Abstract
Objectives
To investigate the impact of computed tomography (CT)-based, artificial intelligence-driven waist skeletal muscle volume on survival outcomes in patients with endometrial cancer.
Methods
We retrospectively identified endometrial cancer patients who received primary surgical treatment between 2014 and 2018 and whose pre-treatment CT scans were available (n = 385). Using an artificial intelligence-based tool, the skeletal muscle area (cm2) at the third lumbar vertebra (L3) and the skeletal muscle volume (cm3) at the waist level were measured. These values were converted to the L3 skeletal muscle index (SMI) and volumetric SMI by normalisation with body height. The relationships between L3, volumetric SMIs, and survival outcomes were evaluated.
Results
Setting 39.0 cm2/m2 of L3 SMI as cut-off value for sarcopenia, sarcopenia (< 39.0 cm2/m2, n = 177) and non-sarcopenia (≥ 39.0 cm2/m2, n = 208) groups showed similar progression-free survival (PFS; p = 0.335) and overall survival (OS; p = 0.241). Using the median value, the low-volumetric SMI group (< 206.0 cm3/m3, n = 192) showed significantly worse PFS (3-year survival rate, 77.3% vs. 88.8%; p = 0.004) and OS (3-year survival rate, 92.8% vs. 99.4%; p = 0.003) than the high-volumetric SMI group (≥ 206.0 cm3/m3, n = 193). In multivariate analyses adjusted for baseline body mass index and other factors, low-volumetric SMI was identified as an independent poor prognostic factor for PFS (adjusted HR, 1.762; 95% CI, 1.051–2.953; p = 0.032) and OS (adjusted HR, 5.964; 95% CI, 1.296–27.448; p = 0.022).
Conclusions
Waist skeletal muscle volume might be a novel prognostic biomarker in patients with endometrial cancer. Assessing body composition before treatment can provide important prognostic information for such patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Key points
-
Waist skeletal muscle volume might be a new prognostic biomarker in endometrial cancer.
-
Assessment of body composition before treatment can provide prognostic information.
-
Volumetric quantification of skeletal muscle appears feasible in patients with endometrial cancer.
Introduction
Endometrial cancer is a global burden, with 417,367 new cases estimated to occur annually [1]. In the USA, endometrial cancer ranks as the fourth most common female cancer and the sixth leading cause of cancer-related deaths in 2021 [2]. In Korea, the incidence of endometrial cancer has increased progressively, and nowadays, it is the most common gynaecologic malignancy [3, 4]. The Western lifestyle and a significant increase in the incidence of obesity in women caused the rapid increase in the incidence of endometrial cancer and other obesity-related cancers in Korea [4, 5].
Obesity is a well-known risk factor for endometrial cancer, and it is strongly correlated with type 1 endometrial cancer. The risk of endometrial cancer reportedly increased 1.5 times for overweight and over 2.5 times for obese women [6]. Body mass index (BMI) has been widely used as an indicator of excess body fat. In a large cohort study in the USA, a significant trend was observed between higher BMI and increased risk of death from endometrial cancer [7]. Recently, not only excess body fat but also lack of muscle mass, known as sarcopenia, has attracted the attention of researchers for causing adverse survival outcomes in many malignancies, including breast [8, 9], lung [10], and gastric cancers [11].
Studies investigating the prognostic role of pre-treatment sarcopenia in endometrial cancer have shown conflicting results [12,13,14]. To determine sarcopenia, these studies commonly measured skeletal muscle area from a single computed tomography (CT) scan image, based on previous findings that the third lumbar vertebra (L3)-level cross-sectional image reflects total body muscle mass and adipose tissues well [15, 16]. Beyond the areal measurement, recent technological advances enable the volumetric measurement of a specific body composition component, such as skeletal muscle, visceral fat, and subcutaneous fat, from the CT scans that were not feasible due to the requirement of substantial time and human effort [17]. The volumetric measurement of body composition may contain more abundant and precise information than areal measurements in a single cross-sectional image [18]. Moreover, the artificial intelligence-based tool can process a large amount of imaging data by automatic segmentation and calculation of volumes shorter than a few minutes.
