Abstract
Background
Toxoplasmosis was recently included as a neglected disease by the Center for Disease Control. Ocular toxoplasmosis is one clinical presentation of congenital or acquired infection. The laboratory diagnosis is being used worldwide to support the clinical diagnosis and imaging. The aim of this study was to evaluate the use of serology and molecular methods to monitor acute OT in immunocompetent patients during treatment.
Methods
Five immunocompetent patients were clinically diagnosed with acute OT. The clinical evaluation was performed by ophthalmologic examination using the Early Treatment Diabetic Retinopathy Study, best-corrected visual acuity, slit lamp biomicroscopy, fundoscopic examination with indirect binocular ophthalmoscopy color fundus photography, fluorescein angiography and spectral optical coherence tomography (OCT). Serology were performed by ELISA (IgA, IgM, IgG) and confirmed by ELFA (IgG, IgM). Molecular diagnoses were performed in peripheral blood by cPCR using the Toxoplasma gondii B1 gene as the marker. Follow-up exams were performed on day +15 and day +45.
Results
Only five non-immunocompromised male patients completed the follow up and their data were used for analysis. The mean age was 41.2 ± 11.3 years (median: 35; range 31–54 years). All of them were positive for IgG antibodies but with different profiles for IgM and IgA, as well as PCR. For all patients the OCT exam showed active lesions with the inner retinal layers being abnormally hyper-reflective with full-thickness disorganization of the retinal reflective layers, which assumed a blurred reflective appearance and the retina was thickened.
Conclusions
The presence of IgA and IgM confirmed the acute infection and thus was in agreement with the clinical evaluation. Our results show the adopted treatment modified the serological profile of IgM antibodies and the PCR results, but not the IgG and IgA antibodies and that imaging is a good tool to follow-up patients.
Similar content being viewed by others
Background
Toxoplasmic retinochoroiditis is a major cause of posterior uveitis [1–3], it was considered the disease of the year in 2011 [4] and included in the list of neglected diseases by the Centers for Disease Control (CDC) of the United States [5].
Toxoplasma gondii infection, the cause of this disease, may also occur during pregnancy during childhood or in adulthood. The clinical symptoms may appear soon after infection or delay with varying degrees of ocular involvement [6, 7]. T. gondii form latent cysts directly on the retina, which may be reactivated several years after the primary infection giving rise to retinochoroiditis. Although many episodes of retinochoroiditis are asymptomatic, some result in loss of vision, pain, photophobia, either in isolation or together [8].
For a long time, toxoplasmic retinochoroiditis has been considered the most common eye lesion caused by T. gondii infection [9, 10]. Its prevalence appears to be quite variable in different countries, but estimates suggest that from 0.3 to 1 % of Europeans and North Americans develop this disease within 1–2 years of contracting the infection [9, 11, 12]. It is believed that the risk of developing this disease ranges from 18 in 100,000 individuals in the UK to as many as 382 in 100,000 individuals in West Africa [8, 13]. Furthermore, the risk of developing retinochoroiditis among individuals who contracted congenital infection is as high as 20 % before the age of 6 years and that new eye lesions may first appear in adolescence [3, 12, 14–16].
Studies in Brazil have shown that the prevalence of toxoplasma retinochoroiditis is variable but very high in adolescents and adults in some regions of the country; the disease ranges from 2 % in the southeast to 25 % in the southern region [10, 17–20]. A study in the State of Rio Grande do Sul revealed a prevalence of ocular toxoplasmosis of 21.3 % in over 13-year-old individuals and concluded that the disease is a consequence of postnatal infection [10]. In the State of Pernambuco, it was observed that 56.2 % of cases of posterior uveitis were due to T. gondii infection [21] and in the State of Rio Grande do Norte, 56.9 % of the patients analyzed in one investigation had bilateral lesions [22]. These studies make it clear that toxoplasmic retinochoroiditis is common in Brazil and thus an analysis of host risk associated with factors of this disease is justified. Furthermore, our recent study showed that the prevalence of toxoplasma retinochoroiditis is approximately 27 % among patients with eye diseases [23].
