Abstract
Background
Echocardiography has become an integral part of the management of critically ill patients. It helps to diagnose and treat various conditions. COVID-19 patients can develop cardiac dysfunction. We planned to study the echocardiographic parameters in COVID-19 patients.
Methods
We conducted a prospective observational multicenter study after institutional ethical committee approval. COVID-19 pneumonia patients admitted to the intensive care unit (ICU) were enrolled. The echocardiographic evaluation was done within 24–48 hours of admission. Assessment of the left and right heart with systolic and left ventricular diastolic function evaluation was done. The primary outcome was ICU mortality. The secondary outcomes were the length of ICU stay and duration of mechanical ventilation.
Results
Among 573 patients mean age was 57.17 (14.67) with 68.60% being males. On day 1 of ICU, invasive mechanical ventilation was used in 257 (45%) patients. One hundred and forty-eight (25.83%) patients were on vasopressors when echocardiography was performed. Severe left ventricle (LV) systolic dysfunction was seen in 8.7% of patients and had higher odds of mortality [2.48(1.058–5.807), p = 0.037] followed by E and e′ with odds ratio of [0.984(0.971–0.998), p = 0.021] and 0.897 (0.805–0.998), p = 0.046], respectively. E/e′ indicative of filling pressure of the LV was not found to be significant. Troponin I, E/A, and RV dilatation were similar among survivors and non-survivors.
Conclusion
Echocardiographic evaluation in COVID-19 patients showed severe LV systolic dysfunction was associated with ICU mortality. E/e′ was not found to be significant but lower e′ was associated with higher mortality.
Trial registration IEC 131/2020, CTRI/2020/06/025858 date 13th June 2020
Similar content being viewed by others
Background
Echocardiography plays a vital role in diagnosing and treating critically ill patients. Majority of the hospitals use echocardiographic evaluation as an initial screening tool. Echocardiography has various advantages such as availability at the bedside, real-time evaluation, and repeatability as and when necessary [1, 2]. Assessment of COVID-19 patients is not an exception to this.
During the pandemic, it was difficult to perform detailed echocardiography and the focus was shifted to point-of-care echocardiography [3]. Earlier studies have reported difficulties in echocardiographic assessment in acute respiratory distress syndrome (ARDS) patients [4,5,6]. Technical challenges in image acquisition for mechanically ventilated patients, the effect of positive end-expiratory pressure (PEEP) on echo parameters, and apprehension among the healthcare workers in using ultrasound during COVID-19 were some of the difficulties [4,5,6].
The global survey from 63 countries studied confirmed or suspected COVID-19 patients and showed cardiac abnormalities in 50% of patients [7]. There are various studies describing systolic dysfunction of the right and left ventricles and biomarkers [8, 9]. Huang et al. observed LV and RV (right ventricle) systolic dysfunction in one-third of COVID-19 patients. Acute cor pulmonale and age were the predictors of ICU and hospital mortality [8]. Jansson et al. showed COVID-19 patients who developed acute myocardial injury diagnosed with elevated high sensitivity troponin I (hsTnT), a small subset of these patients had LV and RV dysfunction [9]. There is limited data on the assessment of diastolic dysfunction in COVID-19 patients [10, 11]. We aimed to evaluate the association between the echo parameters (right and left ventricular systolic function, LV diastolic function including Troponin I), and ICU mortality.
Methods
A prospective observational study across the 4 centers was conducted. Institutional ethical committee approval was obtained (IEC 131/2020). The echocardiographic evaluation was a routine practice in the majority of ICUs. The waiver of consent was given for all the centers except one. This is a detailed echocardiographic assessment of EPIC19 study patients [12]. Patients were admitted to the ICU for the need for organ support like ventilation, vasopressor, renal replacement therapy, neuromonitoring, or anticipated worsening of the clinical condition. The admission criteria were as per the treating physician. Patients in whom echocardiographic evaluation was done were included in the study. Patients with poor echo window were excluded. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed. The inclusion criteria were laboratory-confirmed cases of COVID-19 ICU patients. The primary outcome was ICU mortality. The secondary outcomes were the length of ICU stay and duration of mechanical ventilation.
