Abstract
Background
Although few studies have shown that risk factors for Alzheimer’s disease (AD) are associated with cognitive decline in AD, not much is known whether the impact of risk factors differs between early-onset AD (EOAD, symptom onset < 65 years of age) versus late-onset AD (LOAD). Therefore, we evaluated whether the impact of Alzheimer’s disease (AD) risk factors on cognitive trajectories differ in EOAD and LOAD.
Methods
We followed-up 193 EOAD and 476 LOAD patients without known autosomal dominant AD mutation for 32.3 ± 23.2 months. Mixed-effects model analyses were performed to evaluate the effects of APOE ε4, low education, hypertension, diabetes, dyslipidemia, and obesity on cognitive trajectories.
Results
APOE ε4 carriers showed slower cognitive decline in general cognitive function, language, and memory domains than APOE ε4 carriers in EOAD but not in LOAD. Although patients with low education showed slower cognitive decline than patients with high education in both EOAD and LOAD, the effect was stronger in EOAD, specifically in frontal-executive function. Patients with hypertension showed faster cognitive decline than did patients without hypertension in frontal-executive and general cognitive function in LOAD but not in EOAD. Patients with obesity showed slower decline in general cognitive function than non-obese patients in EOAD but not in LOAD.
Conclusions
Known risk factors for AD were associated with slower cognitive decline in EOAD but rapid cognitive decline in LOAD.
Similar content being viewed by others
Background
The characteristics of Alzheimer’s disease (AD) differ according to the age of onset in several aspects [1]. Early-onset AD (EOAD) is defined as having an age of onset younger than 65 years old and comprises approximately 5–6% of all AD cases [2]. EOAD patients are reported to show a more atypical (non-amnestic) presentation than do late-onset AD (LOAD) patients, with more hippocampal sparing or posterior cortical atrophy, increased tau burden, and more rapid cognitive decline [3, 4]. Although EOAD differs substantially from LOAD, most AD research is focused on LOAD.
The genetic and environmental risk factors for LOAD have been studied extensively. However, these factors may have different effects on EOAD patients. Apolipoprotein E ε4 (APOE ε4), low education, and vascular risk factors are well-known risk factors for AD development [5, 6]. APOE ε4 is the strongest genetic risk factor for AD, with an odds ratio of approximately 3 in heterozygotes and 9 to 34 in homozygotes compared to individuals with the ε3/ε3 genotype [3, 7]. Low education also increases the risk of dementia. According to the cognitive reserve theory, less educated individuals do not cope well with pathological burden and have a lower threshold for dementia symptoms [8]. It is also well-established that vascular risk factors such as hypertension, diabetes, dyslipidemia, and obesity are associated with AD [9,10,11,12,13].
Despite the well-established effects of AD risk factors, it is controversial whether these risk factors affect the speed of cognitive decline after symptom onset. Controversy exists about whether the APOE polymorphisms are associated with the rate of cognitive decline in AD patients [14,15,16,17]. Some studies showed that APOE ε4 was associated with more rapid cognitive decline [14, 18, 19] while others showed APOE ε4 non-carriers have more rapid cognitive decline [4, 20]. Current evidence indicates that the link between diabetes and the rate of cognitive decline in AD patients is uncertain [21]. Obesity is reported to contribute to cognitive decline by facilitating systemic inflammation [22]. Hypertension is also reported to be associated with cognitive decline in dementia overall [23], but there is limited data in AD specifically. Furthermore, the impact of the aforementioned risk factors on cognitive trajectories according to the age of onset is not well understood, because most longitudinal cohorts consisted of LOAD patients [17, 24, 25].
Therefore, we evaluated the impact of known AD risk factors (APOE ε4, low education, hypertension, diabetes, dyslipidemia, and obesity) on cognitive trajectories in EOAD and LOAD patients. We tested our hypothesis that the detrimental effect of the risk factors on cognitive decline would be stronger in LOAD patients than in EOAD patients.
