Abstract
Background
Skeletal muscle satellite cell-derived myoblasts are mainly responsible for postnatal muscle growth and injury-induced regeneration. Many intracellular signaling pathways are essential for myogenic differentiation, while a number of kinases are involved in this modulation process. Type I phosphatidylinositol 4-phosphate 5-kinase (PIP5KI) was identified as one of the key kinases involved in myogenic differentiation, but the underlying molecular mechanism is still unclear.
Methods
PIP5K1α was quantified by quantitative reverse transcriptase PCR and western blot assay. Expression levels of myogenin and myosin heavy chain, which showed significant downregulation in PIP5K1α siRNA-mediated knockdown cells in western blot analysis, were confirmed by immunostaining. Phosphatidylinositol 4,5-bisphosphate in PIP5K1α siRNA-mediated knockdown cells was also measured by the PI(4,5)P2 Mass ELISA Kit. C2C12 cells were overexpressed with different forms of AKT, followed by western blot analysis on myogenin and myosin heavy chain, which reveals their function in myogenic differentiation. FLIPR assays are used to test the release of calcium in PIP5K1α siRNA-mediated knockdown cells after histamine or bradykinin treatment. Statistical significances between groups were determined by two-tailed Student’s t test.
Results
Since PIP5K1α was the major form in skeletal muscle, knockdown of PIP5K1α consistently inhibited myogenic differentiation while overexpression of PIP5K1α promoted differentiation and rescued the inhibitory effect of the siRNA. PIP5K1α was found to be required for AKT activation and calcium release, both of which were important for skeletal muscle differentiation.
Conclusions
Taken together, these results suggest that PIP5K1α is an important regulator in myoblast differentiation.
Similar content being viewed by others
Background
Adult mammalian skeletal muscle could induce a rapid and extensive regeneration in response to severe damage. This muscle repair process occurs through the activation of muscle satellite cells quiescent in the basal lamina and the muscle fiber membrane of normal muscles. The activated satellite cells will move outside the basal lamina and differentiate to accelerate the muscle repair. The differentiation of skeletal muscle is required by the myogenic regulatory genes. The myogenic regulatory factors (MRFs), a family of basic helix–loop–helix (bHLH) transcription factors, consist of myogenic differentiation antigen (MYOD) [1], myogenic factor 5 (MYF5) [2], myogenin [3], and MRFs (MRF4) [4,5,6]. Another group of muscle regulatory transcription factors belong to the myocyte enhancer factor-2 (MEF2) family. There are four MEF2 genes in mammals, including MEF2A, MEF2B, MEF2C, and MEF2D [7,8,9,10,11]. Protein kinases are also key regulators of signal transduction essential for myogenic differentiation, such as the phosphatidylinositol 3-kinase (PI3K)/Akt and the p38 mitogen-activated protein kinase (MAPK)-mediated pathways [12, 13].
Type I phosphatidylinositol 4-phosphate 5-kinase (PIP5KI) is a kinase critical in synthesizing phosphatidylinositol 4,5-bisphosphate (PIP2) through phosphorylating phosphatidylinositol-4-phosphate (PI4P). PIP2 is a substrate of phospholipase C (PLC), which generates the lipid second messengers diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) [14]. DAG activates protein kinase C and IP3 increases the intracellular calcium level by releasing Ca2+ from the endoplasmic reticulum. PIP2 can also be phosphorylated by PI3K to generate PIP3, which is another lipid second messenger involved in cell growth, survival, and apoptosis [15]. In addition, PIP2 can act as a second messenger in many cellular processes such as cell migration, adhesion, and division [16, 17]. Thus, PIP5K1 essentially regulates these processes by modulating the production of the multifunctional lipid messenger PIP2.
In mammalians, three isoforms of PIP5K1 have been identified as PIP5K1α, PIP5K1β, and PIP5K1γ [18,19,20]. In this study, PIP5K1α was considered the major isoform of PIP5K1 in skeletal muscle and required for myogenic differentiation. PIP5K1α was upregulated during myoblast differentiation, while knockdown of PIP5K1α inhibited C2C12 cell differentiation. PIP5K1α promoted myoblast differentiation by regulating the PIP2-mediated AKT pathway and cytoplasmic calcium release. Together, our work shows that PIP5K1α promoted myogenic differentiation via the activation of AKT signaling and modulation of the cytoplasmic calcium level.
