, Sun J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–43.
Damodharan S, Walker K, Forsberg M, McDowell K, Bouchlaka M, Drier D, Sondel P, DeSantes K, Capitini C. Analysis of ex vivo expanded and activated clinical grade human NK cells after cryopreservation. Forthcoming: Cytotherapy; 2020. https://doi.org/10.1016/j.jcyt.2020.05.001.
Mehta RS, Shpall EJ, Rezvani K. Cord blood as a source of natural killer cells. Front Med. 2016;2. https://doi.org/10.3389/fmed.2015.00093.
Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol. 2007;4:377–82.
Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, Saudemont A. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum Immunol. 2012;73:248–57.
Cheng M, Zhang J, Jiang W, Chen Y, Tian Z. Natural killer cell lines in tumor immunotherapy. Front Med China. 2012;6:56–66.
Yang HG, Kang MC, Kim TY, Hwang I, Jin HT, Sung YC, Eom K-S, Kim SW. Discovery of a novel natural killer cell line with distinct immunostimulatory and proliferative potential as an alternative platform for cancer immunotherapy. J Immunother Cancer. 2019;7:138. https://doi.org/10.1186/s40425-019-0612-2.
Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy. 2008;10:625–32.
Williams BA, Law AD, Routy B, DenHollander N, Gupta V, Wang XH, Chaboureau A, Viswanathan S, Keating A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget. 2017;8:89256–68.
Tang X, Yang L, Li Z, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8:1083–9.
Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70.
Gong J-H, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8:652–8.
Tam YK, Martinson JA, Doligosa K, Klingemann HG. Ex vivo expansion of the highly cytotoxic human natural killer cell line NK-92 under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy. 2003;5:259–72.
Pasley S, Zylberberg C, Matosevic S. Natural killer-92 cells maintain cytotoxic activity after long-term cryopreservation in novel DMSO-free media. Immunol Lett. 2017;192:35–41.
Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–83.
NantKwest - Technology. https://nantkwest.com/technology/#tank. Accessed 30 Apr 2020.
Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–63.
Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10:222–9.
Van Der Vliet HJJ, Koon HB, Yue SC, Uzunparmak B, Seery V, Gavin MA, Rudensky AY, Atkins MB, Balk SP, Exley MA. Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res. 2007;13:2100–8.
Ghiringhelli F, Ménard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–85.
Valamehr B, Robinson M, Abujarour R, et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep. 2014;2:366–81.
Hermanson DL, Bendzick L, Pribyl L, McCullar V, Vogel RI, Miller JS, Geller MA, Kaufman DS. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells. 2016;34:93–101.
Zeng J, Tang SY, Toh LL, Wang S. Generation of “off-the-shelf” natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. Stem Cell Rep. 2017;9:1796–812.
Woll PS, Martin CH, Miller JS, Kaufman DS. Human embryonic stem cell-derived NK cells acquire functional receptors and cytolytic activity. J Immunol. 2005;175:5095–103.
Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502.
Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–9.
Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–33.
Minagawa A, Yoshikawa T, Yasukawa M, et al. Enhancing T cell receptor stability in rejuvenated iPSC-derived T cells improves their use in cancer immunotherapy. Cell Stem Cell. 2018;23:850–8.
Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii SI, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Cell. 2013;12:31–6.
Zhu H, Kaufman DS. An improved method to produce clinical-scale natural killer cells from human pluripotent stem cells. Methods Mol Biol. 2019;2048:107–19.
Hermanson D, Ni Z, Kaufman DS. Human pluripotent stem cells as a renewable source of natural killer cells. In: Cheng T, editor. Hematop. Differ. Hum. Pluripotent Stem Cells. Dordrecht: Springer; 2015. p. 69–79.
Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–12.
Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012;11:147–52.
Knorr DA, Ni Z, Hermanson D, Hexum MK, Bendzick L, Cooper LJN, Lee DA, Kaufman DS. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–83.
Larbi A, Gombert JM, Auvray C, et al. The HOXB4 homeoprotein promotes the ex vivo enrichment of functional human embryonic stem cell-derived NK cells. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0039514.
Ni Z, Knorr DA, Clouser CL, Hexum MK, Southern P, Mansky LM, Park I-H, Kaufman DS. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms. J Virol. 2011;85:43–50.
Woll PS, Grzywacz B, Tian X, Marcus RK, Knorr DA, Verneris MR, Kaufman DS. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113:6094–101.
Bock AM, Knorr D, Kaufman DS. Development, expansion, and in vivo monitoring of human NK cells from human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). J Vis Exp. 2013;74. https://doi.org/10.3791/50337.
Dege C, Fegan KH, Creamer JP, et al. Potently cytotoxic natural killer cells initially emerge from Erythro-myeloid progenitors during mammalian development. Dev Cell. 2020;53:239. https://doi.org/10.1016/j.devcel.2020.02.016.