The growing interest in the investigation of toxoplasmosis has helped to develop strategies for early laboratory diagnosis and clinical intervention [24–30]. Moreover, it has been found that imaging tests such as fundus photography, fluorescein angiography and optical coherence tomography (OCT) help in the assessment, registration and documentation of eye disease, thereby showing whether it is in the acute phase or quiescent, and can also be used to monitor eye involvement in respect to T. gondii infection [3, 31–39]. OCT is a noninvasive test that provides data to evaluate morphological changes that occur in the retina, vitreous, and choroid during ocular toxoplasmosis and other ocular diseases. The objective of this study was to monitor the evolution of active ocular disease or acute relapse of ocular toxoplasmosis using serology and molecular tests and evaluate their applicability.
Methods
Ethical aspects of the study
This study was approved by the Ethics Committee of the Medicine School in São José do Rio Preto—FAMERP and the selected patients signed informed consent forms after being informed about the nature of the study including the objectives and laboratory procedures that would be performed.
Patients
In the period between August 2013 and July 2014, patients with clinical suspicion of active lesions or acute relapse of T. gondii infection were evaluated. Of a total of 31 patients enrolled and treated, five were selected because they adhered to the treatment regimen and were followed up for a period of 45 days (day 0; day +15;day +45). The and the start of the treatment were performed at day 0. New blood samples were collected for analysis at day +15 and day +45.
The five patients received clinical care and were submitted to all the proposed tests (serological diagnosis of toxoplasmosis, cPCR to detect T. gondii in peripheral blood; OCT and fundus photography) at each return consultation.
The inclusion criteria were patients with seropositive samples for toxoplasmosis, scarring of the retina characteristic of toxoplasmosis, and active retinochoroiditis lesion (satellite). Under 18-year-old patients were excluded as were immunosuppressed patients, patients receiving immunosuppression drugs and those with retinochoroiditis with clinical characteristics of other causes.
Blood sampling
Blood samples were collected in tubes with and without EDTA in all consultations. An investigation of T. gondii infection (IgM, IgG and IgA) was made and DNA was extracted from leukocytes.
Eye exams
Clinical examination
All patients underwent detailed eye examinations including visual acuity [the logMAR Early Treatment Diabetic Retinopathy Study (ETDRS) chart] with best correction according to standardization recommended by ETDRS [40], measurement of intraocular pressure by Goldmann applanation tonometry, biomicroscopy using a slit lamp, and stereoscopic biomicroscopy performed using a 78 diopters lens (Volk) and classified according to the criteria determined by the ETDRS.
The OCT was performed using the RTVue-100 scanner with an axial image resolution and speed of five axial velocity of 26,000 frames per second using a program to measure the retinal thickness with the cursor placed according to the fixation of the patient or manually in the center of the fovea, when the foveal depression was visible. The analysis strategy utilized the macular thickness map measured in the central region of the retina.
The evaluation was performed using radial cuts (horizontal and vertical of the line and cross line program) within the fundus area encompassing the toxoplasmosis ocular lesion. Whenever possible, three-dimensional OCT was conducted covering the entire retinochoroiditis lesion. In addition, standardized image acquisition programs such as MM5 (5 × 5 mm2 horizontal grid 11 by 11 vertical lines with 668 A-scans each and a 3 × 3 mm2 grid of six internal vertical and horizontal lines with 400 A-scans each), MM6 (12 radial lines with 1024 A-scans each within 6 mm in diameter) and macula 3D (128 scan lines with 512 A-scans each within 6 × 6 mm2) protocols were used, with all checks with signal strength of at least 40 (range 40.4–79.4).
Colored fundus photographs and fluorescent photographs were taken using a digital retinal camera (TRC-50DX, Topcon Medical Systems) in order to document the macula region and optic nerve. Areas of progressive hyperfluorescence (leakage), impregnation of contrast (staining) and transmitted hyperfluorescence (window effect) were observed by fluorescein angiography. The progressive hyperfluorescence with delayed leakage of contrast was considered a sign of lesion activity.
Therapy regimen
After evaluating the patient at baseline and collecting blood samples, ocular toxoplasmosis was treated using the following protocol: sulfadiazine 1 g four times per day, pyrimethamine 50 mg daily, folinic acid 7.5 mg daily and prednisone 0.5 mg/kg/day for 4 weeks.
