Abstract
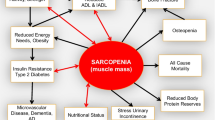
Sarcopenia is a loss of muscle protein mass and loss of muscle function. It occurs with increasing age, being a major component in the development of frailty. Current knowledge on its assessment, etiology, pathogenesis, consequences and future perspectives are reported in the present review. On-going and future clinical trials on sarcopenia may radically change our preventive and therapeutic approaches of mobility disability in older people.
Similar content being viewed by others
References
Landi, F., et al., Body mass index and mortality among older people living in the community. J Am Geriatr Soc, 1999.47(9): p. 1072–6.
Rosenberg, Summary comments. Am J Clin Nutr, 1989(50): p. 1231–3.
Schwartz, R.S., Sarcopenia and physical performance in old age: introduction. Muscle Nerve Suppl, 1997.5: p. S10–2.
Hughes, V.A., et al., Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr, 2002. 76(2): p. 473–81.
Vandervoort, A.A., Aging of the human neuromuscular system. Muscle Nerve, 2002. 25(1): p. 17–25.
Roubenoff, R. and V.A. Hughes, Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci, 2000.55(12): p. M716–24.
Morley, J.E., et al., Sarcopenia. J Lab Clin Med, 2001.137(4): p. 231–43.
Evans, W., Functional and metabolic consequences of sarcopenia. J Nutr, 1997. 127(5 Suppl): p. 998S-1003S.
Gallagher, D., et al., Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol, 1997. 83(1): p. 229–39.
Baumgartner, R.N., et al., Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol, 1998.147(8): p. 755–63.
Baumgartner, R., In vivo body composition studies. Ann NY Acad Sci ed. Body composition in healthy aging, ed. W.J. Yasumura S, Pierson RN Jr. Vol. 904. 2000. 437–448.
Rolland, Y., et al., Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc, 2003.51(8): p. 1120–4.
Janssen, I., et al., Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol, 2004.159(4): p. 413–21.
Melton, L.J., 3rd, et al., Epidemiology of sarcopenia. J Am Geriatr Soc, 2000.48(6): p. 625–30.
Kallman, D.A., C.C. Plato, and J.D. Tobin, The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol, 1990. 45(3): p. M82–8.
Janssen, I., et al., The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc, 2004. 52(1): p. 80–5.
Thomas, D.R., Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr, 2007.26(4): p. 389–99.
Morley, J.E., Weight loss in the nursing home. J Am Med Dir Assoc, 2007. 8(4): p. 201–4.
Morley, J.E., D.R. Thomas, and M.M. Wilson, Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr, 2006. 83(4): p. 735–43.
Friedman, P.J., A.J. Campbell, and T.H. Caradoc-Davies, Prospective trial of a new diagnostic criterion for severe wasting malnutrition in the elderly. Age Ageing, 1985. 14(3): p. 149–54.
Girasole, G., et al., Oestrogens prevent the increase of human serum soluble interleukin-6 receptor induced by ovariectomy in vivo and decrease its release in human osteoblastic cells in vitro. Clin Endocrinol (Oxf), 1999.51(6): p. 801–7.
Waters, D.L., et al., Skeletal muscle mitochondrial function and lean body mass in healthy exercising elderly. Mech Ageing Dev, 2003.124(3): p. 301–9.
van Kan, G.A., et al., Frailty: toward a clinical definition. J Am Med Dir Assoc, 2008. 9(2): p. 71–2.
Abellan Van Kan, A., et al., The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging, 2008.12(1): p. 29–37.
Chumlea, W.C., et al., Techniques of assessing muscle mass and function (sarcopenia) for epidemiological studies of the elderly. J Gerontol A Biol Sci Med Sci, 1995.50 Spec No: p. 45–51.
Chumlea, W.C., A.F. Roche, and P. Webb, Body size, subcutaneous fatness and total body fat in older adults. Int J Obes, 1984. 8(4): p. 311–7.
Chumlea, W.C. and R.N. Baumgartner, Status of anthropometry and body composition data in elderly subjects. Am J Clin Nutr, 1989.50(5 Suppl): p. 1158–66; discussion 1231–5.
Baumgartner RN, W.D., Sarcopenia and sarcopenic-obesity. Pathy MSJ ed. Principles and Practice of Geriatric Medicine. Vol. 2. 2006, United Kingdom: John Wiley and Sons Ltd. 909–933.
Heymsfield, S.B., et al., Appendicular skeletal muscle mass: measurement by dualphoton absorptiometry. Am J Clin Nutr, 1990.52(2): p. 214–8.
Chen, Z., et al., Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr, 2007.137(12): p. 2775–80.
Kim, J., et al., Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol, 2004.97(2): p. 655–60.
Newman, A.B., et al., Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc, 2003.51(11): p. 1602–9.
Janssen, I., S.B. Heymsfield, and R. Ross, Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc, 2002.50(5): p. 889–96.
Delmonico, M.J., et al., Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc, 2007.55(5): p. 769–74.