Methods
Participants
We retrospectively collected 713 AD dementia patients who underwent two or more neuropsychological tests (with at least 1 year interval between each test) and APOE genotyping from 2006 to 2013 in the Memory Clinic at the Samsung Medical Center, Seoul, Korea. All patients were of Korean ethnicity. All patients underwent detailed clinical interviews, neurological examinations, neuropsychological tests, and brain MRI at the time of diagnosis. All of the patients met core clinical criteria for probable AD dementia according to the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria [26]. The patients did not meet other neurodegenerative disease criteria such as those for frontotemporal dementia [27], dementia with Lewy bodies, Parkinson’s disease [28, 29], or subcortical vascular dementia which exhibit severe white matter hyperintensities [30]. We excluded 36 patients who showed stroke or traumatic brain injury that was temporally related to the onset or worsening of cognitive impairment. We also excluded 8 patients who carried causative genetic mutations (PSEN1, PSEN2, or APP). Indications for screening causative mutation were as follows: (1) very early disease onset (< 50 years old), (2) early disease onset (< 60 years old) with two or more affected relatives, or (3) early disease onset (< 60 years old) with one or more affected first-degree relatives with early onset dementia (< 60 years old) [31].
Then, we stratified AD dementia patients according to age of symptom onset based on self-report and/or caregiver-report [32]. The final number of patients included in the analysis was 193 EOAD (onset age < 65 years) and 476 LOAD (onset age ≥ 65 years) patients. The proportion of EOAD (199/669, 28.8%) was larger than known percentage of EOAD among all AD cases (5–6%) [2] since we recruited participants from a referral center. This study was approved by the Institutional Review Board of Samsung Medical Center.
APOE genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the Wizard Genomic DNA Purification kit following the manufacturer’s instructions (Promega, Madison, WI). Two single nucleotide polymorphisms (SNP; rs429358 for codon 112 and rs7412 for codon 158) in the APOE gene were genotyped using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) on a 7500 Fast Real-Time PCR System (Applied Biosystems) according to the manufacturer’s instructions.
Longitudinal follow-up with annual neuropsychological tests
All patients underwent the Seoul Neuropsychological Screening Battery [33, 34] at baseline and one or more times during the follow-up period with at least 1 year interval between each test. The EOAD patients had an average of 3.03 and the LOAD patients had an average of 3.23 longitudinal assessments. Language function was assessed using the Korean version of the Boston Naming Test score (0–60). Visuospatial function was assessed using the Rey Osterrieth Complex Figure Test (RCFT) copy score (0–36). Memory function was assessed by summing the scores of the verbal memory (SVLT recall [0–36], SVLT delayed recall [0–12], and SVLT recognition [0–24]) and visual memory (RCFT immediate recall [0–36], RCFT delayed recall [0–36], and RCFT recognition score [0–12]). Frontal-executive function (0–55) was assessed by summing the scores of the category word generation test (0–20), phonemic word generation test (0–15), and the Stroop color reading test (0–20). General cognition was assessed using the Korean version of the Mini-Mental Status Examination (K-MMSE) (0–30) and the clinical dementia rating sum of boxes (CDR-SB) [35]. These clinical tests were conducted by experienced staffs and supervised by board-certified neuropsychologists.
Statistical analyses
For the comparison of demographic and clinical data between EOAD and LOAD patients, a two-sample t test and Mann-Whitney test were used for continuous variables, and a chi-square test was used for categorical variables.
For all univariable and multivariable analyses, the linear mixed effect model was used, which was adjusted for random intercept, random slope of time, and baseline age. When we estimated models, the cognitive outcome that did not show a normal distribution was analyzed after natural log transformation. We excluded outliers with an absolute standardized residual > 3. We performed a step-by-step approach to identify the risk factors that have differential effects on the cognitive trajectories of EOAD and LOAD.
First, to evaluate the effect of each risk factor on the rate of cognitive decline in EOAD or LOAD patients, we performed univariable analyses for two-way interactions (risk factor*time). We included age, time, risk factors, and risk factors*time for each risk factor.
Second, to evaluate whether the risk factor affected the rate of cognitive decline in EOAD or LOAD patients when other risk factors were controlled for, we performed multivariable analysis for two-way interactions (risk factors*time). In the multivariable analysis, we included age, time, risk factors, and the interaction between risk factors*time for the risk factors that showed significance in univariable analyses (P < .05).
Third, to evaluate whether the risk factors had differential effects on the rate of cognitive decline between EOAD and LOAD patients, we performed univariable analysis for three-way interactions (risk factor*time*group). In the univariable analysis, we included age, time, risk factor, group, two-way interaction effects (risk factor*time, risk factor*group, time*group), and three-way interaction effects (risk factor*time*group) for each risk factor.
