Abstract
Background
Increasing physical activity is one of the most promising and challenging interventions to delay or prevent cognitive decline and dementia.
Methods
We conducted a randomized controlled trial to assess the effects of a physical activity intervention, aimed at increasing step count, in elderly with low levels of physical activity on measures of strength, balance, aerobic capacity, and cognition. Participants were assigned to 9 months of exercise counseling or active control.
Results
The intention-to-treat analyses show that the intervention, compared to control, increases the level of physical activity, but has no significant effect on physical fitness and cognition. Those who increased their physical activity with 35% or more show significant improvements in aerobic capacity, gait speed, verbal memory, executive functioning, and global cognition, compared to those who did not achieve a 35% increase.
Limitations
The number of participants that achieved the intended improvement was lower than expected.
Conclusion
Responder analyses suggest an improvement of physical fitness and cognition in those who achieved an increase in physical activity of at least 35%.
Trial registration
The trial protocol is registered at the Dutch Trial Register NL5675, August 1, 2016.
Similar content being viewed by others
Background
The Lancet commission on dementia prevention, intervention, and care has indicated 12 modifiable risk factors for dementia and estimates that these factors account for 40% of all dementias worldwide that could potentially be prevented or delayed [1]. Physical activity is a promising intervention target to delay cognitive decline and dementia [1,2,3]. Yet, there is also substantial evidence of reverse causality [4, 5]. Subclinical neuropathological changes that proceed the diagnosis of dementia can gradually affect behavior, reducing physical activities as much as 9 years before the diagnosis of dementia [5]. Meta-analyses aggregating the effect of experimental trials report that physical activity interventions have modest positive effects on cognition [6,7,8,9,10]. Heterogeneity among these studies have led others to conclude the evidence is inconsistent or insufficient [11,12,13,14].
Important unanswered questions concern the parameters, mediators, and moderators of successful physical activity interventions. The most consistent positive results are observed in interventions of moderate intensity [8, 11, 15, 16], with a duration of at least 6 months [7, 11, 13, 17], aimed at people with low levels of physical activity [18, 19]. Intervention potential could be enhanced by targeting “at risk” individuals, defined by various health parameters including level of physical activity, genetic susceptibility, cardiovascular risk profile, and age.
Although physical activity is known to have an inverse association with several chronic diseases associated with cognitive impairment and dementia, such as cardiovascular disease, hypertension, diabetes mellitus, and obesity [20,21,22,23,24,25,26,27,28,29,30,31], it is unclear whether the temporary upregulation of the level of physical activity is enough to elicit the desired cardiovascular and neurocognitive changes. Observational data from epidemiological studies indicate that physical activity may take years to impact cardiovascular brain health [32, 33], and systematic reviews of RCTs fail to show consistent evidence of a relationship between physical fitness and cognitive performance [16, 34].
The relatively short-term improvements in cognitive functioning observed in intervention studies may be driven by mechanisms other than the improvement of physical fitness, like the promotion of cerebral angiogenesis and synaptic plasticity elicited by stimulation of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), insulin growth factor 1 (IGF-I), and vascular endothelial growth factor (VEGF) [14, 16, 34,35,36].
Furthermore, observational studies investigating effect moderation by APOE genotype have indicated that higher levels of physical activity might help attenuating both cognitive deficits and risk for Alzheimer’s disease more so in carriers than in non-carriers of the ApoE-ε4 allele [32, 37,38,39,40,41,42,43,44].
Improving physical activity in those who fail to meet the public health recommendations for older adults of 150 min of moderate intensity aerobic activity per week [13] independently, requires a tailored approach. The present study is aimed at durable behavior modification using the COACH method. The COACH method is an individually tailored exercise counseling method based on the Motivational Interviewing Technique and goal-setting theory, both considered to be effective instruments for behavioral modification [45, 46]. Application of this method has proven feasible in various patient groups [47,48,49,50,51,52,53] and increased the physical activity of older adults with an average of 33% after 3 months and 41% after 12 months [50].
In the present study, we conducted a 9 month randomized trial to assess whether an increase in physical activity of 35% or more, generated by the COACH method, can lead to an improvement in physical fitness, and subsequent improvements in cognitive functioning, and well-being of healthy elderly with and without a genetic vulnerability for dementia, based on their APOE genotype. We targeted those with low levels of physical activity.
Methods
Design
The current study was a 9-month randomized controlled trial (RCT) with one intervention group and one control group. Participants could participate in the baseline measurement only (T0) or continue with participation in the trial, including follow-up measurements at 6 months (T1) and at 9 months (T2).
Recruitment
Recruitment took place between September 1, 2015, and August 31, 2019. Participants were recruited among older adults (55 +) who previously participated in the Erasmus Rucphen Family Study (ERF) [54] and among the general population. Study information was spread via various media, including www.hersenonderzoek.nl. Four out of the 102 participants in the intervention study previously participated in the ERF-study.
Participants
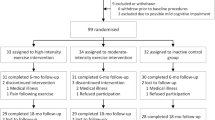
Eligibility criteria were as follows: aged ≥ 55 years, able to perform the Timed Up and Go Test (TUG) within 20 seconds or less [55], and a Mini-Mental Status Examination (MMSE) [56] score ≥ 25. Exclusion criteria were as follows: wheelchair-bound, cardiovascular problems that limit physical activity based on the Physical Activity Readiness Questionnaire (PARQ) [57], epilepsy, diagnosis of dementia or mild cognitive impairment, progressive or terminal disease, depression, history of alcoholism, severe visual or auditory problems, or difficulty with the Dutch language. Only those participants who reported low levels of physical activity at baseline were recruited for participation in the trial. Low levels of physical activity were defined as < 7000 steps a day on average. This number was calculated according to the Tudor-Locke translation of public health recommendations in minutes a day to steps a day for older adults [58]. Figure 1 displays a flow-chart of participant selection.
Randomization and masking
Participants were assigned to the intervention or control condition by an independent researcher using a random number generator per block with an allocation of 2:1 in favor of the intervention. Although research assistants, exercise counselors, nor participants were informed of the existence of a control intervention, the trial could not be blinded as the difference in content and aims of the coaching and stretching sessions could not be concealed for participants, exercise counselors, or research assistants.
Procedure
Eligibility screening was followed by the baseline assessment of all outcome measures including physical activity using a pedometer over two consecutive weeks (T0). Assessments of all outcomes were repeated at T1 and T2. Participant procedures are depicted in Fig. 2.
Intervention
The intervention is an adapted version of the COACH protocol (www.coachmethode.nl), a pedometer-based exercise counseling strategy, aimed at enhancing low-to-moderate daily physical activity [51]. Physical activity goals are quantified in steps per day and pedometers are used as feedback instrument to monitor progress [59,60,61,62,63]. The original version of the COACH program spans a period of 3 to 4 months, including four to six coaching sessions. In the present study, the protocol was extended to seven coaching sessions, spanning 6 months and a follow-up session 9 months after the start of the intervention. In the coaching sessions of 45 minutes each, the counselor encourages participants to enhance daily physical activity such as walking, cycling, housekeeping, and gardening. The follow-up session (C8) is aimed at the evaluation and consolidation of the newly learned physical activity behavior. By stimulating low-to-moderate daily physical activity, the program is feasible for elderly people with varying levels of physical fitness. Adherence is encouraged by allowing participants to freely choose physical activity behaviors that match their preferences. The focus on step count facilitates the process of goal setting and goal monitoring.