Thus, we aimed to ascertain the impact of CT-based, artificial intelligence-driven waist skeletal muscle volume on survival outcomes in patients with endometrial cancer. Additionally, we investigated the prognostic role of each body composition volume, automatically measured using an artificial intelligence-based tool.
Materials and methods
Study population
This single-centre retrospective cohort study was approved by the Institutional Review Board (No. H-2012-027-1178) and performed according to the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
From the institution’s Endometrial Cancer Cohort, we identified patients who met the following criteria: (1) diagnosed with endometrial cancer at the age of 20 years or more; (2) received primary surgical treatment between January 2014 and December 2018; and (3) whose pre-treatment CT scans, obtained less than a month before the primary surgery, were stored in the Picture Archiving and Communication System. Meanwhile, we excluded the patients who (1) were not able to retrieve or did not undergo pre-treatment CT scans; (2) had other active malignancies before and at the time of endometrial cancer diagnosis; (3) received hormone therapy, chemotherapy, or radiation prior to surgical treatment; (4) had insufficient clinicopathologic data; and (5) were lost to follow-up during adjuvant treatment or within 3 months without disease recurrence.
Data collection
Reviewing medical records and pathologic reports, we collected the patients’ clinicopathologic data, including age, comorbidities, serum CA-125 levels, histologic type and grade, and the 2009 International Federation of Gynaecology and Obstetrics (FIGO) stage. Histological grade 3 tumours were considered high-grade disease. We also collected data on pathologic risk factors, such as myometrial invasion and lymphovascular space invasion (LVSI), and post-operative adjuvant treatment. Based on the pre-treatment BMI, all patients were classified into four categories according to the World Health Organization’s (WHO) criteria for Asian populations [19]: < 18.5 kg/m2 (underweight), 18.5–22.9 kg/m2 (normal), 23.0–24.9 kg/m2 (overweight), and ≥ 25.0 kg/m2 (obese).
The patients underwent a physical examination and a blood test for serum cancer antigen 125 (CA-125) levels every 3 to 4 months for the first 2 years, every 6 months for the next 2 years, and annually thereafter. Imaging studies were conducted according to physician preference or when symptoms or examination findings were suspicious for recurrence. Progression-free survival (PFS) and overall survival (OS) were defined as the time interval from the date of surgery to the date of disease progression confirmed by the Response Evaluation Criteria in Solid Tumours version 1.1 [20] and cancer-related death or the end of the study, respectively.
CT image analysis
For body composition analysis, we uploaded the anonymised digital imaging and communications in medicine images of pre-treatment CT scans to the commercially available artificial intelligence-based software (DEEPCATCH v1.0.0.0; MEDICALIP Co. Ltd., Seoul, Korea). This software automatically executes the following procedures: (1) measurement of L3-level skeletal muscle area (cm2); (2) volumetric segmentation of skeletal muscle, abdominal visceral fat, and subcutaneous fat, providing a Dice similarity score of 97%, compared with manual segmentation [17]; (3) labelling the abdominal waist between the top of the iliac crest and the lower border of the rib cage, according to the WHO guidelines for measurement of waist circumference [21]; and (4) quantification of each volumetric segmentation (cm3). One radiologist (S.H.Y.), expertise in body composition analysis, confirmed all the procedures and results.
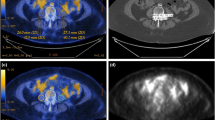
Consequently, we obtained each patient’s L3 skeletal muscle area (cm2) and waist skeletal muscle, visceral fat, and subcutaneous volume (cm3). We added visceral fat and subcutaneous fat volumes to produce total fat volume (cm3). The L3 skeletal muscle area was normalised to height in m2 and reported as the L3 skeletal muscle index (SMI), and waist volume was normalised to the height in m3 and reported as a volumetric index. We also calculated other body composition indices and the skeletal muscle-to-visceral fat ratio (Fig. 1).