Song, M.Y., et al., Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr, 2004.79(5): p. 874–80.
Visser, M., et al., Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci, 2005. 60(3): p. 324–33.
Chumlea, W.C. and A.F. Roche, Ultrasonic and skinfold caliper measures of subcutaneous adipose tissue thickness in elderly men and women. Am J Phys Anthropol, 1986.71(3): p. 351–7.
Reeves, N.D., C.N. Maganaris, and M.V. Narici, Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol, 2004.91(1): p. 116–8.
Chumlea, W.C., R.N. Baumgartner, and A.F. Roche, Specific resistivity used to estimate fat-free mass from segmental body measures of bioelectric impedance. Am J Clin Nutr, 1988.48(1): p. 7–15.
Chumlea, W.C., et al., Bioelectric and anthropometric assessments and reference data in the elderly. J Nutr, 1993.123(2 Suppl): p. 449–53.
Baumgartner, R.N., W.C. Chumlea, and A.F. Roche, Estimation of body composition from bioelectric impedance of body segments. Am J Clin Nutr, 1989.50(2): p. 221–6.
Janssen, I., et al., Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol, 2000. 89(2): p. 465–71.
Narici, M. and P. Cerretelli, Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol, 1998.5(1): p. P73–4.
Martin, A.D., et al., Anthropometric estimation of muscle mass in men. Med Sci Sports Exerc, 1990. 22(5): p. 729–33.
Heymsfield, S.B., et al., Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr, 1982. 36(4): p. 680–90.
Baumgartner, R.N., et al., Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res, 2004.12(12): p. 1995–2004.
Heymsfield, S.B., et al., A radiographie method of quantifying protein-calorie undernutrition. Am J Clin Nutr, 1979. 32(3): p. 693–702.
Rantanen, T., et al., Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc, 2003. 51(5): p. 636–41.
Overend, T.J., et al., Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol, 1992.47(6): p. M204–10.
Visser, M., et al., Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci, 2000.904: p. 456–61.
Bassey, E.J., et al., Leg extensor power and functional performance in very old men and women. Clin Sci (Lond), 1992. 82(3): p. 321–7.
Lauretani, F., et al., Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol, 2003. 95(5): p. 1851–60.
Callahan, D., et al., Assessment of lower extremity muscle power in functionallylimited elders. Aging Clin Exp Res, 2007.19(3): p. 194–9.
Cunningham, D.A., et al., Ageing and isokinetic plantar flexion. Eur J Appl Physiol Occup Physiol, 1987.56(1): p. 24–9.
Larsson, L., G. Grimby, and J. Karlsson, Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol, 1979.46(3): p. 451–6.
Poulin, M.J., et al., Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci, 1992.17(1): p. 3–7.
Vandervoort, A.A., J.F. Kramer, and E.R. Wharram, Eccentric knee strength of elderly females. J Gerontol, 1990.45(4): p. B125–8.
Roubenoff, R., Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci, 2003. 58(11): p. 1012–7.
Rolland, Y.M., et al., Loss of appendicular muscle mass and loss of muscle strength in young postmenopausal women. J Gerontol A Biol Sci Med Sci, 2007. 62(3): p. 330–5.
Baumgartner, R.N., et al., Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev, 1999. 107(2): p. 123–36.
Malbut, K.E., S. Dinan, and A. Young, Aerobic training in the ‘oldest old’: the effect of 24 weeks of training. Age Ageing, 2002. 31(4): p. 255–60.
Zachwieja, J.J. and K.E. Yarasheski, Does growth hormone therapy in conjunction with resistance exercise increase muscle force production and muscle mass in men and women aged 60 years or older? Phys Ther, 1999.79(1): p. 76–82.
Morley, J.E. and R.N. Baumgartner, Cytokine-related aging process. J Gerontol A Biol Sci Med Sci, 2004. 59(9): p. M924–9.
Goodpaster, B.H., et al., Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol, 2001.90(6): p. 2157–65.
Kortebein, P., et al., Effect of 10 days of bed rest on skeletal muscle in healthy older adults. Jama, 2007.297(16): p. 1772–4.
Lee, J.S., et al., Associated Factors and Health Impact of Sarcopenia in Older Chinese Men and Women: A Cross-Sectional Study. Gerontology, 2007. 53(6): p. 166–172.
Sheffield-Moore, M., et al., Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab, 2004.287(3): p. E513–22.
Coggan, A.R., et al., Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol, 1992.72(5): p. 1780–6.
Charifi, N., et al., Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve, 2003.28(1): p. 87–92.
Yarasheski, K.E., J.J. Zachwieja, and D.M. Bier, Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol, 1993. 265(2 Pt 1): p. E210–4.
Hasten, D.L., et al., Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab, 2000. 278(4): p. E620–6.
Jozsi, A.C., et al., Changes in power with resistance training in older and younger men and women. J Gerontol A Biol Sci Med Sci, 1999.54(11): p. M591–6.
Welle, S., C. Thornton, and M. Statt, Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol, 1995. 268(3 Pt 1): p. E422–7.