Detection of IgM, IgG and IgA anti-T. gondii antibodies
IgM, IgG and IgA anti-T. gondii antibodies were investigated using the ELISA test with the ETI—TOXOK-M (IgM), ETI—TOXOK-G (IgG) and ETI—TOXOK-A (IgA) commercial kits (DiaSorin, Italy); the results were confirmed by enzyme-linked fluorescence immunoassay (ELFA) Vidas Toxo IgG and Vidas Toxo IgM (bioMérieux, France) according to manufacturer’s directions.
Molecular diagnosis of T. gondii infection
Extraction of genomic DNA
Genomic DNA was extracted from 5 mL of peripheral blood samples collected in EDTA as previously described [41] using the commercial QIAamp® DNA Blood Mini Kit (QIAGEN, The Netherlands) .
Molecular analysis to identify T. gondii
cPCR
Toxoplasma gondii was identified in blood samples using a previously described technique [41] that amplifies a final volume of 25 uL per reaction tube using the reagent GoTaq Hot Start Green Master Mix (Promega, USA). Each reaction tube (mix) contained 25 pmol of each primer, 1 U of Taq DNA polymerase, 10 mM Tris–HCl at pH 8.5, 50 mM KCl, 1.5 mM MgCl2, and 200 mM of each dNTP. Two negative controls (ultrapure water and genomic DNA negative for T. gondii) and a positive control (DNA extracted from the RH strain of T. gondii) were included in each amplification reaction.
The B22 and B23 primers, which amplify a 115-base pair (bp) fragment of the repeat region of the B1 gene, were utilized [42]. The HGH primers that amplify a 400-bp fragment of the human growth hormone gene were used as a control of amplification and detection of PCR inhibitors. The amplicons were analyzed by electrophoresis in 2 % agarose gel, stained with ethidium bromide and viewed under ultraviolet light.
Data analysis
Data were analyzed descriptively to determine the importance of OCT to monitor the progression of active ocular disease or acute relapse due to infection by T. gondii.
Results
From the 31 patients enrolled in this study, only five completed the proposed follow up; all of them were male. The mean age was 41.2 ± 11.3 years (range 31–54; median: 35). At the time of inclusion, all of them had positive serology for toxoplasmosis IgG antibodies.
Table 1 presents the data from serological tests for IgM, IgA and IgG anti-T. gondii antibodies of the five patients enrolled in this study and Table 2 shows the cPCR results.
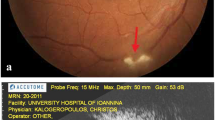
Figure 1 illustrates the results of color retinography imaging (a) fluorescein angiography (b) and OCT (c).
Photodocumentation of pretreatment phase of one patient (case 1). a Fundus photography showing a satellite lesion (yellow square) of activity suggestive of retinochoroiditis toxoplasmosis in the macula region and a healed retinochoroiditis lesion (blue circle); b fluorescein angiography showing a satellite lesion suggestive of activity of toxoplasmosis (yellow rectangle) in the macula region and a healed retinochoroiditis lesion (blue circle); c increases in the thickness of the inner retinal layers in perimacular regions (arrows) seen by optical coherence tomography
Table 3 shows the pattern of eye involvement of the patients with suspicion of acute ocular toxoplasmosis by color retinography imaging, fluorescein angiography, and OCT.
Additional informations are described in Additional file 1.
Discussion
The aim of this study was to evaluate the use of serological, molecular and imaging methods to monitor the status and symptoms of patients clinically diagnosed with acute ocular toxoplasmosis. The B1 gene of T. gondii was used as target for molecular analysis since it has been described as more appropriated to characterize clinical samples in Brazil in contrast with observations from other countries [24, 28, 30, 43–51].
The mean age of the five patients enrolled in this study was about 45-years- old, supporting reports that ocular toxoplasmosis affects young individuals [3, 52, 53]. It is possible that the disease is a result of congenitally acquired infection as the results do not allow us to verify the manner in which the toxoplasmosis was acquired.
The ELISA assay showed that one of the patients (case-01) had acute infection due to the presence of IgM and IgA antibodies at the time of enrollment. This condition remained throughout the follow up which suggests that serological evidence of acute infection may continue even after treatment. This observation is in agreement with literature reports indicating that IgM antibodies persist in patients under treatment for ocular toxoplasmosis. It is also possible that this patient is a special case of individuals who have persistent IgM antibodies (IgM residual); this situation creates additional difficulties in laboratory diagnosis and in the continuation of treatment for long periods of time as it may lead to the impression that an infection is acute or a reactivation of infection [54–60].
