Abstract
Background
The pathogenesis of necrosis of the femoral head (NFH) remains elusive. Limited studies were conducted to investigate the molecular mechanism of hip articular cartilage damage in NFH. We conducted genome-wide gene expression profiling of hip articular cartilage with NFH.
Methods
Hip articular cartilage specimens were collected from 18 NFH patients and 18 healthy controls. Gene expression profiling of NFH articular cartilage was carried out by Agilent Human 4x44K Gene Expression Microarray chip. Differently expressed genes were identified using the significance analysis of microarrays (SAM) software. Gene Ontology (GO) enrichment analysis of differently expressed genes was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID). Significantly differently expressed genes in the microarray experiment were selected for quantitative real-time PCR (qRT-PCR) and immunohistochemical validation.
Results
SAM identified 27 differently expressed genes in NFH articular cartilage, functionally involved in extracellular matrix, cytokines, growth factors, cell cycle and apoptosis. The expression patterns of the nine validation genes in qRT-PCR were consistent with that in proteinaceous extracellular matrix (false discovery rate (FDR) = 3.22 × 10-5), extracellular matrix (FDR = 5.78 × 10-5), extracellular region part (FDR = 1.28 × 10-4), collagen (FDR = 3.22 × 10-4), extracellular region (FDR = 4.78 × 10-4) and platelet-derived growth factor binding (FDR = 5.23 × 10-4).
Conclusions
This study identified a set of differently expressed genes, implicated in articular cartilage damage in NFH. Our study results may provide novel insight into the pathogenesis and rationale of therapies for NFH.
Similar content being viewed by others
Background
Necrosis of the femoral head (NFH) is a debilitating disease, mainly affecting young adults aged between 35 and 55 years [1]. NFH leads to rapid destruction and dysfunction of the hip joints. About 65–70 % of patients with advanced NFH need total hip replacement [2, 3]. The etiology and pathogenesis of NFH remains elusive, and there is a lack of effective approaches to the prevention and early treatment of NFH.
In the early stages NFH is mainly characterized by the death of osteocytes and bone marrow cells [1, 4]. The reparative reaction of necrotic bone is then initiated. During the repair process the imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone reformation results in structural damage and collapse of the femoral head. Because osteonecrosis is the representative pathological change in NFH, most studies of NFH have focused on the mechanism of damage to the bone and the bone marrow in the femoral head.
There is significant destruction of the hip articular cartilage during the development of NFH [5, 6]. Degeneration and cracking of the hip articular cartilage increases the instability of hip and accelerates the development of NFH [5, 6]. Prevention and early treatment of hip articular cartilage damage has the potential to slow the development of NFH and relieve hip dysfunction. However, few studies have been conducted to investigate the molecular mechanism of hip articular cartilage damage in NFH. To the best of our knowledge, to date no gene expression profiling of hip articular cartilage has been conducted in NFH, limiting our efforts to clarify the pathogenesis of NFH.
In this study, we conducted genome-wide gene expression profiling of hip articular cartilage in four patients with NFH and four healthy controls. A set of genes differently expressed in hip articular cartilage were identified for NFH. Quantitative real-time PCR (qRT-PCR) was conducted to validate the gene expression profiling results using an independent sample of eight patients with NFH and eight healthy controls. Our results provide novel clues for understanding the molecular mechanism of NFH.
Methods
Ethics statement
This study was approved by the Institutional Review Board of Xi’an Jiaotong University. Written informed consent was obtained from all subjects.
Articular cartilage specimens
Hip articular cartilage specimens were collected from 18 patients with non-traumatic NFH and 18 healthy control subjects at the Second Affiliated Hospital of Xi’an Jiaotong University. All study subjects were Chinese Han. The NFH patients and control subjects were diagnosed according to clinical manifestations and radiography of the hip assessed by at least two NFH experts [7, 8]. NFH articular cartilage was collected from patients with NFH classified by the the Ficat system as grade III, who were undergoing total hip replacement [7]. Articular cartilage was also obtained from subjects without NFH, who were undergoing total hip replacement within 24 hours of traumatic femoral neck fracture. All cartilage specimens were collected from the antero-superior portions of the femoral head, where the cartilage had collapsed (Fig. 1). Articular cartilage was only used in this study if it had an intact gross appearance and was graded below histological grade 2 [9, 10]. Clinical data for each participant was recorded by doctor-administered questionnaire, including self-reported ethnicity, lifestyle characteristics, health status, and family and medical history. Subjects were excluded if they were identified by assessment of clinical manifestations and radiologic imaging of the hip as having osteoarthritis, rheumatoid arthritis, or other hip disorders. Four, eight, and six NFH-control pairs, matched for age and sex, were used for microarray, qRT-PCR and immunohistochemical analysis, respectively (Table 1).
