Abstract
Cardiovascular disease is the leading cause of death in women at advanced age, who are affected a decade later compared to men. Cardiovascular risk factors in women are not properly investigated nor treated and events are frequently lethal. Both menopause and type 2 diabetes substantially increase cardiovascular risk in the female sex, promoting modifications on lipid metabolism and circulating lipoproteins. Lipoprotein subfractions suffer a shift after menopause towards a more atherogenic lipid profile, consisted of hypertriglyceridemia, lower levels of both total high density lipoprotein (HDL) and its subfraction HDL2, but also higher levels of HDL3 and small low-density lipoprotein particles. This review discusses the impact of diabetes and menopause to the lipid profile, challenges in lipoprotein subfractions determination and their potential contribution to the cardiovascular risk assessment in women. It is still unclear whether lipoprotein subfraction changes are a major driver of cardiometabolic risk and which modifications are predominant. Prospective trials with larger samples, methodological standardizations and pharmacological approaches are needed to clarify the role of lipoprotein subfractions determination on cardiovascular risk prediction and intervention planning in postmenopausal women, with or without DM.
Similar content being viewed by others
Background
Cardiovascular disease (CVD), particularly coronary artery disease (CAD) [1], is a major cause of death in women, who develop it about 10 years later then men [2]. Traditional risk factors are present at a high frequency in individuals with CAD but are lacking in a not negligible proportion. Risk calculators usually underestimate the real CVD risk in women and their CAD episodes are frequently fatal [3–5].
Hypercholesterolemia is the major driven cause for CVD in both sexes [6, 7] and its treatment has been associated with significant reductions in morbidity and mortality [8–10]. Postmenopausal women tend to deteriorate lipid profile that becomes more atherogenic than their premenopausal counterpart [11, 12]. After menopause, total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) usually increase, and these changes are accompanied by a decrease in high-density lipoprotein cholesterol (HDL-c) and an increase in triglycerides (TG) [13, 14]. In addition to these major lipid abnormalities, also modifications in size and density of these lipoprotein particles are expected to happen after the loss of ovarian hormonal production [15–18]. This partially explains the increased cardiovascular risk in postmenopausal women [2, 19], particularly among those with an earlier onset of menopause [20].
Hyperglycemia contributes to the elevation of cardiovascular risk of populations. Increasing prevalence rates of type 2 diabetes mellitus (DM) have been attributed to aging, modern lifestyle and obesity epidemic, which predisposes to several metabolic disturbances linked by the insulin resistance [21–23]. In men and women with DM a typical dyslipidemia was described, characterized by hypertriglyceridemia, low levels of HDL-c and increased proportion of small-dense LDL particles, known to be more prone to oxidation [24–26]. Elevated glucose levels have also been associated with dysfunctional lipoprotein subfractions, contributing to a more atherogenic lipid profile in both sexes [27, 28]. Despite sharing these lipid abnormalities with the male sex, the diabetic woman has a more aggressive form of CAD and is more susceptible to death from CVD, mainly coronary events [29, 30], suggesting that her lipid profile should be even more deleterious. These observations indicate the need of additional quantitative and/or qualitative laboratory procedures—such as determinations of lipoproteins subfractions—to clarify some sex-related differences.
To date, there is paucity of data describing lipoprotein subfractions in postmenopausal diabetic women [1, 31, 32]. It is unclear whether accurate analysis of subfractions of the several lipoproteins could be associated with improved identification of women at higher risk, before and after menopause, with or without DM. In addition, menopausal hormonal replacement therapy (HRT) may impose unique risk to women. We review and discuss the differences in cardiovascular risk and lipoprotein subfractions in pre- and postmenopausal women and in diabetic ones. Understanding sex-related differences in lipid metabolism, as well as the impact of menopause and DM in women, may contribute to improve cardiovascular risk assessment in women. The keywords postmenopausal and menopause, lipoprotein, lipoprotein subclass and subfractions, type 2 diabetes, analysis, cardiovascular risk were selected for search in PubMed database, from 1980 to 2017, in English and/or Portuguese language.
Cardiovascular risk in women
CAD and stroke have been the leading causes of death in both sexes accounting for 25.1% of the total mortality [33]. Even in the younger women, high mortality rates following myocardial infarction (MI) have been reported [34]. In recent years, improvements in hospital treatment [4] have contributed to a 30% decrease in the number of women dying from cardiovascular events in USA [35] although these still cause more deaths than all other causes combined. Estimates of cardiovascular risk and clinical trials are commonly based on unbalanced samples and selection bias has limited gender comparisons of outcomes. Female sex is notably under-represented in clinical trials which frequently have a predominance of the men [36]. Also, there is evidence that women are undertreated and have cardiovascular risk factors less controlled compared to men [37], specially the diabetic population [38].
Apart from methodological concerns, atherogenesis per se could affect men and women distinctly. It is known that atherosclerosis involves inflammatory and thrombotic processes. In premenopausal women, smaller lipid cores, less calcium, and fewer thin-capped atheromas were described, and estrogen-related anti-inflammatory effects on atherosclerotic plaques seem to contribute to their stabilization [39]. The plaque in women is shown to have less inflammatory components than in men which can implicate in slower development of vulnerable plaques. Young women with acute coronary syndromes often present plaque erosion, while men and older women frequently show the classical pattern of ruptured plaque followed by thrombosis [39]. In carotid arteries, lower atheroma burden and more stable plaques were described in women. Despite the ability of estrogen to stabilize the atheroma, prothrombotic effects of this hormone were reported. The reasons for sex-related differences in the development and progression of atherosclerosis are not completely understood [39–42].
Several scores have been proposed for cardiovascular risk assessment and the Framingham risk score is one of the mostly used [43–46]. It has been recognized that the Framingham risk score underestimates risk in women since those with subclinical atherosclerosis are often classified as at low risk [47]. In an update of this score, it was proposed that women should be classified as ‘‘high risk’’, ‘‘at risk’’ and ‘‘at ideal cardiovascular health’’. High-risk was defined by clinical evidence of CAD, peripheral artery disease and abdominal aortic aneurysm, or the presence of coronary risk equivalents, such as chronic kidney disease and DM, together with a 10-year predicted cardiovascular risk of ≥10%. At-risk women are those with at least one major risk factor [cigarette smoking, hypertension, dyslipidemia, obesity, poor diet, physical inactivity, family history of premature CVD, metabolic syndrome, evidence of advanced subclinical atherosclerosis (coronary calcification, carotid plaque, or increased carotid intima-media thickness), poor exercise capacity on treadmill test and/or abnormal heart rate recovery after stopping exercise, systemic autoimmune collagen-vascular disease (lupus or rheumatoid arthritis), history of preeclampsia, gestational diabetes, or pregnancy-induced hypertension]. “Ideal cardiovascular health” was defined by adequate total cholesterol and blood pressure levels, fasting plasma glucose and body mass index, with heart-healthy behaviours including healthy diet, smoking abstinence and regular physical activity [47, 48].
