Abstract
Background
Phosphodiesterase type 5 inhibitors restore nitric oxide signaling, that plays a significant role in erectile function, and appears to counteract insulin resistance in animal and human models. This study was aimed to evaluate the glycemic and metabolic effects of low-dose tadalafil once daily in patients with type 2 diabetes and erectile dysfunction.
Methods
A 6-month, randomized, double-blind, placebo-controlled pilot trial was conducted. Eligible patients were randomly assigned in a ratio of 2:1 to the tadalafil 5 mg and placebo groups; all patients received either tadalafil or placebo once a day. The primary efficacy endpoint was the absolute change in glycated hemoglobin (HbA1c) levels during the 6-month study period. The secondary efficacy endpoints included metabolic parameters and erectile function.
Results
Of the 68 patients who completed this study, 45 and 23 patients were allocated to the tadalafil and placebo groups, respectively. The mean HbA1c level was significantly different between the groups over the 6-month study period (P = 0.021). After 6 months of treatment, the HbA1c decrement in the tadalafil group was greater than that in the placebo group (− 0.14 ± 0.53% vs. 0.20 ± 0.69%, P = 0.030). The International Index of Erectile Function-5 scores improvement was significantly greater in the tadalafil group than in the placebo group at 6 months (P = 0.003).
Conclusion
This prospective pilot study showed that low-dose tadalafil administered once a day was effective in improving glycemic control and erectile function in patients with type 2 diabetes and erectile dysfunction.
Trial registration KCT0005666
Similar content being viewed by others
Background
Erectile dysfunction (ED) is a common complication of diabetes that is underdiagnosed and mostly left untreated [1]. Based on the International Index of Erectile Function (IIEF) score, the prevalence of ED is estimated to be > 50% in men with diabetes and approximately 3.5 times higher in men with diabetes than in those without [2]. Diabetic ED involves vascular and neurological mechanisms [3]. Nitric oxide (NO) plays a significant role in normal penile erection, and a lack of NO in diabetes triggers ED [4]. Phosphodiesterase type 5 (PDE-5) inhibitors selectively block the hydrolysis of cyclic guanosine monophosphate (cGMP) in the penile corpus cavernosum, thus enhancing NO-mediated smooth muscle relaxation, increasing blood flow to the penis, and facilitating erection [5]. PDE-5 inhibitors result in increased levels of cGMP and NO [6]. Exploratory studies have reported beneficial effects of PDE-5 inhibitors on ED in patients with type 2 diabetes (T2D) [7, 8].
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin sensitivity, or both [9]. Insulin resistance (IR) is associated with the development of endothelial dysfunction and a reduction in NO bioavailability [10]. In humans, endothelial dysfunction is linked with diabetes mellitus via a mechanism that interrupts intracellular signaling pathways of insulin and NO production [11]. Endothelial NO mediates the insulin-induced effects by increasing intracellular levels of cGMP in human vascular smooth muscle cells [12]. The increase in glucose transport is stimulated by insulin via the endothelium-derived NO/cGMP pathway [13]. Reduced NO synthesis in endothelial cells contributes to impaired insulin action in patients with T2D [14]. Therefore, treatments that amplify NO/cGMP signaling could improve microvascular recruitment and muscle glucose uptake [15]. Moreover, recent evidence from animal models and in men with ED suggests that PDE-5 inhibition may have metabolic benefits [16]. Nevertheless, a meta-analysis of PDE-5 inhibitors studies showed little benefit in improving glycemic control [17].
Tadalafil, a drug used to treat ED, selectively inhibits PDE-5 in the penile corpus cavernosum; and is an important regulator of smooth muscle relaxation by elevating intracellular NO/cGMP levels [18]. Treatment with tadalafil 5 mg once a day (OAD) is highly effective, safe, and well-tolerated in patients with ED associated with T2D [19]. A recent meta-analysis confirmed that the chronic use of PDE-5 inhibitors is effective in improving endothelial function, measured using brachial artery flow-mediated dilation and endothelial markers in patients with T2D [20]. Furthermore, in a pilot study, tadalafil OAD increased basal insulin secretion in men with ED [21]. In another study, tadalafil improved peripheral microcirculation and glucose uptake in patients with T2D [22]. Overall, these preliminary studies suggest a therapeutic effect of chronic daily use of tadalafil on glycemic control.