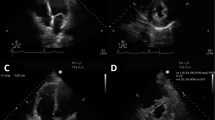
The transthoracic echocardiography (TTE) was performed with Sonosite Edge II or Philips HD11XE machine within 24–48 hours of ICU admission. The cardiac or phased array probe of 2.5 MHz was used. 2D (2 dimensional) echocardiography was done by the intensivists who have received training in focused echocardiography and ultrasound and who are routinely performing screening echocardiography. The majority of ICUs are teaching hospitals hence the echocardiographic evaluation was done by the trainees and supervised by the senior faculty. The echocardiographic evaluation included looking for the heart chamber enlargement and assessment of systolic and diastolic function. The systolic function was assessed by the visual gestalt method. This method evaluates systolic function qualitatively based on the endocardial thickening and also looks into regional wall motion abnormalities. The heart function was assessed by eyeballing using an apical four-chamber view (A4C), parasternal short and long axis, or subcostal view. Left ventricular systolic function was classified as hyperdynamic, good, mildly reduced, moderately reduced, and severely reduced. The presence of any regional wall motion abnormalities or dilatation of the left or right ventricle was captured based on the qualitative evaluation. Right ventricular systolic function was assessed by tricuspid annular pulmonary systolic excursion (TAPSE). RV dysfunction was defined as TAPSE < 1.7 cm [13].
The diastolic function was evaluated by transmitral flow velocity. Early diastolic wave (E) due to rapid filling of the left ventricle (LV), and late systolic wave (A) due to late filling of LV were measured. E/A was calculated. The mitral annular flow velocities were measured. The diastolic waves e′ and a′ were measured at the lateral mitral annulus. E/e′ indicative of the filling pressure of the LV was calculated [8, 9]. We used lateral e′ for calculation for the uniform data capture. For patients with mitral valve pathology or having atrial fibrillation, a doppler assessment was not performed. The classification of diastolic dysfunction was done based on the lateral e′ velocity of < 10 cm/s and E/e` values further categorized based on the E/e′ [14, 15].
The E/e′ of < 8, E/e′ 8–12, and E/e′ ratio > 12 are graded as grade I, II, and III. It was based on the study by Lanspa, et al [16]. As we measured lateral e′ velocity, we used standard cut-offs applicable to lateral e′ [15, 16]. We reported data according to the PRICES statement mentioning (1) baseline characteristics and comorbidities; (2) vasopressors requirement and need for invasive ventilator support, plateau pressure, and positive end-expiratory pressure (PEEP); (3) information on LV systolic, RV systolic, and LV diastolic function including biomarker Troponin I [17].
Statistics
The mean (standard deviation SD), or median (interquartile range IQR) were used as indicated. The categorical variables were presented as (%) percentages. The quantitative data with parametric and nonparametric distribution were analyzed with the ‘Independent t test’ and ‘Mann–Whitney U’ test, respectively. The Chi-square test was used for qualitative data analysis. To assess the association between various echocardiographic parameters with ICU mortality, considering the collinearity, each echocardiographic parameter was analyzed separately. Univariate and multivariable logistic regression analysis was performed. Clinical relevance and variables that had a p-value less than 0.10 in the univariate analysis were considered for multivariable logistic regression and an adjusted p-value less than 5% was considered statistically significant. We used STATA 15, College Station, TX software.
Results
Among 667 patients, 30 patients with poor echo window were excluded as per the exclusion criteria, and for 64 (10.04%) patients data were unavailable. Total of 573 patients were included with 459, 48, 35, and 31 patients from each center, and 95% of echo evaluation was performed within 24 hours of ICU admission. The mean age was 57.17 (14.67) and 68.60% were males. The Acute Physiology, Age, and Chronic Health Evaluation (APACHE II) score was 30.10 (5.96) and the Sequential Organ Failure Assessment (SOFA) score was 7 (4–11). Two seventy-six patients (48.16%) were transferred from the ward to ICU. Among these noninvasive ventilation (NIV) was used in 82 (29.71%) patients before ICU admission. On day1 of ICU, invasive mechanical ventilation was used in 257 (45%), NIV in 148 (25.83%), oxygen therapy in 141 (24.60%), HFNC in 15 (2.62%), and 12 (2.09%) patients were on room air. One hundred and forty-eight (25.83%) patients were on vasopressors when echocardiography was performed. Electrocardiogram (ECG) on day 1 was recorded. The predominant ECG rhythm was sinus. The mean heart rate was 97 (20) beats per minute on day 1 of ICU. The maximum PEEP (PEEPmax) was 10.52 (3.43) cm of H2O. Maximum plateau pressure was 30.46 (6.53) cm of H2O. Among the comorbidities, diabetes mellitus (DM) and ischemic heart disease (IHD) were significantly associated with mortality (Table 1). The elderly population and patients with higher APACHE II and SOFA scores were associated with mortality (p < 0.001). Requirement of invasive ventilation support, vasopressors, and PEEPmax were significantly higher in non-survivors (p < 0.01) (Table 1).