The stimulation of daily physical activities by the exercise counselors was applied in four phases: (1) need to change; (2) benefits versus effort; (3) plan of action including specific, measurable, attainable, realistic, and time-bound (SMART) goals; (4) implementation plan. Participants were required to monitor their daily physical activity by means of a pedometer and record the total number of steps per day in a paper or online diary. For activities other than walking, participants were required to register duration, activity type, and intensity. The duration of these activities was converted into steps, according to the criteria of Tudor-Locke and Basset [58, 64], by using the adult cadence of 100 steps per minute. Based on previously described metabolic equivalents of task, the total number of steps assigned to vigorous activities was multiplied by factor 1.5 [65]. We converted non-ambulatory activity to steps as (1) locomotor activity constitutes the bulk of physical activity for adults [66] and (2) the use of step count facilitates the process of goal-setting and monitoring for the participants and (3) increases comparability across participants. Participants in the intervention group are instructed to use the pedometer during the 6 months of the intervention and at least 1 month before the last measurement 9 months after the start of the intervention. Adherence was determined by active participation in the coaching sessions and daily registration of step count. Table 1 presents a further specification of the content of the COACH sessions. Figure 2b specifies the study procedures per week for participants in the intervention and the control group.
Control condition
Participants in the control group received seven individually guided muscle stretching sessions, of 45 minutes each, scheduled over 6 months. Participants were offered a set of stretching exercises spanning the muscle groups of all body parts. During the sessions, participants were guided through the exercises of their choice and encouraged to perform these exercises outside of the session as desired. Figure 2c specifies the study procedures per week for participants in the control group. The follow-up session (S8) is aimed at the evaluation and consolidation of the newly learned stretching habit. Controls were requested to use the pedometer during 1 month before each follow-up measurement, as average daily step count is known to increase significantly in the first week of measurement when using unsealed pedometers and self-registration, due to reactivity [67]. The coaching sessions and the stretching and toning sessions were offered by a coach trained by the CBO (Centrum voor Beweging en Onderzoek Groningen).
Baseline characteristics
Participants were asked about their health status, medication use, lifestyle habits, and sociodemographic factors. Education level was defined as low, middle, and high according to the Dutch classification of educational attainment [68]. Buccal cells were collected using a buccal swab and stored at − 20 °C. APOE genotyping of DNA samples was performed by the Department of Clinical Chemistry of the Amsterdam UMC. DNA was purified manually from buccal swabs [SOP 011036] and amplified through PCR [SOP 011909]. Cycle sequencing of amplified DNA samples [SOP 032929] (ABI prism Big Dye Terminator, Applied Biosystems by Life Technologies, Austin Texas, USA) and sequencing analysis were performed [SOP 011183] (Applied Biosystems 3130XL Genetic Analyzer Applied Biosystems by Life Technologies, Foster City, CA, USA). Determination of allele status occurred on basis of codons 112 and 158 of the APOE gene on chromosome 19. APOE genotypes were categorized into ApoE-ɛ4 carriers and non-carriers.
Outcomes
Primary outcomes
Physical activity
Level of physical activity was expressed in average number of steps per day as assessed by a hip-worn pedometer. Participants were provided with one of the four following types of pedometers: the Omron walking style II, IV, and One 2.0 (Omron Healthcare Co. Ltd., Dallian, China), and the Yamax Digiwalker (SW-200, Yamasa Tokei Keiki Co. Ltd., Tokyo, Japan). All models use 3-axis technology to register ambulatory motion and have demonstrated good and comparable validity in free-living conditions [69]. Pedometers have a high construct validity [70] and are considered a valid and accessible option to assess physical activity. Pedometer measurements have been shown to correlate strongly (r = 0.86) with different accelerometers and with time in observed activity (r = 0.82) and moderately with different measures of energy expenditure (r = 0.68) [71]. A self-report questionnaire, the Physical Activity Scale for the Elderly (PASE) [72] was used to assess self-reported level of physical activity. The total PASE score is based upon the self-report of the frequency and duration of various physical activities performed in the past seven days, categorized according to their intensity. Higher scores indicate higher levels of physical activity.
Cognitive function
Cognitive function was assessed by neuropsychological tests summarized in composite scores for global cognition, executive functioning, learning, and verbal memory, based on theoretical considerations [73]. Higher scores indicate better cognitive ability. The global cognition score was created using the sum of the standardized scores of the 15 Word Test delayed recall (15-WT) [74], the Trail Making Test (TMT) B [75], the Stroop Color Word Test Interference score [76], the total scores on the Letter Fluency Test [77], and the Digit Span backward test [78, 79]. An executive functioning score was created using the sum of the standardized scores of the TMT AB-ratio, the Stroop Color Word Test Interference score, the total score on the Letter Fluency Test, and the Digit Span Test backward. Verbal episodic memory was assessed with the delayed recall condition of the 15-WT. A domain score learning was created for short-term memory performance using the Location Learning Test [80, 81] and the 15 Word Test. The ability to encode and imprint visual information was reflected in the total number of placement errors on the five trials of the Location Learning Test. The ability to encode and imprint verbal information was reflected in the number of correctly recalled words after five trials of the 15 Word Test. To create the learning index, both scores are standardized and the total number of placement errors on the LLT is inverted, so that for both measures higher scores indicate better imprinting ability.
Physical fitness
The Short Physical Performance Battery (SPPB) [82] was used to assess balance, lower body strength, and gait speed. The total balancing time in seconds is used as an indication of balance. Handgrip strength in kilograms was measured by the hydraulic JAMAR dynamometer [83,84,85]. A combined muscle strength score was created using the sum of the standardized scores of the SPPB sit-to-stand chair test and grip strength. The Six Minute Walk Test (6MWT) [86] is used to assess aerobic capacity [87]. Higher scores indicate better physical fitness. Six-meter walking speed in seconds is used as an indication of gait speed. Lower scores indicate better performance.
Secondary outcomes
Serum concentrations of the cardiovascular risk factor profile
Fasting blood samples are collected and centrifuged at 1800 g for 10 minutes, directly on site or after transport on the same day. Serum is frozen at − 80 °C until assessment. In all serum samples, total cholesterol, HDL-cholesterol, triglycerides, insulin, and IGF-I were assessed by the Department of Clinical Chemistry of the Amsterdam UMC.
Activities of daily living, frailty, and mental health
The Rand-36 mental health sub-scale [88] was used to assess psychological well-being. Higher scores indicate a more positive emotional state. Depressive symptoms were assessed with the Centre for Epidemiological Studies Depression Scale (CES-D) [89, 90]. Higher scores indicate more depressive symptoms. Limitations in the activities of daily living (ADL) were assessed using the Katz-15 scale [91], part of the TOPICS-MDS (www.topics-mds.eu). In this questionnaire, the need for assistance on 15 activities of daily living is inventoried. Higher scores indicate more ADL limitations.