Finally, to evaluate whether the risk factors had differential effects on the rate of cognitive decline between EOAD and LOAD patients when other risk factors were controlled, we performed multivariable analysis for three-way interactions (risk factors*time*group). In the final multivariable analyses, we included age, time, risk factors, group, two-way interaction effects (risk factors*times, risk factors*group, time*group), and three-way interaction effects (risk factors*time*group) that showed significance in univariable analyses for three-ways interaction (P < .05).
All reported p values were two-sided, and a p value < .05 was considered to indicate statistical significance. All analyses were performed using SAS software, version 9.4 (SAS Institute, NC, USA) and R version 3.6.1 (R Project for Statistical Computing).
Results
Demographics of participants in the longitudinal study
The demographics of participants included in the longitudinal study are described in Table 1. LOAD patients were significantly less educated than the EOAD patients (74.4% vs. 64.8%, P < .001). LOAD patients had higher percentage of hypertension and diabetes compared with the EOAD patients (47.9% vs. 28.0%, P < .001 and 26.3% vs. 18.7%, P = .037, respectively). However, the two groups did not significantly differ in dyslipidemia, sex, APOE ε4 carrier proportion, or BMI level.
Overall, baseline cognitive performance was not different between the EOAD and LOAD patients as there was no significant difference in K-MMSE score or CDR-SB score.
Although the follow-up durations varied among participants, it did not differ between EOAD (31.1 ± 22.3 months) and LOAD (33.0 ± 23.5 months) at the group level (P = .35).
The effect of risk factors on cognitive decline in EOAD and LOAD
In EOAD, univariable analyses showed that patients with risk factors demonstrated slower cognitive decline than did those without risk factors. Compared to APOE ε4 noncarriers, APOE ε4 carriers had slower cognitive decline in general cognitive function (MMSE, and CDR-SB, P = .009 and 0.019), language (P = .017), memory (P = .011), and frontal-executive function (P = .001). EOAD patients with dyslipidemia showed slower cognitive decline in memory function (P = .011) than did those without dyslipidemia. Patients with hypertension or obesity demonstrated slower cognitive decline in general cognitive function (MMSE, P = .002 and CDR-SB, P = .021) than did those without these comorbidities. EOAD patients with lower education demonstrated slower cognitive decline in memory (P = .029) and frontal-executive function (P < .001) than did those with higher levels of education (Table 2).
In EOAD, multivariable analyses showed that APOE ε4 carriers demonstrated slower cognitive decline in general cognitive function (MMSE and CDR-SB, P = .011 and .010), language (P = .010), memory (P = .027), and frontal-executive function (P = .002) than did APOE ε4 non-carriers. The EOAD patients with higher levels of education demonstrated steeper cognitive decline in frontal-executive function (P < .001) than did those with lower levels of education. EOAD patients with dyslipidemia showed slower cognitive decline in memory function (P = .044) than did those without dyslipidemia. In addition, patients with hypertension (P = .002) or obesity (P = .012) demonstrated slower cognitive decline in general cognitive function than did patients without these comorbidities (Table 3).
In LOAD, univariable analyses showed that patients with vascular risk factors demonstrated more rapid cognitive decline than did those without vascular risk factors. LOAD patients with diabetes showed more rapid decline in memory function (P = .029) and frontal-executive function (P = .026) than did those without diabetes. LOAD patients with hypertension showed rapid decline in frontal-executive function (P = .020) and general cognitive function (MMSE, P = .021). There was no significant impact of APOE ε4 carrier status on general cognitive function, memory, or frontal-executive function. LOAD patients with lower education demonstrated slower cognitive decline in frontal-executive (P = .038) and general cognitive function (MMSE, P = .044). In addition, LOAD patients who were underweight showed rapid cognitive decline in frontal-executive function (P = .007) than did those with normal BMI (Table 2).
In LOAD, multivariable analyses, the patients with higher levels of education demonstrated steeper cognitive decline in frontal and general cognitive function (MMSE, P = .017) than did those with lower levels of education. LOAD patients with diabetes showed steeper decline in memory function (P = .021) and frontal-executive function (P = .032) than did those without diabetes. In addition, patients with hypertension showed steeper decline in frontal-executive function (P = .016) than did those without hypertension. Underweight patients showed rapid cognitive decline in frontal-executive function (P = .006) (Table 3).
