Abstract
Background
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is a multisystem fibroinflammatory disease. We previously reported that a circulating cell population expressing CD19+CD24−CD38hi was increased in patients with IgG4-RD. In this study, we aimed to document that this cell population represented circulating plasmablasts/plasma cells, to identify the detailed phenotype and gene expression profile of these IgG4-secreting plasmablasts/plasma cells, and to determine whether this B-cell lineage subset could be a biomarker in IgG4-related disease (IgG4-RD).
Methods
A total of 42 untreated patients with IgG4-RD were evaluated. Peripheral B-cell subsets, including CD19+CD24−CD38hi plasmablasts/plasma cells, CD19+CD24+CD38− memory B cells, CD19+CD24intCD38int naïve B cells, and CD19+CD24hiCD38hi regulatory B cells, were assessed and sorted by flow cytometry. Microarray analysis was used to measure gene expression of circulating B-cell lineage subsets. Further characterization of CD19+CD24−CD38hi plasmablasts/plasma cells was carried out by evaluating additional surface markers, including CD27, CD95, and human leukocyte antigen (HLA)-DR, by flow cytometric assay. In addition, various B-cell lineage subsets were cultured in vitro and IgG4 concentrations were measured by cytometric bead array.
Results
In untreated patients with IgG4-RD, the peripheral CD19+CD24−CD38hi plasmablast/plasma cell subset was increased and positively correlated with serum IgG4 levels, the number of involved organs, and the IgG4-related Disease Responder Index. It decreased after treatment with glucocorticoids. Characterization of the plasmablast/plasma cell population by gene expression profiling documented a typical plasmablast/plasma cell signature with higher expression of X-box binding protein 1 and IFN regulatory factor 4, but lower expression of paired box gene 5 and B-cell lymphoma 6 protein. In addition, CD27, CD95, and HLA-DR were highly expressed on CD19+CD24−CD38hi plasmablasts/plasma cells from patients with IgG4-RD. Furthermore, CD19+CD24−CD38hi plasmablasts/plasma cells secreted more IgG4 than other B-cell populations.
Conclusions
Circulating CD19+CD24−CD38hi plasmablasts/plasma cells are elevated in active IgG4-RD and decreased after glucocorticoid treatment. This IgG4-secreting plasmablast/plasma cell population might be a potentially useful biomarker for diagnosis and assessing response to treatment.
Similar content being viewed by others
Background
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is a newly recognized fibroinflammatory disease characterized by infiltration of IgG4+ plasma cells together with distinct storiform fibrosis in involved lesions and significantly increased serum IgG4 levels [1–3]. There are various clinical manifestations in patients with IgG4-RD, including Mikulicz’s disease, autoimmune pancreatitis, IgG4-related retroperitoneal fibrosis, pulmonary involvement, kidney disease, sclerosing cholangitis, sinusitis, sclerosing thyroiditis, and others [4–12].
IgG4-RD is characterized by a number of abnormalities in the differentiation of cells of the B-cell lineage, including increased serum levels of IgG, IgG4, and often IgE; infiltration of affected tissues by IgG4-secreting plasma; and the presence of increased frequencies of circulating plasma cells/plasmablasts [13–15]. B cells and plasma cells may play important roles in the development of this disease [16]. Our previous studies revealed that, compared with healthy control subjects and patients with primary Sjögren’s syndrome, patients with IgG4-RD expressed an increased circulating population of CD19+CD24−CD38hi cells that appeared to be circulating plasmablasts/plasma cells and correlated positively with serum IgG4 levels [17]. The full characterization of this circulating population was not carried out, but its appearance and correlation with serum IgG4 suggested that it might be an important biomarker of IgG4-RD. To begin to address these questions, we initially characterized the circulating plasmablasts/plasma cells in IgG4-RD in greater detail.
Methods
Patients
Forty-two untreated patients with IgG4-RD fulfilling the 2011 comprehensive IgG4-RD diagnostic criteria were enrolled in this study. The diagnosis of IgG4-RD was based on the following three manifestations: (1) clinical examination showing characteristic diffuse/localized swelling or masses in single or multiple organs; (2) hematological examination showing elevated serum IgG4 concentration (>135 mg/dl); and (3) histopathologic examination showing (a) marked lymphocyte and plasma cell infiltration and fibrosis or (b) infiltration of IgG4+ plasma cells (ratio of IgG4+/IgG+ cells >40% and >10% IgG4+ plasma cells per high-power field). Patients with cancer or lymphoma and other autoimmune diseases were excluded.
Clinical data and inflammatory parameters
Clinical data, including age, gender, disease duration, and manifestations, were obtained for all patients. Laboratory findings were recorded, including erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); and serum immunoglobulin IgG, IgA, IgM, and IgG subsets. IgG4-related Disease Responder Index (IgG4-RD RI) was calculated for each patient [18].
