Abstract
Introduction
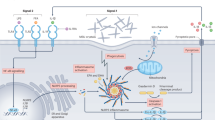
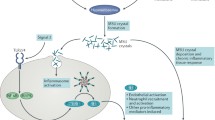
The acute gout flare results from a localised self-limiting innate immune response to monosodium urate (MSU) crystals deposited in joints in hyperuricaemic individuals. Activation of the caspase recruitment domain-containing protein 8 (CARD8) NOD-like receptor pyrin-containing 3 (NLRP3) inflammasome by MSU crystals and production of mature interleukin-1β (IL-1β) is central to acute gouty arthritis. However very little is known about genetic control of the innate immune response involved in acute gouty arthritis. Therefore our aim was to test functional single nucleotide polymorphism (SNP) variants in the toll-like receptor (TLR)-inflammasome-IL-1β axis for association with gout.
Methods
1,494 gout cases of European and 863 gout cases of New Zealand (NZ) Polynesian (Māori and Pacific Island) ancestry were included. Gout was diagnosed by the 1977 ARA gout classification criteria. There were 1,030 Polynesian controls and 10,942 European controls including from the publicly-available Atherosclerosis Risk in Communities (ARIC) and Framingham Heart (FHS) studies. The ten SNPs were either genotyped by Sequenom MassArray or by Affymetrix SNP array or imputed in the ARIC and FHS datasets. Allelic association was done by logistic regression adjusting by age and sex with European and Polynesian data combined by meta-analysis. Sample sets were pooled for multiplicative interaction analysis, which was also adjusted by sample set.
Results
Eleven SNPs were tested in the TLR2, CD14, IL1B, CARD8, NLRP3, MYD88, P2RX7, DAPK1 and TNXIP genes. Nominally significant (P < 0.05) associations with gout were detected at CARD8 rs2043211 (OR = 1.12, P = 0.007), IL1B rs1143623 (OR = 1.10, P = 0.020) and CD14 rs2569190 (OR = 1.08; P = 0.036). There was significant multiplicative interaction between CARD8 and IL1B (P = 0.005), with the IL1B risk genotype amplifying the risk effect of CARD8.
Conclusion
There is evidence for association of gout with functional variants in CARD8, IL1B and CD14. The gout-associated allele of IL1B increases expression of IL-1β – the multiplicative interaction with CARD8 would be consistent with a synergy of greater inflammasome activity (resulting from reduced CARD8) combined with higher levels of pre-IL-1β expression leading to increased production of mature IL-1β in gout.
Similar content being viewed by others
Introduction
The immediate cause of gout is the deposition of monosodium urate (MSU) crystals in and around body tissues, particularly joints [1]. Initially, these deposits trigger a localised and self-limiting inflammatory response (acute gouty arthritis), which becomes increasingly frequent and severe, involving multiple joints and associated with fever. Monosodium urate crystals form under hyperuricaemic conditions when serum urate levels exceed the physiological saturation level (approximately 6.8 mg/dL; approximately 0.41 mM). The most significant biological cause of hyperuricaemia is relatively low renal clearance of uric acid [2, 3]. This is consistent with findings from genome-wide association studies in which 28 loci associated with serum urate levels have been identified, some of which are in genes involved in renal uric acid handling [4, 5]. Predictably most, but not all, of the 28 loci have been associated with gout [4, 6].
Although hyperuricaemia is a prerequisite for MSU formation, only a relatively small proportion of individuals with hyperuricaemia develop gout [7]. This indicates that beside genetic variants associated with urate metabolism and excretion, other factors contribute to the pathogenesis of gout. MSU crystals play an important role in activation of the innate immune system [8] and the recognition of gout as an auto-inflammatory disorder is consistent with the results of functional studies [9, 10]. Variations within genes of the innate immune system may therefore determine whether MSU crystals trigger an inflammatory reaction in susceptible individuals, leading to acute gout; while in others, no inflammation is elicited. Genetic variants that influence the activation and function of the NOD-like Receptor Pyrin containing 3 (NLRP3) inflammasome are candidate genes in this context [11]. The multi-protein inflammasome complex, comprising the NLRP3 polypeptide, ASC or PYCARD (apoptosis-associated speck-like protein containing a CARD) and caspase-1 [12] forms when monocytes and macrophages encounter damaged and pathogen-associated molecular pattern proteins (DAMPs and PAMP; e.g., bacterial lipopolysaccharide or MSU crystals) and leads to activation of caspase-1. Active caspase-1 processes the pro-interleukin (IL)-1β to the mature pro-inflammatory cytokine IL-1β that is then secreted [12].