CVD incidence in premenopausal women is significantly lower than men at the same age (1 woman: 3–10 men), but increases to an extent that the rate becomes similar at the age 65 years and higher by the age 75 years [49]. Among the epidemiological studies that examined cardiovascular risk in women, the Nurses’ Health Study included one of the biggest sample [50]. This reported that 82% of coronary events could be attributed to the absence of a low-risk lifestyle. The INTERHEART study [6] revealed that nine risk factors accounted for 94% of the population attributable risk, including smoking, dyslipidemia, hypertension, DM, abdominal obesity, physical inactivity, low daily fruit and vegetable consumption, alcohol overconsumption, and a low psychosocial index (depression, locus of control, perceived stress, and life events). These are shown to be important risk factors for the development of CVD in both sexes.
In clinical settings, health care professionals commonly underestimate cardiovascular risk in women who are not as properly treated for CVD as men [47, 51]. Comparing sexes after MI, in every age, women are more likely to have a history of hypertension; however, concerning other risk factors, sex differences exist only before the age of 55, when women were more likely to have medical insurance, history of DM, heart failure or stroke, and higher Killip class on hospital admission [4]. Clinical symptoms of CAD also differ between sexes; men express classical symptoms such as angina, with pressure or squeezing to the chest, which can extend to the arms. Meanwhile, women tend to feel sharp, burning chest pain that can extend to neck, jaw, throat, abdomen or back and more frequently have atypical symptoms [52].
Sex differences could be raised concerning the efficacy of lipid lowering treatment. Statins have long been associated with reductions in total cholesterol, LDL-c as well as some increase in HDL-c concentration. Several meta-analyses reported significant reductions in cardiovascular outcomes with statins use for each 1 mmol/L decrease in plasma LDL-c [8, 9]. Accumulated evidence has consistently shown that statins are equally effective in both sexes in the control of dyslipidemia and reduction of cardiovascular morbidity and mortality [53, 54].
The deleterious impact of DM in cardiovascular morbidity and mortality is greater in women compared to men. In 2011, DM was responsible for 281,000 deaths in men and 317,000 in women, the majority from cardiovascular causes [55]. Despite being a strong risk factor for both sexes, a greater impact in mortality from CAD is seen in women than in men [56]. Its presence almost eliminated the sex-related difference in cardiovascular morbidity and mortality, approximating the risk level of the diabetic woman to the non-diabetic men [57]. Therefore, the diabetic woman needs special attention and optimized treatment of comorbidities to control risk factors and to decrease excessive cardiovascular mortality.
CVD is a major issue for women’s health most predominantly at older age, although the younger women have a higher chance of fatality following coronary events. Despite lower absolute incidence compared to men, high mortality rates indicate the need to improve risk prediction, early diagnosis and adequate treatment of risk factors and comorbidities to enhance women quality of life and survival. The increased mortality rates conferred by presence of DM are more prominent in the female sex. A careful analysis of these disparities between sexes is necessary.
Lipoprotein subfractions: determinations and potentialities
Routinely, lipoproteins have been determined according to their molecular density (VLDL, LDL, and HDL) to assess cardiovascular risk. They have been classified by their size, charge, function, lipid core and apolipoprotein composition, and the resulting subgroups are called lipoprotein subfractions [58, 59].
A considerable proportion of individuals that suffer from cardiovascular events shows either few or none of the traditional risk factors [58, 60]. The assessment of lipoprotein subfractions and apolipoproteins (apo) represents a way to improve the cardiovascular risk prediction; in addition, they may enhance the accuracy of atherosclerosis detection, assist in treatment selection, and be useful for counselling first-degree relatives of patients with atherosclerosis [61].
Numerous methods for lipoprotein subfractions determination have been described, mostly for research purposes [61], such as analytic ultracentrifugation, vertical auto profile-II (VAP-II), density gradient ultracentrifugation, gradient gel electrophoresis, nuclear magnetic resonance (NMR) spectroscopy, immunoaffinity chromatography, 2-dimensional gel electrophoresis and ion-mobility analysis (Table 1). Heterogeneous techniques and nomenclature of lipoprotein subfractions limit data interpretation and study comparisons [59].
Analytic ultracentrifugation has been considered the gold standard of lipoprotein subclass analyses due to its precision and reproducibility, and used for validation of other techniques, but it is unfeasible for clinical practice [61]. This method is based on the lipoprotein ability to float when exposed to high gravitational forces. According to flotation rates, four LDL subfractions are grouped whose densities range from 1.025 to 1.060 g/mL [62].
The VAP-II uses a non-segmented continuous flow analyser for the enzymatic analysis of cholesterol in lipoprotein classes, allowing a profile analysis with only 40 µL of plasma [63, 64]. Five subclasses for HDL, four for Lp(a), four for LDL, two for IDL and three for VLDL can be identified. The absorbance curve provides the density distribution of lipoprotein classes and subclasses in the centrifuge tube [65]. The procedures are simple and sensitivity for the lipoprotein density classification is high. However, some studies have shown low correlation of VAP with NMR and electrophoresis [66].
The gradient gel electrophoresis determines LDL and HDL size distribution directly from blood samples. According to major peaks size and percent distribution, seven LDL subclasses, from larger buoyant LDL1, LDL2a and LDL2b to the smaller and less dense LDL3a, LDL3b, LDL4a and LDL4b can be detected [61]. Also, five HDL subclasses, ranging from small dense HDL3c, HDL3b, and HDL3a to larger HDL2a and HDL2b, can be determined. This method does not provide concentrations but the size of predominant species or average size [67]. The two-dimensional gel electrophoresis improved the ability of the gradient gel electrophoresis in recognizing new HDL subfractions: α1, α2, and α3, with sizes of 11.2, 9.51, and 7.12 nm, respectively [68]. Its use has been limited to specialized labs [61].
Lipoproteins subfractions determination can also be based on size and charge using linear polyacrylamide gel. The technique is simple and fast but expensive [69, 70].
NMR spectroscopy allows quantification of lipoprotein subfractions given that each lipoprotein particle in plasma has its own characteristic lipid methyl signal. NMR uses a library of lipoprotein spectra reference in a linear least-square fitting computer program [71]. From the shape of the composite plasma methyl signal, the program computes the subclass signal amplitudes. Particle sizes derive from the sum of the diameter of each subclass multiplied by its relative mass percentage [59, 61]. There is no need to physically separate the subfractions, which is a major advantage of the method. Lipoprotein subfractions identified are [71]:
-
for VLDL: large VLDL/chylomicrons, medium VLDL, small VLDL
-
for LDL, IDL, large LDL, medium small LDL, very small LDL
-
for HDL, large HDL, medium HDL, small HDL
Immunoaffinity chromatography and the ion-mobility have been used for research purposes. The former is able to isolate two HDL subfractions through their content of apolipoprotein A-I and apolipoprotein A-II [61], while the latter determines concentrations of lipoprotein subfractions based on gas-phase differential electric mobility [59, 72].