Differential effects of risk factors on cognitive decline between EOAD and LOAD patients
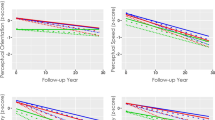
We evaluated whether the risk factors that were found to have significant effects on the rate of cognitive decline in multivariable analyses in each onset age group (P < .05) had differential effects between EOAD and LOAD patients. The results showed that the effects of APOE ε4, hypertension, low education, and obesity on cognitive trajectories were significantly different in EOAD vs. LOAD patients. APOE ε4 carriers showed slower cognitive decline in EOAD but not in LOAD in terms of language (P = .005), memory (P = .011), and general cognitive function (MMSE, P = .026), in which language and memory remained to be significant (P < .05) after Bonferroni correction for four multiple tests (Table 3, Fig. 1A). Although low education associated with slower cognitive decline in both EOAD and LOAD patients, the effect was stronger in EOAD patients, and specifically for frontal-executive function (P = .030) (Table 3, Fig. 1B). Hypertension associated with faster cognitive decline in LOAD patients but not in EOAD patients in frontal-executive (P = .016) and general cognitive function (MMSE, P < .001), in which both remained to be significant (P < .05) after Bonferroni correction for two multiple tests (Table 3, Fig. 1C). Obesity associated with slower decline general cognitive function in EOAD but not in LOAD patients (CDR-SB, P = .022) (Table 3, Fig. 1D).
Discussion
In this longitudinal study, we evaluated the impact of AD risk factors on cognitive trajectory in EOAD vs. LOAD patients. The major findings were as follows: (1) APOE ε4 carriers associated with slower cognitive decline in EOAD but not in LOAD patients. (2) Although low education was associated slower cognitive decline in both EOAD and LOAD patients, the effect was stronger in EOAD patients, specifically for frontal-executive function. (3) Patients with vascular risk factors showed slower cognitive decline in EOAD patients but faster cognitive decline in LOAD patients.
Our first major finding was that in EOAD patients, APOE ε4 noncarriers demonstrated a steeper decline in multiple cognitive domains, namely language, memory, frontal-executive and general cognitive function. Meanwhile, in LOAD patients, there was no significant association between APOE ε4 status and cognitive decline. According to previous studies, there is controversy about the effect of APOE ε4 on rate of cognitive decline in AD. Most published studies used data form the Alzheimer's Disease Neuroimaging Initiative (ADNI) where the cohort consists of predominantly LOAD patients: APOE ε4 accelerated hippocampal atrophy in AD, however, there is not enough evidence for the relationship between APOE ε4 and cognitive decline [16, 36]. In EOAD, there are studies that show APOE ε4 to accelerate [37], decelerate [20], or have no effect [38] on cognitive decline. The controversial results may be due to relatively short follow-up periods and small sample sizes [37,38,39]. Recent studies further showed that the APOE ε4 effect differs according to the cognitive stage [17]. Our current results suggest that the effect of APOE ε4 on the cognitive trajectory of language, memory, and MMSE might differ according to the individual’s age of onset. Our result is in line with our previous report showing that in EOAD APOE ε4 carriers had less severe brain atrophy in the frontal and perisylvian areas compared to APOE ε4 noncarriers while in LOAD APOE ε4 carriers showed more severe brain atrophy in the medial temporal area compared to APOE ε4 noncarriers [3]. The reason why EOAD showed more rapid cognitive decline in the absence of APOE ε4 needs further studies. In attempt to identify genetic risk or cause in these patients, we previously performed whole-exome sequencing in 60 EOAD APOE ε4 non-carriers. However, we found only few pathogenic or likely pathogenic variants that may be associated with dementia [40]. There have been several other reports on whole-exome sequencing among a group of patients with EOAD but no novel risk genes were found [41,42,43]. Collectively, these data suggest that additional and perhaps yet unknown genetic risk factors may be identified by genotyping analyses of larger EOAD cohorts.