A composite measure of frailty was calculated using the number of deficits in activities of daily living, social functioning, emotional well-being, and self-reported health on the TOPICS-MDS Higher index scores indicate more frailty.
Statistical analyses
Sample size calculations are based on the assessment of the neuropsychological outcome measures using “G-Power 3.1.” Based on an expected effect size of f = 0.20 (equals d = 0.4), power = 0.80, alpha = 0.05, 2 groups, 3 measurements, and an estimation of the test–retest reliability = 0.6 [92,93,94], the required sample size for within-between interaction in a repeated measures analysis is (33.5) 34 subjects per group. We additionally calculated the power for unequal sample sizes by using the following equation \(N=\frac{{(Z}_{\left(1-\propto \right)}+ {Z}_{(1-\beta )}{)}^{2}* {\sigma }^{2}*(\mathrm{r }+ 1) [1+T-1)\rho ]}{{v}^{2}T}\). Using an allocation ratio of r = 0.5, standard deviation of the outcome measurement \(\sigma =1\), the number of follow-up measurements T = 2, correlation between measurements \(\rho =0.6\), and the number of participants in the smallest group N = (33.5) 34 participants, the study is powered to detect a \(v=0.47\) difference in mean value of the standardized outcome variable, corresponding to a medium effect size (d = 0.47).
The effect of the intervention relative to the control condition was analyzed using linear mixed-effects models. Compound outcome measures were standardized over time. Group (intervention versus control) was included as a factor. A random intercept was included in all models. Percentage of missingness of all outcome variables varied between 0.37 and 2.21%. APOE genotype information was missing for 5.89% of the participants. Missing values were not imputed as mixed-effects models adequately deal with missing data and multiple imputations provide no gain, regardless of the missing data mechanism [95]. Analyses were performed per protocol and according to the intention-to-treat (ITT) principle [96]. For the ITT analyses, participants were included if they had completed at least one measurement. Per-protocol analyses included only those participants who completed the intervention and all three measurements. For each outcome variable, we estimated the overall effect of the intervention after 9 months.
The linear mixed model analyses were repeated using the increase in physical activity between T0 and T2 as grouping variable, i.e., ≥ 35%, increase in average number of steps per day versus no increase, or < 35% in average number of steps per day at T2, 9 months after the start of the intervention. All analyses were adjusted for baseline performance, age, and sex (model A) and additionally for education level and APOE genotype (model B). Interactions between APOE, sex, and group on primary outcomes were investigated using interaction terms. Stratified analyses should be regarded as exploratory. Effect sizes (Cohen’s d) were calculated by dividing the regression coefficient by the pooled standard deviation of the outcome variable at baseline [97]. Significance level for all analyses was 0.05. Data were analyzed using IBM SPSS Statistics version 24 [98] and Stata version 16 [99].
Results
Numbers analyzed
Data collection was discontinued because of the COVID-19 outbreak. At this time, all participants had finalized the 6-month intervention. Eight participants (intervention n = 7, control n = 1) could no longer participate in the measurement 9 months after the start of the intervention (T2). At T2, data on the primary outcomes were available for 54/69 (78.26%) participants in the intervention group and 27/33 (81.82%) controls. There were no significant differences in baseline characteristics between the randomized participants who discontinued the intervention and those who completed the intervention. On average, the randomized participants who discontinued the intervention were slightly older than those who completed the intervention (M = 73.75, SD = 10.02; M = 69.90, SD = 8.81; p = 0.09). Figure 1 reviews the participant inclusion, withdrawals, and missing data. Serum concentrations were assessed at T0 and T1 only. At T0, serum was available for 64/69 (92.75%) participants in the intervention group and 30/33 (90.90%) participants in the control group. At T1, serum was available for 50/69 (72.46%) participants in the intervention group and for 26/33 (78.79%) controls.
Baseline characteristics
Baseline characteristics are provided in Table 2. The mean age of the total sample was 70.69 years (SD = 9.16), and most participants were female (75.49%). The average age in the controls was 73.3 years (SD = 9.01) which is significantly higher than in the intervention group 69.45 years (SD = 9.02), p = 0.05.
Intention to treat analyses
Primary outcomes
The ITT analysis (Table 3) shows a significant improvement in the average number of steps per day in the intervention group (B = 589.58; p = .047; d = 0.45) compared to the control group. However, the intervention has no significant effect on self-reported physical activity, physical fitness, or cognition. The correlation between the average number of steps and self-reported physical activity across all participants was low (r = 0.24). The intervention decreases balance. However, this difference is explained by an improvement in balance in controls over time (B = − 0.76 [95% CI − 1.47 to − 0.05]; p = .04; d = 0.18). No effect moderation by ApoE-ε4 was observed for the primary outcomes.
The effect of the intervention on gait speed is significantly moderated by sex (B = − 0.82 [95% CI − 1.30 to − 0.33]; p = .001). At baseline, the average time in seconds to walk 6 m is higher for women (M = 4.33, SD = 1.04) than for men (M = 3.93, SD = 1.33, p = 0.03), which indicates a slower gait speed in women. The women in the intervention group show an overall decrease in time in seconds, indicating an increase in gait speed. This increase in gait speed is significantly relative to the control group (B = − 0.82 [95% CI − 1.30 to − 0.33]; p = .04; d = 0.73). The men in the intervention group show a slight overall increase in time in seconds. This increase occurs at T1, followed by a decrease at T2. See Fig. 3. This overall increase in time in seconds is not significant compared to the control group (B = 0.53 [95% CI − 0.01 to 1.07]; p = .06; d = 0.47).
Secondary outcomes—indicators of physical and mental health
The indices of frailty and depressive symptoms were unaffected by the intervention. The intervention decreased the number of ADL limitations (B = − 0.11, p = .03; d = 0.30). The mental health score increased over time in the control group, indicating improved mental health, and remained constant in the intervention group (B = − 2.48, p = .03, d = 0.21) (Table 4).
Secondary outcomes—serum concentrations assessed at 6 months (T1)
Serum concentrations of biomarkers of diabetes (insulin, IGF-I) and lipid metabolism (total cholesterol, HDL-cholesterol, and triglycerides) assessed at T1 were not significantly affected by the intervention (Table 5).
Per-protocol analyses
The relative increase in the average number of steps per day in the intervention group did not reach significance in the per-protocol analyses (B = 679.16, p = .06). No significant intervention effects were observed for self-reported physical activity, physical fitness, and cognition. Significant moderation of the intervention effect by APOE was observed for learning and verbal memory. Stratification by genotype showed mixed results. In the controls, a relative improvement in performance on the learning index is observed only in those with ApoE-ε4 (B = − 0.90, p = .01, d = 0.59) (Table 9, Appendix). Similar to the ITT analyses, significant effect moderation by sex was observed for the measure of gait speed. The results of the secondary outcomes correspond to those of the ITT analyses (Tables 10 and 11, Appendix). A description of the per-protocol analyses can be found in the Appendix.