Evaluation of body composition using CT image. Despite similar baseline BMI, two patients showed different body composition profiles as follows: A a 66-year-old, non-sarcopenic woman with high-volumetric SMI; B a 60-year-old, sarcopenic woman with low-volumetric SMI. Red, skeletal muscle; green, abdominal visceral fat; yellow, subcutaneous fat; blue lines indicate the waist; light green line indicates L3 level. L3 level cross-sectional images are also presented
Up to our knowledge, no studies have validated the cut-off value of L3 SMI for sarcopenia in healthy Korean women. Moreover, it was inappropriate to adopt cut-off values from previous studies conducted in other countries, as body composition varies among geographic regions and ethnicity. Instead, we defined sarcopenia when an individual’s L3 SMI was less than 39.0 cm2/m2, per the cut-off value proposed by an international consensus [22]. This cut-off value was also used in our previous studies on ovarian cancer [23] and cervical cancer [24]. Because there is no established cut-off value of the volumetric index, we used the median value of the volumetric index for each body composition component and divided patients into two groups accordingly. Thereafter, the relationships between sarcopenia, volumetric indices, and survival outcomes were evaluated.
Statistical analysis
Baseline clinicopathologic characteristics and survival outcomes were compared between the sarcopenia and non-sarcopenia groups and between the low- and high-volumetric SMI groups. Categorical variables were compared using the Pearson’s Chi-squared test or Fisher’s exact test, while continuous variables were compared using the Student’s t test or Mann–Whitney U test. The Pearson’s correlation coefficient test was used to measure the relationship between continuous variables. For survival analyses, we used the Kaplan–Meier method with a log-rank test. Cox proportional hazards regression models were used for multivariate analyses, and adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were calculated for each variable. All statistical analyses were conducted using the SPSS software version 25.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5 software (GraphPad Inc., La Jolla, CA, USA). Statistical significance was set at p < 0.05.
Results
Characteristics of the study population
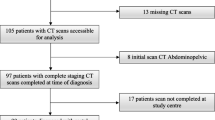
Additional file 1: Figure S1 depicts the selection of the study population. In total, 385 patients were included in the analysis. The patient clinicopathologic characteristics are presented in Additional file 1: Table S1. The mean patient age was 55.5 years. Based on BMI, the proportions of overweight and obese patients were 22.6% and 43.1%, respectively. The endometrioid type was the most common histologic type (81.8%), and high-grade disease was identified in 27.5% of the study population. Two-thirds (76.6%) of the patients had early stage disease (2009 FIGO stage I–II). Pelvic lymphadenectomy and para-aortic lymphadenectomy were performed in 97.7% and 74.0% of the patients, respectively. Myometrial invasion ≥ 50% and LVSI were identified in 28.8% and 29.1% of the patients, respectively. Table 1 shows the baseline body composition of all patients. The median values for L3 SMI and volumetric SMI were 39.8 cm2/m2 (interquartile range [IQR], 33.8–46.6) and 206.0 cm3/m3 (IQR, 179.9–240.3), respectively.
The patients’ L3 SMI was significantly correlated with the volumetric SMI, but the relationship was weak (Pearson’s correlation coefficient r = 0.266; p < 0.001) (Fig. 2A). A significant positive correlation was observed between BMI and L3 SMI (r = 0.284; p < 0.001) and between BMI and volumetric SMI (r = 0.516; p < 0.001) (Fig. 2B, C). BMI was also correlated with volumetric total fat, visceral fat, and subcutaneous fat indices (Fig. 2D–F).
Survival outcomes according to the various body composition indices
Of the 385 patients, 71 (18.4%) experienced disease recurrence, and 15 (3.9%) died during a median observation period of 42.7 months. Based on the L3 SMI, the sarcopenia group (< 39.0 cm2/m2; n = 177) and non-sarcopenia group (≥ 39.0 cm2/m2; n = 208) showed similar PFS (3-year PFS rate, 80.4% vs. 85.5%; p = 0.335) and OS (3-year OS rate, 94.3% vs. 97.8%; p = 0.241) (Fig. 3A, B). In contrast, the low-volumetric SMI group (< 206.0 cm3/m3; n = 192) showed significantly worse PFS (3-year PFS rate, 77.3% vs. 88.8%; p = 0.004) and OS (3-year OS rate, 92.8% vs. 99.4%; p = 0.003), compared to the high-volumetric SMI group (≥ 206.0 cm3/m3; n = 193) (Fig. 3C, D). Divided by each median value, no differences in PFS and OS were observed according to the volumetric total fat, visceral fat, and subcutaneous fat indices and skeletal muscle-to-visceral fat ratio (Additional file 1: Fig. S2).