We conducted a randomized clinical trial to evaluate the effect of the chronic use of low-dose tadalafil OAD on glycemic control, assessed its effect according to glycated hemoglobin (HbA1c) levels and other metabolic effects in patients with T2D and ED. Additionally, we assessed the long-term efficacy and safety of tadalafil in treating erectile function.
Methods
Study design
A randomized, double-blind, placebo-controlled pilot study was conducted to examine the glycemic and metabolic effects of 6-month treatment with tadalafil 5 mg OAD in patients with T2D and ED. Statistical criteria for sample size are not required in the pilot study. All patients were randomized to receive tadalafil or placebo and were instructed to use it OAD for 6 months at the same time every day. The allocation list was produced using dedicated software (ID-net™) via permuted-block randomization with 2:1 allocation and randomly sized blocks. The allocation details were blinded until statistical analysis was completed. All the patients were monitored during the entire study period. Treatment compliance was defined as the administration of at least 70% of the required dose between visits.
The study protocol was reviewed and approved by the institutional review board of Myongji Hospital (Approval no. MJH-16-038). Informed consent was obtained from all the participants when they were enrolled. The study was conducted following the protocol, ethical principles stated in the Declaration of Helsinki (revised in 2000), and applicable laws. This randomized clinical trial was registered at the Clinical Research Information Service (CRIS, http://cris.nih.go.kr), number KCT0005666.
Study participants
Eligible men were recruited from the outpatient clinic of Myongji Hospital, Goyang-si, Gyeonggi-do, the Republic of Korea between January 2017 and November 2018. Men aged 35–75 years who had a regular sexual partner and had intercourse ≥ 1 time in the last month, patients with T2D and ED for > 1 year, patients with HbA1c level < 9%, and patients with no history of PDE-5 inhibitor use in the last 3 months were included in this study. ED was defined as a history of persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance. The exclusion criteria were as follows: use of exogenous insulin, thiazolidinediones, or nitrate; history of malignancy, high-risk cardiovascular disease, and chronic liver or kidney failure; and contraindications to tadalafil. In addition, concomitant medications (such as oral anti-hyperglycemic medications, anti-hypertensive drugs, and statins) were not permitted to change between 3 months before study initiation and 1 month after study completion.
Measurements
The selection criteria were evaluated at baseline and a complete clinical record was obtained from each subject, including demographic characteristics, medical history, alcohol consumption, smoking status, and other medical conditions. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. A single examiner measured waist circumference (WC) in the standing position, and blood pressure (BP) was measured twice using a standardized sphygmomanometer with a 5-min rest period.
Venous blood samples were collected in the morning after an overnight fast of > 8-h. The concentrations of fasting plasma glucose (FPG), insulin, C-peptide, HbA1c, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), blood urea nitrogen (BUN), creatinine, aspartate transaminase (AST), and alanine transaminase (ALT) were measured. Homeostatic model assessment (HOMA) is a method for assessing β-cell function and IR from basal FPG and insulin or C-peptide concentrations [23]. The equations were simplified as HOMA-IR = (fasting plasma insulin × FPG)/22.5 [24]. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation. The hexokinase method, enzymatic method, and homogenous enzymatic colorimetric test were used to measure FPG, TC, TG, HDL-C and LDL-C levels, respectively. HbA1c levels were measured using turbidimetric inhibition immunoassay. Biochemical variables were measured using a Cobas Modular 6000 analyzer series (Roche Diagnostics, Basel, Switzerland). These variables were checked at baseline and 6 months. Weight, WC, BP, FPG, insulin, C-peptide, and HbA1c levels were measured at 3 months.