Responder analyses of those who achieved a 35% increase in daily physical activity
As a significant number of people in the intervention group did not reach the intended improvement in physical activity of 35%, we performed additional analyses comparing participants who achieved an increase in physical activity of ≥ 35% (N = 35), to those who showed no increase in physical activity or less than 35% (N = 45). Those who achieved the intended improvement in physical activity of at least 35% were on average younger (M = 67.87, SD = 8.06; M = 71.67, SD = 9.09; p = .05) and had a higher level of cognitive functioning at baseline (Global cognition score M = 1.03, SD = 2.75; M = − 0.87, SD = 3.04, p = .01) than those who did not achieve a 35% improvement in physical activity.
When comparing responders to non-responders, analyses show significant improvements in physical fitness and cognition. An increase in physical activity of ≥ 35% over 9 months resulted in an improvement of aerobic capacity (B = 24.59, p = .02, d = 0.26) and gait speed (B = − 0.29, p = .02, d = 0.24). Balance, muscle strength, and self-reported level of physical activity were unaffected by the 35% increase in physical activity (B = − 0.45, P value = .23; B = 0.18, p = .11 and B = 2.70, p = .77; B = 2.70, p = .77, respectively). In addition, we find improvements in global cognition (B = 0.65, p = .002, d = 0.23), executive functioning (B = 0.72, p < .001, d = 0.30), and verbal memory (B = 0.23, p = .03, d = 0.23) compared to those that showed no increase or an increase in physical activity of less than 35%, in the fully adjusted models. Learning was unaffected by an increase in physical activity (B = 0.17, p = .27) (Table 6).
Secondary outcomes—indicators of mental and physical health
An increase in physical activity of ≥ 35% at 9 months decreased the number ADL limitations (B = − 0.13, p = .02, d = 0.24). No differences were observed in experienced frailty (B = − 0.01, p = .18), mental health (B = 0.79, p = .55), or depressive symptoms (B = − 0.86, p = .28) (Table 7).
Secondary outcomes—serum concentrations assessed after 6 months (T1)
At 6 months, serum concentrations of biomarkers of diabetes (insulin, IGF-I) and lipid metabolism (total cholesterol, HDL-cholesterol, and triglycerides) were not significantly affected by the increase in physical activity (Table 8).
Effect moderation by APOE
The significant interaction between the 35% increase in physical activity and APOE on executive functioning (B = 1.22, p = .003, d = 0.51) indicates that increasing physical activity with ≥ 35% leads to greater improvement in executive functioning for those with ApoE-ε4 (B = 1.50, p = .003, d = 0.63) than for non-carriers (B = 0.41, p = .04, d = 0.17). No significant effect moderation by APOE was observed for global cognition, learning, and verbal memory. Stratification by genotype shows significant effects of a ≥ 35% increase in physical activity on gait speed, global cognition, learning, and verbal memory only in ApoE-ε4 non-carriers and shows a large variance in the smaller group of ApoE-ε4 carriers. Figure 4 shows the effects of a ≥ 35% increase in physical activity, stratified by ApoE-ε4. Of note is that overall improvements in cognitive functioning were more robust and consistent in non-carriers.
Forest plot of overall effect sizes of those who showed an increase in physical activity of at least 35% versus those who showed no increase in daily physical activity or an increase that was lower than 35% of their original level of physical activity at 9 months, stratified by genotype. Effect sizes are represented by Cohen’s d
Discussion
The ITT analyses of primary outcomes show that the COACH method, aimed at the promotion of low-to-moderate physical activity, increases the level of physical activity of healthy elderly. No significant effects of the intervention are seen on self-reported physical activity, physical fitness, and cognition. Although the indices of self-reported physical activity, physical fitness, and cognition in the intervention group improve over time, a similar trend is seen in the controls. Stratification by sex shows opposing effects of the intervention in gait speed for men (lower gait speed) and women (higher gait speed) relative to controls. The secondary outcomes show a decrease in the number of ADL limitations in the intervention group and a relative improvement in the mental health of those in the control group. The intervention did not affect serum levels or measures of frailty or depression.
Additional responder analyses, focusing on the group that achieved the intended exposure of an increase in physical activity of ≥ 35%, reveal that this group showed significant improvements in aerobic capacity and gait speed as well as verbal memory, executive functioning, and global cognition, compared to those who did not. The responder group did not show any significant improvements in the measures of balance, muscle strength, learning, frailty, mental health, serum levels, or depressive symptoms.
The null results of the ITT analyses of the intervention effect on cognition are at odds with the results from several meta-analyses that indicate that physical activity interventions have modest positive effects on cognition [6,7,8,9,10]. However, these trials show considerable heterogeneity in intervention type, outcome measures, and population attributes, which has lead several authors to conclude the experimental evidence is inconsistent or insufficient [11,12,13,14]. We hypothesize that the overall intensity of the promoted physical activity behaviors in the intervention group (low-to-moderate) in the present study was not high enough to achieve positive effects on physical fitness or cognition, relative to the active control group. Moderator analyses indicate that intensity and training duration are important effect moderators across studies, and the most successful interventions include the promotion of aerobic exercise of at least moderate intensity [7, 8, 11, 12, 15, 16, 100, 101].
The improvement in mental health observed in the control condition cannot be attributed to group differences in mental health score at baseline and might therefore be speculatively interpreted as a beneficial effect of the stretching exercises. A review of previous trials investigating the effects of yoga, a combination between stretching exercises and regulation of respiration, on mental health, has reported similar improvements in perceived stress and mental health [102]. However, this hypothesis remains to be confirmed in an independent study. While physical activity has been found to improve mental health in multiple studies, we do not see evidence for that in the intervention group.
The positive effects of the responder analyses on physical fitness and cognition suggest a threshold effect and substantiate the role of intensity and duration of physical activity behavior as important moderators of success. Based on the current findings, it remains unclear whether the effects of moderate-intensity aerobic exercise could also be achieved by equal energy expenditure during prolonged low-to-moderate physical activity. In addition, studies with a control condition with no contact or lifestyle education only more often resulted in significant intervention effects, whereas the presence of an active control or social group led to positive but no longer significant effects of the intervention, compared to controls [8].
The significant increase in average number of steps per day following the intervention was not reflected in a significant change in the self-reported physical activity score. Previous comparisons have found moderate correlations between pedometer assessments and self-reported activity (r = 0.33) [71]. In the present study, we found that both steps and self-reported physical activity increased after the intervention, but the correlation between both measures was lower than expected based on the literature (r = 0.24). Self-reported energy expenditure has been shown to be more strongly associated with psychological variables, like depression, attitudes toward physical activity, self-efficacy, and locus of control, than with health and anthropometric measures that predict engagement in physical activity [70]. In addition, the use of self-report questionnaires is more sensitive to response and recall bias, compared to more direct means of measurement. Self-report measures of physical activity might, therefore, also reflect more stable participant characteristics that are less likely to change as a consequence of a physical activity intervention.
Compared to controls and their male counterparts, the women in the intervention group show significantly more gain in average gait speed. This sex difference in the effect of the intervention on gait speed was not reported previously in the literature. Our finding could have been the result of regression to the mean or a higher compliance with the intervention in women. However, none of the other primary outcome measures suggested a difference in compliance between men and women. The relevance of this finding remains to be confirmed.