RNA preparation
The obtained cartilage specimens were rapidly dissected and frozen in liquid nitrogen, and subsequently stored at –80 °C until RNA extraction. Frozen cartilage samples were first rapidly ground in liquid nitrogen using a freezer mill. Total RNA were then isolated from cartilage samples using the Agilent Total RNA Isolation Mini kit (Agilent Technologies, Santa Clara, CA, USA) following the manufacturer’s recommended protocol. The integrity of isolated total RNA was evaluated with 1 % agarose gel electrophoresis. The concentration of isolated total RNA was determined by Agilent ND-1000 (Agilent Technologies) (Additional file 3: Table S1).
Microarray hybridization
Total RNA was translated into complementary RNA (cRNA) and labeled with Cy3 using the Agilent Quick Amp Labeling kit (Agilent Technologies). Following the Agilent One-Color Microarray-Based Gene Expression Analysis protocol (Agilent Technology), the labeled cRNA was purified using RNeasy Mini Kit (Qiagen, Germantown, MD, USA)). The concentration and specific activity of labeled cRNA were measured by Agilent ND-1000: 1 μg of labeled cRNA was mixed with hybridization buffer and hybridized to the Agilent Human 4x44K Gene Expression Microarray (v2, Agilent Technologies). Hybridization signals were recorded using the Agilent microarray scanner (G2505C), and analyzed by Feature Extraction v11.0 and Agilent GeneSpring GX v12.1 software (Agilent Technologies). The quality of fluorescent spots was evaluated, and the fluorescent spots failing to pass the quality control procedures were excluded for further analysis. Linear and locally weighted scatterplot smoothing (LOWESS) normalization were conducted. The microarray data have been deposited in the Gene Expression Omnibus database [GEO: GSE74089].
Identification of differently expressed genes
Differently expressed genes were identified using the Significance Analysis of Microarrays (SAM) software, Excel plug-in version 4.01 (http://statweb.stanford.edu/~tibs/SAM/) [11]. To ensure the accuracy of microarray data analysis, the genes presenting both fold changes >3.0 and false discovery rate (FDR) <0.01 were considered as being significantly differentially expressed. The FDR values were calculated by the permutation-based analysis algorithm of SAM [11].
Gene Ontology enrichment analysis
Gene Ontology (GO) enrichment analysis of differently expressed genes was performed using the functional annotation tool Database for Annotation, Visualization and Integrated Discovery (DAVID) 6.7 (http://david.abcc.ncifcrf.gov/home.jsp) [12]. GO enrichment analysis can integrate the information about disease-related genes and known functional relationships of multiple genes, and can help identify disease-relevant gene sets with known biological functions. In this study significant GO terms were identified at a FDR <0.01.
Quantitative real-time PCR
qRT-PCR was conducted to validate the accuracy of microarray data using an independent sample of eight patients with NFH and eight healthy controls (Table 1). Based on gene function and results from previous study of joint diseases, nine cartilage development and damage-related differently expressed genes in the microarray experiment were selected for qRT-PCR validation, including ANGPTL4, ASPN, COL1A1, COL3A1, CRTAC1, OGN, P4HA2, SPP1 and VKORC1 [13–20]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous invariant control for data normalization. Total RNA was isolated from cartilage specimens, and prepared in the same way as used by the microarray experiment. The isolated total RNA was converted into cDNA using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). The ABI Gene Amp PCR System 9700 (Applied Biosystems) was used for cDNA amplification and detection following the manufacturer’s recommended protocol. The expression levels of the nine genes were normalized to the amount of GAPDH. Relative fold changes of genes were calculated using the comparative cycle threshold (Ct) equation (2-△△Ct): t tests were conducted to assess the significance of gene expression differences between articular cartilage in NFH and healthy articular cartilage.