The availability of several techniques and different parameters to express lipoprotein subfractions (concentrations, percent distribution of the HDL subclasses relative to the total or by average particle diameter) should explain part of the contrasting results on their association with CVD. The most consistent finding is the association of gradient gel electrophoresis-determined HDL subfractions [73]. The amount of large HDL identified by NMR has been correlated with the gradient gel electrophoresis HDL2b results, but other NMR HDL components have shown weaker correlations [73].
Regarding LDL phenotype, substantial agreements among gradient gel electrophoresis, VAP, NMR, and ion-mobility have been described [74]. Using any of these four methods, association of small, dense LDL with coronary atherosclerosis progression was demonstrated [75]. Furthermore, gradient gel electrophoresis, NMR and ion-mobility confirmed that the associations were independent of standard lipid measurements. A recent study on the comparison of ultracentrifugation, a novel electrophoretic method and two independent methods of NMR indicated ultracentrifugation as the most precise method for LDL particle determination with the lowest coefficient of variation. The electrophoresis showed a close precision, whereas NMR showed the highest coefficient of variation [76].
Meanwhile, lipoproteins are heterogeneous even within each subclass and differ not only in size, charge and density, but also in their lipid and protein composition. Lipidomics and proteomics use mass spectrometry to identify and quantify lipid and protein content in a cell, tissue or organ, respectively [77–79]. These methods involve the use of complex technology in several research settings and may even help determine typical and abnormal lipoprotein composition [80, 81]. Changes in key components of lipoproteins under unusual circumstances, such as chronic inflammation and subclinical atherosclerosis, cause their remodelling, affect their functionality and contribute to the atherosclerotic process [82–84].
Evidence that certain lipoprotein subfractions enhance atherogenesis and increase cardiovascular risk emphasizes the importance of their determinations to improve the identification of those at higher risk [85, 86]. Determination methods differ by their basic principles, technology, complexity and accuracy. Such diversity limits to compare results and to assure the real contribution for the improvement in cardiovascular risk prediction.
Also, apolipoprotein determination has shown to improve cardiovascular risk assessment. Apo B100 concentration reflects the atherogenic lipoproteins (VLDL, IDL and LDL), while apo A-I has been considered a HDL surrogate. Apo B-to-apo A-I ratio provides a balance between the atherogenic and anti-atherogenic cholesterol particles and its usefulness as a predictor of cardiovascular events was demonstrated [87–89]. Lower apo B-to-apo A-I ratio was reported in premenopausal compared to postmenopausal women and men [90]. Lipoprotein (a) has a similar structure to LDL, containing one apo-B molecule combined with an apo (a), known to diminish plasminogen activation and fibrin degradation, favouring thrombosis. It has been considered an independent cardiovascular risk factor [91, 92]. There is no gender-related differences in lipoprotein (a) concentration, and a predictive value was observed only in men [93].
Standardization and cost reduction will be necessary for lipoprotein subfractions and apolipoprotein determinations reaching the clinical practice.
Lipid changes following menopause and hormonal replacement therapy
Women experience modifications on lipid profile and metabolism from child to adult life, during pregnancies and following menopause. Aging itself is associated with an increase in LDL-c, in part due to a reduction in its catabolism by the liver. However, the higher levels of total cholesterol, LDL-c and apo-B found after menopause compared to premenopausal ones are not completely explained by aging [94]. A cross-sectional analysis of the Framingham Offspring Study [14], including 1597 women and 1533 men, showed higher LDL-c concentration in male sex, as expected. Additionally, in the postmenopausal compared to premenopausal women, increased LDL-c concentration was maintained after adjustments for age and several confounders.
Smaller denser Apo-B rich LDL particles are more frequent in postmenopausal women, while larger and buoyant LDL are decreased [16]. It is estimated that 14–30% of postmenopausal women have predominance of small dense LDL particles in contrast to only 5–7% in premenopausal counterpart [16, 95]. Lower HDL-c/total cholesterol and apo-AI/apo-B ratios [16, 95], as well as direct association of small LDL-c particles with TG levels, and inverse associations of HDL-c and Apo-AI with Apo-B were reported following menopause [95]. Increased TG rich lipoproteins are associated with higher proportions of small dense LDL. In postmenopausal period, affinity to the hepatic LDL receptor is reduced in small dense LDL-c that is more susceptible to oxidation, transendothelial transport and deposition in artery wall. This LDL subfraction has long been considered by the scientific community as an independent risk factor for CVD, although this is still controversial as some studies have failed to determine this association after several adjustments for confounding factors [58, 96–104]. Small dense LDL is also considered an independent risk factor for the development of type 2 DM [105], particularly in women [106]. Meanwhile, large HDL particles—also named HDL2—play an essential role on reverse cholesterol transport and are considered cardioprotective [66, 85, 107]. In postmenopausal women, the latter seemed to be diminished, with a predominance of cholesterol-depleted smaller HDL particles [18, 108–112]. These are not able to adequately transport cholesterol esters back to the liver, contributing to increased cholesterol concentrations in the blood.
In men, low levels of HDL2 particles (larger buoyant particles) have been associated with CAD indicating worse and diffuse lesions [113]. A cross-sectional analysis of more than 1000 women in UK showed that postmenopausal ones tended to decrease their total HDL-c concentrations together with a decrease in HDL2, without any difference in the HDL3 concentrations when compared to the premenopausal women [18]. Similar reductions in HDL2 were reported in high-risk postmenopausal women with untreated breast cancer [114]. Other studies have confirmed lower levels of large HDL2 particles following menopause suggesting that HDL2 concentrations might be influenced by the drop in female hormonal levels.
The role of sex hormones on lipid metabolism is supported by the demonstration that estrogenic therapy prevents decrease in LDL-c and increases in TG and VLDL-c concentration after menopause. Mechanisms by which female hormones interfere on lipid metabolism have been largely investigated. Estrogen is shown to increase both LDL receptor population in the liver, together with hepatic production of TG rich lipoproteins. Some authors have proposed that the lack of estrogen after menopause contributes to hypertriglyceridemia, low HDL-c and a predominance of small dense LDL particles, like the abnormalities seen in the metabolic syndrome [115]. This lipid profile is found in 15–25% of postmenopausal women and might in part be responsible for their increased cardiovascular risk [115]. The very large lipid database (VLDL 10B) study [116], in which more than a million-people had their lipoprotein subfractions measured by density gradient ultracentrifugation, supported that, after middle age, women presented a shift towards a more atherogenic lipid profile.
These findings have raised questions about the utility of hormonal replacement therapy (HRT) to prevent lipid metabolism abnormalities following menopause which could help in the prevention of CVD. Several clinical trials were conducted to investigate the effects of different schemes of HRT on the lipid profile after menopause [117–120], but those using accurate methods for the determination of subfractions of lipoproteins are less numerous [121, 122]. In one study, 38 postmenopausal Brazilian women with formal indication for HRT were treated with continuous doses of 0.625 mg of conjugated equine estrogen (CEE) with (if they had uterus) or without 2.5 mg of medroxyprogesterone for 12 weeks. Lipoprotein subfractions were measured using an NMR spectroscopy at baseline and after treatment. Significant increases in larger VLDL and HDL particles, together with a decrease in the smaller HDL and VLDL particles were observed, but treatment did not induce significant differences in LDL subfractions [123].