Looking at effect modification by APOE, the effects of an increase in physical activity of ≥ 35% on cognition are most robust and consistent in ApoE-ε4 non-carriers. However, a significant group-by-genotype interaction indicates that a ≥ 35% increase in physical activity leads to greater improvement in executive functioning for ApoE-ε4 carriers. This finding is in line with the idea that protective associations between physical activity and brain health might be more pronounced in ApoE-ε4 carriers than in non-carriers [32, 37, 38, 40, 103,104,105,106,107].
The increase in physical activity following the intervention did not lead to an increase in the level of circulating IGF-I or to a significant improvement in the biomarkers of the cardiovascular risk profile, the number of depressive symptoms, and levels of experienced frailty and mental health as expected based on the literature [108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123]. We hypothesize that the power, duration, and intensity of the present study were insufficient to detect the expected protective effects. The assessment of the serum biomarkers, in particular, might have been too early in the intervention to detect any effects. Observational data from epidemiological studies indicate that moderate physical activity, when sufficiently sustained, can slow the age-related progression of frailty and depressive symptoms [110,111,112,113,114, 122] and mitigation of cardiovascular risk factors and diabetes mellitus largely depends on sustained regular physical activity throughout the lifespan [124].
The strength of our trial is the accessibility of the intervention for those vulnerable to accelerated cognitive decline. Dropout rates were under 25%. Comprehensive measurement led to the detection of modest changes in a wide range of functions. The effects of an improvement in physical activity of ≥ 35% on cognition are small (Cohen’s d 0.17–0.30) but span a wide range of highly relevant cognitive functions.
Limitations
The present trial has some limitations. First, the number of participants in the control group that completed the study was lower than indicated in the power calculations. As a consequence, the power might have been insufficient to detect the expected effects and effect estimates might be biased. Stratified analyses should be regarded as exploratory. Second, the number of participants in the intervention group that achieved the intended improvement in physical activity was too low, suggesting the intervention was feasible but not effective enough to establish a significant change in physical activity in most of the participants. Third, converting non-ambulatory activities to steps, according to the criteria of Tudor-Locke and Basset [64, 65, 125], might have introduced subjectivity. Fourth, the duration of pedometer use was not standardized across groups. The difference in the length of the registration period might have led to an overestimation of the levels of physical activity in the control group, due to reactivity in the first week of measurement [67]. Lastly, because of the long pre-dementia stage of neuropathological changes, we cannot fully exclude bias by reverse causation, in that those with the highest level of cognitive functioning might profit more from exercise counseling, than those with lower levels of cognitive functioning at baseline.
Conclusion
In summary, although not all participants achieved the intended improvement in physical activity, the tailored intervention was easily accessible and well appreciated in a group of elderly with low levels of physical activity. The ITT analyses did not show intervention effects on physical fitness or cognition. Responder analyses suggest benefits on physical fitness and cognition for those who managed to achieve an increase in physical activity ≥ 35% over 9 months. In light of the potential benefits of physical activity on physical fitness and cognitive functioning, we highly recommend an ambitious approach towards the prevention of cognitive decline and dementia by stimulating sustained engagement in moderate to high levels of physical activity, over the course of the lifetime. Future studies could optimize intervention potential by aiming at a sustained increase in physical activity of ≥ 35% in a population of “at risk” individuals defined by various health parameters including age, level of physical activity, and genetic susceptibility. This study also reveals several difficulties in physical activity trials, which are difficult to blind for participants and researchers. Intervention potential could be further enhanced by replacing the active control condition with a no-contact or waitlist control condition and further standardization of the assessment of physical activity by means of actigraphy or accelerometry.
Availability of data and materials
All data collected for the study are under embargo until 2024, unless permission is granted by the principal investigator ES. After the expiration of the embargo access to the deidentified participant data, informed consent, study protocol, and a data dictionary defining each field can be requested via the principal investigator. Access to the data will be granted after approval of the research proposal, with a signed data access agreement.
Abbreviations
- 15 WT:
-
15 Word test
- ADL:
-
Activities of daily living
- APOE :
-
Apolipoprotein gene
- ApoE-ε4:
-
Apolipoprotein E ε4 allele
- BDNF:
-
Brain-derived neurotropic factor
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CES-D:
-
Centre for Epidemiological Studies depression scale
- DNA:
-
Deoxyribonucleic acid
- HDL:
-
High-density lipoprotein
- IGF-I:
-
Insulin-like growth factor I
- ITT:
-
Intention to treat
- LLT:
-
Location learning test
- MMSE:
-
Mini-Mental Status Examination
- PA:
-
Physical activity as measured in daily number of steps taken
- PARQ:
-
Physical Activity Readiness Questionnaire
- PASE:
-
Physical activity scale for the elderly
- SPPB:
-
Short physical performance test
- SD:
-
Standard deviation
- TMT:
-
Trail making test
- TUG:
-
Timed Up and Go Test
References
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46.
Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera M. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3448908&tool=pmcentrez&rendertype=abstract. cited 2015 Mar 25.
Prakash RS, Voss MW, Erickson KI, Kramer AF. Physical activity and cognitive vitality. Annu Rev Psychol. 2015;66(1):769–97. Available from: https://doi.org/10.1146/annurev-psych-010814-015249.
Floud S, Simpson RF, Balkwill A, Brown A, Goodill A, Gallacher J, et al. Body mass index, diet, physical inactivity, and the incidence of dementia in 1 million UK women. Neurology. 2020;94(2):e123–32.
Sabia S, Dugravot A, Dartigues J-F, Abell J, Elbaz A, Kivimäki M, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. Bmj. 2017;2709(June):j2709. Available from: https://doi.org/10.1136/bmj.j2709.
Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Front Neuroendocrinol. 2017;46(March):71–85.
de Asteasu MLS, Martínez-Velilla N, Zambom-Ferraresi F, Casas-Herrero Á, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–34. Available from: https://doi.org/10.1016/j.arr.2017.05.007.
Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-Analysis. Br J Sports Med. 2018;52(3):154–60.
Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, et al. Effect of Tai Chi on cognitive performance in older adults: systematic review and meta-analysis. J Am Geriatr Soc. 2014;62(1):25–39. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24383523. cited 2019 Nov 29.
Wu Y, Wang Y, Burgess EO, Wu J. The effects of Tai Chi exercise on cognitive function in older adults: a meta-analysis. J Sport Heal Sci. 2013;2(4):193–203. Available from: https://www.sciencedirect.com/science/article/pii/S2095254613000720. cited 2019 Nov 29.
Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2015;2015(4):CD005381.
van Uffelen JGZ, Chin A Paw MJM, Hopman-Rock M, van Mechelen W. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18:486–500.
Snowden M, Steinman L, Mochan K, Grodstein F, Prohaska TR, Thurman DJ, et al. Effect of exercise on cognitive performance in community-dwelling older adults: Review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21438861. cited 2019 Nov 29.
Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;16(1):12–31. Available from: https://doi.org/10.1016/j.arr.2014.05.002.
Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019;51:1242–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31095081. cited 2019 Oct 21.
Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(2):CD005381. https://doi.org/10.1002/14651858.CD005381.pub2. Update in: Cochrane Database Syst Rev. 2008;(3):CD005381. PMID: 18425918.