By ELISA, one patient (case-02) was positive for IgM antibodies on day 0 but did not have evidence of IgA antibodies in subsequent analyzes. Given that he was positive for IgG antibodies at enrollment in the study, it appears that he seroconverted to a chronic infection. However, these data are not enough to predict the time that the infection occurred. This condition seems to be common in patients infected with T. gondii irrespective of clinical evidence of ocular disease [25, 54, 56, 61, 62]. The other patients were not positive for IgM and IgA antibodies at inclusion in the study but the identification of IgG antibodies was used to infer a chronic infection.
Except for one patient (case-01), all responded adequately to the treatment regimen and the imaging tests showed a reduction of ocular inflammation during the follow-up period. This patient shows a different profile since he presented serological evidence of acute infection but the cPCR was negative during the follow up. Maybe this is a case of atypical strain infection not detected by B1 gene as previously described and the cPCR should be done using other set of primer as being reported in other regions of the world [3, 26, 44, 47, 63–67], but not in South American studies [30, 45, 48–50, 68–72].
There were no variations in the serological profile of three patients (case-03–05) but the cPCR results were positive at day 0 (cases 04 and 05), day 15 (case-03) and day 45 (case-05). These conditions of infection revealed by cPCR in the peripheral blood may be the result of two possible situations: the presence of residual DNA or a false-positive result [25, 43, 48, 56]. These data are in agreement with that reported by Novais et al. 2014 [49] which found positive PCR in Brazilian patients presenting inactive toxoplasmic retinochoroidits lesions and with our previous report [41]. Additionally our study observed that one patient (case-05) had clinical evidence of reactivation of ocular disease based on the PCR results.
Fluorescein angiography showed progressive hyperfluorescence with delayed leakage of contrast and OCT showed that the locations of the lesions of all patients had abnormal inner layers of the retina with hyper-reflective thickened and blurred areas. The level of resolution of this imaging method is well suited to show the characterization of ocular lesions, including those resulting from T. gondii infection [36, 39, 73].
The presence of specific antibodies against T. gondii (IgM and IgA) identifies acute infection and confirms the clinical evaluation. Furthermore, our data suggest that the treatment used in this study may modify the serological profile of IgM antibodies and the result of cPCR, but not the serological profile of IgG and IgA antibodies. Exceptions to this observation can be seem among patients with tendency to remain with residual IgM specific antibodies [25, 49, 56, 58, 59, 74, 75].
The present investigation was limited by the small number of patients evaluated and included. Would be desirable studies with a large amount of patients around the world and in Brazil which could confirm the results reported here.
Conclusion
In conclusion, blood tests are useful to monitor ocular toxoplasmosis and to determine whether the infection is acute or chronic. Molecular analysis by PCR helps to identify possible parasitemia and monitor the effectiveness of treatment as therapy together with the immune response should eliminate parasites circulating in the peripheral blood. Finally, this study shows that imaging tests are excellent noninvasive tools of photo documentation and to monitor inflammation and subsequent scarring of areas damaged by the parasite during ocular toxoplasmosis.
Abbreviations
- CDC:
-
Center for Disease Control
- OT:
-
ocular toxoplasmosis
- T. gondii :
-
Toxoplasma gondii
- IgG:
-
immunoglobulin G
- IgM:
-
immunoglobulin M
- IgA:
-
immunoglobulin A
- cPCR:
-
conventional polymerase chain reaction
- ELFA:
-
enzyme-linked fluorescente assay
- ELISA:
-
enzyme-linked immunosorbent assay
- OCT:
-
optical coherence tomography
- FAMERP:
-
Faculdade de Medicina de São José do Rio Preto
- IAL:
-
Instituto Adolfo Lutz
References
Nussenblatt RB. Ocular toxoplasmosis. an old disease revisited. JAMA, J Am Med Assoc. 1994;271:304–7.
Hay J, Dutton GN. Toxoplasma and the eye. BMJ (Clinical research ed). 1995;310:1021–2.
Maenz M, Schlüter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retinal Eye Res. 2014;39:77–106.