Immunohistochemical analysis
Hip cartilage specimens were collected from six patients with NFH (three male and three female, age 53.2 ± 5.1 years) and 6 healthy control subjects (three male and three female, age 59.2 ± 4.3 years). The paraformaldehyde-fixed cartilage tissues from patients with NFH and control subjects were rinsed with phosphate-buffered saline (PBS), decalcified and embedded in paraffin. Paraffin-embedded cartilage tissues were sectioned (approximately 5–8 μm thick), and placed on glass slides. For histochemical analysis the cartilage tissue slides were dewaxed in xylene, hydrated with graded ethanol, and stained respectively by hematoxylin and eosin (H&E), toluidine blue (TU) and Safranin O (SO) (Additional file 2: Figure S1). For immunohistochemical analysis, the dewaxed and hydrated cartilage sections were treated with 3 % hydrogen peroxide solution for 10 minutes, rinsed with PBS, and incubated with P4HA2, SPP1 and CRTAC1 antibody (1:50 dilution, Abcam plc, Cambridge, MA, USA) at 4 °C overnight. After washing with PBS, the cartilage sections were incubated with secondary antibody (ZHONGSHAN Golden Bridge Biotechnology, Beijing, China) at 37 °C for 15 minutes, exposed to streptavidin-horseradish peroxidase at 37 °C for 15 minutes, and stained with 3,3-diaminobenzidine (DAB). Four cartilage sections prepared from each cartilage specimen were used for immunohistochemical analysis. In the superficial zone, middle zone and deep zone of the cartilage (Additional file 3: Figure S2), the percentages of positive chondrocytes in 1000 chondrocytes were calculated separately for each cartilage section. Finally, the mean percentage of positive chondrocytes in the four cartilage sections was reported for each cartilage specimen. Significant differences in the expression of the P4HA2, SPP1 and CRTAC1 proteins in cartilage specimens from the six patients with NFH and the six control subjects were assessed using the t test. The methods used for the negative control groups were the same as described previously, except that the P4HA2, SPP1 and CRTAC1 antibodies were replaced by PBS.
Results
Differently expressed genes in articular cartilage from patients with NFH
SAM identified 24 genes that were significantly upregulated (FDR <0.01) in articular cartilage from patients with NFH (Table 2). The biological function of the 24 upregulated genes mainly includes extracellular matrix (11 genes), cytokines (3 genes), growth factors (2 genes), cell cycle (2 genes) and apoptosis (1 gene). The average gene expression ratio of the 24 upregulated genes was 16.77. Additionally, SAM identified three significantly downregulated genes in articular cartilage from patients with NFH, including TMEM171 (FDR = 5.61 × 10-5), MDK (FDR = 4.38 × 10-4) and VKORC1 (FDR = 4.02 × 10-3). The average gene expression ratio of the three downregulated genes was 0.42.
GO enrichment analysis
GO enrichment analysis was performed to investigate the molecular mechanism of differently expressed genes involved in damage to the articular cartilage in NFH. We detected six GO terms significantly enriched in the differently expressed genes in articular cartilage from patients withg NFH (Table 3). They are proteinaceous extracellular matrix (FDR = 3.22 × 10 -5), extracellular matrix (FDR = 3.22 × 10-5), extracellular region part (FDR = 1.28 × 10-4), collagen (FDR = 3.22 × 10-4), extracellular region (FDR = 4.78 × 10-4) and platelet-derived growth factor binding (FDR = 5.23 × 10-4).
qRT-PCR validation
Nine significantly differently expressed genes in the microarray experiment were selected for qRT-PCR using an independent sample of eight patients with NFH and eight healthy controls (Fig. 2). The expression patterns of the nine validation genes in qRT-PCR were consistent with that in the microarray experiment, including ANGPTL4 (ratio = 4.89, P = 0.05), ASPN (ratio = 6.69, P value = 3.90 × 10-5), COL1A1 (ratio = 17.43, P value = 0.01), COL3A1 (ratio = 5.33, P value = 0.02), CRTAC1 (ratio = 5.08, P value = 4.24 × 10-3), OGN (ratio = 5.99, P value = 9.14 × 10-4), P4HA2 (ratio = 3.12, P value = 1.30 × 10-5), SPP1 (ratio = 3.20, P value = 2.14 × 10-3), and VKORC1 (ratio = 0.56, P value = 1.76 × 10-3).
Immunohistochemical analysis
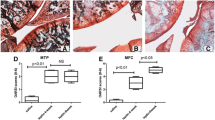
Immunohistochemical experiments were performed to evaluate the expression levels of the P4HA2, SPP1 and CRTAC1 proteins in NFH and normal cartilage. As shown in Fig. 3, expression of the P4HA2, SPP1 and CRTAC1 proteins in the superficial zone, middle zone, and deep zone of cartilage from patients with NFH was significantly higher than in normal hip cartilage (all P values <0.05). Additionally, we also observed decreased expression levels of the P4HA2, SPP1 and CRTAC1 proteins in the superficial zone, and middle zone to deep zone of cartilage from both patients with NFH and normal controls.