Baker LD, Skinner JS, Craft S, Sink KM, Montine T, Hansen A, et al. O5–04–05: Aerobic exercise reduces phosphorylated tau protein in cerebrospinal fluid in older adults with mild cognitive impairment. Alzheimers Dement. 2015;11(7S_Part_7):P324–P324.
Guiney H, Machado L. Benefits of regular aerobic exercise for executive functioning in healthy populations. Psychon Bull Rev Springer. 2013;20:73–86. Available from: https://doi.org/10.3758/s13423-012-0345-4. cited 2021 Jun 14.
Langlois F, Vu TTM, Chassé K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol Ser B Psychol Sci Soc Sci. 2012;68(3):400–4. Available from: https://academic.oup.com/psychsocgerontology/article/68/3/400/557671.
Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325(3):147–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2052059. cited 2018 Jun 14.
Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132(4):612–28.
Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46(4):667–75. https://doi.org/10.1161/01.HYP.0000184225.05629.51. Epub 2005 Sep 12. PMID: 16157788.
Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Scand J Med Sci Sport. 2006;16(6):470–470. Available from: https://doi.org/10.1111/j.1600-0838.2006.00610_1.x.
Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose–response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–400. Available from: https://doi.org/10.1093/ije/dyr112. cited 2018 Jun 14.
U.S. Department of Health and Human Services. 2008 Physical activity guidelines for Americans. Pres Counc Phys Fit Sport Res Dig. 2008;9(4):1–8. Available from: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/previous-guidelines/2008-physical-activity-guidelines.
Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. Available from: https://pubmed.ncbi.nlm.nih.gov/17762377/. cited 2020 Dec 14.
Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70.
McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older Adults: a brief review. Brain Behav Immun. 2004;18:214–20 (Academic Press).
Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur studies of successful aging. J Am Geriatr Soc. 2003;51(8):1125–30. https://doi.org/10.1046/j.1532-5415.2003.51380.x (cited 2020 Dec 14).
Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17765329. cited 2019 Oct 21.
Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21(1):21–4.
Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(November):705–11.
Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38(6):1766–73. Available from: http://ahajournals.org. cited 2021 May 31.
Etnier JL, Nowell PM, Landers DM, Sibley BA. Brain Res Rev. 2006;52:119–30.
Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30:493–503. Available from: https://pubmed.ncbi.nlm.nih.gov/20041290/. cited 2021 Apr 6.
Kramer AF, Hahn S, McAuley E, Cohen NJ, Banich MT, Harrison C, et al. Exercise, aging, and cognition: healthy body, healthy mind? In: Rogers WA, Fisk AD (Eds.). Human Factors Interventions for the Health Care of Older Adults (1st ed.). CRC Press; 2001. https://doi.org/10.1201/b12459.
Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, et al. Cognitive performance in older women relative to ApoE-ε4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(35):199–207.
Niti M, Yap K-B, Kua E-H, Tan C-H, Ng T-P. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20(2):237–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18190728. cited 2015 Feb 27.
Shih IF, Paul K, Haan M, Yu Y, Ritz B. Physical activity modifies the influence of apolipoprotein E ε4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimers Dement. 2018;14(1):1–9.
Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population based study. JCell MolMed. 2008;12(6):2762–71.
Schiepers OJG, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Molecular Psychiatry. 2012;17:315–24. Available from: https://doi.org/10.1038/mp.2010.137 Nature Publishing Group.
Schuit AJ, Feskens EJM, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–7.
Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15(1):99–108. Available from: https://www.dialogues-cns.org. cited 2020 Dec 14.
Chieffi S, Messina G, Villano I, Messina A, Esposito M, Monda V, et al. Exercise influence on hippocampal function: Possible involvement of Orexin-A. Front Physiol. 2017;8:85. https://doi.org/10.3389/fphys.2017.00085. PMID: 28261108; PMCID:PMC5306252.
Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. A 35-year odyssey. Am Psychol. 2002;57(9):705–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12237980).
Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305–12. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1463134&tool=pmcentrez&rendertype=abstract. cited 2015 Feb 2.
Blaauwbroek R, Bouma MJ, Tuinier W, Groenier KH, De Greef MHG, Meyboom-De Jong B, et al. The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Support Care Cancer. 2009;17(8):1041–8.
Sprenger SR, Houët BJ, de Greef MHG. De COACH methode : State of the Art Doelgroep : werknemers De COACH methode : State of the Art Doelgroep : werknemers. 2011.
Houët BJ, Sprenger SR, de Greef MHG. Eindevaluatie COACH methode bij COPD patiënten in Gezondheidscentrum Slochteren. 2012.
Sprenger SR, de Greef MHG. DRASTIC: Een evidence based methode om patiënten met een chronische aandoening meer te stimuleren om meer te bewegen. 2006.
Hospes G, Bossenbroek L, ten Hacken NHT, van Hengel P, de Greef MHG. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: Results of an exercise counseling program. Patient Educ Couns. 2009;75(2):274–8. Available from: https://doi.org/10.1016/j.pec.2008.10.005.
Mutsaerts MAQ. Randomized trial of a lifestyle program in obese infertile women. Ned Tijdschr Geneeskd. 2016;160(20):D916. Available from: https://pubmed.ncbi.nlm.nih.gov/27192672/. cited 2021 Oct 4.
Altenburg WA, Ten Hacken NHT, Bossenbroek L, Kerstjens HAM, De Greef MHG, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: A randomized controlled trial. Respir Med. 2015;109(1):112–21. Available from: https://pubmed.ncbi.nlm.nih.gov/25499548/. cited 2021 Oct 4.
Sleegers K, de Koning I, Aulchenko YS, van Rijn MJE, Houben MPWA, Croes EA, et al. Cerebrovascular risk factors do not contribute to genetic variance of cognitive function. The ERF study. Neurobiol Aging. 2007;28(5):735–41.
Richardson S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J Am Geriatr Soc. 1991;39(2):142–8. Available from: https://doi.org/10.1111/j.1532-5415.1991.tb01616.x.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”, A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Thomas S, Reading J, sport RS-C journal of, 1992 undefined. Revision of the physical activity readiness questionnaire (PAR-Q). psycnet.apa.org. Available from: https://psycnet.apa.org/record/1993-24047-001. cited 2022 Feb 28
Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):80. Available from: http://www.ijbnpa.org/content/8/1/80.
Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296–304. Available from: http://jama.jamanetwork.com/article.aspx?articleid=209526. cited 2014 Dec 15.
Calfas KJ, Long BJ, Sallis JF, Wooten WJ, Pratt M, Patrick K. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med (Baltim). 1996;25(3):225–33. Available from: http://www.sciencedirect.com/science/article/pii/S009174359690050X. cited 2015 Feb 24.
Croteau KA. A preliminary study on the impact of a pedometer-based intervention on daily steps. Am J Health Promot. 18(3):217–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14748310. cited 2015 Apr 10
Swartz AM, Strath SJ, Bassett DR, Moore JB, Redwine BA, Groër M, et al. Increasing daily walking improves glucose tolerance in overweight women. Prev Med (Baltim). 2003;37(4):356–62.