Questionnaires
The secondary measures of efficacy of erectile and voiding function included the IIEF-5 score and the International Prostate Symptom Score (IPSS). The IIEF-5, the abridged five-item version of the IIEF, was used to evaluate erectile function [25]. The possible IIEF-5 scores ranged from 5 to 25. The IPSS is a validated seven‐item questionnaire used to assess lower urinary tract symptoms (LUTS). All participants completed self-administered questionnaires at baseline and 3 and 6 months of treatment.
Safety assessment
Safety and tolerability were evaluated through adverse event (AE) monitoring throughout the study. The study investigators obtained and recorded all observed or self-reported AEs and their severities (mild, moderate, or severe). The relationships between AEs and study medication were established prior to breaking the blind. All patients underwent laboratory tests, vital sign examinations, physical examinations, and 12-lead electrocardiography at the baseline and end of the trial. Drug adherence was measured using the medication possession ratio, defined as the total number of days covered by filled prescriptions divided by the total number of observation days.
Statistical analysis
All analyses were prespecified. The study outcomes of both groups were compared using the Student’s t-test for continuous variables and Pearson’s Chi-square test for categorical variables. Data are expressed as mean ± SD or number (proportion). Repeated measures analysis of variance (ANOVA) was used to monitor the differences in HbA1c levels between the groups over the 6 months. Exploratory data analysis was used to investigate the absolute changes in HbA1c levels from baseline at 3 and 6 months in both groups. Absolute change in HbA1c was defined as the difference between baseline HbA1c levels and HbA1c levels at 3 and 6 months. Pearson’s correlation analysis was used to explore the correlations between absolute changes in HbA1c levels and other variables. Statistical significance was set at P < 0.05. All statistical analyses were performed using IBM SPSS (version 18.0; IBM, Armonk, NY, USA).
Results
Study execution and baseline characteristics of participants
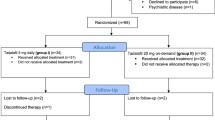
81 patients with T2D and ED were randomized to receive tadalafil (n = 50) or placebo (n = 25) for 6 months. Subsequently, 5 and 2 patients from tadalafil and placebo groups, respectively, discontinued treatment. In the tadalafil group, 3 patients were lost to follow-up, and two discontinued treatment. In the placebo group, one patient was lost to follow-up, and one patient was discontinued. Overall, 45 and 23 subjects in the tadalafil and placebo groups completed the trial (Fig. 1). Baseline characteristics were well-matched between the groups at randomization and study completion (Table 1).
In the tadalafil group, 3 patients were lost to follow-up and 2 patients discontinued treatment. One patient was lost to follow-up in the placebo group, while another discontinued therapy. Overall, 45 and 23 participants in the tadalafil and placebo groups, respectively, completed the trial (Fig. 1). Baseline characteristics were well-matched between groups at randomization and study completion.
At baseline, the mean HbA1c level in the tadalafil and placebo groups was 6.83 ± 0.77% and 6.77 ± 0.58%, respectively (P = 0.747). There were no significant differences between the groups with regard to age, WC, BMI, BP, FBS level, insulin level, C-peptide level, TC level, LDL-C level, eGFR, AST and ALT levels, diabetes duration, smoking status, and alcohol consumption. The groups were comparable with respect to concomitant medications, such as antihyperglycemic and antihypertensive drugs.
Changes in HbA1c levels
Repeated-measures ANOVA revealed that the mean HbA1c levels significantly differed between groups over the 6-month study period (P = 0.021; Fig. 2). The mean absolute changes in baseline HbA1c levels were − 0.138 ± 0.527% and 0.204 ± 0.492% at 3 months (P = 0.012) and − 0.137 ± 0.52% and 0.196 ± 0.691% at 6-months (P = 0.030) in the tadalafil and placebo groups, respectively (Table 2).