Flow cytometric analysis and separation of B-cell subpopulations
Peripheral blood mononuclear cells (PBMCs) from patients with IgG4-RD were separated by Ficoll gradient centrifugation. Different B-cell populations were stained with phycoerythrin (PE)-cyanine 7 (Cy7)-anti-CD19, fluorescein isothiocyanate-anti-CD24, allophycocyanin-anti-CD38, peridinin chlorophyll protein-CY5.5-anti-CD27, PE-anti-CD40, PE-anti-CD80, PE-anti-CD86, PE-anti-B-cell activating factor receptor (BAFF-R), PE-anti-transmembrane activator CAML (calcium modulator and cyclophilin ligand) interactor (TACI), PE-anti-CD95, PE-anti-CD138, PE-anti-interleukin-6 receptor (IL-6R), PE-anti-IgD, and PE-anti-CD59 monoclonal antibodies (mAbs) (BD Biosciences, San Jose, CA, USA), as well as PE-anti-B-cell maturation antigen (BCMA) mAb (BioLegend, San Diego, CA, USA), or isotype-matched controls. After incubation for 30 minutes at 4 °C, the cells were washed and resuspended in fluorescence-activated cell sorting staining buffer (BD Biosciences). All experiments were analyzed by gating on lymphocytes according to forward scatter/side scatter; dead or dying cells or granulocytes were excluded. B-cell subsets were gated on CD19 and then gated on CD24 and CD38. Flow cytometric analysis was performed immediately after sample preparation. All samples were analyzed using a BD FACSAria II system (BD Biosciences), and data were analyzed by FlowJo version 7.6.4 software (FlowJo, Ashland, OR, USA).
PBMCs were stained with CD19, CD24, and CD38 antibodies and then sorted with a MoFlo high-performance cell sorter (Beckman Coulter Life Sciences, Indianapolis, IN, USA). Different B-cell subsets were separated, including CD19+CD24+CD38− memory B cells, CD19+CD24intCD38int naïve B cells, CD19+CD24hiCD38hi regulatory B cells (Bregs), and CD19+CD24−CD38hi plasmablasts/plasma cells. Sorted B-cell populations with purity greater than 95% were used for in vitro culture and RNA extraction.
In vitro cell culture
Purified B-cell populations from patients with IgG4-RD were resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics (penicillin 100 IU/ml, streptomycin 100 μg/ml; Life Technologies, Carlsbad, CA, USA) in 96-well U-bottomed plates (Nunc, Langenselbold, Germany) in a humidified atmosphere of 5% CO2 at 37 °C with 1 × 105 cells in each well. For each group, 100 ng/ml recombinant human CD40L (Abcam, Cambridge, MA, USA) and 0.1 μg/ml cytosine phosphate guanosine oligodeoxynucleotide 2006 (CpG ODN 2006; InvivoGen, San Diego, CA, USA) was added at the beginning. After 7 days of culture, the levels of Ig secretion in collected supernatants were tested.
Cytometric bead array analysis
Culture supernatant samples from different B-cell subsets were collected and stored at −80 °C until used. Cytometric bead array (CBA) analysis for IgG, IgA, IgM, IgG4, and IgE in supernatants was performed according to the manufacturer’s instructions (BD Biosciences). Data were analyzed using CBA analysis software obtained from BD Biosciences. The concentrations of IgG, IgA, IgM, IgG4, and IgE in supernatants were determined by reference to a standard curve.
Microarray analysis of gene expression
Sorted B-cell subpopulations were placed in TRIzol reagent (Life Technologies) for RNA extraction following the manufacturer’s instructions. Isolated RNA was further purified with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and processed for microarray analysis using the standard Affymetrix protocols (www.affymetrix.com; Affymetrix, Santa Clara, CA, USA). Briefly, 1–10 μg of RNA was reverse-transcribed into complementary DNA (cDNA) (Life Technologies). The template cDNA was purified for amplification and in vitro transcription to cRNA using the BioArray™ HighYield™ RNA Transcript Labeling Kit (T7) (Enzo Life Sciences, Inc., Farmingdale, NY, USA). cRNA was biotin-labeled, purified, and hybridized to Human Genome U133A GeneChips® (Affymetrix). GeneChips® were scanned on a high-resolution scanner using GCOS version 1.2 software (Affymetrix). Data analysis was conducted after standard Affymetrix algorithm analysis (MAS5).
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics version 19.0 software (IBM, Armonk, NY, USA) and Prism software version 5.0 (GraphPad Software, La Jolla, CA, USA). A P value <0.05 was considered significantly different. Data are reported as mean ± SD. Normal distribution data between two groups were analyzed using independent-samples t tests or paired-samples t tests, and one-way analysis of variance (ANOVA) was used to compare groups. The relationships between CD19+CD24−CD38hi plasmablasts/plasma cells and clinical features were analyzed by Pearson’s rank correlation test, and a P value <0.05 was considered significant. The level of gene expression was standardized by robust multiarray average and detection above background. One-way ANOVA was used to test different levels of gene expression. The Benjamini-Hochberg method was employed to determine the false discovery rate after multiple hypothesis testing. A false discovery rate <0.3 was used.