Our second major finding was that although patients with higher education demonstrated steeper cognitive decline in both EOAD and LOAD patients, this effect was greater in EOAD patients. The cognitive reserve theory posits that highly educated individuals cope better with AD pathology [8, 44] and do not show dementia symptoms until they have substantial amount of pathological burden in the brain. However, when more educated individuals started to show dementia symptoms, they showed steeper cognitive decline [45]. Likewise, young individuals have greater neural reserve and need greater pathological burden to show dementia symptoms than old individuals [46]. This is supported by pathological studies showing that there were more significant burden of amyloid-ß plaques and neurofibrillary tangles in EOAD compared to LOAD patients [47, 48]. We observed that the impact of cognitive reserve was greater in EOAD compared to LOAD patients, especially in frontal-executive function. This agrees with one previous study showing that AD patients with higher cognitive reserve had better scores on frontal-executive function tests than subjects with lower cognitive reserve [49].
Our third major finding was that EOAD patients without vascular risk factors showed a steeper cognitive decline. EOAD patients without dyslipidemia showed steeper cognitive decline in memory function and EOAD patients without hypertension or obesity demonstrated steeper cognitive decline in general cognitive function. Meanwhile, the reverse association was observed in LOAD patients. LOAD patients with diabetes demonstrated steeper decline in memory function, and LOAD patients with hypertension or diabetes demonstrated steeper decline in frontal function. Diabetes, hypertension, and dyslipidemia are vascular risk factors that are well-known to be associated with the development of AD [6, 9, 10, 21]. However, there is limited published evidence for the association between these vascular risk factor and cognitive decline in AD patients [21]. One previous study showed that vascular risk factors were associated with accelerated brain amyloid-ß accumulation in AD patients [50] via increasing APP expression, reducing clearance of amyloid-ß peptide, inducing oxidative stress, and increasing inflammatory response [51]. However, those studies were generally based on LOAD patients and more studies are needed in EOAD patients. Also, a likely cause for LOAD experiencing more negative effects from vascular risk factors might be that age has reduced overall brain vitality in these subjects, which leads them to be less resilient to the effects of vascular risk factors.
The reason why known risk factors for AD were associated with slower cognitive decline in EOAD patients but rapid cognitive decline in LOAD patients might be explained in several ways. First, it might be related to different pathomechanism of accumulation and clearance of amyloid-ß or tau in EOAD and LOAD [52, 53]. As cognitive decline in dementia stage is more correlated with tau [54], further studies on how the risk factors contribute to tau according to age might be able to provide underlying mechanisms of our results. Second, it is possible that although the pathobiological detrimental effects of APOE ε4, hypertension, diabetes, and dyslipidemia still exist at younger ages, they might be overshadowed by other yet unknown genetic or environmental factors. Our findings may encourage the search for AD-associated genes in a larger EOAD study sample or in a more homogenous subtype of EOAD, such as young APOE ε4 noncarriers without conventional risk factors. Third, the degree of pathological burden might differ according to the presence of risk factors. EOAD patients without risk factor might have needed more amyloid-ß or tau burden to show dementia symptoms [3], which might have led to rapid disease progression thereafter. Lastly, it is possible that the biological effect of vascular risk factors may differ according to age. Vascular risk factors such as hypertension or diabetes might indirectly lead to increased pathological burden in old ages which may result in rapid cognitive decline.
Strengths and limitations
The strength of our study is that we specifically characterized the impact of APOE ε4, education, and vascular risk factors in EOAD and LOAD patients in a large longitudinal cohort. To the best of our knowledge, this is the first study to compare the impact of risk factors on cognitive decline in EOAD and LOAD patients.
However, this study also has several limitations. First, AD dementia was diagnosed based on clinical criteria and not confirmed by amyloid or tau biomarkers. Further studies are needed to evaluate whether analysis including amyloid or tau biomarker confirmed AD show similar results. Second, the patients were from a single referral center and our results are not representative of the total AD population. Further multicenter studies including other ethnicities are needed to increase the generalizability of the study results. Third, although we excluded patients when known AD causing mutations (PSEN1, PSEN2, or APP) were identified, those mutations were not completely ruled out in every AD patient. Lastly, we could not explore the unique pattern of cognitive trajectories of patients who developed AD dementia at oldest old ages due to paucity of data. More studies are needed to explore the genetic or environmental factors that drive rapid cognitive decline in EOAD patients without conventional risk factors.