Analyses between high- and low-volumetric SMI groups
Comparisons of clinicopathologic characteristics between the high- and low-volumetric SMI groups are presented in Table 2. Patients in the low-volumetric SMI group were significantly older (mean, 58.0 vs. 53.1 years; p < 0.001) and had significantly lower baseline BMI (median, 22.9 vs. 26.0 kg/m2; p < 0.001) than those in the high-volumetric SMI group. The proportion of patients with high-grade disease was significantly higher in the low-volumetric SMI group (34.9% vs. 20.2%; p = 0.001). Other characteristics, including comorbidities, serum CA-125 levels, histologic type, 2009 FIGO stage, pathologic risk factors, and adjuvant treatment, were similar between both groups.
The baseline body composition of the two groups is presented in Additional file 1: Table S2. The low-volumetric SMI group had significantly lower L3 SMI (median, 33.8 vs. 44.4 cm2/m2; p < 0.001) and a higher proportion of L3 SMI-determined sarcopenia (64.1% vs. 28.0%; p < 0.001) than the high-volumetric SMI group. Volumetric total fat, visceral fat, and subcutaneous fat indices were also lower in the low-volumetric SMI group. However, the skeletal muscle-to-visceral fat ratio was higher in the low-volumetric SMI group (1.340 vs. 1.046; p < 0.001) than in the high-volumetric SMI group (Additional file 1: Table S2).
Associations between clinicopathologic characteristics and volumetric SMI were investigated. As shown in Additional file 1: Table S3, patients aged ≥ 55 years (median, 196.6 vs. 217.3 cm3/m3; p < 0.001) and patients included in underweight–normal BMI categories (median, 189.4 vs. 222.7 cm3/m3; p < 0.001) had significantly lower volumetric SMI, compared to those aged < 55 years and those included in overweight–obese BMI categories, respectively. However, 2009 FIGO stage was not associated with baseline volumetric SMI (four categories, p = 0.608; and 2009 FIGO stage I–II vs. III–IV, p = 0.359).
Next, we conducted multivariate analyses adjusting for patient age, FIGO stage, and other clinicopathologic factors. While BMI category was not associated with survival outcomes, low-volumetric SMI was identified as an independent poor prognostic factor for PFS (aHR, 1.762; 95% CI, 1.051–2.953; p = 0.032) and OS (aHR, 5.964; 95% CI, 1.296–27.448; p = 0.022) (Table 3 and Additional file 1: Table S4).
Discussion
In this single-institution, retrospective cohort study, we demonstrated the impact of pre-treatment sarcopenia and waist body composition on survival outcomes in patients with endometrial cancer. While CT-determined sarcopenia, defined as L3 SMI < 39.0 cm2/m2, did not affect patients’ disease recurrence and mortality rates, low-volumetric SMI (< 206.0 cm3/m3) was significantly associated with worse PFS and OS.
To date, poor survival outcomes from sarcopenia have been reported in various malignancies [8,9,10,11]. While the relationship between BMI and prognosis in endometrial cancer has been well studied, studies on the prognostic impact of sarcopenia are relatively limited. Moreover, the study population (e.g. geographic regions, ethnicities, and disease setting) and definition of sarcopenia differed among the studies, which makes interpretation difficult, apart from the conflicting results.