Fiatarone, M.A., et al., Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med, 1994.330(25): p. 1769–75.
Yarasheski, K.E., et al., Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am J Physiol, 1999.277(1 Pt 1): p. El 18–25.
Ivey, F.M., et al., Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci, 2000. 55(11):p. M641–8.
Cress, M.E., et al., Exercise: effects on physical functional performance in independent older adults. J Gerontol A Biol Sci Med Sci, 1999.54(5): p. M242–8.
Hikida, R.S., et al., Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol A Biol Sci Med Sci, 2000. 55(7): p. B347–54.
Hagerman, F.C., et al., Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci, 2000.55(7): p. B336–46.
Hameed, M., S.D. Harridge, and G. Goldspink, Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev, 2002. 30(1): p. 15–9.
Trappe, S., Master athletes. Int J Sport Nutr Exerc Metab, 2001. 11 Suppl: p. S196–207.
Raguso, CA., et al., A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr, 2006.25(4): p. 573–80.
Edstrom, E., et al., Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav, 2007. 92(1-2): p. 129–35.
Lauretani, F., et al., Axonal degeneration affects muscle density in older men and women. Neurobiol Aging, 2006.27(8): p. 1145–54.
Belanger, A.Y. and A.J. McComas, Extent of motor unit activation during effort. J Appl Physiol, 1981.51(5): p. 1131–5.
Andersen, J.L., G. Terzis, and A. Kryger, Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve, 1999. 22(4): p. 449–54.
Essen-Gustavsson, B. and O. Borges, Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand, 1986. 126(1): p. 107–14.
Oertel, G., Changes in human skeletal muscles due to ageing. Histological and histochemical observations on autopsy material. Acta Neuropathol (Berl), 1986. 69(3-4): p. 309–13.
Lexell, J., Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci, 1995.50 Spec No: p. 11–6.
McComas, A.J., 1998 ISEK Congress Keynote Lecture: Motor units: how many, how large, what kind? International Society of Electrophysiology and Kinesiology. J Electromyogr Kinesiol, 1998. 8(6): p. 391–402.
Doherty, T.J., Invited review: Aging and sarcopenia. J Appl Physiol, 2003. 95(4): p. 1717–27.
Doherty, T.J., et al., Effects of motor unit losses on strength in older men and women. J Appl Physiol, 1993. 74(2): p. 868–74.
Larsson, L., B. Sjodin, and J. Karlsson, Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand, 1978. 103(1): p. 31–9.
Lee, C.E., A. McArdle, and R.D. Griffiths, The role of hormones, cytokines and heat shock proteins during age-related muscle loss. Clin Nutr, 2007.
Porter, M.M., A.A. Vandervoort, and J. Lexell, Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports, 1995.5(3): p. 129–42.
Fargnoli, J., et al., Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci USA, 1990. 87(2): p. 846–50.
Edstrom, E., et al., Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci, 2006.61(7): p. 663–74.
Volpi, E., et al., The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab, 2000. 85(12): p. 4481–90.
Boirie, Y., et al., Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab, 2001. 86(2): p. 638–44.
Goulet, E.D., et al., No difference in insulin sensitivity between healthy postmenopausal women with or without sarcopenia: a pilot study. Appl Physiol Nutr Metab, 2007. 32(3): p. 426–33.
Boirie, Y., et al., Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes, 2001.50(12): p. 2652–8.
Guillet, C. and Y. Boirie, Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab, 2005.31 Spec No 2: p. 5S20–5S26.
Guillet, C., et al., Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J, 2004. 18(13): p. 1586–7.
Roubenoff, R., Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care, 2003.6(3): p. 295–9.
Rasmussen, B.B. and S.M Phillips, Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev, 2003. 31(3): p. 127–31.
Dionne, I.J., K.A. Kinaman, and E.T. Poehlman, Sarcopenia and muscle function during menopause and hormone-replacement therapy. J Nutr Health Aging, 2000. 4(3): p. 156–61.
Phillips, S.K., et al., The weakness of old age is not due to failure of muscle activation. J Gerontol, 1992.47(2): p. M45–9.
Kramer, P.R., S.F. Kramer, and G. Guan, 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum, 2004.50(6): p. 1967–75.
Jacobsen, D.E., et al., Postmenopausal HRT and tibolone in relation to muscle strength and body composition. Maturitas, 2007.58(1): p. 7–18.
Taaffe, D.R., et al., Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med Sci Sports Exerc, 2005. 37(10): p. 1741–7.
Gower, B.A. and L. Nyman, Associations among oral estrogen use, free testosterone concentration, and lean body mass among postmenopausal women. J Clin Endocrinol Metab, 2000. 85(12): p. 4476–80.
Volpi, E., R. Nazemi, and S. Fujita, Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care, 2004.7(4): p. 405–10.
Brown, M., S.J. Birge, and W.M. Kohrt, Hormone replacement therapy does not augment gains in muscle strength or fat-free mass in response to weight-bearing exercise. J Gerontol A Biol Sci Med Sci, 1997.52(3): p. B166–70.