Conclusions
In the present study, we evaluated whether the impact of AD risk factors on cognitive trajectories differ in EOAD and LOAD. Our study results suggested that known risk factors for AD were associated with slower cognitive decline in EOAD but rapid cognitive decline in LOAD. More studies are needed to explore the factors that drive rapid cognitive decline in EOAD patients without known risk factors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- APOE:
-
Apolipoprotein E
- CDR-SB:
-
Clinical dementia rating sum of boxes
- CIs:
-
Confidence intervals
- EOAD:
-
Early-onset AD
- LOAD:
-
Late-onset AD
- MMSE:
-
Mini-Mental State Exam
- NP:
-
Neuropsychological
- PD:
-
Parkinson’s disease
- SD:
-
Standard deviations
- SMC:
-
Samsung Medical Center
- TBI:
-
Traumatic brain injury
References
Cho H, Seo SW, Kim JH, Kim C, Ye BS, Kim GH, et al. Changes in subcortical structures in early- versus late-onset Alzheimer’s disease. Neurobiol Aging. 2013;34(7):1740–7. https://doi.org/10.1016/j.neurobiolaging.2013.01.001.
Zhu XC, Tan L, Wang HF, Jiang T, Cao L, Wang C, et al. Rate of early onset Alzheimer’s disease: a systematic review and meta-analysis. Ann Transl Med. 2015;3(3):38. https://doi.org/10.3978/j.issn.2305-5839.2015.01.19.
Kim J, Park S, Yoo H, Jang H, Kim Y, Kim KW, et al. The impact of APOE ɛ4 in Alzheimer’s Disease differs according to age. J Alzheimers Dis. 2018;61(4):1377–85. https://doi.org/10.3233/JAD-170556.
van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM. Most rapid cognitive decline in APOE epsilon4 negative Alzheimer’s disease with early onset. Psychol Med. 2009;39(11):1907–11. https://doi.org/10.1017/S0033291709005492.
Chartier-Harlin MC, Parfitt M, Legrain S, Perez-Tur J, Brousseau T, Evans A, et al. Apolipoprotein E, epsilon 4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer’s disease: analysis of the 19q13.2 chromosomal region. Hum Mol Genet. 1994;3(4):569–74. https://doi.org/10.1093/hmg/3.4.569.
Campos-Peña V, Toral-Rios D, Becerril-Pérez F, Sánchez-Torres C, Delgado-Namorado Y, Torres-Ossorio E, et al. Metabolic syndrome as a risk factor for Alzheimer’s disease: is Aβ a crucial factor in both pathologies? Antioxid Redox Signal. 2017;26(10):542–60. https://doi.org/10.1089/ars.2016.6768.
Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–56.
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313(19):1924–38. https://doi.org/10.1001/jama.2015.4668.
Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19(1):61–70. https://doi.org/10.1016/S1474-4422(19)30393-X.
Reitz C. Dyslipidemia and the risk of Alzheimer’s disease. Curr Atheroscler Rep. 2013;15(3):307. https://doi.org/10.1007/s11883-012-0307-3.
Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60(2):223–8. https://doi.org/10.1001/archneur.60.2.223.
Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol. 1997;145(4):301–8. https://doi.org/10.1093/oxfordjournals.aje.a009106.
Lloret A, Monllor P, Esteve D, Cervera-Ferri A, Lloret A. Obesity as a risk factor for Alzheimer’s disease: implication of leptin and glutamate. Front Neurosci. 2019;13. Article 508. https://doi.org/10.3389/fnins.2019.00508.
Saunders AM. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J Neuropathol Exp Neurol. 2000;59(9):751–8. https://doi.org/10.1093/jnen/59.9.751.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Rimmler JB, et al. Apolipoprotein E, survival in Alzheimer’s disease patients, and the competing risks of death and Alzheimer’s disease. Neurology. 1995;45(7):1323–8. https://doi.org/10.1212/WNL.45.7.1323.
Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–52. https://doi.org/10.1016/S1474-4422(10)70325-2.
Suzuki K, Hirakawa A, Ihara R, Iwata A, Ishii K, Ikeuchi T, et al. Effect of apolipoprotein E ε4 allele on the progression of cognitive decline in the early stage of Alzheimer’s disease. Alzheimers Dement (N Y). 2020;6(1):e12007.
Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology. 1998;51(1):149–53. https://doi.org/10.1212/WNL.51.1.149.