Results
Characteristics of patients with IgG4-RD
All 42 patients were newly diagnosed, untreated patients with IgG4-RD. Their demographic features as well as clinical and laboratory manifestations are listed in Table 1. Their average age was 55 (41.5–60) years old, and the male-to-female ratio was 2.23:1. Thirty-two (76.2%) of the patients were characterized as definite IgG4-RD, 1 (2.4%) was classified as probable IgG4-RD, and 9 (21.4%) were categorized as possible IgG4-RD. The majority of patients had multiple organ involvement. Forty-one (97.6%) patients had elevated serum IgG4.
Correlations of CD19+CD24−CD38hi plasmablasts/plasma cells with clinical and laboratory parameters in patients with IgG4-RD
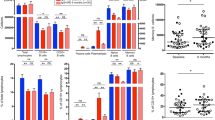
Our previous studies revealed that CD19+CD24−CD38hi plasmablasts/plasma cells were significantly increased in the peripheral blood of patients with IgG4-RD (6.99 ± 6.24%), higher than that in patients with Sjögren’s syndrome (2.39 ± 2.64%, P < 0.001) and healthy control subjects (2.16 ± 1.65%, P < 0.001) [17]. The plot with the gating strategy for identifying the different B-cell subpopulations is shown in Fig. 1a. In this study, we further analyzed the association of these B-lineage populations with clinical and laboratory parameters of IgG4-RD. Figure 1b–h illustrates the correlations between CD19+CD24−CD38hi plasmablasts/plasma cells and clinical/laboratory parameters and shows that the ratio of CD19+CD24−CD38hi plasmablasts/plasma cells/CD19+ B cells correlated positively with serum IgG4 (R = 0.4852, P = 0.0001), the numbers of involved organs (R = 0.5039, P < 0.0001), and ESR (R = 0.3557, P = 0.0097), but that it had no correlation with serum IgG (P = 0.0874), serum IgE (P = 0.6020), or CRP (P = 0.2580). In addition, the absolute number of CD19+CD24−CD38hi cells in patients with IgG4-RD was markedly elevated at 7100/ml (2711–16,297/ml), compared with 1475/ml (1021–2792/ml) in healthy subjects (P < 0.001). Importantly, the absolute number of CD19+CD24−CD38hi plasmablasts/plasma cells significantly correlated with serum IgG4 as well (R = 0.3088, P = 0.0466) (Fig. 1c).
The correlations of CD19+CD24−CD38hi plasmablasts/plasma cells with clinical and laboratory parameters. a Gating strategy of CD19+CD24−CD38hi plasmablasts/plasma cells. Correlations of the ratio of CD19+CD24−CD38hi plasmablasts/plasma cells and serum (b) immunoglobulin G4 (IgG4), (d) erythrocyte sedimentation rate (ESR), (e) IgG, (f) IgE, (g) C-reactive protein (CRP), and (h) involved organs are shown. c Correlation of the absolute number of CD19+CD24−CD38hi plasmablasts/plasma cells and serum IgG4. SSC Side scatter
Differences in gene expression profiling among CD19+C24−CD38hi plasmablasts/plasma cells, naïve B cells, memory B cells, and regulatory B cells
To further analyze the characteristics of CD19+CD24−CD38hi plasmablasts/plasma cells, we collected PBMCs from 15 newly diagnosed patients with IgG4-RD, and the following B cell subsets were sorted by flow cytometry: Bregs (CD19+CD24hiCD38hi), memory B cells (CD19+CD24+CD38−), naïve B cells (CD19+CD24intCD38int), and plasmablasts/plasma cells (CD19+CD24−CD38hi). Because the numbers of each B-cell subset were small, the subsets of B cells from individual patients were pooled. Total RNA was extracted for microarray analysis. Hierarchical clustering visualized by heat mapping indicated significant differences in gene expression by the four B-cell subsets (Fig. 2). Importantly, CD19+CD24−CD38hi plasmablasts/plasma cells were the most different from the other populations. Among genes that were differentially upregulated in CD19+CD24−CD38hi plasmablasts/plasma cells compared with naïve B cells and memory B cells, we found those that are known to alter the B-cell program and commit a B cell to plasma cell differentiation, such as interferon regulatory factor 4 (IRF4), PR domain containing 1 (PRDM1), and X-box binding protein 1 (XBP1). Ig heavy and light chains as well as J chain were also significantly upregulated, in addition to those previously reported to be upregulated in plasma cell genes, such as VDR, CAV1, SDC1, SSR4, ERP70, PPIB, ITGA6, CD38, and others listed in Tables 2 and 3 [19–26]. Both IGK and IGL genes were upregulated, implying the polyclonal nature of the plasma cell expansion. Genes involved in plasma cell homing to tissue niches such as SDC1 were also upregulated, although CXCR4, which is also involved in homing of plasma cells to niches, was downregulated. Finally, tumor necrosis factor receptor superfamily member 17 (TNFRSF-17), which encodes BCMA, a BAFF-R involved in plasma cell survival, was greatly upregulated in CD19+CD24−CD38hi plasmablasts/plasma cells. As expected, BCR complex, CD19, CD20, CIITA, human leukocyte antigen (HLA)-DRA, and HLA-DRB1 gene expression was downregulated in CD19+CD24−CD38hi plasmablasts/plasma cells. Likewise, the genes characteristic of earlier stages of B-cell development or differentiation, such as SPIB, BCL6, BLK, FYN, BCL11A, CD37, CD1C, VAV3, CCR6, and CD22, were downregulated significantly.