Morley, J.E., Growth hormone: fountain of youth or death hormone? J Am Geriatr Soc, 1999.47(12): p. 1475–6.
Musaro, A., et al., IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature, 1999. 400(6744): p. 581–5.
Chen, Y., J.D. Zajac, and H.E. MacLean, Androgen regulation of satellite cell function. J Endocrinol, 2005. 186(1): p. 21–31.
Blackman, M.R., et al., Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. Jama, 2002. 288(18): p. 2282–92.
Lange, K.H., et al., GH administration and discontinuation in healthy elderly men: effects on body composition, GH-related serum markers, resting heart rate and resting oxygen uptake. Clin Endocrinol (Oxf), 2001. 55(1): p. 77–86.
Papadakis, M.A., et al., Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med, 1996. 124(8): p. 708–16.
Thompson, J.L., et al., The effects of recombinant human insulin-like growth factor-I and growth hormone on body composition in elderly women. J Clin Endocrinol Metab, 1995. 80(6): p. 1845–52.
Rudman, D., et al., Effects of human growth hormone in men over 60 years old. N Engl J Med, 1990. 323(1): p. 1–6.
Yarasheski, K.E., et al., Effect of growth hormone and resistance exercise on muscle growth and strength in older men. Am J Physiol, 1995.268(2 Pt 1): p. E268–76.
Clavel, S., et al., Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev, 2006. 127(10): p. 794–801.
Morley, J.E., et al., Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism, 1997. 46(4): p. 410–3.
Morley, J.E., et al., Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci USA, 1997.94(14): p. 7537–42.
Galvao, D.A., et al., Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis, 2007.
Tenover, J.S., Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab, 1992. 75(4): p. 1092–8.
Morley, J.E., et al., Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc, 1993.41(2): p. 149–52.
Katznelson, L., et al., Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab, 1996. 81(12): p. 4358–65.
Sih, R., et al., Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab, 1997. 82(6): p. 1661–7.
Ly, L.P., et al., A double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab, 2001. 86(9): p. 4078–88.
Kenny, A.M., et al., Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci, 2001. 56(5): p. M266–72.
Wittert, G.A., et al., Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci, 2003.58(7): p. 618–25.
Emmelot-Vonk, M.H., et al., Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. Jama, 2008.299(1): p. 39–52.
Borst, S.E., Interventions for sarcopenia and muscle weakness in older people. Age Ageing, 2004. 33(6): p. 548–55.
Genazzani, A.D., C. Lanzoni, and A.R. Genazzani, Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging, 2007.24(3): p. 173–85.
Percheron, G., et al., Effect of 1-year oral administration of dehydroepiandrosterone to 60-to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med, 2003.163(6): p. 720–7.
Perry, H.M., 3rd, et al., Longitudinal changes in serum 25-hydroxyvitamin D in older people. Metabolism, 1999.48(8): p. 1028–32.
Szulc, P., et al., Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr, 2004. 80(2): p. 496–503.
Volpi, E., et al., Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest, 1998.101(9): p. 2000–7.
Volpi, E., et al., Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol, 1999. 277(3 Pt 1): p. E513–20.
Boirie, Y., P. Gachon, and B. Beaufrere, Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr, 1997.65(2): p. 489–95.
Bischoff-Ferrari, H.A., et al., Effect of Vitamin D on falls: a meta-analysis. Jama, 2004. 291(16): p. 1999–2006.
Visser, M, DJ. Deeg, and P. Lips, Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab, 2003. 88(12): p. 5766–72.
Bischoff, H.A., et al., In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J, 2001.33(1): p. 19–24.
Boland, R., Role of vitamin D in skeletal muscle function. Endocr Rev, 1986.7(4): p. 434–48.
Wassner, S.J., et al., Vitamin D Deficiency, hypocalcemia, and increased skeletal muscle degradation in rats. J Clin Invest, 1983.72(1): p. 102–12.
Jacques, P.F., et al., Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr, 1997.66(4): p. 929–36.
Stein, M.S., et al., Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc, 1999.47(10): p. 1195–201.
Drinka, P.J., et al., Determinants of vitamin D levels in nursing home residents. J Am Med Dir Assoc, 2007. 8(2): p. 76–9.
Hamid, Z., et al., Vitamin D deficiency in residents of academic long-term care facilities despite having been prescribed vitamin D. J Am Med Dir Assoc, 2007. 8(2): p. 71–5.
Morley, J.E., Should all long-term care residents receive vitamin D? J Am Med Dir Assoc, 2007. 8(2): p. 69–70.
Roubenoff, R., et al., Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci, 1998.53(1): p. M20–6.
Schaap, L.A., et al., Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med, 2006.119(6): p. 526 e9–17.
Payette, H., et al., Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc, 2003.51(9): p. 1237–43.
Morey, M.C., et al., Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc, 1989.37(4): p. 348–54.
Visser, M., et al., Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci, 2002. 57(5): p. M326–32.