Outcomes
The ICU mortality was 60% (95% CI 55–63%). The secondary outcomes were similar between survivors and non-survivors (Table 2).
Echocardiographic evaluation
The systolic dysfunction was classified into different categories (Table 3). Severe systolic dysfunction was significantly associated with mortality (p = 0.007). The regional wall motion abnormalities (RWMA) were higher in non-survivors than survivors (p = 0.005) (Table 3). Among patients having RWMA (90 patients), 85.55% of patients had LV systolic dysfunction.
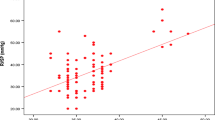
The diastolic function evaluation was routinely performed in one of the centers [18]. It showed significantly lower E/A values among non-survivors than in survivors [0.91 (0.70–1.14) vs 1.14 (0.94–1.42), p < 0.01]. The e′ indicative of relaxing properties of the LV was significantly lower in non-survivors than in survivors [9.88 (3.03) vs 10.84 (2.75) vs, p = 0.021]. The E/e′ was not a significant parameter (Table 3). Grade I, II, and III diastolic dysfunction was seen in 30%, 53%, and 17%, of patients, respectively, was not statistically significant (p = 0.562) (Fig. 1). Troponin I was significantly higher in non-survivors than survivors. (p = 0.015) (Table 3).
We did a logistic regression analysis of the echocardiographic parameters after adjusting for the variables like age, gender, history of DM, hypertension, IHD, need for invasive ventilator support, and requirement of vasopressors (Table 4). The adjusted odds ratio showed severe LV systolic dysfunction, E and e′ were the significant parameters predictive of mortality with the odds ratio of 2.48 (1.058–5.807), 0.984 (0.971–0.998) and 0.897 (0.805–0.998), respectively.
Discussion
In COVID-19 patients among the various echocardiographic parameters, severe LV systolic dysfunction was associated with ICU mortality and had a higher odds ratio (Table 4). A recent study describing patients with sepsis and septic shock showed an association of LV systolic dysfunction with hospital mortality. It showed a “U” shaped association suggesting patients with severe systolic dysfunction with ejection fraction of < 25% as well as hyperdynamic LV function with EF > 70% had higher hospital mortality [19].
The echocardiographic evaluation is feasible in patients of ARDS requiring invasive ventilator support. During COVID-19 pandemic, initial ultrasound evaluation helped to understand the disease severity better [20]. However, we did not collect information on changes in the treatment plan based on the echocardiography. In our study, 45% of the patients were on invasive ventilator support. The echocardiographic assessment was done by the intensivists (94.44%, 543/573) as against cardiologists in the Szekeley et al. study [4, 5, 10].
There are various studies describing echocardiographic parameters in COVID-19 patients. The initial study by Ceriani, et al., described elevated systolic pulmonary artery pressure as one of the significant parameters in patients with severe pneumonia [30.67 (5.16) vs 26.24 (4.34), p = 0.006] but it was not associated with the adverse outcomes. This study used mortality and or the need for invasive ventilation as the adverse outcomes [21]. Schott et al., mentions severe RV dilatation observed in non-survivors was not statistically significant [5].
A systematic review describing echocardiographic parameters observed normal echocardiographic findings in 50% of the patients with preserved LV ejection fraction [22]. The patient population described was a general population irrespective of the severity of the illness. In our study, the ICU population was included. In the ECHO-COVID study, the majority of the patients had normal LV systolic function with abnormal LV and or RV dysfunction in one-third of patients [8]. Similarly in our study, LV systolic function was normal in 63.15% of patients. The proportion of patients having severe LV dysfunction was similar in our study 8.7% vs 6.5% in the ECHO-COVID study.