Methods
Cell culture
C2C12 cells (ATCC, Manassas, VA, USA) were maintained in growth medium (GM; Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin) in a 37 °C incubator with 5% CO2. To induce differentiation, cells were grown in differentiation medium (DM; DMEM with 2% horse serum).
Preparation of mouse primary myoblasts
The mice limb muscles were isolated and incubated with 0.1% Pronase in DMEM at 37 °C for 1 hour. After centrifuge at 1500 rpm for 5 min the supernatant was removed, while the pellet was resuspended in 10 ml DMEM and passed through a 40-μm filter to remove muscle debris. The cells were collected by centrifugation, resuspended in 10 ml growth media (Ham’s F-10 medium with 20% FBS and 5 ng/ml beta-fibroblast growth factor (β-FGF)), and transferred to noncoated plates to allow fibroblasts to attach. The floating cells were then transferred to 2% Matrigel-coated (BD Biosciences) plates to facilitate attachment of myoblasts. The growth medium was changed after 24 hours. Myoblasts were trypsinized and transferred to a new Matrigel-coated plate for the following experiments.
siRNA and plasmid transfection
For siRNA transfection, cells were plated into 12-well plates. For each well, 100 nM siRNA was added with the Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the instructions. The sequences of siRNA were listed as follows: PIP5K1α#1, AGAAGUGGGUGGCGUGAAU; and PIP5K1α#2, TCAGAAAGAACGAGAGAAA. For plasmid transfection, cells were plated into 12-well plates and plasmids were added with the Lipofectmine Plus reagents (Invitrogen) according to the instructions.
Extraction of PI(4,5)P2 from cells and measurement
After siRNA treatment, cells were collected with 1 ml ice-cold 0.5 M TCA and incubated on ice for 5 min. After centrifuge, the pellet was washed twice with 1 ml of 5% TCA/1 mM EDTA. Neutral lipids were extracted with 1 ml MeOH:CHCl3 (2:1). Then acidic lipids were extracted with 750 μl MeOH:CHCl3:12 N HCl (80:40:1). The supernatant was transferred to a new 2-ml centrifuge tube, and 250 μl CHCl3 and 450 μl of 0.1 N HCl added. After vortex and centrifuge, the organic phase were collected into a clean 1.5-ml vial and dried in a vacuum dryer. The measurement of PI(4,5)P2 from cells is a 96-well ELISA assay for detection and quantification of PI(4,5)P2 according to the instructions of the PI(4,5)P2 Mass ELISA Kit (Echelon, USA).
Antibodies, immunostaining, and western blotting
Anti-myogenin and anti-PIP5K1α were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), anti-GAPDH was purchased from Ambion (Austin, TX, USA), anti-phospho-AKT (T308/S473) and anti-total-AKT were purchased from Cell Signaling (Danvers, MA, USA), and anti-MHC was purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA, USA). For immunostaining, C2C12 cells were plated on six-well plates with glass coverslips. After cotransfection with siRNA for 24-hour and then 48-hour treatment of differential medium, cells were fixed in 4% paraformaldehyde for 15 min and permeabilized by 0.2% Triton X-100 for 15 min. Cells were rinsed in PBS, blocked in 5% BSA for 1 hour, and then incubated with anti-myogenin and anti-MHC antibody (1:500) overnight. Cells were washed three times in PBS and incubated with fluorescein-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 1 hour. Then 100 ng/ml of DAPI was added for another 10 min to stain the nuclei. After three washes in PBS, the coverslips were mounted and cells visualized using an Olympus IX70 fluorescence microscope. Western blot analysis was performed according to procedures described previously [21].