Cesari, M., et al., Sarcopenia, obesity, and inflammation-results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr, 2005. 82(2): p. 428–34.
Ferrucci, L., et al., Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc, 1999.47(6): p. 639–46.
Mitch, W.E. and A.L. Goldberg, Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med, 1996.335(25): p. 1897–905.
Vincent, K.R., et al., Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc, 2002.50(6): p. 1100–7.
Yudkin, J.S., et al., Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis, 2000.148(2): p. 209–14.
Ryan, A.S. and B.J. Nicklas, Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care, 2004.27(7): p. 1699–705.
Visser, M., et al., Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc, 2002.50(5): p. 897–904.
Sipila, S. and H. Suominen, Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol, 1994. 14(4): p. 433–42.
Corcoran, M.P., S. Lamon-Fava, and R.A. Fielding, Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr, 2007. 85(3): p. 662–77.
Roubenoff, R., Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci, 2000.904: p. 553–7.
Dirks, A.J., et al., Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev, 2006.5(2): p. 179–95.
Stump, C.S., et al., Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA, 2003. 100(13): p. 7996–8001.
Bua, E.A., et al., Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol, 2002.92(6): p. 2617–24.
Kent-Braun, J.A., A.V. Ng, and K. Young, Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol, 2000. 88(2): p. 662–8.
Jubrias, S.A., et al., Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol, 2001.90(5): p. 1663–70.
Melov, S., et al., Resistance exercise reverses aging in human skeletal muscle. PLoS ONE, 2007.2(5): p. e465.
Dupont-Versteegden, E.E., Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol, 2005.40(6): p. 473–81.
Giresi, P.G., et al., Identification of a molecular signature of sarcopenia. Physiol Genomics, 2005. 21(2): p. 253–63.
Solomon, A. and P. Bouloux, Endocrine therapies for sarcopenia in older men. Br J Hosp Med (Lond), 2006. 67(9): p. 477–81.
Marzetti, E. and C. Leeuwenburgh, Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol, 2006.41(12): p. 1234–8.
Leeuwenburgh, C., Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci, 2003. 58(11): p. 999–1001.
Reed, T., et al., Genetic influences and grip strength norms in the NHLBI twin study males aged 59–69. Ann Hum Biol, 1991.18(5): p. 425–32.
Arden, N.K. and T.D. Spector, Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res, 1997. 12(12): p. 2076–81.
Huygens, W., et al., Linkage of myostatin pathway genes with knee strength in humans. Physiol Genomics, 2004.17(3): p. 264–70.
Frederiksen, H., et al., Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid-and late-life physical functioning. Genet Epidemiol, 2002. 23(2): p. 110–22.
Carmelli, D. and T. Reed, Stability and change in genetic and environmental influences on hand-grip strength in older male twins. J Appl Physiol, 2000. 89(5): p. 1879–83.
Christensen, K., et al., Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol A Biol Sci Med Sci, 2000.55(8): p. M446–52.
Sayer, A.A., et al., Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J Gerontol A Biol Sci Med Sci, 2004.59(9): p. M930–4.
Yliharsila, H., et al., Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond), 2007. 31(9): p. 1392–9.
Huygens, W., et al., Quantitative trait loci for human muscle strength: linkage analysis of myostatin pathway genes. Physiol Genomics, 2005.22(3): p. 390–7.
Roth, S.M., et al., CNTF genotype is associated with muscular strength and quality in humans across the adult age span. J Appl Physiol, 2001.90(4): p. 1205–10.
Schrager, M.A., et al., Insulin-like growth factor-2 genotype, fat-free mass, and muscle performance across the adult life span. J Appl Physiol, 2004. 97(6): p. 2176–83.
Delmonico, M.J., et al., Alpha-actinin-3 (ACTN3) R577X polymorphism influences knee extensor peak power response to strength training in older men and women. J Gerontol A Biol Sci Med Sci, 2007. 62(2): p. 206–12.
Pfeifer, M., B. Begerow, and H.W. Minne, Vitamin D and muscle function. Osteoporos Int, 2002. 13(3): p. 187–94.
Roth, S.M., et al., Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci, 2004.59(1): p. 10–5.
Grundberg, E., et al., Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol, 2004. 150(3): p. 323–8.
Geusens, P., et al., Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res, 1997.12(12): p. 2082–8.
Fisher, A.L., Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc, 2004.52(7): p. 1185–90.
Herndon, L.A., et al., Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature, 2002.419(6909): p. 808–14.
Kenyon, C., et al., A C. elegans mutant that lives twice as long as wild type. Nature, 1993. 366(6454): p. 461–4.
Chaput, J.P., et al., Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J Nutr Health Aging, 2007.11(4): p. 363–9.
Lord, C., et al., Dietary animal protein intake: association with muscle mass index in older women. J Nutr Health Aging, 2007.11(5): p. 383–7.
Campbell, W.W., et al., Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am J Clin Nutr, 1999.70(6): p. 1032–9.
Campbell, W.W., et al., Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr, 1994. 60(2): p. 167–75.