Ni Z, Knorr DA, Bendzick L, Allred J, Kaufman DS. Expression of chimeric receptor CD4ζ by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells. 2014;32:1021–31.
Tartaglia C. Fate therapeutics announces FDA clearance of landmark IND for FT500 iPSC-derived, Off-the-Shelf NK Cell Cancer Immunotherapy https://ir.fatetherapeutics.com/news-releases/news-release-details/fate-therapeutics-announces-fda-clearance-landmark-ind-ft500. Accessed 26 Dec 2019.
FT500 - Fate Therapeutics. https://fatetherapeutics.com/pipeline/immuno-oncology-candidates/ft500/. Accessed 26 Dec 2019.
Guven H, Konstantinidis KV, Alici E, Aints A, Abedi-Valugerdi M, Christensson B, Ljunggren HG, Dilber MS. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33:1320–8.
Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–83.
Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, Kiessling R, Blankenstein T, Abken H, Charo J. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. PNAS USA. 2008;105:17481–6.
Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, Campana D, Juergens H, Pule M, Rossig C. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–66.
Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–86.
Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–31.
Boissel L, Betancur M, Lu W, Wels WS, Marino T, Van Etten RA, Klingemann H. Comparison of mRNA and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53:958–65.
Figueiredo C, Seltsam A, Blasczyk R. Permanent silencing of NKG2A expression for cell-based therapeutics. J Mol Med. 2009;87:199–210.
Sahm C, Schönfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified vectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother. 2012;61:1451–61.
Micucci F, Zingoni A, Piccoli M, Frati L, Santoni A, Galandrini R. High-efficient lentiviral vector-mediated gene transfer into primary human NK cells. Exp Hematol. 2006;34:1344–52.
Baeriswyl V, Wodnar-Filipowicz A, Kalberer CP. The effect of silencing NKG2D through RNA interference on receptor functions in interleukin-2-activated human natural killer cells. Haematologica. 2006;91:1538–41.
Han J, Chu J, Keung Chan W, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. 2015;5. https://doi.org/10.1038/srep11483.
Chu J, Deng Y, Benson DM, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–27.
Shimasaki N, Fujisaki H, Cho D, Masselli M, Lockey T, Eldridge P, Leung W, Campana D. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy. 2012;14:830–40.
Boissel L, Betancur M, Wels WS, Tuncer H, Klingemann H. Transfection with mRNA for CD19 specific chimeric antigen receptor restores NK cell mediated killing of CLL cells. Leuk Res. 2009;33:1255–9.
Li L, Liu LN, Feller S, et al. Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010;17:147–54.
Chu Y, Flower A, Cairo MS. Modification of expanded NK cells with chimeric antigen receptor mRNA for adoptive cellular therapy. In: Somanchi S, editor. Nat. Kill. Cells. Methods Mol. Biol. New York: Humana Press, New York; 2016. p. 215–30.
Wilber A, Linehan JL, Tian X, Woll PS, Morris JK, Belur LR, McIvor RS, Kaufman DS. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. Stem Cells. 2007;25:2919–27.
Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, Kan YW. Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24:1526–33.
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–92.
Jing Y, Ni Z, Wu J, Higgins LA, Markowski TW, Kaufman DS, Walcheck B. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS One. 2015;10:e0121788. https://doi.org/10.1371/journal.pone.0121788.
Blum R, Arumugam A, Wu J, Walcheck B, Kaufman D. Engineering human pluripotent stem cell-derived natural killer cells to prevent CD16a shedding for enhanced anti-tumor killing. Blood. 2016;128:1336. https://doi.org/10.1182/blood.v128.22.1336.1336.
Zhu H, Blum R, Wu Z, Meng Z, Guan K-L, Kaufman DS. Deletion of CISH in human pluripotent stem cell-derived natural killer cells enhances anti-tumor activity via metabolic reprogramming. Blood. 2019;134:619. https://doi.org/10.1182/BLOOD-2019-124446.
Snyder KM, Hullsiek R, Mishra HK, Mendez DC, Li Y, Rogich A, Kaufman DS, Wu J, Walcheck B. Expression of a recombinant high affinity IgG fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front Immunol. 2018;9:1–11.
FT516 - Fate Therapeutics. https://fatetherapeutics.com/pipeline/immuno-oncology-candidates/ft516/. Accessed 26 Dec 2019.
Zhu H, Blum RH, Bjordahl R, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135:399–410.
De Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8.
Casneuf T, Xu XS, Adams HC, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1:2105–14.
FT538 - Fate Therapeutics. https://fatetherapeutics.com/pipeline/immuno-oncology-candidates/ft538/. Accessed 26 Dec 2019.