Gene expression of CD19+CD24−CD38hi plasmablasts/plasma cells, regulatory B cells (Bregs), memory B cells, and naïve B cells in patients with immunoglobulin G4-related disease (IgG4-RD). B-cell subsets were sorted from the peripheral blood of patients with IgG4-RD, and total RNA was extracted for microarray analysis. The differences among CD19+CD24−CD38hi plasmablasts/plasma cells and other B-cell subsets are shown. Columns from left to right represent CD19+CD24−CD38hi plasmablasts/plasma cells, CD19+CD24intCD38int naïve B cells, CD19+CD24+CD38− memory B cells, and CD19+CD24hiCD38hi Bregs, respectively. Red represents overexpressed genes, and blue represents underexpressed genes
B-cell differentiation-related transcription factor genes in CD19+CD24−CD38hi plasmablasts/plasma cells in patients with IgG4-RD
The transcription factors regulating B-cell differentiation into plasmablasts/plasma cells were analyzed in the various B-cell subsets in greater detail (Fig. 3). In patients with IgG4-RD, B-cell lymphoma 6 protein (BCL6) and PRDM1, a pair of mutually restrictive transcription factors involved in B-cell differentiation, showed opposite expression in CD19+CD24−CD38hi plasmablasts/plasma cells compared with the other three B-cell subsets, with BCL6 expression being lower and PRDM1 expression higher in CD19+CD24+CD38hi plasmablasts/plasma cells. In addition, compared with the other three B-cell subsets, the expression of paired box gene 5 (PAX5) in CD19+CD24−CD38hi plasmablasts/plasma cells was much lower. Genes related to PAX5, including IRF8, SPIB, Bach2, EBF, ID3, and CIITA, were also downregulated. These results confirm that CD19+CD24−CD38hi cells are plasmablasts/plasma cells.
The major transcription factors regulating B-cell differentiation into plasma cells. Analysis of transcription factor expression regulating B-cell differentiation into plasma cells. Bars A to D indicate CD19+CD24−CD38hi plasmablasts/plasma cells, regulatory B cells, memory B cells, and naïve B cells, respectively. *Difference of more than twofold by comparison of CD19+CD24−CD38hi plasmablasts/plasma cells with other B-cell subsets
Next, we analyzed other important genes related to the differentiation of B cells. Compared with the other three B-cell subsets, CD22 was markedly downregulated in CD19+CD24−CD38hi plasmablasts/plasma cells, whereas HLA class II genes (HLA-DRA, HLA-DRB1), CIITA, and CD19 were significantly downregulated, compared with memory and naïve B cells (Fig. 4, Tables 2 and 3). High expression of IGHD, IGJ, IGK, and IGHM are important indicators for B-cell differentiation into plasma cells. In CD19+CD24−CD38hi plasmablasts/plasma cells from patients with IgG4-RD, IGH gene expression was also markedly increased, as was that of IGK and IGL genes. In addition, the expression of CD27 and Fas/CD95 genes was significantly higher than in the other three B-cell subsets, whereas CD40 gene expression was decreased. CD23 was also markedly decreased in CD19+CD24−CD38hi plasmablasts/plasma cells of patients with IgG4-RD.
Analysis of B-cell proliferation-regulating genes and homing factor genes
Marker of proliferation Ki-67 (MKI67), encoding the Ki-67 protein and denoting recent cellular proliferation, was markedly increased in CD19+CD24−CD38hi plasmablasts/plasma cells compared with the other three B-cell subsets (Fig. 5). We also found that a number of genes involved in cell homing, including SELL, which encodes L-selectin (CD62L), was markedly increased, whereas expression of CCR7, CXCR5, and CXCR4 was greatly decreased, in CD19+CD24−CD38hi plasmablasts/plasma cells compared with the other three B-cell subsets. CCR10 expression was also markedly increased compared with memory B cells and naïve B cells (Fig. 5).
Expression of proliferation genes, homing genes, and immunoglobulin genes in different B-cell subsets. B-cell proliferation genes, homing genes, and immunoglobulin genes were analyzed in B-cell subsets in patients with immunoglobulin G4-related disease. a to l illustrate genes differently expressed in B cell subsets. Bars A to D indicate CD19+CD24−CD38hi plasmablasts/plasma cells, regulatory B cells, memory B cells, and naïve B cells, respectively. *Difference of more than two fold by comparison of CD19+CD24−CD38hi plasmablasts/plasma cells with other B-cell subsets
Expression of activating factors of CD19+CD24−CD38hi plasmablasts/plasma cells
By flow cytometric analysis, we compared the expression of B-cell-activating factors and homing factors in different B-cell subsets in patients with IgG4-RD (Fig. 6). This analysis revealed that CD86, CD62L, HLA-DR, and CD95 were highly expressed in CD19+C24−CD38hi plasmablasts/plasma cells compared with other B-cell subsets. In particular, the majority of CD19+CD24−CD38hi B cells expressed CD27 (76.46 ± 7.16%), which demonstrated that the CD19+CD24−CD38hi population was a larger population that included CD19+CD20−CD27+ plasmablasts/plasma cells. Whereas CD138, BAFF-R, and BCMA were expressed by a subset of CD19+CD24−CD38hi plasma/plasma cells, there was almost no expression of TACI, CD23, CD40, IgE, and IgD by this population.