Campbell, W.W. and W.J. Evans, Protein requirements of elderly people. Eur J Clin Nutr, 1996. 50 Suppl 1: p. S180–3; discussion S183–5.
Campbell, W.W., et al., The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci, 2001.56(6): p. M373–80.
Rooyackers, O.E., et al., Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA, 1996. 93(26): p. 15364–9.
Roberts, S.B., et al., Effects of age on energy expenditure and substrate oxidation during experimental underfeeding in healthy men. J Gerontol A Biol Sci Med Sci, 1996.51(2):p. B158–66.
Wilson, M.M. and J.E. Morley, Invited review: Aging and energy balance. J Appl Physiol, 2003. 95(4): p. 1728–36.
Bennet, W.M., et al., The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest, 1990. 20(1): p. 41–50.
Volpi, E., et al., Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr, 2003. 78(2): p. 250–8.
Campbell, W.W., et al., Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol, 1995.268(6 Pt 1): p. E1143–53.
Welle, S. and C.A. Thornton, High-protein meals do not enhance myofibrillar synthesis after resistance exercise in 62-to 75-yr-old men and women. Am J Physiol, 1998. 274(4Pt 1): p. E677–83.
Katsanos, C.S., et al., Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr, 2005. 82(5): p. 1065–73.
Dardevet, D., et al., Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr, 2000.130(11): p. 2630–5.
Rasmussen, B.B., et al., Insulin resistance of muscle protein metabolism in aging. Faseb J, 2006. 20(6): p. 768–9.
Fujita, S., et al., Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes, 2007.56(6): p. 1615–22.
Ferrucci, L., et al., Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc, 2004.52(4): p. 625–34.
Rantanen, T., Muscle strength, disability and mortality. Scand J Med Sci Sports, 2003. 13(1): p. 3–8.
Rosenberg, I.H., Sarcopenia: origins and clinical relevance. J Nutr, 1997. 127(5 Suppl):p. 990S-991S.
Fleg, J.L. and E.G. Lakatta, Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol, 1988. 65(3): p. 1147–51.
Janssen, I., Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc, 2006.54(1): p. 56–62.
Jensen, G.L., Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med, 2005.21(4): p. 677–87, v.
Ensrud, K.E., et al., Correlates of impaired function in older women. J Am Geriatr Soc, 1994.42(5): p. 481–9.
Sarkisian, C.A., et al., Modifiable risk factors predict functional decline among older women: a prospectively validated clinical prediction tool. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc, 2000.48(2): p. 170–8.
Friedmann, J.M., T. Elasy, and G.L. Jensen, The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc, 2001.49(4): p. 398–403.
Visser, M., et al., High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr, 1998. 68(3): p. 584–90.
Zamboni, M., et al., The relationship between body composition and physical performance in older women. J Am Geriatr Soc, 1999.47(12): p. 1403–8.
Davison, K.K., et al., Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J Am Geriatr Soc, 2002.50(11): p. 1802–9.
Sternfeld, B., et al., Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol, 2002. 156(2): p. 110–21.
Zoico, E., et al., Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord, 2004.28(2): p. 234–41.
Villareal, D.T., et al., Physical frailty and body composition in obese elderly men and women. Obes Res, 2004.12(6): p. 913–20.
Rolland, Y., et al., Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am J Clin Nutr, 2004.79(4): p. 552–7.
Ford, E.S., A.H. Mokdad, and W.H. Giles, Trends in waist circumference among U.S. adults. Obes Res, 2003.11(10): p. 1223–31.
Lebrun, C.E., et al., Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause, 2006.13(3): p. 474–81.
Janssen, I., P.T. Katzmarzyk, and R. Ross, Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc, 2005. 53(12): p. 2112–8.
Bigaard, J., et al., Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obes Res, 2003.11 (7): p. 895–903.
Kanaya, A.M., et al., Association of total and central obesity with mortality in postmenopausal women with coronary heart disease. Am J Epidemiol, 2003.158(12): p. 1161–70.
Griffiths, R.D., Muscle mass, survival, and the elderly ICU patient. Nutrition, 1996. 12(6): p. 456–8.
Cosqueric, G., et al., Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr, 2006.96(5): p. 895–901.
Newman, A.B., et al., Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci, 2006. 61(1): p. 72–7.
Newman, A.B., et al., Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc, 2003. 51(3): p. 323–30.
Buchner, D.M., et al., Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing, 1996.25(5): p. 386–91.
Rantanen, T., et al., Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc, 2001.49(1): p. 21–7.
Janssen, I., et al., Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol, 2000. 89(1): p. 81–8.
Lynch, N.A., et al., Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol, 1999.86(1): p. 188–94.
Singh, A.S., et al., Cross-sectional relationship between physical fitness components and functional performance in older persons living in long-term care facilities. BMC Geriatr, 2006.6: p. 4.
Guralnik, J.M., et al., Progressive versus catastrophic loss of the ability to walk: implications for the prevention of mobility loss. J Am Geriatr Soc, 2001. 49(11): p. 1463–70.