RNA preparation and quantitative real-time PCR
Total RNA from cells was extracted with TRIzol reagent (Invitrogen). Following the manufacturer’s instructions, the expression level of PIP5K1 was detected by SYBR Green-based qRT-PCR with FastStart Universal SYBR Green Master mix (Roche). The sequences of primers are listed as follows: PIP5K1A forward primer, 5′-CTGATGATTACTTGTACTCCCT-3′; PIP5K1A reverse primer, 5′-CATCACTGGACACATAGAAG-3′; PIP5K1B forward primer, 5′-AGTTCCTGCAGAAGCTGCTG-3′; PIP5K1B reverse primer, 5′-CCTGACTGCATGCAATACAG-3′; PIP5K1C forward primer, 5′-GAGTTCATCATCAAGACTGT-3′; PIP5K1C reverse primer, 5′-GTTGAGATTCATGTAGTAGC-3′; GAPDH forward primer, 5′-TGCACCACCAACTGCTTAGC-3′; and GAPDH reverse primer, 5′-GGCATGGACTGTGGTCATGAG-3′.
FLIPR assay
After siRNA transfection, the C2C12 cells were seeded into 96-well microtiter plates and incubated with 300 μM bradykinin for 16 hours. On the following day, the cells were labeled with 100 μl labeling medium containing Opti-MEM/Hanks’ balanced salt solution (HBSS), 2.5% FBS, 20 mM HEPES (pH 7.4), 2.5 nM probenecid, and 2 μM Fluo-4 at 37 °C for 60 min. Then 70 μl 3× drugs were prepared and aliquotted into the corresponding wells in the V-well drug plate. Changes in fluorescence were detected in the FLIPR 96 (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Statistical significances between groups were determined by two-tailed Student’s t test. p < 0.05 was considered statistically significant.
Results
PIP5K1α was upregulated during myoblast differentiation
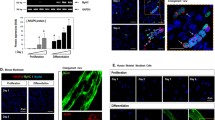
To explore the potential role of PIP5K1 isoforms in myogenic differentiation, we first examined their expression patterns. As shown in Fig. 1a, PIP5K1α was dominantly expressed in skeletal muscle and accumulated in C2C12 and primary myoblast cells (Fig. 1b). Moreover, the protein level of PIP5K1α was gradually increased during C2C12 differentiation (Fig. 1c). This suggested PIP5K1α might have a role in muscle differentiation.
PIP5K1α is the major isoform of PIP5KI in skeletal muscle. a Expression of three PIP5KI isoforms in mature muscles. b Expression of PIP5K1α in C2C12 cells, primary myoblasts (Satellite cell), and mature muscles. c Protein levels of PIP5K1α in C2C12 cells harvested at different time points during differentiation. Data presented as mean ± SD. *p < 0.05, ***p < 0.001. PIP5KI phosphatidylinositol 4-phosphate 5-kinase, GM growth medium, DM differentiation medium
Knockdown of PIP5K1α inhibited C2C12 differentiation
To further reveal the role of PIP5K1α in C2C12 differentiation, the RNAi technique was introduced. Two siRNAs were designed to target different regions of PIP5K1α, both of which inhibited the expression of PIP5K1α efficiently at the mRNA and protein levels (Fig. 2a, b). Cells transfected with these siRNAs showed decreased expression of both myogenin and myosin heavy chain (MHC), which were early and late differentiation markers respectively (Fig. 2b). Similar results were obtained by immunostaining (Fig. 2c). MHC-positive multinucleated myotubes were reduced in the cells transfected with the PIP5K1α-targeting siRNA (Fig. 2c). Consistently, knockdown of PIP5K1α also inhibited the primary myoblast differentiation (Fig. 2d). Thus, the results demonstrated that PIP5K1α was required for myogenic differentiation. As shown in Fig. 2e, the efficient inhibited expression of PIP5K1α by its targeting siRNA was rescued by overexpression of PIP5K1α. As a result, the effect of siRNA on muscle differentiation was partially rescued by the overexpression of PIP5K1α, which indicated that the changed differentiation status was not due to offtarget effects of siRNA. Furthermore, overexpression of PIP5K1α increased expression of myogenin and MHC, which suggested that PIP5K1α promoted myogenic differentiation (Fig. 2e).