Another trial evaluated the effect of estrogen alone or combined with medroxyprogesterone (1 mg of 17β-estradiol and/or 0.625 mg of CEE) for 3 months in 43 postmenopausal women [124]. Combined therapy resulted in a significant increase in the proportion of bigger HDL particles in circulation, also diminishing the absolute amount of smaller HDL particles. Other trials with estrogen alone in surgically induced menopause have shown a tendency for an increase in HDL and HDL2, but a variety of results were found for LDL particles [118–121]. Different HRT regimens, such as natural vs synthetic, transdermal vs oral, cyclic vs continuous, different progestogens or estrogens and doses have also been tested, but modifications in both lipid and lipoprotein subclasses are inconsistent across trials.
An interesting analysis of 243 postmenopausal women from the Healthy Women Study confirmed higher levels of large HDL particles measured by NMR spectroscopy between HRT users as compared to nonusers [125]. Despite lower levels of LDL-c, there were no differences in LDL subclasses or in coronary artery calcification (CAC) between the groups. As expected, having detectable CAC was associated with worse traditional lipid profile and increased atherogenic subfractions. Although an HRT-dependent shift on the proportions of lipoprotein subfractions could be expected in postmenopausal women, trials have not shown any benefit in cardiovascular morbidity or mortality [126–128]. Only in a subset of younger women who initiated on HRT immediately after menopause some beneficial effects were detected [129]. Scientific societies have not recommended estrogen replacement aiming at treating dyslipidemia or reducing cardiovascular risk in postmenopausal women [130–132].
Since aging and menopause provoke lipid changes (decreased HDL, especially HDL2, increased small dense LDL and TG) that elevate cardiovascular risk in women partially controlled by HRT, several open questions need to be addressed to improve the prognosis of the atherosclerotic disease.
Disturbances in lipid profile and lipoprotein subfractions in diabetes and in postmenopausal diabetic women
Type 2 DM commonly coexists with obesity and both are characterized by states of low-grade inflammation and insulin resistance. Type 1 macrophages accumulated in the hypertrophic adipose tissue potentiate the pro-inflammatory cytokines secretion. Efflux of free fatty acids into circulation and the hepatic insulin resistance are responsible for the dyslipidemia in this condition [133, 134]. Molecular mechanisms of the lipid metabolism disturbances in DM involve microRNAs, that are non-coding RNA molecules which regulate gene expression post-transcriptionally [135]. When microRNAs bind to their complementary sites at the 3′-untranslated regions of the target messenger RNAs (mRNAs) results in mRNA translational and repression or transcript degradation [136, 137]. They have been proven to play important role on insulin resistance and on the regulation of liver metabolism affecting circulating lipids (miR-122, miR-33a, miR-33b) and lipoprotein receptor. The relationship between insulin resistance and hypertriglyceridemia has been recognized, whereas through microRNA miR-34a, hypertriglyceridemia seems to favor the onset of DM [138, 139].
Obesity and impairment in glucose tolerance are frequent pathophysiological conditions that generate lipid-related cardiovascular risk in women following menopause. As chronic inflammatory states, these conditions contribute to lipoprotein remodelling, compromising its function. Meanwhile, reduced estrogen levels contribute to a decrease in insulin sensitivity and aggravate metabolic disturbances [140]. Therefore, postmenopausal obese type 2 diabetic individuals are prone to a combination of disorders that markedly increases the risk of dying from cardiovascular events [141, 142]. Obesity-induced efflux of free fatty acids provokes insulin-mediated skeletal uptake of free fatty acids and increased liver exposure, which results in a rise in hepatic secretion of VLDL, together with a retarded clearance of VLDL and chylomicrons, contributing to hypertriglyceridemia. This pattern of large VLDL, named VLDL1, results in increased precursors of small dense LDL-c [143].
The typical pattern of dyslipidemia in DM—characterized by hypertriglyceridemia, low HDL-c and high small dense LDL-c levels—does not differ between sexes [144]. The HDL-c catabolism that occurs by the hepatic lipase and TG enrichment is elevated in conditions of insulin resistance [145]. Consequently, there is a reduction in HDL-c—that is predominantly from the HDL2b subclass—as well as a relative or absolute increase in the smaller denser HDL3b and HDL3c [143]. Elevated non-HDL-c and predominance of small dense LDL particles to large buoyant LDL, known as phenotype B [143, 146], raise atherogenicity even in near-normal limits of LDL-c. As these particles are prone to oxidative modification, oxidized LDL is more frequently found in diabetic individuals, contributing to accelerate atherogenesis.
Small dense LDL particles have reduced affinity to LDL receptors and a prolonged plasma residence time, which could result in an increment in LDL3a and LDL3b and a decrement in LDL1 and LDL2a [143]. Of note, the opposite and desirable profile, with higher concentration of large buoyant LDL, has been called phenotype A [143, 146]. TG enrichment of these particles (VLDL and LDL) is due to the action of cholesteryl ester transfer protein (CETP), and hepatic lipase hydrolysis of TG and phospholipids [143, 147].
In addition, abnormalities on scavenger receptor class BI (SR-BI), that promotes selective uptake of HDL cholesteryl esters (HDL-CEs) into cells, have been described in the type 2 DM. An overexpression of SR-BI in the liver accompanied by a reduction of HDL-c levels were reported [148]. In contrast, genetic deletion of SR-BI resulted in increased HDL-c and atherosclerosis. These HDL-c molecules seemed to have an altered composition, including a shift toward large, buoyant HDL particles, and a significant increase in plasma apo A-I, but not apo A-II in HDL particle [149].
Consequences of insulin resistance can be present in individuals with the metabolic syndrome even before the clinical diagnosis of DM [143, 145]. Hyperglycaemia and hypoadiponectinemia are involved in the pathophysiology of the diabetic dyslipidemia, but several questions remain unanswered [145].
Incidence of type 2 DM elevates after menopause [150] and that postmenopausal diabetic women are at increased cardiovascular risk compared to nondiabetic women at the same age and hormonal status [30]. Such risk is strongly related to modifications in the lipid metabolism which are dependent of both, menopause per se as well as the diabetic condition. For our best knowledge, the deleterious impact on lipid metabolism due to the presence of DM is similar in men and postmenopausal women.
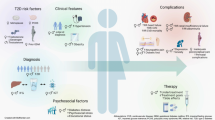
The increased risk for atherosclerosis in postmenopausal diabetic women depends on low HDL-c levels, hypertriglyceridemia and predominance of small dense LDL particles [151]. Additionally, type 2 DM clusters with other disturbances from the spectrum of the metabolic syndrome, contributing to an elevated cardiovascular mortality [152]. Interestingly, the deleterious impact of DM in the LDL particle size seems to be greater in the diabetic women than in men [153, 154] and postmenopausal diabetic women exhibited decreased large HDL particles (HDL2) levels together with increased small HDL particles compared to normoglycemic women after menopause [31]. Figure 1 summarizes the main characteristics of structural and functional abnormalities of lipid metabolism during atherogenic process and aging and the impact of diabetes mellitus.