Subauste CS, Ajzenberg D, Kijlstra A. Review of the series “disease of the year 2011: toxoplasmosis” pathophysiology of toxoplasmosis. Ocul Immunol Inflamm. 2011;19:297–306.
CDC - Toxoplasmosis. http://www.cdc.gov/parasites/toxoplasmosis/. Accessed 13 Dec 2014.
Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol. 1999;128:502–5.
Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–42.
Gilbert RE, Dunn DT, Lightman S, Murray PI, Pavesio CE, Gormley PD, et al. Incidence of symptomatic toxoplasma eye disease: aetiology and public health implications. Epidemiol Infect. 1999;123:283–9.
Perkins ES. Ocular toxoplasmosis. Br J Ophthalmol. 1973;57:1–17.
Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier M Jr, Silveira S, et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992;114:136–44.
Burnett AJ, Shortt SG, Isaac-Renton J, King A, Werker D, Bowie WR. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology. 1998;105:1032–7.
Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol. 2000;84:224–6.
Dineen B, Gilbert CE, Rabiu M, Kyari F, Mahdi AM, Abubakar T, et al. The Nigerian national blindness and visual impairment survey: rationale, objectives and detailed methodology. BMC ophthalmol. 2008;8:17.
Koppe JG, Loewer-Sieger DH, De Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–6.
Wallon M, Garweg JG, Abrahamowicz M, Cornu C, Vinault S, Quantin C, et al. Ophthalmic outcomes of congenital toxoplasmosis followed until adolescence. Pediatrics. 2014;133:e601–8.
Furtado JM, Lansingh VC, Carter MJ, Milanese MF, Peña BN, Ghersi HA, et al. Causes of blindness and visual impairment in Latin America. Surv Ophthalmol. 2011;57:149–77.
Garcia JL, Navarro IT, Ogawa L, de Oliveira RC, E Kobilka. Seroprevalence, epidemiology and ocular evaluation of human toxoplasmosis in the rural zone Jauguapitã (Paraná) Brazil. Revista Panamericana de Salud Pública = Pan Am J Publ Health. 1999;6:157–63.
Silveira C, Belfort R, Muccioli C, Abreu MT, Martins MC, Victora C, et al. A follow-up study of Toxoplasma gondii infection in southern Brazil. Am J Ophthalmol. 2001;131:351–4.
Portela RWD, Bethony J, Costa MI, Gazzinelli A, Vitor RWA, Hermeto FM, et al. A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis, and levels of glycoinositolphospholipid-specific immunoglobulin A. J Infect Dis. 2004;190:175–83.
Furtado JM, Winthrop KL, Butler NJ, Smith JR. Ocular toxoplasmosis I: parasitology, epidemiology and public health. Clin Experiment Ophthalmol. 2013;41:82–94.
Lynch MI, de Moraes LFL, Malagueño E, Ferreira S, Cordeiro F, Oréfice F. Características clínicas de 64 indivíduos portadores de uveítis posterior activa presumiblemente toxoplásmica en Pernambuco. Arq Bras Oftalmol. 2008;71:43–8.
Mendes NHD, Oliveira CBS, Garcia CA, Holanda CMXC, Andrade-Neto VF. Epidemiological and serological profiles of ocular toxoplasmosis in the municipality of Natal, northeastern Brazil. Trans R Soc Trop Med Hyg. 2014;108:656–61.
Ferreira AIC, De Mattos CCB, Frederico FB, Meira CS, Almeida GC, Nakashima F, et al. Risk factors for ocular toxoplasmosis in Brazil. Epidemiol Infect. 2014;142:142–8.
Blanco PJ, Assia YM, Montero YM, Orozco KE. ELFA IgG anti-Toxoplasma y PCR anidada para el diagnóstico de toxoplasmosis en mujeres gestantes de Sincelejo, Colombia. Infectio. 2011;15:253–8.
Robert-Gangneux F, Dardé MML. Epidemiology of an diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96.
Dubey JP, Lago EG, Gennari SM, Su C, Jones JL. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 2012;139:1375–424.
Ferrara DC, Costa RA, Tsang S, Calucci D, Jorge R, Freund KB. Multimodal fundus imaging in Best vitelliform macular dystrophy. Graefes Arch Clin Exp Ophthalmol. 2010;248:1377–86.