We observed LV dilatation was more prevalent (17.04%) than in the ECHO-COVID study (8%) and among the patients with septic shock requiring vasopressors (148, 25.82%), LV dilatation was observed in 47.30%. LV dilatation present in shock patients was pre-existing as acute dilatation of the left ventricle in shock is rare based on the available literature [23]. However, repeat echocardiographic assessment after the resolution of shock would have helped to confirm the pre-existing vs acute reversible LV dilatation as a result of viral myocarditis [24, 25].
In a study by Szekeley et al., systolic function was preserved and 3 patients were on vasopressor support [10]. As against in our study although the majority of the patients had normal LV systolic function, the proportion of patients having systolic dysfunction were higher (36.85%, 199/540) with 25.82% being on vasopressors. In comparison with the LV diastolic function, our patients had a lower mean E/e′ ratio (8.09 vs 10.5). This could be due to the effect of PEEP on LV preload and afterload. Also among the comorbidities, we had a lesser number of patients with IHD 14.3% as against 23% (including IHD 16%, and congestive heart failure 7%) in Szekeley’s study.
The study by Luigi La via et al. describes a single-center experience on the diastolic function evaluation in 35 patients. It showed non-survivors had lower ‘s’ wave and higher E/e′ measured at the lateral mitral leaflet [11]. As against we did not find any difference in E/e′ in non-survivors and survivors [8.11 (2.69) vs 8.05 (2.59), p = 0.872]. We found e′ was lower in non-survivors than survivors [9.88 (3.03) vs 10.84 (2.75), p = 0.021], suggesting impaired LV relaxation was associated with the non-survivors.
There are studies describing different phenotypic patterns of RV, classified into 3 types as preserved RV function, dilatation of RV with preserved systolic function, and class 3 as RV dilatation with severely impaired systolic function. It was a single-center retrospective study [26]. In our study, evaluation of RV systolic function by TAPSE was available only in 26 patients (Table 3).
The strengths of our study include, it is one of the few prospective multicenter studies describing biventricular echocardiographic parameters. The study describes both systolic as well as diastolic function assessment of the LV and limited information on RV systolic function and Troponin I in COVID-19 patients.
The limitations of the study are only a single echocardiographic evaluation was performed at the time of admission. Hence the subsequent effect of PEEP and different therapeutic maneuvres such as recruitment or proning could not be studied. We suggest monitoring the trend of RV function will help in implementing therapeutic strategies such as proning or extracorporeal membrane oxygenation (ECMO) as the early interventions. The echocardiography was performed by different operators, hence the interobserver variability could not be ruled out. With the training experience of the operators, only screening evaluation was possible during the pandemic. We did not include the left atrial volume index and tricuspid regurgitation velocity parameters as suggested by the 2016 guidelines [27]. The left atrial volume index will have limited applicability as an acute increase in diastolic pressure may not result in dilatation of the left atrium [28]. We used Doppler-based parameters for diastolic dysfunction evaluation (E/A, e′ at the lateral mitral annulus, and E/e′) based on the study by Lanspa et al. [16]. We strongly believe that qualitative evaluation is rapid and can provide valuable information, as compared to quantitative evaluation in ICU patients [29]. Although there are limitations with the use of qualitative evaluation, it is a rapid screening tool for ICU patients when clinicians have varied skills in echocardiography such as basic to advanced training. Echocardiographic parameters need careful interpretation for each patient’s clinical characteristics such as age, gender, comorbidities, invasive ventilation, and fluid status for deciding patient management. Ultrasound has a vital role in the management of COVID-19 patients [30]. Integration of the lung ultrasound with echocardiographic parameters and diaphragm will be useful in understanding disease severity and deciding patient management as suggested by Dell’Aquila, et al [31]. In our study, lung ultrasound was performed in one of the centers and lung ultrasound score was similar in survivors and non-survivors [12].