Meanwhile, the hypothesis that estrogen therapy could alter lipids and improve cardiovascular risk profile and outcomes has been tested in both, diabetic and non-diabetic women [123, 155–168]. Despite many studies that investigated the HRT effects on cardiovascular risk factors in postmenopausal diabetic women, just a few evaluated lipoprotein subfractions with conflicting results. Some authors described a significant increase in total HDL, predominantly on the HDL2 subfraction, after intervention with combined HRT [168], while others failed to demonstrate any impact on HDL or LDL subfractions [32, 166]. Due to the limited sample size and different HRT schemes used, studies available only generated hypothesis.
The effect of HRT on glucose homeostasis remains questionable [158]. A systematic review which included 16 trials with 17,971 postmenopausal women with type 2 DM demonstrated that estrogen replacement diminishes DM incidence and improves glycemic control [169], but there is no consensus yet.
To summarize, limited data on lipoprotein subfractions distribution in postmenopausal diabetic women, with or without dyslipidemia, are available. Different pharmacological approaches to ovarian failure still deserve comparisons, as well as different analytical methods to measure lipoprotein subfractions. Glycemic control level may add a confounding factor among comparisons contributing partially for inconsistent results.
Worth of measurement of lipoprotein subfractions to the cardiovascular risk assessment in women
To date, there is insufficient evidence to recommend lipoprotein subfractions determination in clinical practice in both sexes at lower or higher cardiovascular risk [169]. Evidence that this measurement would impact on lipid-lowering treatment strategies is lacking either [170].
A small prospective nested case–control study in normal middle-aged women has previously demonstrated that baseline particle concentration was more predictive of future cardiovascular events than LDL particle size [171]. On the other hand, an analysis of 286 postmenopausal women from the Healthy Women Study confirmed an independent association of small dense LDL with higher CAC scores, suggesting a benefit from the addition of lipoprotein subfraction measurement for CVD prediction in this subset of individuals [172].
The largest prospective trial available included 27,673 healthy women followed for 11 years [173]. Traditional lipid profile and NMR-determined lipoprotein subclass number and size were measured at baseline. No extra benefit on cardiovascular risk prediction with lipoprotein subfractions measurement after adjustment for non-lipid risk factors was obtained [173]. Finally, a recent systematic review of 24 studies, in which the impact of LDL particles for cardiovascular outcomes was examined in both sexes, reported similar findings [174].
In summary, controversies in this matter persist [175] and it is questionable whether determination of lipoprotein subfractions could be useful in clinical settings. Several techniques for measurement are available, costs of the assays are high and the incremental benefit beyond traditional lipid measures may be minimal. Prospective studies demonstrating that advantages of lipoprotein subfractions to traditional lipid profile in the context of primary and secondary prevention of cardiovascular outcomes are needed.
Final remarks
Despite the lower incidence of CVD in adult women compared to men, their sex-related protective effect vanishes after menopause. This phase of women life per se imposes deterioration of their lipid profile and weight gain is a frequent manifestation that could aggravate their predisposition to metabolic disturbances. The cardiovascular risk scores and health care professionals commonly underestimate their risk, and higher mortality and morbidity after coronary events have been reported in women. Consequently, women are less properly treated for CVD than men.
The deleterious impact of type 2 DM in cardiovascular risk may be superior in women compared to men, emphasizing the importance of improving the risk assessment, especially in postmenopausal diabetic women.
Since plasma lipoproteins constitute a major cardiovascular risk factor, a deeper analysis of their subfractions might contribute to understanding why lipid-dependent cardiovascular risk in women is increased. A more atherogenic lipid profile—hypertriglyceridemia, lower levels of both HDL-c and HDL2, higher levels of both HDL3 and small dense LDL—are usual after menopause, and modifications in lipoprotein subfractions are also expected in the presence of hyperglycemia. Therefore, postmenopausal diabetic women should be aggressively treated against dyslipidemia as well as against other risk factors.
Nowadays, no evidence supports that replacement of ovarian hormones has benefits in reducing cardiovascular events and mortality in different subgroups of women.
Finally, prospective trials including large samples of postmenopausal women, with or without DM, at different treatments and metabolic control, should be conducted to clarify whether lipoprotein subclass analysis would improve identification of higher-risk individuals. Considering that these determinations are expensive, cost-effectiveness studies are also necessary to address the worth of the addition of lipoprotein subfraction analysis in clinical practice.
Abbreviations
- CVD:
-
cardiovascular disease
- CAD:
-
coronary artery disease
- TC:
-
total cholesterol
- HDL-c:
-
high density lipoprotein cholesterol
- LDL-c:
-
low density lipoprotein cholesterol
- TG:
-
triglycerides
- DM:
-
diabetes mellitus
- HRT:
-
hormone replacement therapy
- MI:
-
myocardial infarction
- VAP:
-
vertical auto profile method
- NMR:
-
nuclear magnetic resonance
- Apo:
-
apolipoprotein
- CEE:
-
conjugated equine estrogen
- CETP:
-
cholesteryl ester transfer protein
References
Roger VL, Go AS, Lloyd-Jones DM, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–209.
Kannel W, Hjortland M, McNamara P. Menopause and risk of cardiovascular disease: the Framingham Study. Ann Intern Med. 1976;85:447–52.
Vaccarino V, Parsons L, Every NR, et al. Sex-based differences in early mortality after myocardial infarction: National Registry of Myocardial Infarction 2 participants. N Engl J Med. 1999;34:217–25.
Vaccarino V, Parsons L, Peterson ED, et al. Sex differences in mortality after acute myocardial infarction. Arch Intern Med. 2009;169:1767–74.
Berger JS, Elliott L, Gallup D, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–82.
Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–52.
McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case–control study. Lancet. 2008;372:224–33.
Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
Cholesterol Treatment Trialists (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Cifkova R, Krajcoviechova A. Dyslipidemia and cardiovascular disease in women. Curr Cardiol Rep. 2015;17:609.
Bonithon-Kopp C, Scarabin PY, Darne B, et al. Menopause related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19:42–8.
Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas. 1990;12:321–31.
Schaefer EJ, Lamon-Fava S, Cohn SD, et al. Effects of age, gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res. 1994;35:779–92.
Li Z, McNamara JR, Fruchart JC, et al. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37:1886–96.
Campos H, McNamara JR, Wilson PW, et al. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 1988;67:30–5.
Stevenson J, Crook D, Godsland I. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90.
Anagnostis P, Stevenson JP, Crook D, et al. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas. 2015;81:62–8.
Colditz G, Willett W, Stampfer M, et al. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–10.
Rosenberg L, Hennekens CH, Rosner B, et al. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981;139:47–51.
Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9.
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88.
Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–6.