The phenotypic analysis of CD19+CD24−CD38hi plasmablasts/plasma cells determined by flow cytometry. CD19+CD24−CD38hi plasmablasts/plasma cells were gated and then analyzed by flow cytometry. Red represents isotype control, and blue represents the expression level by CD19+CD24−CD38hi plasmablasts/plasma cells. BAFF B-cell activating factor, BCMA B-cell maturation antigen, Ig Immunoglobulin, IL-6R Interleukin-6 receptor, TACI Transmembrane activator CAML (calcium modulator and cyclophilin ligand) interactor
Immunoglobulin secretion by different B-cell subsets in IgG4-RD
The CD19+C24−CD38hi plasmablasts/plasma cells, Bregs, memory B cells, and naïve B cells from patients with IgG4-RD were isolated by cell sorting and cultured in vitro. At day 7, the supernatants were collected, and the secretion of immunoglobulin was tested.
The results showed that the IgG secreted by CD19+CD24−CD38hi plasmablasts/plasma cells was 618.4 ± 163.3 ng/ml, much higher than that of Bregs and naïve B cells (0.72 ± 0.59 ng/ml and 231.2 ± 44.83 ng/ml, respectively; (P = 0.003 and P = 0.02, respectively), but that it was not different from that of memory B cells (541.0 ± 252.1 ng/ml, P = 0.678). In addition, the IgG4 secreted by CD19+C24−CD38hi plasmablasts/plasma cells was 176.1 ± 28.5 ng/ml, significantly higher than that secreted by Bregs, memory B cells, and naïve B cells (0.87 ± 0.59 ng/ml, P = 0.0005; 26.24 ± 7.08 ng/ml, P = 0.0009; and 5.34 ± 1.50 ng/ml, P = 0.0005, respectively). CD19+CD24−CD38hi plasmablasts/plasma cells also secreted more IgE than other B-cell subsets (3.14 ± 0.99 ng/ml, 2.01 ± 0.01 ng/ml, 2.01 ± 0.01 ng/ml, and 2.00 ± 0.01 ng/ml, respectively; P = 0.054). Regarding other immunoglobulins, there was no significant difference in secreted IgA (20,573 ± 22,215 ng/ml, 12.39 ± 1.96 ng/ml, 5209 ± 1066 ng/ml, and 12105 ± 16970 ng/ml, respectively; P = 0.37) and secreted IgM (2090 ± 2401 ng/ml, 8.34 ± 1.75 ng/ml, 2385 ± 2402 ng/ml, and 2810 ± 2387 ng/ml, respectively; P = 0.41) compared with CD19+C24−CD38hi plasmablasts/plasma cells and the other three subsets (Fig. 7).
The secretion of immunoglobulin by regulatory B cells (Bregs), CD19+C24−CD38hi plasmablasts/plasma cells, memory B cells, and naïve B cells. B-cell subsets, including CD19+CD24−CD38hi plasmablasts/plasma cells, Bregs, memory B cells, and naïve B cells from peripheral blood of patients with immunoglobulin G4-related disease (IgG4-RD) were sorted by flow cytometry and underwent cell culture in vitro. After 7 days of cell culture with CD40L and CpG ODN 2006, the supernatants were collected, and immunoglobulin secretion was analyzed. Horizontal histogram illustrates (a) IgG4, (b) IgG, (c) IgE, and (d) IgM secreted by four subsets of B cells. Columns A–D represent Bregs, CD19+C24−CD38hi plasmablasts/plasma cells, memory B cells, and naïve B cells, respectively. e Flow diagram of a representative patient with IgG4-RD. (*: P <0.05, **: P <0.01, ***: P <0.001)
Correlations of CD19+CD24−CD38hi plasmablasts/plasma cells with disease activity and changes with treatment
Forty-two patients with IgG4-RD were assessed before treatment with glucocorticoids, and 15 patients with IgG4-RD were assessed both before and after treatment with glucocorticoids. Before treatment, both the ratio of CD19+CD24−CD38hi cells/CD19+ B cells and the absolute number of CD19+CD24−CD38hi cells correlated positively with disease activity in terms of IgG4-RD RI (R = 0.575, P < 0.0001; R = 0.373, P = 0.015, respectively) (Fig. 8a, b). After treatment, along with the decrease of IgG4-RD RI (from 13.48 ± 5.60 to 5.06 ± 2.38, P < 0.009) (Fig. 8f), serum IgG (from 25.74 g/L ± 12.55 g/L to 14.06 g/L ± 6.23 g/L, P < 0.001), and IgG4 (from 1740 mg/dl [7160–58,700] to 938 mg/dl [2900–17,300], P < 0.0001) (Fig. 8g, h), both the absolute number (from 5914 [1888–19,487]/ml to 2157 [822–3582]/ml, P = 0.005) and the ratio (from 7.25% ± 6.35% to 2.55% ± 1.50%, P < 0.001) of CD19+C24−CD38hi plasmablasts/plasma cells decreased significantly (Fig. 8c–e). By contrast, circulating memory B cells, which were also increased in untreated patients [17], did not show significant changes after steroid treatment. Notably, however, there was no significant correlation between the magnitude of decrease of the ratio of CD19+CD24−CD38hi plasma cells and the decrease of the IgG4-RD RI (R = 0.505, P = 0.055) (data not shown).