Future studies describing serial echocardiographic assessment are required. To observe the effect of PEEP on routine echocardiographic parameters and ventricular interdependence as well as on ventilator parameters (PaO2/FiO2 ratio, PCO2, and pH) will be useful. Patients with pre-existing LV diastolic dysfunction with elevated LV filling pressure can develop ARDS due to lung pathology. Diastolic function assessment is as important as systolic function. It can help in optimization of the PEEP and fluid balance in managing interstitial and hydrostatic edema for these patients and can assist in weaning [32]. We need a comprehensive approach in managing ARDS patients. Evaluation of cardiac, lung, and diaphragm ultrasound can help in managing these patients in different phases of illness and improving outcomes [33].
Conclusion
The study describing echocardiographic parameters in COVID-19 patients showed that left ventricular systolic dysfunction assessed by the visual gestalt method is one of the parameters to predict the ICU mortality.
Availability of data and materials
Data will be available after the reasonable request.
Abbreviations
- APACHE II:
-
Acute Physiology, Age, and Chronic Health Evaluation
- ARDS:
-
Acute respiratory distress syndrome
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CVA:
-
Cerebrovascular accident
- DM:
-
Diabetes Mellitus
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- IEC:
-
Institutional ethical committee
- IHD:
-
Ischemic heart disease
- LV:
-
Left ventricle
- PEEP:
-
Positive end-expiratory pressure
- RV:
-
Right ventricle
- RWMA:
-
Regional wall motion abnormalities
- SOFA:
-
Sequential Organ Failure Assessment
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Vieillard-Baron A, Millington SJ, Sanfilippo F, Chew M, Diaz-Gomez J, McLean A et al (2019) A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 45:770–788
Beaulieu Y (2007) Specific skill set and goals of focused echocardiography for critical care clinicians. Crit Care Med 35:S144–S149
Picard MH, Weiner RB (2020) Echocardiography in the time of COVID-19. J Am Soc Echocardiogr 33:674–675
Lazzeri C, Cianchi G, Bonizzoli M, Batacchi S, Peris A, Gensini GF (2016) The potential role and limitations of echocardiography in acute respiratory distress syndrome. Ther Adv Respir Dis 10:136–148
Schott JP, Mertens AN, Bloomingdale R, O’Connell TF, Gallagher MJ, Dixon S et al (2020) Transthoracic echocardiographic findings in patients admitted with SARS-CoV-2 infection. Echocardiography 37:1551–1556
Zwaenepoel B, Dhont S, Hoste E, Gevaert S, Schaubroeck H (2021) The prognostic value of cardiac biomarkers and echocardiography in critical COVID-19. Front Cardiovas Med. https://doi.org/10.3389/fcvm.2021.752237
Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE (2020) Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 21(9):949–958
Huang S, Vignon P, Mekontso-Dessap A, Tran S, Prat G, Chew M, Balik M, Sanfilippo F, Banauch G, Clau-Terre F, Morelli A (2022) Echocardiography findings in COVID-19 patients admitted to intensive care units: a multi-national observational study (the ECHO-COVID study). Intensive Care Med 48:667–678
Jansson S, Blixt PJ, Didriksson H, Jonsson C, Andersson H, Hedström C, Engvall J, Aneq MÅ, Chew MS (2022) Incidence of acute myocardial injury and its association with left and right ventricular systolic dysfunction in critically ill COVID-19 patients. Ann Intensive Care 12(1):56
Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y (2020) Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 142(4):342–353
La Via L, Dezio V, Santonocito C, Astuto M, Morelli A, Huang S et al (2022) Full and simplified assessment of left ventricular diastolic function in covid-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 39:1391–1400
Havaldar AA, Kumar MV, Vijayan B, Prakash J, Kartik M, Sangale A (2022) Epidemiology and ventilation characteristics of confirmed cases of severe COVID-19 pneumonia admitted in intensive care unit (EPIC19): A multicentre observational study. Indian J Anaesth 66:724–733. https://doi.org/10.4103/ija.ija_179_22
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28(1):1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imag 17:1321–1360
Mitter SS, Shah SJ, Thomas JD (2017) A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 69:1451–1464