Roth, S.M., R.F. Ferrell, and B.F. Hurley, Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging, 2000.4(3): p. 143–55.
Nelson, M.E., et al., Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation, 2007.116(9): p. 1094–105.
Hakkinen, K., et al., Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol, 1998. 84(4): p. 1341–9.
Taaffe, D.R., et al., Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc, 1999.47(10): p. 1208–14.
Fielding, R.A., et al., Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc, 2007.39(11): p. 1997–2004.
Strength training among adults aged >65 years — United States 2001., in MMWR. 2004, US department of health and human services. p. 25–28.
Cardinale, M., et al., Whole-body vibration can reduce calciuria induced by high protein intakes and may counteract bone resorption: A preliminary study. J Sports Sci, 2007.25(1):p. 111–9.
Cardinale, M. and J. Rittweger, Vibration exercise makes your muscles and bones stronger: fact or fiction? J Br Menopause Soc, 2006.12(1): p. 12–8.
Bogaerts, A., et al., Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci, 2007.62(6): p. 630–5.
Morley, J.E., Anorexia and weight loss in older persons. J Gerontol A Biol Sci Med Sci, 2003.58(2): p. 131–7.
Heiat, A., V. Vaccarino, and H.M. Krumholz, An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med, 2001. 161(9): p. 1194–203.
Elia, M., Obesity in the elderly. Obes Res, 2001.9 Suppl 4: p. 244S-248S.
Morais, J.A., S. Chevalier, and R. Gougeon, Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging, 2006.10(4): p. 272–83.
Houston, D.K., et al., Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr, 2008. 87(1): p. 150–5.
Fujita, S. and E. Volpi, Amino acids and muscle loss with aging. J Nutr, 2006. 136(1 Suppl): p. 277S-80S.
Timmerman, K.L. and E. Volpi, Amino acid metabolism and regulatory effects in aging. Curr Opin Clin Nutr Metab Care, 2008.11(1): p. 45–9.
Drummond, M.J., et al., Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol, 2008.
Hayes, A. and P.J. Cribb, Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care, 2008.11(1): p. 40–4.
Rieu, I., et al., Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition, 2007. 23(4: p. 323–31.
Kim, J.H., et al., Lifelong exercise and mild (8%) caloric restriction attenuate ageinduced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol, 2008.43(4): p. 317–29.
Dirks Naylor, A.J. and C. Leeuwenburgh, Sarcopenia: the role of apoptosis and modulation by caloric restriction. Exerc Sport Sci Rev, 2008. 36(1): p. 19–24.
Arnal, M.A., et al., Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr, 1999.69(6): p. 1202–8.
Boirie, Y., et al., Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA, 1997.94(26): p. 14930–5.
Dangin, M., et al., Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr, 2002.132(10): p. 3228S-33S.
Wolfson, L., et al., Training balance and strength in the elderly to improve function. J Am Geriatr Soc, 1993.41(3): p. 341–3.
Sayer, A.A., et al., Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol, 2006.164(7): p. 665–71.
Szulc, P., et al., Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab, 2002. 87(2): p. 666–74.
Bhasin, S., et al., The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med, 1996. 335(1): p. 1–7.
Ottenbacher, K.J., et al., Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc, 2006. 54(11): p. 1666–73.
Bhasin, S. and J.G. Buckwalter, Testosterone supplementation in older men: a rational idea whose time has not yet come. J Androl, 2001. 22(5): p. 718–31.
Borst S.E., et al., Anabolic effects of testosterone are preserved during inhibition of 5alpha-reductase. Am J Physiol Endocrinol Metab, 2007. 293(2): p. E507–14.
Parsons, J.K., et al., Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev, 2005. 14(9): p. 2257–60.
Venken, K., et al., Bone and muscle protective potential of the prostate-sparing synthetic androgen 7alpha-methyl-19-nortestosterone: evidence from the aged orchidectomized male rat model. Bone, 2005. 36(4): p. 663–70.
Li, J.J., et al., Discovery of Potent and Muscle Selective Androgen Receptor Modulators through Scaffold Modifications. J Med Chem, 2007. 50(13): p. 3015–3025.
Takala, J., et al., Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med, 1999. 341(11): p. 785–92.
Frost, R.A., G.J. Nystrom, and C.H. Lang, Regulation of IGF-I mRNA and signal transducers and activators of transcription-3 and -5 (Stat-3 and -5) by GH in C2C12 myoblasts. Endocrinology, 2002. 143(2): p. 492–503.
Leroith, D. and P. Nissley, Knock your SOCS off! J Clin Invest, 2005. 115(2): p. 233–6.
Leger, B., et al., Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res, 2008. 11(1): p. 163–175B.
Barbieri, M., et al., Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab, 2003. 284(3): p. E481–7.
Cappola, A.R., et al., Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab, 2001. 86(9): p. 4139–46.
Onder, G., et al., Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab, 2006. 291(4): p. E829–34.
Thompson, J.L., et al., Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab, 1998. 83(5): p. 1477–84.