Tudor-Locke CE, Myers AM, Bell RC, Harris SB, Wilson RN. Preliminary outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type 2 diabetes. Patient Educ Couns. 2002;47(1):23–8.
Tudor-Locke C, Bassett DRB. How Many Steps/Day Are Enough? Sport Med. 2004;34(1):1–8. Available from: https://doi.org/10.2165/00007256-200434010-00001/fulltext.html.
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81.
Welk G. Use of accelerometry-based activity monitors to assess physical activity. In: Physical activity assessments for health-related research. 2002. p. 125–42.
Clemes SA, Deans NK. Presence and duration of reactivity to pedometers in adults. Med Sci Sports Exerc. 2012;44(6):1097–101.
Verhage F. Intelligence and age in a dutch sample. Human Dev. 1965;8. Available from: https://about.jstor.org/terms. cited 2020 May 8
Fokkema T, Kooiman TJ, Krijnen WP, VAN DER Schans CP, DE Groot M. Reliability and validity of ten consumer activity trackers depend on walking speed. Med Sci Sports Exerc. 2017;49(4):793–800. https://doi.org/10.1249/MSS.0000000000001146.
Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc. 2009;41(7):1392–402.
Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Med. 2002;32(12):795–808. Available from: https://pubmed.ncbi.nlm.nih.gov/12238942/. cited 2022 Feb 13.
Schuit, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50(5):541–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9180646. cited 2015 Apr 1.
Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G. Endpoints for trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol. 2008;7:436–50. Available from: https://europepmc.org/article/med/18420157. cited 2021 May 11.
Saan RJ, Deelman BG. De 15-Woordentests A en B. Groningen: Een voorlopige handleiding; 1986.
Reitan RM. V alidity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–6. https://doi.org/10.2466/pms.1958.8.3.271. cited 2015 Apr 2.
Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–62.
Barelds D. Groninger Intelligentie Test 2 (GIT-2). In: Resing WCM, editor. Handboek intelligentietheorie en testgebruik. Pearson; 2015.
Wechsler D. Wechsler adult intelligence scale. 3rd ed. (WAIS-III); 1997.
Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio: The Psychological Corporation; 1987.
Bucks RS, Willison JR. Development and validation of the Location Learning Test (LLT): A test of visuo-spatial learning designed for use with older adults and in dementia. Clin Neuropsychol. 1997;11(April 2015):273–86.
Kessels RPC, Nys GMS, Brands AMA, van Zandvoort MJE. De Location Learning Test als maat voor het ruimtelijk geheugen: Bruikbaarheid van een nieuwe afnameprocedure en normgegevens. / The Location Learning Test as a measure of spatial memory: Applicability of a modified administration procedure and no. 2004.
Hoeymans N, Wouters ER, Feskens EJ, van den Bos GA, Kromhout D. Reproducibility of performance-based and self-reported measures of functional status. J Gerontol A Biol Sci Med Sci. 1997;52(6):M363–8. Available from: http://biomedgerontology.oxfordjournals.org/content/52A/6/M363.short. cited 2015 Apr 2.
Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51(6):321–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5423802. cited 2016 Feb 8.
Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18489802. cited 2016 Feb 5.
Härkönen R, Harju R, Alaranta H. Accuracy of the Jamar Dynamometer. J Hand Ther. 1993;6(4):259–62. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0894113012803267).
Tappen RM, Roach KE, Buchner D, Barry C, Edelstein J. Reliability of physical performance measures in nursing home residents with Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1997;52(1):M52–5.
Rikli, Roberta E, Jones CJ. The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act. 1998;6:363–75.
van der Zee KI, Karen I, Sanderman R. Het meten van de algemene gezondheidstoestand met de RAND-36: een handleiding. Noordelijk Centrum voor Gezondheidsvraagstukken, Rijksuniversiteit Groningen. 1993.
Beekman ATF, Deeg DJH, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27(1):231–5.
Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
Laan W, Zuithoff NPA, Drubbel I, Bleijenberg N, Numans ME, De Wit NJ, et al. Validity and reliability of the Katz-15 scale to measure unfavorable health outcomes in community-dwelling older people. J Nutr Health Aging. 2014;18(9):848–54. Available from: https://pubmed.ncbi.nlm.nih.gov/25389963/. cited 2022 Feb 14.
Schmand B, Groenink SC, Van Den Dungen M. Letter fluency: psychometric properties and Dutch normative data. Tijdschr Gerontol Geriatr. 2008;39(2):64–76. Available from: https://pubmed.ncbi.nlm.nih.gov/18500167/. cited 2022 Feb 15.
Dikmen SS, Heaton RK, Grant I, Temkin NR. Test-retest reliability and practice effects of expanded Halstead-Reitan Neuropsychological Test Battery. J Int Neuropsychol Soc. 1999;5(4):346–56. Available from: https://www.unboundmedicine.com/medline/citation/10349297/Test_retest_reliability_and_practice_effects_of_expanded_Halstead_Reitan_Neuropsychological_Test_Battery_. cited 2022 Feb 15.
Bouma A, Johanna M. Handboek neuropsychologische diagnostiek. 2012.
Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23790725. cited 2019 Oct 21.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32 (American College of Physicians).
Cohen J. Statistical power analysis for the behavioral sciences (2nd edition). Hillsdale: Lawrence Earlbaum Associates; 1988. p. 286.
Statistical Package for the Social Sciences. Armonk, NY: IBM Corp.
Stata Statistical Software. College Station TX: StataCorp LLC; 2019.
Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–7.
Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults. Psychol Sci. 2003;14:125. Available from: http://pss.sagepub.com/content/14/2/125.full.pdf+html).
Chu AHY, Koh D, Moy FM, Müller-Riemenschneider F. Do workplace physical activity interventions improve mental health outcomes? Occup Med (Chic Ill). 2014;64(4):235–45. Available from: https://academic.oup.com/occmed/article/64/4/235/1465341. cited 2022 Apr 16.
Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–51.
Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–87.
Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(20):772–7.
Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69(5):636–43.
Smith JC, Nielson KA, Woodard JL, Seidenberg M, Verber MD, Durgerian S, et al. Does physical activity influence semantic memory activation in amnestic mild cognitive impairment? Psychiatry Res - Neuroimaging. 2011;193(1):60–2.
Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med. 2001;31:1033–62. Available from: https://pubmed.ncbi.nlm.nih.gov/11735685/. Adis International Ltd; cited 2021 Jan 26.
Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med. 2007;167:999–1008. Available from: https://pubmed.ncbi.nlm.nih.gov/17533202/. cited 2021 Jan 26.
Catalan-Matamoros D, Gomez-Conesa A, Stubbs B, Vancampfort D. Exercise improves depressive symptoms in older adults: an umbrella review of systematic reviews and meta-analyses. Psychiatry Res. 2016;244:202–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0165178116301809. Elsevier Ireland Ltd; . cited 2021 Feb 1.
Herring MP, Puetz TW, O’Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101–11.
Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175(7):631–48. Available from: https://doi.org/10.1176/appi.ajp.2018.17111194. cited 2021 Feb 1.