Immunohistochemistry results for P4HA2 (a), SPP1 (b) and CRTAC1 (c) proteins in cartilage from patients with necrosis of the femoral head (NFH) and normal hip cartilage. Original magnification × 400 of the superficial zone (SZ), middle zone (MZ) and deep zone (DZ). The expression of the P4HA2, SPP1 and CRTAC1 proteins in cartilage from patients with NFH was significantly higher than in normal hip cartilage in the SZ, MZ and DZ: n = 6 in each group. *P values <0.05; &P values <0.001
Discussion
Previous studies have implicated degeneration and cracking of hip articular cartilage in the development of NFH [5, 6]. We compared the gene expression profiles of articular cartilage from patients with NFH in articular cartilage from subjects without NFH, to try and understand the mechanism of damage to the articular cartilage in NFH. We identified 24 upregulated genes and 3 downregulated genes in articular cartilage in NFH. The 27 differently expressed genes are functionally involved mainly in the extracellular matrix, cytokines, growth factors, cell cycle and apoptosis. To the best of our knowledge, this is the first gene expression profile study of articular cartilage in NFH. Our study results may provide novel insight into the pathogenesis of NFH and a rationale for therapies.
We found that 11 extracellular-matrix-related genes were significantly upregulated in NFH, including ASPN, COL1A1, COL3A1, COL5A1, COL6A1, CRTAC1, MXRA7, OGN, P4HA2, and SPP1: 4 of these are collagen genes. COL1A1 encodes pro-alpha1 chains of type I collagen, which is abundant in bone. COL1A1 mutations are one of the major causes of osteogenesis imperfecta [21]. COL3A1 encodes the pro-alpha1 chains of type III collagen, which is widely expressed in the vascular system. Loeser et al. observed significant upregulation of COL3A1 in an osteoarthritis mice model [22]. COL5A1 encodes the alpha chain of type V collagen, which is a minor component of connective tissue. In an animal study dysfunction of COL5A1 was found to generate an abnormal joint phenotype, such as joint laxity and early-onset osteoarthritis [23]. COL6A1 encodes the alpha 1 subunit of type VI collagen, which is a major structural component of microfibrils. COL6A1 mutations have been linked to Bethlem myopathy with joint contractures [24]. P4HA2 encodes procollagen-proline, 2-oxoglutarate 4-dioxygenase, which is a key collagen synthesis enzyme [25]. P4HA2 knock-out mice have defects in skeletal growth and development [26]. In patients with NFH the upregulated expression of collagen and the collagen synthesis enzyme may be explained by enhanced repairing activity in articular cartilage defects with fibrous tissue. ASPN encodes cartilage extracellular protein asporin, which is able to negatively regulate the chondrogenesis of articular cartilage through blocking transforming growth factor (TGF)-beta/receptor interaction in chondrocytes [27]. It has also been found to be involved in damage to the articular cartilage in osteoarthritis and rheumatoid arthritis [13, 28–30]. SPP1 encodes secreted phosphoprotein 1 (also named osteopontin), which is implicated in the attachment of osteoclasts to mineralized bone matrix. The association between SPP1 and osteoarthritis has been demonstrated [31]. SPP1-deficient mice exhibit accelerated development of osteoarthritis [32]. Yamamoto et al. found that SPP1 contributes to osteoclast-mediated bone resorption and joint inflammatory responses in the mouse model of rheumatoid arthritis [33]. OGN encodes osteoglycin, which is capable of inducing ectopic bone formation and regulating cardiovascular development [34, 35]. CRTAC1 encodes cartilage acidic protein 1, which is expressed in the deep zone in articular cartilage. CRTAC1 acts as a biomarker for distinguishing chondrocytes from osteoblasts and mesenchymal stem cells [17].
With respect to cytokines and growth factors, we observed significant upregulation of the PRG4 and ANGPTL4 genes in articular cartilage from patients with NFH. PRG4 encodes proteoglycan 4, which acts as a boundary lubricant at the surface of articular cartilage [36, 37]. Using transgenic mice and intra-articular adenoviral virus gene transfer, Ruan et al. demonstrated that PRG4 protected against the development of osteoarthritis in mice [38]. The ANGPTL4 gene encodes angiopoietin-like 4, which is an important regulator of angiogenesis [39]. Perdiguero observed that ANGPTL4-deficient mice have impaired angiogenesis and increased vascular leakage [40]. ANGPTL4 also simulates endothelial cell growth and tubule formation, and prevents endothelial cell apoptosis [39, 41]. The role of ANGPTL4 in the dysfunctional blood supply in NFH is worthy of further study.