Costa JGL, Carneiro ACAV, Tavares AT, Andrade GMQ, Vasconcelos-Santos DV, Januário JN, et al. Real-time PCR as a prognostic tool for human congenital toxoplasmosis. J Clin Microbiol. 2013;51:2766–8.
Pfaff AW, de-la-Torre A, Rochet E, Brunet J, Sabou M, Sauer A, et al. New clinical and experimental insights into Old World and neotropical ocular toxoplasmosis. Int J Parasitol. 2014;44:99–107.
Regalado Andújar B, Rodríguez Peña MS, Fraga Nodarse J, Rojas Rivero L, Núñez Fernández FÁ, Jerez Puebla LE. Aplicación de herramientas serológicas y moleculares para el diagnóstico de coriorretinitis por Toxoplasma gondii. Rev Cubana Med Trop. 2013;65:13–25.
Sheets CW, Grewal DS, Greenfield DS. Ocular toxoplasmosis presenting with focal retinal nerve fiber atrophy simulating glaucoma. J Glaucoma. 2009;18:129–31.
Cho DY, Nam W. A case of ocular toxoplasmosis imaged with spectral domain optical coherence tomography. Korean J Ophthalmol KJO. 2012;26:58–60.
Diniz B, Regatieri C, Andrade R, Maia A. Evaluation of spectral domain and time domain optical coherence tomography findings in toxoplasmic retinochoroiditis. Clin ophthalmol (Auckland, NZ). 2011;5:645–50.
Khairallah M, Kahloun R, Ben Yahia S, Jelliti B. Clinical, tomographic, and angiographic findings in patients with acute toxoplasmic retinochoroiditis and associated serous retinal detachment. Ocul Immunol Inflamm. 2011;19:307–10.
Alwassia AA, Cho H, Adhi M, Duker JS, Baumal CR. Sequential optical coherence tomography images of retinal necrosis in acute ocular toxoplasmosis. Retin Case Br Rep. 2013;7:98–101.
Oréfice JL, Costa RA, Scott IU, Calucci D, Oréfice F. Spectral optical coherence tomography findings in patients with ocular toxoplasmosis and active satellite lesions (MINAS Report 1). Acta Ophthalmol. 2013;91:e41–7.
Goldenberg D, Goldstein M, Loewenstein A, Habot-Wilner Z. Vitreal, retinal, and choroidal findings in active and scarred toxoplasmosis lesions: a prospective study by spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251:2037–45.
Onal S, Tugal-Tutkun I, Neri P, Herbort CP. Optical coherence tomography imaging in uveitis. Int Ophthalmol. 2014;34:401–35.
Pakzad-Vaezi K, Or C, Yeh S, Forooghian F. Optical coherence tomography in the diagnosis and management of uveitis. Can J Ophthalmol J Can D’ophtalmol. 2014;49:18–29.
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806.
Mattos CCB, Meira CS, Ferreira AIC, Frederico FB, Hiramoto RM, Almeida GC Jr, et al. Contribution of laboratory methods in diagnosing clinically suspected ocular toxoplasmosis in Brazilian patients. Diagn Microbiol Infect Dis. 2011;70:362–6.
Burg JL, Grover CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–92.
Mesquita RT, Vidal JE, Pereira-Chioccola VL. Molecular diagnosis of cerebral toxoplasmosis: comparing markers that determine Toxoplasma gondii by PCR in peripheral blood from HIV-infected patients. Braz J Infect Dis. 2010;14:346–50.
Bourdin C, Busse A, Kouamou E, Touafek F, Bodaghi B, Le Hoang P, Mazier D, Paris L, Fekkar A. PCR-based detection of Toxoplasma gondii dna in blood and ocular samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2014;52:3987–91.
Silva LLA, Andrade RRO, Carneiro ACAV, Vitor RRWA. Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in southeastern Brazil. PLoS ONE. 2014;9:e90237.
Andrade GMQ, Vasconcelos-Santos DV, Carellos EVM, Romanelli RMC, Vitor RWA, Carneiro ACAV, Januario JN. Congenital toxoplasmosis from a chronically infected woman with reactivation of retinochoroiditis during pregnancy—an underestimated event? Jornal de Pediatria. 2009;86:85–8.