Changes in metabolic parameters
Table 3 shows the changes in metabolic parameters from baseline at 3 and 6 months of treatment in both groups. At 3 and 6 months of treatment, the HOMA-IR score decreased from baseline in the tadalafil group; however, the change was not significantly different from that in the placebo group. In addition, there were no significant differences in WC, BMI, or BP between the groups at 3 and 6 months. At 6 months, the reduction in the FPG level from baseline was significantly greater in the tadalafil group than in the placebo group (− 6.40 ± 28.53 mg/dL vs. 5.35 ± 17.77 mg/dL, P = 0.046). At 6 months, the change in the ALT level was − 1.49 ± 12.01 IU/L in the tadalafil group and 6.17 ± 8.39 IU/L in the placebo group (P = 0.008). No significant differences were observed in changes in TC, TG, HDL-C, LDL-C, BUN, creatinine, or eGFR between the groups at 6 months.
Changes in the International Index of Erectile Function Score and International Prostate Symptom Score
The IIEF-5 and IPSS scores at baseline did not differ between the groups (Table 1). However, improvement in the IIEF-5 score was significantly greater in the tadalafil group than in the placebo group at 3 months (5.96 ± 5.26 vs. 0.78 ± 5.82; P = 0.001) and 6 months (6.56 ± 5.32 vs. 2.22 ± 5.73; P = 0.003). Moreover, we noted a numerical reduction in the IPSS of the tadalafil group; however, the difference was not statistically significant when compared with the placebo group.
Adverse events and drug compliance
Tadalafil was well tolerated, and no subject discontinued the study because of treatment-emergent AEs. Overall, a higher percentage of patients reported AEs in the placebo group (n = 2, 8.6%) than in the tadalafil group (n = 2, 4.4%). The reported AEs were myalgia and senile cataracts in the tadalafil group, while lumbar spinal stenosis, and decreased visual acuity were documented in the placebo group. AEs were mild in severity. The study population showed high medication adherence (> 80%) during the study period.
Discussion
In this randomized, double-blind, placebo-controlled study, we found that daily administration of 5 mg tadalafil could be associated with improved glycemic control in patients with T2D and ED. Repeated measures ANOVA revealed that the mean HbA1c level significantly differed between the tadalafil and placebo groups during the 6-month study period. Additionally, absolute changes in the baseline HbA1c level at 3 and 6 months of treatment were significantly greater in the tadalafil group than in the placebo group. In addition, the reduction in FPG level from baseline was greater in the tadalafil group at 6 months of treatment than in the placebo group at the same time point.
PDE-5 enzymes are present in various tissues; they are present in penile erectile tissues and blood vessels, platelets, and smooth muscle tissues [26]. Recently accumulated data on chronic usage of low-dose PDE-5 inhibitors indicate that low-dose therapy can provide additional potential benefits in diverse medical conditions, apart from their well-established erectogenic action [27, 28]. However, there are limited placebo-controlled data regarding the effects of PDE-5 inhibitors on glycemic control in patients with T2D. The current study demonstrates that long-term therapy with low-dose tadalafil has a beneficial effect on glycemic control in patients with T2D and ED, as determined using HbA1c and FPG levels.
Tadalafil was first approved for low-dose daily administration at 2.5 or 5 mg for treating ED, and it has a longer duration of action than other PDE-5 inhibitors [29]. Chronic low-dose tadalafil therapy has favorable effects on systemic endothelial dysfunction [30]. Although we did not examine the effect of tadalafil on biomarkers of endothelial dysfunction, tadalafil was found to improve the levels of circulating inflammatory cytokines and chemokines in a diabetic animal model, while improving FPG levels [31]. Moreover, tadalafil improves insulin action on muscle glucose uptake by prolonging NO/cGMP signaling in women with obesity-linked IR [32]. Tadalafil administration was shown to improve β-cell function in metabolic syndrome independent of insulin sensitivity [33]. A pilot study has reported improvements in β-cell function with tadalafil treatment in individuals with severe obesity [34]. However, the participants in the present study were not severely obese and had good metabolic control, including BMI, WC, and HbA1c values. Therefore, it is difficult to generalize the findings of the present study. Future studies need to evaluate the glycemic effect of daily low-dose tadalafil treatment in obesity.