Conclusion
We conducted a gene expression profile study of the articular cartilage in NFH. We identified a set of differently expressed genes, implicated in the destruction of articular cartilage in NFH. Further biological studies are warranted to confirm our findings and clarify the potential mechanism of the identified genes involved in the development of NFH.
Abbreviations
- cRNA:
-
complementary RNA
- DAVID:
-
Database for Annotation, Visualization and Integrated Discovery
- FDR:
-
false discovery rate
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- GO:
-
Gene Ontology
- H&E:
-
hematoxylin and eosin
- NFH:
-
necrosis of femoral head
- PBS:
-
phosphate-buffered saline
- qRT-PCR:
-
quantitative real-time PCR
- SAM:
-
significance analysis of microarrays
References
Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63(1):16–28.
Johnson AJ, Mont MA, Tsao AK, Jones LC. Treatment of femoral head osteonecrosis in the United States: 16-year analysis of the Nationwide Inpatient Sample. Clin Orthop Relat Res. 2014;472(2):617–23.
Fukushima W, Fujioka M, Kubo T, Tamakoshi A, Nagai M, Hirota Y. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 2010;468(10):2715–24.
Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22(7):455–64.
McCarthy J, Puri L, Barsoum W, Lee JA, Laker M, Cooke P. Articular cartilage changes in avascular necrosis: an arthroscopic evaluation. Clin Orthop Relat Res. 2003;406:64–70.
Magnussen RA, Guilak F, Vail TP. Articular cartilage degeneration in post-collapse osteonecrosis of the femoral head. Radiographic staging, macroscopic grading, and histologic changes. J Bone Joint Surg Am. 2005;87(6):1272–7.
Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg. 1985;67(1):3–9.
Bluemke DA, Zerhouni EA. MRI of avascular necrosis of bone. Top Magn Reson Imaging. 1996;8(4):231–46.
Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–37.
Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. J Orthop Res. 2002;20(1):92–100.
Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98(9):5116–21.
da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57.
Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37(2):138–44.
Loughlin J. Polymorphism in signal transduction is a major route through which osteoarthritis susceptibility is acting. Curr Opin Rheumatol. 2005;17(5):629–33.
Knebelmann B, Deschenes G, Gros F, Hors MC, Grunfeld JP, Zhou J, et al. Substitution of arginine for glycine 325 in the collagen alpha 5 (IV) chain associated with X-linked Alport syndrome: characterization of the mutation by direct sequencing of PCR-amplified lymphoblast cDNA fragments. Am J Hum Genet. 1992;51(1):135–42.
Narcisi P, Richards AJ, Ferguson SD, Pope FM. A family with Ehlers-Danlos syndrome type III/articular hypermobility syndrome has a glycine 637 to serine substitution in type III collagen. Hum Mol Genet. 1994;3(9):1617–20.
Steck E, Braun J, Pelttari K, Kadel S, Kalbacher H, Richter W. Chondrocyte secreted CRTAC1: a glycosylated extracellular matrix molecule of human articular cartilage. Matrix Biol. 2007;26(1):30–41.
Madisen L, Neubauer M, Plowman G, Rosen D, Segarini P, Dasch J, et al. Molecular cloning of a novel bone-forming compound: osteoinductive factor. DNA Cell Biol. 1990;9(5):303–9.
Heppner JM, Zaucke F, Clarke LA. Extracellular matrix disruption is an early event in the pathogenesis of skeletal disease in mucopolysaccharidosis I. Mol Genet Metab. 2015;114(2):146–55.
Lv C, Li Y, Xu J, Cao H, Li X, Ma B, et al. Association of SPP1 promoter variants with hip osteoarthritis susceptibility in Chinese population. Gene. 2015;564(1):9–13.
Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–85.
Loeser RF, Olex AL, McNulty MA, Carlson CS, Callahan M, Ferguson C, et al. Disease progression and phasic changes in gene expression in a mouse model of osteoarthritis. PLoS One. 2013;8(1):e54633.
Sun M, Connizzo BK, Adams SM, Freedman BR, Wenstrup RJ, Soslowsky LJ, et al. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype. Am J Pathol. 2015;185(5):1436–47.