Olariu TR, Remington JS, Montoya JG. Polymerase chain reaction in cerebrospinal fluid for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J. 2014;33:566–70.
Teixeira LE, Kanunfre KA, Shimokawa PT, Targa LS, Rodrigues JC, Domingues W, et al. The performance of four molecular methods for the laboratory diagnosis of congenital toxoplasmosis in amniotic fluid samples. Rev Soc Bras Med Trop. 2013;46:584–8.
Novais EA, Commodaro AG, Santos F, Muccioli C, Maia A, Nascimento H, et al. Patients with diffuse uveitis and inactive toxoplasmic retinitis lesions test PCR positive for Toxoplasma gondii in their vitreous and blood. Br J Ophthalmol. 2014;98:937–40.
Gómez-Marín JE, de-la-Torre A, Barrios P, Cardona N, Álvarez C, Herrera C. Toxoplasmosis in military personnel involved in jungle operations. Acta Trop. 2012;122:46–51.
Vasconcelos-Santos DV. Ocular manifestations of systemic disease. Curr Opin Ophthalmol. 2012;23:543–50.
Jones JL, Parise ME, Fiore AE. Neglected parasitic infections in the United States: toxoplasmosis. Am J Trop Med Hyg. 2014;90:794–9.
Pleyer U, Schlüter D, Mänz M. Ocular toxoplasmosis: recent aspects of pathophysiology and clinical implications. Ophthalmic Res. 2014;52:116–23.
Filisetti D, Candolfi E. Immune response to Toxoplasma gondii. Ann Ist Super Sanita. 2004;40:71–80.
Correa D, Cañedo-Solares I, Ortiz-Alegría LB, Caballero-Ortega H, Rico-Torres CP. Congenital and acquired toxoplasmosis: diversity and role of antibodies in different compartments of the host. Parasite Immunol. 2007;29:651–60.
Fricker-Hidalgo H, Cimon B, Chemla C, Darde ML, Delhaes L, L’ollivier C, et al. Toxoplasma seroconversion with negative or transient immunoglobulin M in pregnant women: myth or reality? A French multicenter retrospective study. J Clin Microbiol. 2013;51:2103–11.
Csep A. Clinical and biological correlations in acute toxoplasmosis. BMC Infect Dis. 2014;14(Suppl 7):P15.
Ribeiro AC, Mutis MS, Fernandes O. Association of the presence of residual anti-Toxoplasma gondii IgM in pregnant women and their respective family groups in Miracema, Northwest Rio de Janeiro Brazil. Memórias do Instituto Oswaldo Cruz. 2008;103:591–4.
Eskandarian AA, Jafarnezghad G-A, Akbari M. Seroprevalence of toxoplasma-specific antibodies in patients suspected to have active toxoplasmosis: a cross-sectional survey. Adv Biomed Res. 2014;3:236.
McLeod R, Lykins J, Noble AG, Rabiah P, Swisher CN, Heydemann PT, et al. Management of congenital toxoplasmosis. Curr Pediatr Rep. 2014;2:166–94.
Furtado JM, Smith JR, Belfort R, Gattey D, Winthrop KL. Toxoplasmosis: a global threat. J Glob Infect Dis. 2011;3:281–4.
Said RN, Zaki MM, Abdelrazik MB. Congenital toxoplasmosis: evaluation of molecular and serological methods for achieving economic and early diagnosis among Egyptian preterm infants. J Trop Pediatr. 2011;57:333–9.
Fekkar A, Ajzenberg D, Bodaghi B, Touafek F, Le Hoang P, Delmas J, et al. Direct genotyping of Toxoplasma gondii in ocular fluid samples from 20 patients with ocular toxoplasmosis: predominance of type II in France. J Clin Microbiol. 2011;49:1513–7.
Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LMG, Tan HK, Wallon M, et al. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis. 2008;2:e277.
Sakamoto N, Maeda T, Mikita K, Kato Y, Yanagisawa N, Suganuma A, et al. Clinical presentation and diagnosis of toxoplasmic encephalitis in Japan. Parasitol Int. 2014;63:701–4.
Malik A, Rizvi M, Khan F, Khan N, Rabbani T, Khan HM. Toxoplasma gondii in women with bad obstetric history and infertility: a five-year study. Asian Pac J Trop Dis. 2014;4:S236–9.