In the present study, we observed that ALT levels were lower in the tadalafil group at 6 months than in the placebo group. In a previous study, ALT levels were associated with T2D, and glucose-lowering drugs decreased ALT levels as tighter blood glucose levels were achieved [35]. Herein, daily administration of low-dose tadalafil improved erectile function, and there was a statistical difference in IIEF-5 scores between tadalafil and placebo groups. Diabetes-associated ED is predominantly attributed to endothelial dysfunction; however, the precise mechanisms remain poorly understood. Daily therapy with low-dose tadalafil has been approved by the Food and Drug Administration to treat LUTS, which is suggestive of clinical benign prostatic hyperplasia. However, the efficacy of tadalafil for LUTS did not significantly differ from that of the placebo, as reported in other studies [36], which might be due to the small sample size. In the safety assessment, tadalafil was well-tolerated, and no subject discontinued the study due to AEs. The AEs reported were mild in severity.
The current study is the largest double-blinded trial with a longer-term follow-up than previous trials. We observed a small but significant decrease in HbA1c levels after 6 months of therapy. However, this study had several limitations. First, as the present study included a small sample size, there was a limit to obtaining statistically significant results. We also did not adjust for confounding variables, such as lifestyle modifications in diet and physical activity, which may have affected the results. Second, the possible mechanisms that support our results remain unclear. Insulin sensitivity was measured as indexed by HOMA-IR. Still, there is a lack of information on insulin secretion capacity, endothelial markers, and brachial artery flow-mediated dilation. Larger sample, long-term randomized controlled trials are needed to support the hypothesized effects of tadalafil on glycemic control.
Conclusions
This prospective clinical study found that the daily use of tadalafil 5 mg resulted in more favorable HbA1c and FPG levels than the daily use of placebo after 6 months of treatment. Low-dose tadalafil may be used effectively and safely to improve glycemic control and erectile function in patients with T2D and ED. Nevertheless, future randomized controlled studies with large sample sizes are required to elucidate the underlying mechanisms that could clarify the effects of tadalafil on glycemic control.
Availability of data and materials
The data used to support the findings of this study are available upon request from the corresponding author.
Abbreviations
- ED:
-
Erectile dysfunction
- IIEF:
-
International Index of Erectile Function
- NO:
-
Nitric oxide
- PDE-5:
-
Phosphodiesterase type 5
- cGMP:
-
Cyclic guanosine monophosphate
- T2D:
-
Type 2 diabetes
- IR:
-
Insulin resistance
- OAD:
-
Once a day
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- BP:
-
Blood pressure
- FPG:
-
Fasting plasma glucose
- TC:
-
Total cholesterol
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- TG:
-
Triglyceride
- BUN:
-
Blood urea nitrogen
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- HOMA:
-
Homeostatic model assessment
- eGFR:
-
Estimated glomerular filtration rate
- IPSS:
-
International Prostate Symptom Score
- ANOVA:
-
Analysis of variance
- AE:
-
Adverse event
References
Romeo JH, Seftel AD, Madhun ZT, Aron DC. Sexual function in men with diabetes type 2: association with glycemic control. J Urol. 2000;163:788–91.
Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185–92.
Penson DF, Wessells H. Erectile dysfunction in diabetic patients. Diabetes Spectr. 2004;17:225–30.
Zheng H, Bidasee KR, Mayhan WG, Patel KP. Lack of central nitric oxide triggers erectile dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1158–64.
Ryu DS, Suh JK. Recent advance in medical treatment of erectile dysfunction. Endocrinol Metab (Seoul). 1998;13:137–44.
Francis SH, Morris GZ, Corbin JD. Molecular mechanisms that could contribute to prolonged effectiveness of PDE5 inhibitors to improve erectile function. Int J Impot Res. 2008;20:333–42.
Vickers MA, Satyanarayana R. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction in patients with diabetes mellitus. Int J Impot Res. 2002;14:466–71.
Hatzichristou D, Gambla M, Rubio-Aurioles E, Buvat J, Brock GB, Spera G, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med. 2008;25:138–46.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7.
Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5–12.
Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74.
Kang YM, Kim F, Lee WJ. Role of NO/VASP signaling pathway against obesity-related inflammation and insulin resistance. Diabetes Metab J. 2017;41:89–95.