Lim YY, Kalinowski P, Pietrzak RH, Laws SM, Burnham SC, Ames D, et al. Association of β-amyloid and apolipoprotein E ε4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 2018;75(4):488–94. https://doi.org/10.1001/jamaneurol.2017.4325.
Smits LL, Pijnenburg YA, van der Vlies AE, Koedam EL, Bouwman FH, Reuling IE, et al. Early onset APOE E4-negative Alzheimer’s disease patients show faster cognitive decline on non-memory domains. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol. 2015;25(7):1010–7. https://doi.org/10.1016/j.euroneuro.2015.03.014.
Li J, Cesari M, Liu F, Dong B, Vellas B. Effects of diabetes mellitus on cognitive decline in patients with Alzheimer disease: a systematic review. Can J Diabetes. 2017;41(1):114–9. https://doi.org/10.1016/j.jcjd.2016.07.003.
Nguyen JCD, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8. Article 275. https://doi.org/10.3389/fnins.2014.00375.
Wysocki M, Luo X, Schmeidler J, Dahlman K, Lesser GT, Grossman H, et al. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriatr Psychiatry. 2012;20(2):179–87. https://doi.org/10.1097/JGP.0b013e31820ee833.
Rajan KB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Evans DA. Race, APOEɛ4, and long-term cognitive trajectories in a biracial population sample. J Alzheimers Dis. 2019;72(1):45–53. https://doi.org/10.3233/JAD-190538.
Watts AS, Loskutova N, Burns JM, Johnson DK. Metabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adults. J Alzheimers Dis. 2013;35(2):253–65. https://doi.org/10.3233/JAD-121168.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. https://doi.org/10.1212/WNL.51.6.1546.
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. https://doi.org/10.1212/WNL.47.5.1113.
Park KW, Kim HS, Cheon SM, Cha JK, Kim SH, Kim JW. Dementia with Lewy bodies versus Alzheimer’s disease and Parkinson’s disease dementia: a comparison of cognitive profiles. J Clin Neurol (Seoul, Korea). 2011;7(1):19–24.
Kim GH, Lee JH, Seo SW, Ye BS, Cho H, Kim HJ, et al. Seoul criteria for PiB(-) subcortical vascular dementia based on clinical and MRI variables. Neurology. 2014;82(17):1529–35. https://doi.org/10.1212/WNL.0000000000000360.
Kim Y-E, Cho H, Kim HJ, Na DL, Seo SW, Ki C-S. PSEN1 variants in Korean patients with clinically suspicious early-onset familial Alzheimer’s disease. Sci Rep. 2020;10(1):3480. https://doi.org/10.1038/s41598-020-59829-z.
Cooper S, Greene JDW. The clinical assessment of the patient with early dementia. J Neurol Neurosurg Psychiatry. 2005;76(suppl 5):v15.
Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25(7):1071–6. https://doi.org/10.3346/jkms.2010.25.7.1071.
Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s continuum. Dement Neurocogn Disord. 2019;18(3):77–95. https://doi.org/10.12779/dnd.2019.18.3.77.
Kang Y. A normative Study of the Korean-Mini Mental State Examination (K-MMSE) in the Elderly. Korean J Psychol. 2006;25(2):1–12.
Li B, Shi J, Gutman BA, Baxter LC, Thompson PM, Caselli RJ, et al. Influence of APOE genotype on hippocampal atrophy over time - an N=1925 surface-based ADNI study. PLoS One. 2016;11(4):e0152901. https://doi.org/10.1371/journal.pone.0152901.
Atkins ER, Bulsara MK, Panegyres PK. The natural history of early-onset dementia: the Artemis Project. BMJ Open. 2012;2:e001764. https://doi.org/10.1136/bmjopen-2012-001764.
Yoon B, Shim YS, Park HK, Park SA, Choi SH, Yang DW. Predictive factors for disease progression in patients with early-onset Alzheimer’s disease. J Alzheimers Dis. 2016;49(1):85–91. https://doi.org/10.3233/JAD-150462.
Wattmo C, Wallin AK. Early- versus late-onset Alzheimer’s disease in clinical practice: cognitive and global outcomes over 3 years. Alzheimers Res Ther. 2017;9(1):70. https://doi.org/10.1186/s13195-017-0294-2.