For example, Kuroki et al. defined sarcopenia based on the median value (4.33 cm2) of average psoas muscle area, measured from L3 level cross-sectional images of the pre-treatment CT scans. In that study, sarcopenia was identified as an independent poor prognostic factor for PFS (aHR, 3.99; 95% CI, 1.42–11.3), but not for OS [12]. Similar to our study, Rodrigues et al. measured L3 SMI on pre-treatment CT scans, but they used the median value (42.45 cm2/m2) to categorise patients into high- and low-L3 SMI groups. The multivariate analyses revealed that a low L3 SMI was not associated with 1-year mortality [13]. Recently, Ganju et al. reported that sarcopenia, defined as L3 SMI < 41.0 cm2/m2 from the CT scans obtained at radiation simulation, did not affect PFS and OS in patients who underwent hysterectomy and pelvic radiation [14]. While these three studies were conducted in Western populations, Lee et al.’s bi-institutional retrospective cohort study was conducted in Taiwanese population [25]. This study included 131 patients with FIGO stage III endometrial cancer who underwent staging surgery and adjuvant chemoradiotherapy. Sarcopenia was defined using L3 SMI, with a cut-off value of 39.3 cm2/m2, a value similar to that in the current study. Consistent with our study, sarcopenia was not associated with either PFS (p = 0.28) or OS (p = 0.37) [25].
To the best of our knowledge, this study is the first to adopt the concept of waist volume measurement of each body component in patients with endometrial cancer. We recognise that the L3 level image analysis is a universal and widely used method. However, the analysis of a single cross-sectional CT image at the L3 level has limitations: the distribution of abdominal muscle and fat in a single cross-sectional image might vary up to twofold and threefold, respectively, owing to the shifting of the gastrointestinal tract [18]. Such variability seemed to result in a weak relationship between the L3 SMI and volumetric SMI in the current study. Thus, the volume measurement in the waist would be more precise and reflective of the whole body composition than the areal measurement in a single cross-sectional image. In particular, the artificial intelligence-based tool executed automatic volumetric quantification of a large amount of imaging data quickly and accurately.
Unlike the L3 SMI, the volumetric SMI was significantly associated with worse survival outcomes in the current study. Compared to the L3 SMI, the low-volumetric SMI might better reflect the presence of sarcopenia. Previous studies have reported that sarcopenia is associated with increased toxicity and resistance to chemotherapy in many malignancies [8,9,10,11]. Among the many features of sarcopenia, increases in pro-inflammatory cytokines, such as IL-6 and TNF-α, may be responsible for the poor prognosis of patients with sarcopenia and endometrial cancer [26, 27]. IL-6 is known to promote tumour proliferation and resistance to chemotherapy and to trigger epithelial-to-mesenchymal transition, leading to cancer metastasis [28]. Nevertheless, some might argue that patients have already suffered cancer cachexia, presenting with sarcopenia at the time of the initial diagnosis of endometrial cancer [29]. However, 76.6% of the study population had early stage disease at the time of diagnosis, and the stage was adjusted in the multivariate analyses.
Here, we also evaluated the prognostic role of other volumetric indices. Based on Calle et al.’s large cohort study of the American population [7], we initially expected that patients with endometrial cancer who have high volumetric total fat, visceral fat, and subcutaneous fat indices, and low skeletal muscle-to-visceral fat ratio would show poor prognosis. However, none of these factors was associated with survival outcomes. These findings may originate from the obesity paradox and ethnic differences. First, researchers pointed out that tumours among obese patients have less aggressive features than those among patients with normal body weight [30]. In endometrial cancer, obese patients tend to have a good prognosis for type 1 tumours, rather than poor prognosis type 2 tumours [31]. Park et al. have reported that a high pre-treatment BMI did not affect PFS and OS in Korean women with endometrial cancer [32]. Similar results were observed in the current study. Next, Asian populations are less obese than Western populations, and generally have a higher body fat percentage than Western populations, even with the same BMI [19]. Thus, studies targeting other ethnic groups may show different results. The optimal cut-off values for the volumetric body component indices may also differ.
In line with the era of precision medicine, early identification of adverse body composition which might influence individuals’ survival outcomes has important clinical implications. Therefore, if an individual has a low-volumetric SMI at a high risk of disease recurrence and mortality, physicians may pay more attention during treatment and surveillance. Based on the assessment results, physicians may prescribe oral or intravenous nutritional support and best symptomatic care [33]. Physical exercise or training intervention may be recommended to increase skeletal muscle mass or prevent further muscle loss during treatment [34, 35]. To identify disease recurrence earlier, visit intervals and surveillance methods may be individualised.