Taskinén MR, Bóren J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239:483–95.
Berneis K, Rizzo M. LDL size: does it matter? Swiss Med Wkly. 2004;134:720–4.
Rydén L, Grant PJ, Anker SD, et al. ESC guidelines on diabetes, pre-diabetes and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34:3035–87.
Nobécourt E, Jacqueminet S, Hansel B, et al. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48:529–38.
Gomez Rosso L, Lhomme M, Meroño T, et al. Poor glycemic control in type 2 diabetes enhances functional and compositional alterations of small, dense HDL3c. Biochim Biophys Acta. 2017;1862:188–95.
Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8.
Ballotari P, Ranieri SC, Luberto F, et al. Sex differences in cardiovascular mortality in diabetic and nondiabetic subjects: a population-based study (Italy). Int J Endocrinol. 2015;2015:914057.
Mascarenhas-Melo F, Marado D, Palavra F. Diabetes abrogates sex differences and aggravates cardiometabolic risk in postmenopausal women. Cardiovasc Diabetol. 2013;12:61.
Aguilar-Salinas CA, Melzer OA, Reyna LS, et al. Effects of estrogen/medrogestone therapy on the apoprotein B-containing lipoproteins in postmenopausal women with type 2 diabetes mellitus under satisfactory and non-satisfactory glycemic control. Isr Med Assoc J. 2001;3:137–43.
World Health Organization. The 10 leading causes of death in the world, 2000 and 2012. In: The top 10 causes of death. Geneva: World Health Organization; 2014. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed 09 Feb 2016.
Zhang Z, Fang J, Gillespie C, et al. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Am J Cardiol. 2012;109:1097–103.
Garcia M, Miller VM, Gulati M, et al. Focused cardiovascular care for women: the need and role in clinical practice. Mayo Clin Proc. 2016;91:226–40.
Melloni C, Berger JS, Wang TY, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–42.
Rossi MC, Cristofaro MR, Gentile S, et al. Sex disparities in the quality of diabetes care: biological and cultural factor may play a different role for different outcomes. Diabetes Care. 2013;36:3162–8.
Russo G, Pintaudi B, Giorda C, et al. Age- and gender-related differences in LDL-cholesterol management in outpatients with type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:957105.
Burke AP, Farb A, Malcom G, et al. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001;141(2 Suppl):S58–62.
Frink RJ. Gender gap, inflammation and acute coronary disease: are women resistant to atheroma growth? Observations at autopsy. J Invasive Cardiol. 2009;21:270–7.
Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–75.
Maas AHEM, Van der Schouw YT, Regitz-Zagrosek V, et al. Red alert for women’s heart: the urgent need for more research and knowledge on cardiovascular disease in women. Eur Heart J. 2011;32:1362–8.
Thomsen T. HeartScore: a new web-based approach to European cardiovascular disease risk management. Eur J Cardiovasc Prev Rehabil. 2005;12:424–6.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III. Circulation. 2002;106(25):3143–421.
Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105:310–5.
Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47.
Wenger NK. What do the 2011 American Heart Association guidelines tell us about prevention of cardiovascular disease in women? Clin Cardiol. 2011;34(9):520–3.
Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–23.
Zhijuan L, Jianxin C, Liping W, et al. Analysis of high risk factors and characteristics of coronary artery in premenopausal women with coronary artery disease. Int J Clin Exp Med. 2015;8:16488–95.
Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22.
Weiss AM. Cardiovascular disease in women. Prim Care. 2009;36:73–102.
National Heart, Lung and Blood Institute. What are the signs and symptoms of heart disease? In: Heart disease in women. National Heart, Lung and Blood Institute. 2014. http://http://www.nhlbi.nih.gov/health/health-topics/topics/hdw/signs. Accessed 09 Feb 2016.
Kostis WJ, Cheng JQ, Dobrzynski JM, et al. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol. 2012;59:572–82.
Kostis WJ. Absolute risk reduction due to statin use according to sex. J Am Coll Cardiol. 2012;60:1580.
Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC), European Association for the Study of Diabetes (EASD). ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD—summary. Diabetes Vasc Dis Res. 2014;11:133–73.
Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes a stronger risk factor for fatal ischemic heart disease in women than in men? JAMA. 1991;265:627–31.
Rivellese AA, Riccardi G, Vaccaro O. Cardiovascular risk in women with diabetes. Nutr Metab Cardiovasc Dis. 2010;20:474–80.
Sacks FM, Campos H. Clinical review 163: cardiovascular endocrinology: low-density lipoprotein size and cardiovascular disease: a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–32.
Mora S. Advanced lipoprotein testing and subfractionation are not (yet) ready for routine clinical use. Circulation. 2009;119:2396–404.
Heidemann C, Hoffmann K, Klipstein-Grobusch K, et al. Potentially modifiable classic risk factors and their impact on incident myocardial infarction: results from the EPIC-Potsdam study. Eur J Cardiovasc Prev Rehabil. 2007;14:65–71.
Superko HR. Advanced lipoprotein testing and subfractionation are clinically useful. Circulation. 2009;119:2383–95.
Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104.
Kulkarni KR, Garber DW, Marcovina SM, et al. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35:159–68.
Kulkarni KR, Marcovina SM, Krauss RM, et al. Quantification of HDL2 and HDL3 cholesterol by the vertical auto profile-II (VAP-II) methodology. J Lipid Res. 1997;38:2353–64.
Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26:787–802.
Hafiane A, Genest J. High density lipoproteins: measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–88.
Pirillo A, Norata GD, Catapano AL. High-density lipoprotein subfractions—what the clinicians need to know. Cardiology. 2013;124:116–25.
Asztalos BF, Sloop CH, Wong L, et al. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169:291–300.
Hirany SV, Othman Y, Kutscher P, et al. Comparison of low-density lipoprotein size by polyacrylamide tube gel electrophoresis and polyacrylamide gradient gel electrophoresis. Am J Clin Pathol. 2003;119:439–45.
Chung M, Lichtenstein AH, Ip S, et al. Comparability of methods for LDL subfraction determination: a systematic review. Atherosclerosis. 2009;205:342–8.
Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–70.
Caulfield MP, Li S, Lee G, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–16.
Superko HR, Pendyala L, Williams PT, et al. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol. 2012;6:496–523.
Sninsky JJ, Rowland CM, Baca AM, et al. Classification of LDL phenotypes by 4 methods of determining lipoprotein particle size. J Investig Med. 2013;61:942–9.
Williams PT, Vranizan KM, Krauss RM. Correlations of plasma lipoproteins with LDL subfractions by particle size in men and women. J Lipid Res. 1992;33:765–74.
Hopkins PN, Pottala JV, Nanjee MN. A comparative study of four independent methods to measure LDL particle concentration. Atherosclerosis. 2015;243:99–106.
Yang K, Han X. Lipidomics: techniques, applications, and outcomes related to biomedical sciences. Trends Biochem Sci. 2016;41:954–69.