Srpan K, Ambrose A, Karampatzakis A, Saeed M, Cartwright ANR, Guldevall K, De Matos GDSC, Önfelt B, Davis DM. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J Cell Biol. 2018;217:3267–83.
Vanherberghen B, Olofsson PE, Forslund E, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–34.
Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382:545–53.
CD19 CAR NK-cell therapy achieves 73% response rate in patients with leukemia and lymphoma. https://www.mdanderson.org/newsroom/cd19-car-nk-cell-therapy-achieves-73-percent-response-rate-in-patients-with-leukemia-and-lymphoma.h00-159379578.html. Accessed 29 Apr 2020.
Development Pipeline | NKX101 | NKX019 | Nkarta Inc. https://www.nkartatx.com/pipeline/. Accessed 30 Apr 2020.
Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6.
FT596 - Fate Therapeutics. https://fatetherapeutics.com/pipeline/immuno-oncology-candidates/ft596/. Accessed 26 Dec 2019.
Cooper MA, Bush JE, Fehniger TA, Vandeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–8.
Delconte RB, Kolesnik TB, Dagley LF, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol. 2016;17:816–24.
Lieberman NAP, DeGolier K, Haberthur K, Chinn H, Moyes KW, Bouchlaka MN, Walker KL, Capitini CM, Crane CA. An uncoupling of canonical phenotypic markers and functional potency of ex vivo-expanded natural killer cells. Front Immunol. 2018;9:150. https://doi.org/10.3389/fimmu.2018.00150.
Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–32.
Cho MM, Quamine AE, Olsen MR, Capitini CM. Programmed cell death protein 1 on natural killer cells: fact or fiction? J Clin Invest. 2020;130(6):2816-19.
Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–6.
Cursons J, Souza-Fonseca-Guimaraes F, Foroutan M, Anderson A, Hollande F, Hediyeh-Zadeh S, Behren A, Huntington ND, Davis MJ. A gene signature predicting natural killer cell infiltration and improved survival in melanoma patients. Cancer Immunol Res. 2019;7:1162–74.
Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–63.
Kumar MP, Du J, Lagoudas G, Jiao Y, Sawyer A, Drummond DC, Lauffenburger DA, Raue A. Analysis of single-cell RNA-Seq identifies cell-cell communication associated with tumor characteristics. Cell Rep. 2018;25:1458–1468.e4.
Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. 2014;64:225–35.
Castriconi R, Cantoni C, Chiesa M, Della VM, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor β1 inhibits expression of NKP30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. PNAS USA. 2003;100:4120–5.
Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, Weller M. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–603.
Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40.
Burga RA, Yvon E, Chorvinsky E, Fernandes R, Cruz CR, Bollard CM. Engineering the TGFb receptor to enhance the therapeutic potential of natural killer cells as an immunotherapy for neuroblastoma. Clin Cancer Res. 2019;25:4400–12.
Powell AB, Yadavilli S, Saunders D, et al. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med. 2019;17:321. https://doi.org/10.1186/s12967-019-2055-4.
Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–13.
Huntington ND, Vosshenrich CAJ, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14.
Acknowledgements
The authors would like to thank Nicole J. Piscopo for providing constructive feedback and help in reviewing the manuscript.
Funding
We acknowledge generous support from the National Science Foundation CBET-1645123 (CMC and KSa), AWD-101645-G3/RJ375-G3 (KSa); National Institutes of Health/National Cancer Institute R01 CA215461 (CMC), National Institute of General Medical Sciences R35 GM119644-01 (KSa); St. Baldrick’s – Stand up to Cancer Pediatric Dream Team Translational Research Grant SU2C-AACR-DT-27-17 (CMC); American Cancer Society Research Scholar grant RSG-18-104-01-LIB (CMC); and the MACC Fund (CMC). Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the Scientific Partner of SU2C. The contents of this article do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. Figures were created with Biorender.com.
Author information
Authors and Affiliations
Contributions
KSh and KSa were involved in designing and drafting the review. KSh conducted the literature review, drafted the manuscript, and designed the tables and figures. CMC contributed content to improve the scope of the review after initial submission. KSa and CMC substantively revised the manuscript. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
CMC reports honorarium from an advisory board for Nektar Therapeutics. The other authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Selected Clinical Trials with Primary NK Cells and NK-92 Cells. The table provides an overview of selected clinical trials with normal and gene modified primary NK and NK-92 cells for hematological and solid tumors.
Additional file 2.
Summary of iPSC-NK Cell Therapies. The table summarizes the iPSC-NK cell therapies reviewed in the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shankar, K., Capitini, C.M. & Saha, K. Genome engineering of induced pluripotent stem cells to manufacture natural killer cell therapies. Stem Cell Res Ther 11, 234 (2020). https://doi.org/10.1186/s13287-020-01741-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-020-01741-4