The association between CD19+CD24−CD38hi plasmablasts/plasma cells and disease activity. a and b Correlations of the ratio of CD19+CD24−CD38hi cells/CD19+ B cells and the absolute number of CD19+CD24−CD38hi plasmablasts/plasma cells with IgG4-related Disease Responder Index (IgG4-RD RI) before treatment. c Change of CD19+CD24−CD38hi plasmablasts/plasma cells before and after treatment. d–h Ratios of CD19+CD24−CD38hi cells/CD19+ B cells, absolute number of CD19+CD24−CD38hi plasmablasts/plasma cells, IgG4-RD RI, IgG, and IgG4 before and after treatment. *** P < 0.001, **** P < 0.0001
Discussion
The data derived from the present study indicate that CD19+CD24−CD38hi plasmablasts/plasma cells are significantly elevated in the peripheral blood of patients with IgG4-RD, and this B-cell population notably correlates with the number of organs involved, serum IgG4, IgG4-RD RI, and the ratio of IgG4/IgG. In addition, peripheral CD19+CD24−CD38hi plasmablasts/plasma cells decreased remarkably after treatment of glucocorticoids, and the clinical manifestations of patients improved accordingly. These findings are consistent with previous observations [17] and indicate that the level of CD19+CD24−CD38hi plasmablasts/plasma cells could be an important biomarker in IgG4-RD.
By gene expression analysis, we found that CD19+CD24−CD38hi plasmablasts/plasma cells differed greatly from the other three subsets of B cells, including naïve B cells, memory B cells, and Bregs, and expressed many genes typical of plasma cells. Considering that the gene expression of CD19+CD24−CD38hi plasmablasts/plasma cells differed from that of other known B-cell subsets, we attempted to use gene expression analysis to determine their stage of plasma cell maturation. A number of typical plasma cell genes were overexpressed, including XBP1, PRDM1, IRF4, and TNFRSF17, whereas BCL6, SPIB, and PAX5 were underexpressed, which is typical of mature plasma cells. However, expression of these genes does not permit a more detailed analysis of the stage of maturation of plasma cells. It is known, however, that expression of HLA-DR can be employed to identify newly generated cells from more mature plasma cells; in this regard, CD19+CD24−CD38hi plasmablasts/plasma cells from patients with IgG4-RD exhibited bright expression of HLA-DR protein, as is typical of newly formed plasma cells [27]. Notably, however, messenger RNA (mRNA) levels of various major histocompatibility complex class II molecules were decreased, as has been noted in systemic lupus erythematosus (SLE) [22], suggesting that these plasma cells were transitioning from newly formed to more mature plasma cells that had decreased mRNA expression but retained protein expression of HLA-DR. Consistent with this, IgG4-RD plasma cells greatly overexpressed Mki-67 mRNA, which encodes the Ki-67 protein, a marker of recently divided immunoglobulin-secreting plasma cells [28] and which is increased in plasmablasts compared with memory B cells [29]. Together, the increased expression of HLA-DR protein but not mRNA and the increase in Mki-67 mRNA identify IgG4-RD plasma cells as newly generated and similar to those found in the circulation of patients with SLE [30].
A number of other mRNAs and proteins were assessed to further understand the maturation status of the IgG4-RD plasma cells. The presence of mRNA for both lambda and kappa light chains suggests that these CD19+CD24−CD38hi plasmablasts/plasma cells are polyclonal, although more detailed analysis on a single-cell level would be required to confirm this. An interesting feature of CD19+CD24−CD38hi plasmablasts/plasma cells was their decreased expression of CXCR4. This chemokine receptor uniquely recognizes CXCL12 and plays an important role in plasma cell homing to bone marrow and other niches [31, 32]. The decreased expression of CXCR4 could contribute to their persistence in the circulation. A similar abnormality has been noted in SLE plasma cells [22].