Our study has several limitations. First, due to its retrospective nature, selection bias is the most problematic. During the pre-treatment workup for endometrial cancer, CT scans have not yet been routinely performed. From the institution’s Endometrial Cancer Cohort, approximately 20% of patients were excluded owing to this reason, suggesting potentially biased. Second, associations between sarcopenia and perioperative or treatment-related complications were not investigated. Third, we did not consider sequential changes in the body composition of each patient. Patients might experience loss of skeletal muscles or gain of abdominal visceral fat during adjuvant treatment. Further studies investigating whether such longitudinal changes worsen the survival outcomes of patients with endometrial cancer are warranted. Lastly, we only measured or quantified muscle area and volume, and not muscle quality, owing to the limitations of the imaging modality.
Despite these limitations, our study is the first to introduce artificial intelligence-based volumetric measurement of body composition in patients with endometrial cancer. Conducted in a single centre with clear inclusion and exclusion criteria, homogeneity in surgery, adjuvant treatment, perioperative care, and surveillance would be strengths of the current study.
Conclusion
In conclusion, our study results suggest that waist volumetric SMI might be a novel prognostic biomarker in patients with endometrial cancer. Considering that CT scans are commonly obtained as part of diagnosis, routine artificial intelligence-based volumetric quantification of waist skeletal muscle appears feasible in patients with endometrial cancer.
Availability of data and material
The data presented in this study are also available on request from the corresponding author.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- BMI:
-
Body mass index
- CA-125:
-
Cancer antigen 125
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- FIGO:
-
International Federation of Gynaecology and Obstetrics
- L3:
-
The third lumbar vertebra
- LVSI:
-
Lymphovascular space invasion
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- SMI:
-
Skeletal muscle index
- WHO:
-
World Health Organization
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG (2021) Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat 53(2):316–322
Lim MC, Won YJ, Ko MJ et al (2019) Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol 30(1):e38
Oh SW (2011) Obesity and metabolic syndrome in Korea. Diabetes Metab J 35(6):561–566
Shaw E, Farris M, McNeil J, Friedenreich C (2016) Obesity and endometrial cancer. Recent Results Cancer Res 208:107–136
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348(17):1625–1638
Caan BJ, Cespedes Feliciano EM, Prado CM et al (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4(6):798–804
Song EJ, Lee CW, Jung SY et al (2018) Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat 172(2):425–436
Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM (2015) Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol 10(12):1795–1799
Lee JS, Kim YS, Kim EY, Jin W (2018) Prognostic significance of CT-determined sarcopenia in patients with advanced gastric cancer. PLoS One 13(8):e0202700
Kuroki LM, Mangano M, Allsworth JE et al (2015) Pre-operative assessment of muscle mass to predict surgical complications and prognosis in patients with endometrial cancer. Ann Surg Oncol 22(3):972–979
Rodrigues CS, Chaves GV (2018) Skeletal muscle quality beyond average muscle attenuation: a proposal of skeletal muscle phenotypes to predict short-term survival in patients with endometrial cancer. J Natl Compr Cancer Netw 16(2):153–160
Ganju RG, TenNapel M, Spoozak L, Chen AM, Hoover A (2020) The impact of skeletal muscle abnormalities on tolerance to adjuvant chemotherapy and radiation and outcome in patients with endometrial cancer. J Med Imaging Radiat Oncol 64(1):104–112
Shen W, Punyanitya M, Wang Z et al (2004) Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 97(6):2333–2338
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006
Lee YS, Hong N, Witanto JN et al (2021) Deep neural network for automatic volumetric segmentation of whole-body CT images for body composition assessment. Clin Nutr 40(8):5038–5046
Weston AD, Korfiatis P, Kline TL et al (2019) Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology 290(3):669–679
WHO Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363(9403):157–163
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Ross R, Neeland IJ, Yamashita S et al (2020) Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 16(3):177–189