PIP5K1α is required for myoblast differentiation. a Knockdown efficiency of PIP5K1α siRNA measured by real-time PCR. b C2C12 cells transfected with PIP5K1α siRNA or GFP siRNA as a control; 24 hours after transfection, cells were induced to differentiate for 48 hours. Cells harvested for western blot analysis. c C2C12 cells transfected with PIP5K1α siRNA or GFP siRNA as a control. After transfection for 24 hours, cells were induced to differentiate for 48 hours. Cells fixed for immunostaining with myogenin and MHC antibodies. d Primary muscle satellite cells isolated from mouse skeletal muscle and cultured for 3 days. Cells transfected with siRNA, and fixed for immunostaining with MHC antibody. e C2C12 cells cotransfected with siRNA and siRNA-resistant PIP5K1α cDNA plasmids or empty vector. After transfection for 48 hours, cells subjected to western blot analysis. Data presented as mean ± SD. *p < 0.05, **p < 0.01. PIP5KI phosphatidylinositol 4-phosphate 5-kinase, MHC myosin heavy chain, MyoG myogenin, DAPI 4′,6-diamidino-2-phenylindole, siRNA small interfering RNA. GFP Green Florescent Protein
PIP5K1α promoted myoblast differentiation by regulating the AKT pathway
The intracellular PIP2 of mammalian cell is mainly catalyzed by PIP5K1 and subsequently affects the PI3K/AKT pathway. After knockdown of PIP5K1α, C2C12 cells suffered a significant decrease in the production of PIP2 (Fig. 3a). The activation of AKT was important to myogenic differentiation [12]. Knockdown of PIP5K1α suppressed the phosphorylation of AKT, which suggested the AKT pathway might play a role in the promyogenic effect of PIP5K1α (Fig. 3b). To examine whether PIP5K1α-mediated myogenic differentiation was specifically affected by the activation of AKT, PIP5K1α siRNA was cotransfected with plasmids carrying a constitutively active AKT (AKT CA) form or a dominant-negative AKT (AKT DN) form. As shown in Fig. 3c, only overexpression of constitutively active AKT promoted C2C12 cell differentiation and rescued the myogenic inhibition of PIP5K1α targeting siRNA. As we know, the MKK6 (EE) form can activate the p38 pathway which is also required for myogenic differentiation [22]. Although MKK6 (EE) could also strongly promote differentiation, it failed to rescue the myogenic inhibition of PIP5K1α-targeting siRNA.
PIP5K1α regulates myoblast differentiation through the AKT pathway. a C2C12 cells transfected with PIP5K1α siRNA show decreased production of PIP2. Data presented as mean ± SD. *p < 0.05. b C2C12 cells transfected with PIP5K1α siRNA show decreased phosphorylation of AKT. c C2C12 cells cotransfected with siRNA and AKT CA, AKT DN, or MKK6 S207E, T211E constitutively active mutant (MKK6 EE) plasmids. Cells harvested after 24-hour transfection and 48-hour treatment of differential medium, followed by western blot analysis. PIP2 phosphatidylinositol 4,5-bisphosphate, PIP5KI phosphatidylinositol 4-phosphate 5-kinase, CA constitutively active, DN dominant-negative, MHC myosin heavy chain, MyoG myogenin, siRNA small interfering RNA. MKK6(EE) MKK6 S207E, T211E constitutively active mutant, GFP Green Fluorescent Protein
PIP5K1α regulated PIP2-mediated cytoplasmic calcium release
PIP2 can be hydrolyzed by PLC and converted to DAG and inositol triphosphate (IP3), which are essential for the intracellular calcium level. Cytoplasmic calcium has been reported important in myogenic differentiation [23, 24]. Several drugs targeting different G protein receptors were tested. Only histamine and bradykinin were found to induce the release of calcium in C2C12 cells (Fig. 4a). Interestingly, histamine and bradykinin receptors were sensitive to PIP2 [25]. To investigate whether PIP5K1α can affect the cytoplasmic calcium level, C2C12 cells transfected with PIP5K1α siRNA were treated with histamine or bradykinin, which exhibited an obvious defect of cytoplasmic calcium release (Fig. 4b).