Lanspa MJ, Gutsche AR, Wilson EL, Olsen TD, Hirshberg EL, Knox DB, Brown SM, Grissom CK (2016) Application of a simplified definition of diastolic function in severe sepsis and septic shock. Crit Care 20:1–8
Sanfilippo F, Huang S, Herpain A, Balik M, Chew MS, Clau-Terré F, Corredor C, De Backer D, Fletcher N, Geri G, Mekontso-Dessap A (2021) The PRICES statement: an ESICM expert consensus on methodology for conducting and reporting critical care echocardiography research studies. Intensive Care Med 47:1–3
Amarja H, Bhuvana K, Sriram S (2019) Prospective observational study on evaluation of cardiac dysfunction induced during the weaning process. Indian J Crit Care Med 23(1):15
Dugar S, Sato R, Chawla S, You JY, Wang X, Grimm R, Collier P, Lanspa M, Duggal A (2023) Is left ventricular systolic dysfunction associated with increased mortality among patients with sepsis and septic shock? Chest 163(6):1437–1447
Yuriditsky E, Saric M, Horowitz JM (2021) Point-of-care ultrasound during the COVID-19 pandemic: a multidisciplinary approach between intensivists and echocardiographers. Echocardiography 38(3):446–449
Ceriani E, Marceca A, Lanfranchi A, De Vita S, Schiavon R, Casella F et al (2021) Early echocardiographic findings in patients hospitalized for COVID-19 pneumonia: a prospective, single center study. Intern Emerg Med 16:2173–2180
Messina A, Sanfilippo F, Milani A, Calabrò L, Negri K, Garcia MI et al (2021) COVID-19-related echocardiographic patterns of cardiovascular dysfunction in critically ill patients: a systematic review of the current literature. J Crit Care 65:26–35
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168(11):1270–1276
Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby JJ (2009) Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit Care Med 37(2):441–447
Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CA (2020) Recognizing COVID-19–related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 17(9):1463–1471
Chotalia M, Ali M, Alderman JE, Patel JM, Parekh D, Bangash MN (2022) Cardiovascular subphenotypes in patients with COVID-19 pneumonitis whose lungs are mechanically ventilated: a single-centre retrospective observational study. Anaesthesia 77:763–771
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur J Echocardiogr 17(12):1321–1360
Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM (2005) Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 45(1):87–92
Vieillard-Baron A, Charron C, Chergui K, Peyrouset O, Jardin F (2006) Bedside echocardiographic evaluation of hemodynamics in sepsis: is a qualitative evaluation sufficient? Intensive Care Med 32:1547–1552
Havaldar AA (2020) Vital role of ultrasound in the era of COVID-19: arriving at the right diagnosis real time. Indian J Crit Care Med 24(7):563–564
Dell’Aquila P, Raimondo P, Racanelli V, De Luca P, De Matteis S, Pistone A, Melodia R, Crudele L, Lomazzo D, Solimando AG, Moschetta A (2022) Integrated lung ultrasound score for early clinical decision-making in patients with COVID-19: results and implications. Ultrasound J 14(1):1–8
Sanfilippo F, Bignami EG, Astuto M, Messina A, Cammarota G, Maggiore SM, Vetrugno L (2022) Understanding left ventricular diastolic dysfunction in anesthesia and intensive care patients: “a glass with progressive shape change.” Minerva Anestesiol 88(11):950–960
Guarracino F, Vetrugno L, Forfori F, Corradi F, Orso D, Bertini P, Ortalda A, Federici N, Copetti R, Bove T (2021) Lung, heart, vascular, and diaphragm ultrasound examination of COVID-19 patients: a comprehensive approach. J Cardiothorac Vasc Anesth 35(6):1866–1874
Acknowledgements
The authors would like to thank staff of Critical care department of each of the hospitals who helped in completing this study.
Author information
Authors and Affiliations
Contributions
AAH—concept, design, conduct, writing and finalizing manuscript. MVK, RK, SPY, SK, MSK, KCM, AS, JP and MK helped in the data collection and participation from the respective centers. SS helped in statistical analysis. All the authors had approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participation were obtained as applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Havaldar, A.A., Kumar, M.V., Kumar, R. et al. Echocardiographic parameters in COVID-19 patients and their association with ICU mortality: a prospective multicenter observational study. Ultrasound J 15, 38 (2023). https://doi.org/10.1186/s13089-023-00336-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13089-023-00336-3