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Kim SI, Kim TM, Lee M et al (2020) Impact of CT-determined sarcopenia and body composition on survival outcome in patients with advanced-stage high-grade serous ovarian carcinoma. Cancers 12(3):559
Han Q, Kim SI, Yoon SH et al (2021) Impact of computed tomography-based, artificial intelligence-driven volumetric sarcopenia on survival outcomes in early cervical cancer. Front Oncol 11:741071
Lee J, Lin JB, Wu MH et al (2019) Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle 10(4):814–826
Muscaritoli M, Anker SD, Argilés J et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr 29(2):154–159
Rolland Y, Czerwinski S, Abellan Van Kan G et al (2008) Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12(7):433–450
Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL (2018) IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res 10:6685–6693
da Silva SP, Santos JM, e Silva MPC, da Costa RMG, Medeiros R (2020) Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 11(3):619–635
Lennon H, Sperrin M, Badrick E, Renehan AG (2016) The obesity paradox in cancer: a review. Curr Oncol Rep 18(9):56
Crosbie EJ, Roberts C, Qian W, Swart AM, Kitchener HC, Renehan AG (2012) Body mass index does not influence post-treatment survival in early stage endometrial cancer: results from the MRC ASTEC trial. Eur J Cancer 48(6):853–864
Park JY, Cho JH, Min JY et al (2014) Impact of body mass index on the prognosis of Korean women with endometrioid adenocarcinoma of the uterus: a cohort study. Obstet Gynecol Sci 57(2):115–120
Seol A, Kim SI, Song YS (2020) Sarcopenia: clinical implications in ovarian cancer, diagnosis, etiology, and management. Sports Med Health Sci 2(4):202–210
Meyerhardt JA, Giovannucci EL, Holmes MD et al (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24(22):3527–3534
Soares Falcetta F, de Araújo Vianna Träsel H, de Almeida FK, Rangel Ribeiro Falcetta M, Falavigna M, Dornelles Rosa D (2018) Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 170(3):455–476
Acknowledgements
We thank all the investigators and research staff involved in this study.
Funding
This work was supported by grants from the Seoul National University Hospital Research Fund (No. 0420200430) and the Korea Medical Device Development Fund grant funded by the Korean government (Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Health & Welfare, and Ministry of Food and Drug Safety) (No. 202011C01).
Author information
Authors and Affiliations
Contributions
SIK was involved in conceptualisation, data curation, formal analysis, investigation, methodology, visualisation, writing—original draft, writing—review & editing. JYC helped in data curation, investigation, writing—original draft, writing—review & editing. HP contributed to data curation, writing—review & editing. AS was involved in data curation, writing—review & editing. SY helped in conceptualisation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualisation, writing—review & editing. TMK contributed to data curation, writing—review & editing. HSK helped in validation, writing—review & editing. HHC was involved in validation, writing—review & editing. JYC contributed to validation, writing—review & editing. J-WK was involved in validation, writing—review & editing. ML helped in conceptualisation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Seoul National University Hospital (No. H-2012-027-1178) and performed according to the principles of the Declaration of Helsinki. The requirement for informed consent was waived.
Consent for publication
Further information is available upon request.
Competing interests
Soon Ho Yoon declares relationships with the following companies: MEDICALIP. He works in the MEDICALIP as a chief medical officer. Other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1. Clinicopathologic characteristics of all patients. Table S2. Baseline body composition of high- and low-volumetric SMI groups. Table S3. Associations between volumetric SMI and clinicopathologic characteristics. Table S4. Factors associated with patients’ overall survival. Figure S1. Flow diagram depicting the selection of the study population. Figure S2. Survival outcomes according to the various body composition indices. Volumetric total fat index (A, E); Volumetric visceral fat index (B, F); Volumetric subcutaneous fat index (C, G); Skeletal muscle-to-visceral fat ratio (D, H). (Upper) Progression-free survival; (Lower) Overall survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.I., Chung, J.Y., Paik, H. et al. Prognostic role of computed tomography-based, artificial intelligence-driven waist skeletal muscle volume in uterine endometrial carcinoma. Insights Imaging 12, 192 (2021). https://doi.org/10.1186/s13244-021-01134-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-021-01134-y