PIP5K1α regulates PIP2-mediated cytoplasmic calcium release. a C2C12 cells treated with drugs targeting different G protein receptors followed by FLIPR® Calcium Assay (FLIPR) assays. b C2C12 cells transfected with siRNA and treated with histamine or bradykinin for another 16 hours. Cells then subjected to FLIPR assays. Data presented as mean ± SD. *p < 0.05, **p < 0.01. PIP5KI phosphatidylinositol 4-phosphate 5-kinase, Ctrl control. FLIPR FLIPR® Calcium Assay
Discussion
In our study, we first found that PIP5K1α was gradually increased during myogenic differentiation, which suggests its role in myogenesis. Calcium signaling is important for differentiation-dependent gene expression. Keratinocyte differentiation involves an intricate pathway involving an acute and sustained rise of the intracellular free calcium level [26]. PIP5K1α activation is also an important step in calcium-induced keratinocyte differentiation [27], which is consistent with its role in myogenic differentiation through regulating the intracellular free calcium level. Interestingly, the expression level of PIP5K1α was much lower in mature muscle than that in satellite cells and C2C12 cells. Similar developmental patterns in the expression of MyoD and myogenin, myogenic transcriptional regulatory proteins, were found during myogenesis [28]. This suggested that these factors play distinct roles in the control of myogenesis.
Our studies have investigated the role of PIP5K1α in inducing muscle differentiation via the activation of AKT signaling and modulation of the cytoplasmic calcium level (Fig. 5). PIP5K1α is the key regulator for the production of PIP2, which is important for various signal transductions in mammalian cells. The activated PIP5K1 synthesizes more PIP2 in the plasma membrane to provide sufficient substrate for PI3K and PLC-γ, which is an essential regulatory step to sustain the activation of PI3K and PLC-γ in muscle. As a result, PIP2 levels are constantly maintained at a high level to enable the generation of IP3 required for the increase in intracellular calcium necessary for initiating muscle differentiation.
PIP5K1α regulates myogenic differentiation through two pathways. Activated PIP5K1α synthesizes more PIP2 to provide sufficient substrate for PI3K and PLC-γ. On one hand, PIP2 levels are constantly maintained at a high level to enable generation of IP3 required for the increase in intracellular calcium, which is necessary for initiating muscle differentiation. On the other, increased intracellular PIP2 of mammalian cell catalyzed by PIP5K1α subsequently affects the PI3K/AKT pathway. Activation of AKT was also important to myogenic differentiation. PI4P phosphatidylinositol-4-phosphate, PIP5KI phosphatidylinositol 4-phosphate 5-kinase, PI3K phosphatidylinositol 3-kinase, PLC phospholipase C, IP3 inositol 1,4,5-triphosphate, MEF2 myocyte enhancer factor-2. PIP(4,5)P2 Phosphatidylinositol 4,5-bisphosphate, PIP(3,4,5)P3 Phosphatidylinositol (3,4,5)-trisphosphate
The crucial role of Akt (also known as protein kinase B) has been demonstrated previously in the proliferation, survival, differentiation, and viability of muscle cells [29]. Akt regulates protein expression through the mammalian target of rapamycin (mTOR) signaling pathway, which plays an important role in muscle cell differentiation. Furthermore, the IGF/PI3K/Akt signaling pathway has been shown to stimulate myogenic differentiation by inducing the expression of myogenin, MyoD, and MEF2 in normal myogenic cells [12, 30]. Therefore, we hypothesize that PIP5K1α promotes myogenic differentiation by regulating Akt signaling through increasing PIP2 production. Further understanding of the regulation of PIP5K1α will be obtained through exploring more downstream genes regulated by PIP5K1α.
Conclusions
Our results demonstrate PIP5K1α is required for the activation of AKT signaling and modulation of the cytoplasmic calcium level, which indicates that PIP5K1α regulates myogenic differentiation through multiple pathways.
Abbreviations
- PI3K:
-
Phosphatidylinositol 3-kinase
- DAG:
-
Diacylglycerol
- IP3:
-
Inositol triphosphate
- PI4P:
-
Phosphatidylinositol-4-phosphate
- PIP2:
-
Phosphatidylinositol 4,5-bisphosphate
References
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000.
Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. Embo J. 1989;8:701–9.
Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–40.
Braun T, Bober E, Winter B, Rosenthal N, Arnold HH. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. Embo J. 1990;9:821–31.
Miner JH, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990;87:1089–93.
Rhodes SJ, Konieczny SF. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–61.
Breitbart RE, Liang CS, Smoot LB, Laheru DA, Mahdavi V, Nadal-Ginard B. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development. 1993;118:1095–106.