To further identify the characteristics of CD19+CD24−CD38hi plasmablasts/plasma cells, we analyzed the surface markers by flow cytometry, and we found that CD19+CD24−CD38hi plasmablasts/plasma cells highly expressed CD86, CD62L, IL-6R, CD27, and CD95, which was consistent with those observed in gene levels. CD86 (B7-2) signaling plays a pivotal role in activating T cells [33]. Expression of CD86 on B-lineage cells has been shown to foster B-cell–T-cell collaboration and facilitate immunoglobulin production, including IgE and IgG4 [34–39]. In IgG4-RD, the CD19+CD24−CD38hi plasmablasts/plasma cell subset highly expressed the CD86 molecule, suggesting the intriguing possibility that this cell subset may maintain some features of earlier B cells and, by virtue of persistent expression of CD86, chronically stimulate T-cell help and in so doing enhance the likelihood of class-switching recombination to the downstream heavy-chain isotypes IgG4 and IgE. The physiologic role of other molecules expressed by CD19+CD24−CD38hi plasmablasts/plasma cells requires delineation.
As previously reported, circulating plasmablasts identified using CD19lowCD38+CD20−CD27+ phenotypic markers are significantly elevated in active IgG4-RD, even in patients with normal serum IgG4 concentrations, suggesting that plasmablast counts are a potentially useful biomarker for diagnosis of IgG4-RD as well as for assessing response to treatment [13]. In our study, we found that 76.46% of CD19+CD24−CD38hi cells expressed CD27, indicating that the CD19+CD24−CD38hi plasmablasts/plasma cell population is a larger one that contained CD19+CD20−CD27+CD38hi cells. Similarly, the ratio of CD19+CD24−CD38hi cells correlated positively with IgG4-RD RI, as did the absolute number of CD19+CD24−CD38hi plasmablasts/plasma cells [17]. Although there was no statistical correlation between the magnitude of change in circulating CD19+CD24−CD38hi plasmablasts/plasma cells and the change in the IgG4-RD RI, this may emerge when more patients are studied. Moreover, the significant correlation between disease activity and circulating plasmablasts/plasma cells, as well as the decrease in circulating plasma cells with therapy, suggests that this might be useful in assessing these patients and their response to treatment.
Conclusions
In this study, we found that CD19+CD24−CD38hi plasmablasts/plasma cells are prominently increased in peripheral blood of untreated patients with IgG4-RD, correlating positively with serum IgG4, IgG4/IgG ratio, IgG4-RD RI, and the number of involved organs. This population decreased significantly after steroid treatment, suggesting that CD19+CD24−CD38hi plasmablasts/plasma cells may be a biomarker of IgG4-RD and potentially could be useful in confirming a diagnosis, monitoring response to therapy, or assessing disease activity.
Abbreviations
- ANOVA:
-
Analysis of variance
- BAFF:
-
B-cell activating factor
- BAFF-R:
-
B-cell activating factor receptor
- BCL-6:
-
B-cell lymphoma 6 protein
- BCMA:
-
B-cell maturation antigen
- BCR:
-
B-cell receptor
- Breg:
-
Regulatory B cell
- CBA:
-
Cytometric bead array
- cDNA:
-
Complementary DNA
- CRP:
-
C-reactive protein
- Cy:
-
Cyanine
- ESR:
-
Erythrocyte sedimentation rate
- HLA:
-
Human leukocyte antigen
- Ig:
-
Immunoglobulin
- IgG4:
-
Immunoglobulin G4
- IgG4-RD:
-
Immunoglobulin G4-related disease
- IgG4-RD RI:
-
IgG4-related Disease Responder Index
- IL:
-
Interleukin
- IRF4:
-
Interferon regulatory factor 4
- mAb:
-
Monoclonal antibody
- MKI67:
-
Marker of proliferation Ki-67
- mRNA:
-
Messenger RNA
- PAX5:
-
Paired box gene 5
- PBMC:
-
Peripheral blood mononuclear cell
- PE:
-
Phycoerythrin
- SLE:
-
Systemic lupus erythematosus
- SPIB:
-
Transcription factor Spi-B
- SSC:
-
Side scatter
- TACI:
-
Transmembrane activator CAML (calcium modulator and cyclophilin ligand) interactor
- TNFRSF:
-
Tumor necrosis factor receptor superfamily member 17
- XBP1:
-
X-box binding protein 1
References
Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–71.
Umehara H, Kawano M. IgG4-related disease. JOP. 2015;16(2):217.
Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol. 2014;9:315–47.
Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466–75.
Lin W, Lu S, Chen H, Wu Q, Fei Y, Li M, et al. Clinical characteristics of immunoglobulin G4-related disease: a prospective study of 118 Chinese patients. Rheumatology (Oxford). 2015;54(11):1982–90.
Kawano M, Saeki T. IgG4-related kidney disease – an update. Curr Opin Nephrol Hypertens. 2015;24(2):193–201.
Lin J, Cummings OW, Greenson JK, House MG, Liu X, Nalbantoglu I, et al. IgG4-related sclerosing cholangitis in the absence of autoimmune pancreatitis mimicking extrahepatic cholangiocarcinoma. Scand J Gastroenterol. 2015;50(4):447–53.
Brito-Zeron P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203–10.
Nath V, Lewin J, Subramony C, Shenoy V. IgG4-associated cholangitis. J Miss State Med Assoc. 2014;55(12):384–8.
Chen Y, Zhao JZ, Feng RE, Shi JH, Li XM, Fei YY, et al. Types of organ involvement in patients with immunoglobulin G4-related disease. Chin Med J (Engl). 2016;129(13):1525–32.