Hashoosh D, Majeed I. Comparison of two assays in the diagnosis of toxoplasmosis: immunological and molecular. East Mediterr Health J. 2014;20:46–50
Carneiro ACAV, Andrade GM, Costa JGL, Pinheiro BV, Vasconcelos-Santos DV, Ferreira AM, et al. Genetic characterization of Toxoplasma gondii revealed highly diverse genotypes for isolates from newborns with congenital toxoplasmosis in southeastern Brazil. J Clin Microbiol. 2013;51:901–7.
Avelino MM, Amaral WN, Rodrigues IMX, Rassi AR, Gomes MBF, Costa TL, et al. Congenital toxoplasmosis and prenatal care state programs. BMC Infect Dis. 2014;14:33.
Anselmo LMP, Vilar FC, Lima JE, Yamamoto AY, Bollela VR, Takayanagui OM. Usefulness and limitations of polymerase chain reaction in the etiologic diagnosis of neurotoxoplasmosis in immunocompromised patients. J Neurol Sci. 2014;346:231–4.
Barbaresco AA, da Costa TL, Avelar JB, Rodrigues IMX, do Amaral WN, et al. Vertical transmission from abortive material and blood with emphasis on Toxoplasma gondii. Revista brasileira de Ginecologia e Obstetrícia. 2014;36:17–22.
Vidigal PVT, Santos DVV, Castro FC, Couto JCF, Vitor RWA, Brasileiro Filho G. Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR. Rev Soc Bras Med Trop. 2002;35:1–6.
Abe K, Shirane J, Sakamoto M, Tanabe F, Kuniyoshi K, Matsumoto C, et al. Optical coherence tomographic findings at the fixation point in a case of bilateral congenital macular coloboma. Clin ophthalmol (Auckland, NZ). 2014;8:1017–20.
Martins LM, Rangel ALP, Peixe RG, Silva-Dos-Santos PPSP, Lemos EM, Martins-Filho OA, et al. Specific IgM, IgG and IgG1 directed against Toxoplasma gondii detected by flow cytometry and their potential as serologic tools to support clinical indirect fundoscopic presumed diagnosis of ocular disease. J Immunol Method. 2015;417:97–106.
Filisetti D, Yera H, Villard O, Escande B, Wafo E, Houfflin-Debarge V, et al. Contribution of neonatal amniotic fluid testing to the diagnosis of congenital toxoplasmosis. J Clin Microbiol. 2015;53:1719–21.
Authors’ contributions
CCBM corresponding author, head of the FAMERP Toxoplasma Research Group, was responsible to concept and design of the study. MP, FBF, RCS, APB, PPMN performed the inclusion of patients with ocular toxoplasmosis, sample collection, and develop the ophthalmological clinical evaluation and clinical analyses. FHAM, AFS, CSM, RG performed the laboratorial tests; VLPC head of the IAL Toxoplasma Research Group; CCBM, RCS, MP, LCM, VLPC performed the data analysis, interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
CCBM declares that received grant to support the publications fees payment from São Paulo Research Foundation (FAPESP #2014/20512-9). This study was supported by research grants (FAPESP #2009/17540-2 to LCM; #2011/13939-8 to VLPC), and by scholarship grants to MP (FAPESP #2013/10050-5), and to FHAM (FAPESP# 2013/15879-8). The opinions, assumptions, and conclusions or recommendations expressed in this material are responsibility of the authors and do not necessarily reflect the views of FAPESP. This study was supported by the Brazilian Ministry of Science, Technology and Innovation—Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to VLPC (#303489/2012-0) and to LCM (#473579/2009-0) and by BAP-FAMERP to LCM and to CCBM.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mariana Previato, Fábio Batista Frederico and Fernando Henrique Antunes Murata contributed equally as first authors
Additional file
13104_2015_1650_MOESM1_ESM.docx
Additional file 1. Clinical data and results of imaging studies (fundus photography, fluorescein angiography and Optical coherence tomography) of the five patients who correctly completed the follow-up.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Previato, M., Frederico, F.B., Henrique Antunes Murata, F. et al. A Brazilian report using serological and molecular diagnosis to monitoring acute ocular toxoplasmosis. BMC Res Notes 8, 746 (2015). https://doi.org/10.1186/s13104-015-1650-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1650-6