Deshmukh AS, Long YC, de Castro BT, Karlsson HKR, Glund S, Zavadoski WJ, et al. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle. Diabetologia. 2010;53:1142–50.
Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62:4030–42.
Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–9.
Aversa A. Systemic and metabolic effects of PDE5-inhibitor drugs. World J Diabetes. 2010;1:3–7.
Poolsup N, Suksomboon N, Aung N. Effect of phosphodiesterase-5 inhibitors on glycemic control in person with type 2 diabetes mellitus: a systematic review and meta-analysis. J Clin Transl Endocrinol. 2016;6:50–5.
Kuan J, Brock G. Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2002;11:1605–13.
Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6.
Santi D, Giannetta E, Isidori AM, Vitale C, Aversa A, Simoni M. Therapy of endocrine disease. Effects of chronic use of phosphodiesterase inhibitors on endothelial markers in type 2 diabetes mellitus: a meta-analysis. Eur J Endocrinol. 2015;172:R103–14.
McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med. 2004;1:292–300.
Jansson P-A, Murdolo G, Sjögren L, Nyström B, Sjöstrand M, Strindberg L, et al. Tadalafil increases muscle capillary recruitment and forearm glucose uptake in women with type 2 diabetes. Diabetologia. 2010;53:2205–8.
Wallace TM, Levy JC, Matthews DR. Use and Abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Rhoden EL, Telöken C, Sogari PR, Vargas Souto CA. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int J Impot Res. 2002;14:245–50.
Lin C-S, Lin G, Xin Z-C, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12:3439–57.
Mostafa T. Oral phosphodiesterase type 5 inhibitors: nonerectogenic beneficial uses. J Sex Med. 2008;5:2502–18.
Sung HH, Lee SW. Chronic low dosing of phosphodiesterase type 5 inhibitor for erectile dysfunction. Korean J Urol. 2012;53:377–85.
Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003;62:121–6.
Rosano GMC, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–22.
Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC. Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS ONE. 2012;7:e45243.
Murdolo G, Sjöstrand M, Strindberg L, Lönnroth P, Jansson PA. The selective phosphodiesterase-5 inhibitor tadalafil induces microvascular and metabolic effects in type 2 diabetic postmenopausal females. J Clin Endocrinol Metab. 2013;98:245–54.
Hill KD, Eckhauser AW, Marney A, Brown NJ. Phosphodiesterase 5 inhibition improves beta-cell function in metabolic syndrome. Diabetes Care. 2009;32:857–9.
Ho JE, Arora P, Walford GA, Ghorbani A, Guanaga DP, Dhakal BP, et al. Effect of phosphodiesterase inhibition on insulin resistance in obese individuals. J Am Heart Assoc. 2014;3(5):e001001.
Harris EH. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23:115–9.
Brock G, Broderick G, Roehrborn CG, Xu L, Wong D, Viktrup L. Tadalafil once daily in the treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) in men without erectile dysfunction. BJU Int. 2013;112:990–7.
Acknowledgements
The authors would like to thank the participants of the study.
Funding
This study was supported by Hanmi Pharmaceutical Corp, Seoul, Korea. However, the funder had no role in the trial’s design, conduct, or analysis.
Author information
Authors and Affiliations
Contributions
MKL and SCK contributed to study design and data analysis. MKL, JHL, SYS, SYL, TYJ, and SCK collected and organized the data. MKL wrote, reviewed, and edited the manuscript. SCK supervised and revised the manuscript. MKL, JHL, SYS, SYL, TYJ, and SCK participated in the analytical discussion of results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study protocol was reviewed and approved by the institutional review board of Myongji hospital (approval no. MJH-16-038). The study was conducted following the protocol, the ethical principles stated in the Declaration of Helsinki (revised in 2000), and the applicable laws.
Consent for publication
The written informed consents for publication were acquired from each participant.
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, MK., Lee, JH., Sohn, SY. et al. Effect of low-dose tadalafil once daily on glycemic control in patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, placebo-controlled pilot study. Diabetol Metab Syndr 14, 56 (2022). https://doi.org/10.1186/s13098-022-00825-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00825-w