Sethi S, Brietzke E. Recent advances in lipidomics: analytical and clinical perspectives. Prostaglandins Other Lipid Mediat. 2016;27(128–129):8–16.
Aslam B, Basit M, Nisar MA, et al. Proteomics: technologies and their applications. J Chromatogr Sci. 2017;55:182–96.
Bancells C, Canals F, Benítez S, et al. Proteomic analysis of electronegative low-density lipoprotein. J Lipid Res. 2010;51:3508–15.
Vaisar T. Proteomics investigations of HDL: challenges and promise. Curr Vasc Pharmacol. 2012;10:410–21.
Salazar J, Olivar LC, Ramos E, et al. Dysfunctional high-density lipoprotein: an innovative target for proteomics and lipidomics. Cholesterol. 2015;2015:296417.
Chang CT, Yang CY, Tsai FJ, et al. Mass spectrometry-based proteomic study makes high-density lipoprotein a biomarker for atherosclerotic vascular disease. Biomed Res Int. 2015;2015:164846.
Davidson WS, Silva RA, Chantepie S, et al. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–6.
Rizzo M, Berneis K. Should we measure routinely the LDL peak particle size? Int J Cardiol. 2006;107:166–70.
Lőrincz H, Katkó M, Harangi M, et al. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin Endocrinol. 2014;81:370–7.
Sierra-Johnson J, Fisher RM, Romero-Corral A, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J. 2009;30:710–7.
Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta-analysis of prospective studies. J Intern Med. 2006;259:481–92.
Huang F, Yang Z, Xu B, et al. Both serum apolipoprotein B and the apolipoprotein B/apolipoprotein A-I ratio are associated with carotid intima-media thickness. PLoS ONE. 2013;8:e54628.
Anagnostis P, Stevenson JC, Crook D, et al. Effects of gender, age and menopausal status on serum apolipoprotein concentrations. Clin Endocrinol. 2016;85:733–40.
Liu L, Boffa MB, Koschinsky ML. Apolipoprotein(a) inhibits in vitro tube formation in endothelial cells: identification of roles for Kringle V and the plasminogen activation system. PLoS ONE. 2013;8:e52287.
The Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23.
Lamon-Fava S, Marcovina SM, Albers JJ, et al. Lipoprotein(a) levels, apo(a) isoform size, and coronary heart disease risk in the Framingham Offspring Study. J Lipid Res. 2011;52:1181–7.
Phan BA, Toth P. Dyslipidemia in women: etiology and management. Int J Women’s Health. 2014;6:185–94.
Campos H, Blijevens E, McNamara JR, et al. LDL particle size distribution. Results from the Framingham Offspring study. Arterioscler Thromb Vasc Biol. 1992;12:1410–9.
Austin MA, King MC, Vranizam KM, et al. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease. Circulation. 1990;82:495–506.
Austin MA, Breslow JL, Hennekens CH, et al. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–21.
Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79.
Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–7.
Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–81.
Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–8.
Liu ML, Ylitalo K, Nuotio I, et al. Association between carotid intima-media thickness and low-density lipoprotein size and susceptibility of low-density lipoprotein to oxidation in asymptomatic members of familial combined hyperlipidemia families. Stroke. 2002;33:1255–60.
Rajman I, Kendall MJ, Cramb R, et al. Investigation of low density lipoprotein subfractions as a coronary risk factor in normotriglyceridaemic men. Atherosclerosis. 1996;125:231–42.
Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75.
Austin MA, Mykkanen L, Kuusisto J, et al. Prospective study of small LDLs as a risk factor for non-insulin dependent diabetes mellitus in elderly men and women. Circulation. 1995;92:1770–8.
Mora S, Otvos JD, Rosenson RS, et al. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–60.
Forti N, Diament J. High-density lipoproteins: metabolic, clinical, epidemiological and therapeutic intervention aspects. An update for clinicians. Arq Bras Cardiol. 2006;87:672–9.
Matthews KA, Wing RR, Kuller LH, et al. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154:2349–55.
Grandjean P, Crouse S, O’Brien B, et al. The effects of menopausal status and exercise training on serum lipids and the activities of intravascular enzymes related to lipid transport. Metabolism. 1998;47:377–83.
Razay G, Heaton KW, Bolton CH. Coronary heart disease risk factors in relation to the menopause. Q J Med. 1992;85:889–96.
Williams P, Feldman D. Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman’s Livermore Cohort. Atherosclerosis. 2011;214:196–202.
Martin SS, Khokhar AA, May TH, et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J. 2015;36:22–30.
Drexel H, Ammann FW, Rentsch K, et al. Relation of the level of high-density lipoprotein subfraction to the presence and extent of coronary artery disease. Am J Cardiol. 1992;70:436–40.
Michalaki V, Koutrolis G, Syrigos K, et al. Evaluation of serum lipids and high-density lipoprotein subfractions (HDL2, HDL3) in postmenopausal patients with breast cancer. Mol Cell Biochem. 2005;268:19–24.
Spencer CP, Godsland IF, Stevenson JC. Is there a menopausal metabolic syndrome? Gynecol Endocrinol. 1997;11:341–55.
Swiger KJ, Martin SS, Blaha MJ, et al. Narrowing sex differences in lipoprotein cholesterol subclasses following mid-life: the very large database of lipids (VLDL-10B). J Am Heart Assoc. 2014;3:e000851.
Ottosson UB. Oral progesterone and estrogen/progestogen therapy. Effects of natural and synthetic hormones on subfractions of HDL cholesterol and liver proteins. Acta Obstet Gynecol Scand Suppl. 1984;127:1–37.
Haines CJ, Chung TK, Masarei JR, et al. An examination of the effect of combined cyclical hormone replacement therapy on lipoprotein(a) and other lipoproteins. Atherosclerosis. 1996;119:215–22.
Walsh BW, Li H, Sacks FM. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J Lipid Res. 1994;35:2083–93.
Rajman I, Lip GY, Cramb R, et al. Adverse change in low-density lipoprotein subfractions profile with oestrogen-only hormone replacement therapy. QJM. 1996;89:771–8.
Griffin B, Farish E, Walsh D, et al. Response of plasma low density lipoprotein subfractions to oestrogen replacement therapy following surgical menopause. Clin Endocrinol. 1993;39:463–8.
Hughes TA, Pace DT, Ke RW, et al. Lipoprotein compositional changes with combination hormone therapy (conjugated estrogen and medroxyprogesterone) in African American women. Endocr Pract. 2004;10:179–86.
Vieira JLC, Gomes MEW, Almeida AB, et al. Changes in the profile of lipoprotein subfractions associated with hormone replacement therapy. Arq Bras Cardiol. 2001;76:177–82.
Tangney CC, Mosca LJ, Otvos JD, et al. Oral 17β-estradiol and medroxyprogesterone acetate therapy in postmenopausal women increases HDL particle size. Atherosclerosis. 2001;155:425–30.
Mackey RH, Kuller LH, Sutton-Tyrrell K, et al. Hormone therapy, lipoprotein subclasses, and coronary calcification. Arch Intern Med. 2005;165:510–5.