Park JE, Kim HJ, Kim YE, Jang H, Cho SH, Kim SJ, et al. Analysis of dementia-related gene variants in APOE ε4 noncarrying Korean patients with early-onset Alzheimer’s disease. Neurobiol Aging. 2020;85:155.e155–8.
Lanoiselee HM, Nicolas G, Wallon D, Rovelet-Lecrux A, Lacour M, Rousseau S, et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 2017;14(3):e1002270. https://doi.org/10.1371/journal.pmed.1002270.
Nicolas G, Charbonnier C, Campion D. From common to rare variants: the genetic component of Alzheimer disease. Hum Hered. 2016;81(3):129–41. https://doi.org/10.1159/000452256.
Nicolas G, Wallon D, Charbonnier C, Quenez O, Rousseau S, Richard AC, et al. Screening of dementia genes by whole-exome sequencing in early-onset Alzheimer disease: input and lessons. Eur J Hum Genet. 2016;24:710–6. https://doi.org/10.1038/ejhg.2015.173.
Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(2):112–7. https://doi.org/10.1097/01.wad.0000213815.20177.19.
Contador I, Bermejo-Pareja F, Pablos DL, Villarejo A, Benito-León J. High education accelerates cognitive decline in dementia: a brief report from the population-based NEDICES cohort. Dement Neuropsychol. 2017;11(3):297–300. https://doi.org/10.1590/1980-57642016dn11-030012.
Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27(6):1176–99. https://doi.org/10.1016/j.cmet.2018.05.011.
Hansen LA, DeTeresa R, Davies P, Terry RD. Neocortical morphometry, lesion counts, and choline acetyltransferase levels in the age spectrum of Alzheimer’s disease. Neurology. 1988;38(1):48–54. https://doi.org/10.1212/WNL.38.1.48.
Ho GJ, Hansen LA, Alford MF, Foster K, Salmon DP, Galasko D, et al. Age at onset is associated with disease severity in Lewy body variant and Alzheimer’s disease. Neuroreport. 2002;13(14):1825–8. https://doi.org/10.1097/00001756-200210070-00028.
Sobral M, Pestana MH, Paúl C. The impact of cognitive reserve on neuropsychological and functional abilities in Alzheimer’s disease patients. Psychol Neurosci. 2015;8(1):39–55. https://doi.org/10.1037/h0101022.
Gomez G, Beason-Held LL, Bilgel M, An Y, Wong DF, Studenski S, et al. Metabolic syndrome and amyloid accumulation in the aging brain. J Alzheimers Dis. 2018;65(2):629–39. https://doi.org/10.3233/JAD-180297.
Chakrabarti S, Khemka VK, Banerjee A, Chatterjee G, Ganguly A, Biswas A. Metabolic risk factors of sporadic Alzheimer’s Disease: implications in the pathology, pathogenesis and treatment. Aging Dis. 2015;6(4):282–99. https://doi.org/10.14336/AD.2014.002.
Mentis A-FA, Dardiotis E, Chrousos GP. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: a conceptual framework. Molecul Psychiatry. 2021;26:1075–97. https://doi.org/10.1038/s41380-020-0731-7.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–70. https://doi.org/10.1038/nrneurol.2015.119.
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–81. https://doi.org/10.1097/NEN.0b013e31825018f7.
Acknowledgements
Not applicable.
Funding
This research was supported by the MSIT (Ministry of Science and ICT), Korea, under the ICT Creative Consilience program (IITP-2020-0-01821) supervised by the Institute for Information & communications Technology Planning & Evaluation (IITP); the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2018R1A1A3A04079255); the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI19C1082); the Research of Korea Disease Control and Prevention Agency (2021-ER1004-00). The study sponsors had no role in the design, collection, analysis, or interpretation of data, and had no role in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
J.K, and H.J.K designed and conceptualized the study. J. K, S.Y.W, S.W.K, and H.J.K analyzed the data. J.K, H.J, J.P.K, J.S.K, S.H.K, D.L.N, J.H.C, S.W.S, and H.J.K collected the data. J.K, L.G.A, and H.J.K drafted and revised the manuscript for intellectual content. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, J., Woo, SY., Kim, S. et al. Differential effects of risk factors on the cognitive trajectory of early- and late-onset Alzheimer’s disease. Alz Res Therapy 13, 113 (2021). https://doi.org/10.1186/s13195-021-00857-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-021-00857-w