Morres ID, Hatzigeorgiadis A, Stathi A, Comoutos N, Arpin-Cribbie C, Krommidas C, et al. Aerobic exercise for adult patients with major depressive disorder in mental health services: a systematic review and meta-analysis. Depress Anxiety. 2019;36:39–53. Available from: https://pubmed.ncbi.nlm.nih.gov/30334597/. Blackwell Publishing Inc. cited 2021 Feb 1.
Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Ann Behav Med. 2010;39(2):128–38. https://doi.org/10.1007/s12160-010-9172-x.
Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. Available from: https://pubmed.ncbi.nlm.nih.gov/26978184/. cited 2021 Feb 1.
Pate RR. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA J Am Med Assoc. 1995;273(5):402–7. Available from: https://pubmed.ncbi.nlm.nih.gov/7823386/. cited 2021 Jan 26.
King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91(10):2596–604. Available from: https://pubmed.ncbi.nlm.nih.gov/7743622/. cited 2021 Jan 26.
Ramakrishnan R, Doherty A, Smith-Byrne K, Rahimi K, Bennett D, Woodward M, et al. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank Cohort study. Paluch A, editor. PLoS Med. 2021;18(1):e1003487. Available from: https://doi.org/10.1371/journal.pmed.1003487 . cited 2021 Jan 26.
Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81(10):3492–7. Available from: https://pubmed.ncbi.nlm.nih.gov/8855791/. cited 2021 Jan 26.
Lazarus NR, Izquierdo M, Higginson IJ, Harridge SDR. Exercise deficiency diseases of ageing: the primacy of exercise and muscle strengthening as first-line therapeutic agents to combat frailty. J Am Med Direct Assoc. 2018;19:741–3. Available from: http://www.jamda.com/article/S1525861018302317/fulltext. cited 2021 Feb 1.
Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017;46(3):401–7. Available from: https://pubmed.ncbi.nlm.nih.gov/28064172/. cited 2021 Feb 1.
Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: Evidence from the english longitudinal study of ageing. PLoS One. 2017;12(2):e0170878. https://doi.org/10.1371/journal.pone.0170878. PMID: 28152084; PMCID: PMC5289530.
Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, Huang E, Dale W, Waite L, et al. The relationship between physical activity and frailty among U.S. older adults based on hourly accelerometry data. J Gerontol Ser A. 2018;73(5):622–9. Available from: https://academic.oup.com/biomedgerontology/article/73/5/622/4587480. cited 2021 Feb 1.
Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11(11):e005263.
Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? for adults. Int J Behav Nutr Phys Act. 2011;8(1):1–17. Available from: http://www.presidentschallenge.org/challenge/active/index.shtml. cited 2021 May 31.
Acknowledgements
The authors would like to thank all participants for their invaluable contribution to this study. We would like to express our gratitude to all people involved in the process of participant recruitment and data collection including research assistants, exercise counselors, health care professionals, and senior service organizations in the municipalities of Amsterdam and Rucphen. Participant recruitment was further extended with the help of Alzheimer Nederland, and with the professional assistance of Hersenonderzoek.nl (www.hersenonderzoek.nl). Hersenonderzoek.nl is funded by ZonMw-Memorabel (project number 73305095003), a project in the context of the Dutch Deltaplan Dementie, Gieskes-Strijbis Foundation, the Alzheimer’s Society in the Netherlands, and Brain Foundation Netherlands. The authors are grateful to the municipality of Rucphen for their assistance in participant recruitment and the facilitation of the local research facility. We would like to thank Star-SHL, Atal Medial, and Amsterdam UMC for the collection and handling of the serum samples and the Department of Clinical Chemistry of the Amsterdam UMC, for the assessment of APOE genotype, cholesterol, HDL-cholesterol, triglycerides, insulin, and insulin-like growth factor 1. Lastly, we are indebted to Prof. Dr. M. de Greef and the CBO for the opportunity to apply the COACH method and benefit from their experience and expertise.
Funding
The work described here is funded in the Netherlands by the Organization for the Health Research and Development (ZonMw) as part of the project Memorabel (Dementia research and innovation program—grant 733050303) and the European Union’s Horizon 2020 Research and Innovation program as part of the CoSTREAM project (Common mechanisms and pathways in Stroke and Alzheimer’s disease www.costream.eu, grant 667375). Additional funding is provided by the Vrije Universiteit Amsterdam and the Erasmus Medical Center and Erasmus University Rotterdam. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing and submission of the report.
Author information
Authors and Affiliations
Contributions
All authors engaged in the conception and design of the study. MdG developed the COACH method and trained the exercise counselors in collaboration with the CBO. Participant recruitment and data collection were organized by SG. SG analyzed the data and wrote the first draft of the report, supervised by CvD and MD. JB and SG accessed and verified the data. All authors had full access to all the data in the study, provided feedback on the manuscript, and share the final responsibility for the decision to submit the report for publication. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Participants gave written informed consent for both parts of the study. The study was conducted in compliance with the Declaration of Helsinki. The Medical Ethics Committee of the Amsterdam UMC, METc VUmc, approved the research protocol (2015.233). The trial protocol is registered at the Dutch Trial Register, registration ID: NL5675.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Per-protocol analyses
Primary outcomes
The per-protocol analyses showed no significant effects of the intervention on the average number of steps per day (B = 679.16 [95% CI − 25.99 to 1384.31]; p = .06).
No effect of the intervention on self-reported physical activity or measures of physical fitness and cognition was observed (Table 9). Significant effect moderation by sex was observed for the measure of gait speed (B = − 0.84 [95% CI − 1.42 to − 0.27]; p = .004). Stratification by sex showed neither men nor women in the intervention group experienced significant changes in gait speed, relative to controls. Significant effect moderation by APOE genotype was observed for measures of verbal memory (B = − 0.60 [95% CI − 1.14 to − 0.06]; p = .03) and learning (B = − 0.89 [95% CI − 1.71 to − 0.08]; p = .03). Stratification by genotype showed a greater improvement in learning in the control group in ApoE-ε4 carriers only (B = − 0.90 [95% CI − 1.62 to − 0.18]; p = .01, d = 0.59). Stratification did not yield significant intervention effects on verbal memory in either group.
Secondary outcomes—indicators of physical and mental health
The per-protocol analyses showed an effect of the intervention on the number of experienced ADL limitations (B = − 0.13, [95% CI − 0.26 to − 0.01], p = .03, d = 0.35). The TOPICS-MDS mental health score increases over time in the control group and remains constant in the intervention group (B = − 2.73, [95% CI − 5.46 to − 0.002], p = .05, d = 0.22) (Table 10).
Secondary outcomes—serum concentrations assessed at 6 months (T1)
Serum concentrations of biomarkers of diabetes (insulin, IGF-1) and lipid metabolism (total cholesterol, HDL-cholesterol, and triglycerides) assessed at 6 months were not significantly affected by the intervention (Table 11).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Galle, S.A., Deijen, J.B., Milders, M.V. et al. The effects of a moderate physical activity intervention on physical fitness and cognition in healthy elderly with low levels of physical activity: a randomized controlled trial. Alz Res Therapy 15, 12 (2023). https://doi.org/10.1186/s13195-022-01123-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-022-01123-3