Perrone G, Brunelli R. Prevention and treatment of cardiovascular disease in women: the obstetric-gynecologist’s point of view. Ther Apher Dial. 2013;17:162–8.
Wenger NK. Menopausal hormone therapy: currently no evidence for cardiac protection. Pediatr Blood Cancer. 2005;44:625–9.
Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–33.
Lobo RA, Pickar JH, Stevenson JC, et al. Back to the future: hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis. 2016;254:282–90.
Wenger NK. Drugs for cardiovascular disease prevention in women: implications of the AHA guidelines—2007 update. Drugs. 2008;68:339–58.
Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–88.
Boardman HM, Hartley L, Eisinga A, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. 1010;2015:CD002229. doi:10.1002/14651858.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Investig. 2011;121:2111–7.
Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–45.
Massart J, Katayama M, Krook A. Micromanaging glucose and lipid metabolism in skeletal muscle: Role of microRNAs. Biochim Biophys Acta. 2016;1861(12 Pt B):2130–8.
Lagos-Quintana M, Rauhaut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8.
Vienberg S, Geiger J, Madsen S, et al. MicroRNAs in metabolism. Acta Physiol. 2016. doi:10.1111/apha.12681 (Epub ahead of print).
Lovis P, Roggli E, Laybutt DR, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57:2728–36.
Kong L, Zhu J, Han W, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61–9.
Alonso-Magdalena P, Ropero AB, Carrera MP, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;30(3):e2069.
Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5:S19–27.
Farmer JA. Diabetic dyslipidemia and atherosclerosis: evidence from clinical trials. Curr Diabetes Rep. 2008;8:71–7.
Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–504.
Haffner SM, American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26(S83–6):104.
Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–99.
Sánchez-Quesada JL, Vinagre I, De Juan-Franco E, et al. Impact of the LDL subfraction phenotype on Lp-PLA2 distribution, LDL modification and HDL composition in type 2 diabetes. Cardiovasc Diabetol. 2013;12:112.
Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–62.
Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–71.
Hildebrand RB, Lammers B, Meurs I, et al. Restoration of high-density lipoprotein levels by cholesteryl ester transfer protein expression in scavenger receptor class B type I (SR-BI) knockout mice does not normalize pathologies associated with SR-BI deficiency. Arterioscler Thromb Vasc Biol. 2010;30:1439–45.
Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Womens Health. 2012;8:155–67.
Russo GT, Giandalia A, Romeo EL, et al. Lipid and non-lipid cardiovascular risk factors in postmenopausal type 2 diabetic women with and without coronary heart disease. J Endocrinol Investig. 2014;37:261–8.
Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72.
Berg G, Muzzio ML, Wikinski R, et al. A new approach to the quantitative measurement of dense LDL subfractions. Nutr Metab Cardiovasc Dis. 2004;14:73–80.
Haffner SM, Mykkanen L, Stern MP, et al. Greater effect of diabetes on LDL size in women than in men. Diabetes Care. 1994;17:1164–71.
Salpeter SR, Walsh JM, Ormiston TM, et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–54.
Thijs A, Stehouwer CD. Hormone replacement therapy in postmenopausal women with diabetes mellitus. Semin Vasc Med. 2002;2:215–9.
Lamon-Fava S, Herrington DM, Horvath KV, et al. Effect of hormone replacement therapy on plasma lipoprotein levels and coronary atherosclerosis progression in postmenopausal women according to type 2 diabetes mellitus status. Metabolism. 2010;59:1794–800.
Bitoska I, Krstevska B, Milenkovic T, et al. Effects of hormone replacement therapy on insulin resistance in postmenopausal diabetic women. Open Access Maced J Med Sci. 2016;4:83–8.
Gooding KM, MacLeod KM, Spyer G, et al. Impact of hormone replacement therapy on microvascular function in healthy and type 2 diabetic postmenopausal women. Diabet Med. 2005;22:536–42.
Khoo CL, Perera M. Diabetes and the menopause. J Br Menopause Soc. 2005;11:6–11.
Scott AR, Dhindsa P, Forsyth J, et al. Effect of hormone replacement therapy on cardiovascular risk factors in postmenopausal women with diabetes. Diabetes Obes Metab. 2004;6:16–22.
Stojanovic ND, Kwong P, Byrne DJ, et al. The effects of transdermal estradiol alone or with cyclical dydrogesterone on markers of cardiovascular disease risk in postmenopausal women with type 2 diabetes: a pilot study. Angiology. 2003;54:391–9.
Crespo CJ, Smit E, Snelling A, et al. Hormone replacement therapy and its relationship to lipid and glucose metabolism in diabetic and nondiabetic postmenopausal women: results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2002;25:1675–80.
Granfone A, Campos H, McNamara JR, et al. Effetcs of estrogen replacement on plasma lipoproteins and apolipoproteins in postmenopausal, dyslipidemic women. Metabolism. 1992;41:1193–8.
Vadlamudi S, MacLean P, Israel RG, et al. Effects of oral combined hormone replacement therapy on plasma lipids and lipoproteins. Metabolism. 1998;47:1222–6.
Manning PJ, Allum A, Jones S, et al. The effect of hormone replacement therapy on cardiovascular risk factors in type 2 diabetes: a randomized controlled trial. Arch Intern Med. 2001;161:1772–6.
Boukhris M, Tomasello SD, Marzà F, et al. Coronary heart disease in postmenopausal women with type II diabetes mellitus and the impact of estrogen replacement therapy: a narrative review. Int J Endocrinol. 2014;2014:413920.
Lilley SH, Spivey JM, Vadlamudi S, et al. Lipid and lipoprotein responses to oral combined hormone replacement therapy in normolipemic obese women with controlled type 2 diabetes mellitus. J Clin Pharmacol. 1998;38:1107–15.
Xu Y, Lin J, Wang S, et al. Combined estrogen replacement therapy on metabolic control in postmenopausal women with diabetes mellitus. Kaohsjung J Med Sci. 2014;30:350–61.
Davidson MH, Ballantyne CM, Jacobson TA, et al. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–67.
Blake GJ, Otvos JD, Rifai N, et al. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–7.
Mackey RH, Kuller LH, Sutton-Tyrrell K, et al. Lipoprotein subclasses and coronary artery calcium in postmenopausal women from the Healthy Women Study. Am J Cardiol. 2002;90:71i–6i.
Mora S, Otvos JD, Rifai N, et al. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–9.
Ip S, Lichtenstein Ah, Chung M, et al. Systematic review: association of low density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med. 2009;150:474–84.
Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21:305–11.
Authors’ contributions
Each of the authors has contributed substantially to the design, planning, analysis, interpretation, preparation of draft and critical content review. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fonseca, M.I.H., da Silva, I.T. & Ferreira, S.R.G. Impact of menopause and diabetes on atherogenic lipid profile: is it worth to analyse lipoprotein subfractions to assess cardiovascular risk in women?. Diabetol Metab Syndr 9, 22 (2017). https://doi.org/10.1186/s13098-017-0221-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-017-0221-5