Lamande SR, Bateman JF, Hutchison W, McKinlay Gardner RJ, Bower SP, Byrne E, et al. Reduced collagen VI causes Bethlem myopathy: a heterozygous COL6A1 nonsense mutation results in mRNA decay and functional haploinsufficiency. Hum Mol Genet. 1998;7(6):981–9.
Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J Biol Chem. 2013;288(15):10819–29.
Aro E, Salo AM, Khatri R, Finnila M, Miinalainen I, Sormunen R, et al. Severe extracellular matrix abnormalities and chondrodysplasia in mice lacking collagen prolyl 4-hydroxylase isoenzyme II in combination with a reduced amount of isoenzyme I. J Biol Chem. 2015;290(27):16964–78.
Nakajima M, Kizawa H, Saitoh M, Kou I, Miyazono K, Ikegawa S. Mechanisms for asporin function and regulation in articular cartilage. J Biol Chem. 2007;282(44):32185–92.
Sakao K, Takahashi KA, Arai Y, Saito M, Honjyo K, Hiraoka N, et al. Asporin and transforming growth factor-beta gene expression in osteoblasts from subchondral bone and osteophytes in osteoarthritis. J Orthop Sci. 2009;14(6):738–47.
Ikegawa S. Expression, regulation and function of asporin, a susceptibility gene in common bone and joint diseases. Curr Med Chem. 2008;15(7):724–8.
Torres B, Orozco G, Garcia-Lozano JR, Oliver J, Fernandez O, Gonzalez-Gay MA, et al. Asporin repeat polymorphism in rheumatoid arthritis. Ann Rheum Dis. 2007;66(1):118–20.
Cheng C, Gao S, Lei G. Association of osteopontin with osteoarthritis. Rheumatol Int. 2014;34(12):1627–31.
Matsui Y, Iwasaki N, Kon S, Takahashi D, Morimoto J, Matsui Y, et al. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009;60(8):2362–71.
Yamamoto N, Sakai F, Kon S, Morimoto J, Kimura C, Yamazaki H, et al. Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. J Clin Invest. 2003;112(2):181–8.
Tanaka K, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287(15):11616–28.
Petretto E, Sarwar R, Grieve I, Lu H, Kumaran MK, Muckett PJ, et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet. 2008;40(5):546–52.
Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254(3):535–41.
Jones AR, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res. 2007;25(3):283–92.
Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra134.
Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295(5):E1056–1064.
Perdiguero EG, Galaup A, Durand M, Teillon J, Philippe J, Valenzuela DM, et al. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J Biol Chem. 2011;286(42):36841–51.
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm CS, et al. Hepatic expression, synthesis and secretion of a novel fibrinogen/angiopoietin-related protein that prevents endothelial-cell apoptosis. Biochem J. 2000;346(Pt 3):603–10.
Acknowledgements
We thank Zengtie Zhang, CuiYan Wu, and Yan Wen for their technical assistance. The study was supported by National Natural Scientific Fund of China (81101337, 81472925) and Fundamental Research Funds for the Central Universities (No. XJJ2014154). Written informed consent was obtained from all study subjects for publication of their individual details and accompanying images in this manuscript. The consent form is held by the authors and is available for review by the Editor-in-Chief.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RYL and FZ conceived and designed the study. RYL, QL, and FZ performed the microarray, PCR and immunohistochemical experiments. FZ performed the statistical analysis. RYL, FZ, QL, XQD, and KZW wrote the paper. All authors read and approved the manuscript.
Additional files
Additional file 1: Table S1.
RNA concentrations of study samples. (DOCX 27 kb)
Additional file 2: Figure S1.
Hematoxylin and eosin (HE), safranin O (SO) and toluidine blue (TU) staining of NFH articular cartilage and normal articular cartilage. (TIF 10334 kb)
Additional file 3: Figure S2.
Cartilage zones for immunohistochemical analysis of P4HA2 (A), SPP1 (B) and CRTAC1 (C) proteins. The arrows indicate respectively the superficial zone (SZ), middle zone (MZ) and deep zone (DZ) of articular cartilage. (TIF 13919 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, R., Liu, Q., Wang, K. et al. Comparative analysis of gene expression profiles in normal hip human cartilage and cartilage from patients with necrosis of the femoral head. Arthritis Res Ther 18, 98 (2016). https://doi.org/10.1186/s13075-016-0991-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-016-0991-4