Martin JF, Schwarz JJ, Olson EN. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci U S A. 1993;90:5282–6.
McDermott JC, Cardoso MC, Yu YT, Andres V, Leifer D, Krainc D, et al. hMEF2C gene encodes skeletal muscle- and brain-specific transcription factors. Mol Cell Biol. 1993;13:2564–77.
Pollock R, Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991;5:2327–41.
Yu YT, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992;6:1783–98.
Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. 2000;275:36750–7.
Xu Q, Yu L, Liu L, Cheung CF, Li X, Yee SP, et al. p38 Mitogen-activated protein kinase-, calcium-calmodulin-dependent protein kinase-, and calcineurin-mediated signaling pathways transcriptionally regulate myogenin expression. Mol Biol Cell. 2002;13:1940–52.
Michell RH. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975;415:81–47.
Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–85.
Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J Cell Sci. 2000;113(Pt 21):3685–95.
Mao YS, Yin HL. Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflugers Arch. 2007;455:5–18.
Ishihara H, Shibasaki Y, Kizuki N, Katagiri H, Yazaki Y, Asano T, et al. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J Biol Chem. 1996;271:23611–4.
Loijens JC, Anderson RA. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J Biol Chem. 1996;271:32937–43.
Oude Weernink PA, Schmidt M, Jakobs KH. Regulation and cellular roles of phosphoinositide 5-kinases. Eur J Pharmacol. 2004;500:87–99.
Chen XF, Zhang LJ, Zhang J, Dou X, Shao Y, Jia XJ, et al. MiR-151a is involved in the pathogenesis of atopic dermatitis by regulating interleukin-12 receptor beta2. Exp Dermatol. 2016;1–6. https://doi.org/10.1111/exd.13276.
Wang H, Xu Q, Xiao F, Jiang Y, Wu Z. Involvement of the p38 mitogen-activated protein kinase alpha, beta, and gamma isoforms in myogenic differentiation. Mol Biol Cell. 2008;19:1519–28.
Al-Shanti N, Stewart CE. Ca2+/calmodulin-dependent transcriptional pathways: potential mediators of skeletal muscle growth and development. Biol Rev Camb Philos Soc. 2009;84:637–52.
Rose AJ, Kiens B, Richter EA. Ca2 + -calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903.
Gutowski S, Smrcka A, Nowak L, Wu DG, Simon M, Sternweis PC. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991;266:20519–24.
Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J Clin Invest. 1996;97:1085–93.
Shrestha C, Tang Y, Fan H, Li L, Zeng Q, Pennypacker SD, et al. Phosphoprotein phosphatase 1 is required for extracellular calcium-induced keratinocyte differentiation. Biomed Res Int. 2016;2016:3062765.
Montarras D, Chelly J, Bober E, Arnold H, Ott MO, Gros F, et al. Developmental patterns in the expression of Myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol. 1991;3:592–600.
Ceci M, Ross Jr J, Condorelli G. Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. J Mol Cell Cardiol. 2004;37:905–12.
Florini JR, Ewton DZ, Roof SL. Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol Endocrinol. 1991;5:718–24.
Acknowledgements
The authors thank the members of the Zhenguo Wu laboratories in the Hong Kong University of Science and Technology for helpful discussions and insights.
Funding
This work was supported by the National Natural Scientific Foundation of China (81402600, 31301126, 81673053) and Shenzhen Technology Research Foundation (JCYJ20170411090739316, JCYJ20170306161807726).
Availability of data and material
All data generated or analyzed for this study are included in this published article.
Author information
Authors and Affiliations
Contributions
YD conceived the experiments. BY carried out the molecular genetic studies. XC carried out the immunoassays and other experiments. JW performed the statistical analysis. WZ analyzed the results and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All studies on animals were performed after approval by the Ethics Committee of Peking University Shenzhen Hospital, in compliance with Guidelines for the Use and Care of Small Laboratory Animals.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, X., Wan, J., Yu, B. et al. PIP5K1α promotes myogenic differentiation via AKT activation and calcium release. Stem Cell Res Ther 9, 33 (2018). https://doi.org/10.1186/s13287-018-0770-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-018-0770-z