Ohyama K, Koike H, Takahashi M, Kawagashira Y, Iijima M, Sobue G. Clinicopathological features of neuropathy associated with IgG4-related disease [in Japanese]. Rinsho Shinkeigaku. 2014;54(12):1047–9.
Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 2014;43(6):806–17.
Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190–5.
Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol. 2014;134(3):679–87.
Akiyama M, Suzuki K, Yamaoka K, Yasuoka H, Takeshita M, Kaneko Y, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol. 2015;67(9):2476–81.
Della-Torre E, Feeney E, Deshpande V, Mattoo H, Mahajan V, Kulikova M, et al. B-cell depletion attenuates serological biomarkers of fibrosis and myofibroblast activation in IgG4-related disease. Ann Rheum Dis. 2015;74(12):2236–43.
Lin W, Jin L, Chen H, Wu Q, Fei Y, Zheng W, et al. B cell subsets and dysfunction of regulatory B cells in IgG4-related diseases and primary Sjögren’s syndrome: the similarities and differences. Arthritis Res Ther. 2014;16(3):R118.
Carruthers MN, Stone JH, Deshpande V, Khosroshahi A. Development of an IgG4-RD Responder Index. Int J Rheumatol. 2012;2012:259408.
Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–30.
De Vos J, Hose D, Reme T, Tarte K, Moreaux J, Mahtouk K, et al. Microarray-based understanding of normal and malignant plasma cells. Immunol Rev. 2006;210:86–104.
Lin KI, Tunyaplin C, Calame K. Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol Rev. 2003;194:19–28.
Lugar PL, Love C, Grammer AC, Dave SS, Lipsky PE. Molecular characterization of circulating plasma cells in patients with active systemic lupus erythematosus. PLoS One. 2012;7(9), e44362.
Piskurich JF, Lin KI, Lin Y, Wang Y, Ting JP, Calame K. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat Immunol. 2000;1(6):526–32.
Roman C, Riggs K, Merrell K, Calame K. Activator proteins which regulate immunoglobulin heavy chain gene transcription in B lymphocytes. Prog Clin Biol Res. 1990;352:241–8.
Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5(3):230–42.
Tarte K, Zhan F, De Vos J, Klein B, Shaughnessy Jr J. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. 2003;102(2):592–600.
Mei HE, Wirries I, Frolich D, Brisslert M, Giesecke C, Grun JR, et al. A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood. 2015;125(11):1739–48.
Rashan AR, Goshn E, Peterson A, Yang Y, Phillips M, Sahaf B, et al. Characterization of immunoglobulin E plasma cells that are elevated in the upper airway mucosa of nonatopic patients with chronic rhinosinusitis without nasal polyps. Int Forum Allergy Rhinol. 2016;6(4):378–84.
Kjeldsen MK, Perez-Andres M, Schmitz A, Johansen P, Boegsted M, Nyegaard M, et al. Multiparametric flow cytometry for identification and fluorescence activated cell sorting of five distinct B-cell subpopulations in normal tonsil tissue. Am J Clin Pathol. 2011;136(6):960–9.
Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J Immunol Methods. 2011;363(2):187–97.
Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–90.
Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194(1):45–56.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48.
Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164(1):79–85.
Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gauchat JF, Ellis J, Bonnefoy JY. CD86 (B7-2) on human B cells: a functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J Biol Chem. 1997;272(25):15613–9.
Podojil JR, Kin NW, Sanders VM. CD86 and β2-adrenergic receptor signaling pathways, respectively, increase Oct-2 and OCA-B expression and binding to the 3′-IgH enhancer in B cells. J Biol Chem. 2004;279(22):23394–404.
Podojil JR, Sanders VM. Selective regulation of mature IgG1 transcription by CD86 and β2-adrenergic receptor stimulation. J Immunol. 2003;170(10):5143–51.
Podojil JR, Sanders VM. CD86 and β2-adrenergic receptor stimulation regulate B-cell activity cooperatively. Trends Immunol. 2005;26(4):180–5.
Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. 2009;183(12):7661–71.
Acknowledgements
We thank Professor Wanli Liu of the Tsinghua University School of Life Sciences for instructions and discussion.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81373190, 81571587).
Availability of data and materials
Yes, the data and materials are available.
Authors’ contributions
WL and PZ participated in cell sorting, cell culturing, gene microarray analysis, and statistical analysis, and they drafted the manuscript. YC and HY carried out flow cytometric analysis. HC, WZhe, XZ, and FZ helped with recruiting patients, study conception and design, and revision of the manuscript. WZha and PEL conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Yes, we agree to publish our study.
Ethics approval and consent to participate
The study was approved by the medical ethics committee of Peking Union Medical College Hospital (Peking, China). Written informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lin, W., Zhang, P., Chen, H. et al. Circulating plasmablasts/plasma cells: a potential biomarker for IgG4-related disease. Arthritis Res Ther 19, 25 (2017). https://doi.org/10.1186/s13075-017-1231-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-017-1231-2