Pollak, M., Insulin-like growth factor physiology and cancer risk. Eur J Cancer, 2000. 36(10): p. 1224–8.
Cobb, L.J., et al., Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J Cell Sci, 2004.117(Pt 9): p. 1737–46.
Artaza, J.N., et al., Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology, 2005.146(8): p. 3547–57.
Schuelke, M., et al., Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med, 2004. 350(26): p. 2682–8.
Siriett, V., et al., Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther, 2007.15(8): p. 1463–70.
Petersen, A.M., et al., Smoking impairs muscle protein synthesis and increases the expression of myostatin and MAFbx in muscle. Am J Physiol Endocrinol Metab, 2007. 293(3): p. E843–8.
Solomon, A.M. and P.M. Bouloux, Modifying muscle mass — the endocrine perspective. J Endocrinol, 2006.191(2): p. 349–60.
Nakatani, M., et al., Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. Faseb J, 2008. 22(2): p. 477–87.
Ohsawa, Y., et al., Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest, 2006.116(11): p. 2924–34.
Haidet, A.M., et al., Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA, 2008. 105(11): p. 4318–22.
Lemoine, S., et al., Estrogen receptor alpha mRNA in human skeletal muscles. Med Sci Sports Exerc, 2003. 35(3): p. 439–43.
Wiik, A., et al., Oestrogen receptor beta is present in both muscle fibres and endothelial cells within human skeletal muscle tissue. Histochem Cell Biol, 2005. 124(2): p. 161–5.
Bischoff-Ferrari, H.A., et al., Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Jama, 2005.293(18): p. 2257–64.
Janssen, H.C., M.M. Samson, and H.J. Verhaar, Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr, 2002.75(4): p. 611–5.
Candow, D.G. and P.D. Chilibeck, Effect of creatine supplementation during resistance training on muscle accretion in the elderly. J Nutr Health Aging, 2007. 11(2): p. 185–8.
Brose, A., G. Parise, and M.A. Tarnopolsky, Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci, 2003.58(1): p. 11–9.
Willoughby, D.S. and J.M. Rosene, Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc, 2003.35(6): p. 923–9.
Bermon, S., et al., Effects of creatine monohydrate ingestion in sedentary and weighttrained older adults. Acta Physiol Scand, 1998. 164(2): p. 147–55.
Chrusch, M.J., et al., Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc, 2001. 33(12): p. 2111–7.
Eijnde, B.O., et al., Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J Appl Physiol, 2003. 95(2): p. 818–28.
Gotshalk, L.A., et al., Creatine supplementation improves muscular performance in older men. Med Sci Sports Exerc, 2002. 34(3): p. 537–43.
Jakobi, J.M., et al., Neuromuscular properties and fatigue in older men following acute creatine supplementation. Eur J Appl Physiol, 2001. 84(4): p. 321–8.
Rawson, E.S., M.X. Wehnert, and P.M. Clarkson, Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol, 1999. 80(2): p. 139–44.
Rawson, E.S. and P.M. Clarkson, Acute creatine supplementation in older men. Int J Sports Med, 2000. 21(1): p. 71–5.
Tarnopolsky, M.A. and A. Safdar, The potential benefits of creatine and conjugated linoleic acid as adjuncts to resistance training in older adults. Appl Physiol Nutr Metab, 2008. 33(1): p. 213–27.
Carter, C.S., et al., Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci, 2005. 60(11): p. 1437–46.
Onder, G., et al., Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet, 2002. 359(9310): p. 926–30.
Han, Y., M.S. Runge, and A.R. Brasier, Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res, 1999. 84(6): p. 695–703.
Vescovo, G., et al., Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation, 1998. 98(17): p. 1742–9.
Payne, G.W., Effect of inflammation on the aging microcirculation: impact on skeletal muscle blood flow control. Microcirculation, 2006. 13(4): p. 343–52.
Onder, G., C.D. Vedova, and M. Pahor, Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des, 2006. 12(16): p. 2057–64.
Haslett, P., et al., The metabolic and immunologie effects of short-term thalidomide treatment of patients infected with the human immunodeficiency virus. AIDS Res Hum Retroviruses, 1997. 13(12): p. 1047–54.
Calabrese, L.H., N. Zein, and D. Vassilopoulos, Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis, 2004. 63 Suppl 2: p. ii18-ii24.
Robinson, S.M., et al., Diet and its relationship with grip strength in communitydwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc, 2008. 56(1): p. 84–90.
Silventoinen, K., et al., Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol, 2008. 32(4): p. 341–9.
Mascher, H., et al., Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab, 2008. 294(1): p. E43–51.
Marzetti, E., et al., Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol, 2008. 294(2): p. R558–67.
Phillips, T. and C. Leeuwenburgh, Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. Faseb J, 2005. 19(6): p. 668–70.
Semba, R.D., F. Lauretani, and L. Ferrucci, Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys, 2007. 458(2): p. 141–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rolland, Y., Czerwinski, S., van Kan, G.A. et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12, 433–450 (2008). https://doi.org/10.1007/BF02982704
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02982704