CARD8 (also known as TUCAN or Cardinal) is a protein with a caspase-domain that interacts with caspase-1 and inhibits its activation [13] and also with a FIIND domain that binds to NLRP3 preventing its recruitment into the active inflammasome complex [14, 15]. Genetic associations between variants of CARD8 and autoimmune diseases have been previously reported (reviewed in [16]). The T allele of CARD8 rs2043211 (C10X) has been associated with increased risk of gout in Chinese [17], and rs2149356 in toll-like receptor 4 (TLR4), a receptor functionally implicated in MSU-stimulated inflammation [18], has also been associated with serum IL-1β levels and the risk of gout in Han Chinese [19]. However, using a haplotype tagging approach, there is no evidence of association between NLRP3 and gout in Chinese [20].Footnote 1
Our aim was to extend the findings from the Han Chinese population [17] and to test genetic variants influencing inflammasome function for association with gout in other population groups. Eleven functional variants were tested in eight genes involved in the MSU crystal-mediated activation of the NLRP3-inflammasome and production of mature IL-1β for association with gout in people of European and New Zealand (NZ) Polynesian (Māori and Pacific Island) ancestry. The prevalence of gout in the NZ Polynesian population is 6–8 % (compared to 3 % in NZ European), exhibiting the highest prevalence worldwide [21, 22].
Methods
Participants, ethics and consent
The study was carried out on sample sets comprising: a NZ Polynesian sample set consisting of 1,893 individuals of Samoan, Tongan, Niuean, Cook Island Māori and NZ Māori descent (Table 1; 863 cases and 1,030 controls); and a sample set consisting of 1,684 cases of European ancestry (957 recruited from NZ and Australia and 727 recruited from Europe (‘Eurogout’) [23]) and 882 NZ European controls. All people with gout met the 1977 preliminary American Rheumatism Association classification criteria for gout [24]. New Zealand gout cases were recruited from the Auckland and Christchurch regions of NZ, Australian gout cases from an outpatient clinic in Adelaide, Australian private practice rheumatologists and from a previously reported pharmacogenetic study [25]. European gout cases were recruited from outpatient clinics in Edinburgh, Lausanne, Dresden, Arnhem and Nijmegen. The NZ controls did not have any self-reported history of arthritis, were >17 years of age and were convenience sampled from the Auckland, Christchurch and Otago regions of NZ. The New Zealand Multi-Region Ethics Committee (MEC/105/10/130) and these institutional committees in Europe and Australia granted ethical approval: Research Ethics Committee, University of New South Wales; Ethikkommission, Technische Universität Dresden (EK 8012012); South East Scotland Research Ethics Committee (04/S1102/41); Commission Cantonale (VD) D'éthique de la Recherche sur l'être Humain, Université de Lausanne; Commissie Mensgebonden Onderzoek regio Arnhem Nijmegen. All subjects gave written and informed consent.
Control subjects of European ancestry, who had been genotyped genome-wide, were also included from the Atherosclerosis Risk in Communities (ARIC) study (n = 6,989) and the Framingham Heart Study (FHS; n = 3,131). Participants with self-reported gout were excluded. The sample sets were also screened to remove all but one representative of any closely related family grouping (full/half siblings and parent/child duos and trios). The ARIC study and FHS analyses (project #834) were approved by the relevant Database of Genotype and Phenotype (dbGaP; [26]) Data Access Committees.
Genotyping and imputation
A Sequenom MassARRAY System was used for genotyping the 4,269 individuals without genome-wide genoytpe data available as previously described [27]. Publicly available genome-wide genotype data from ARIC and FHS were imputed as required to obtain genotypes from the eleven selected SNPs. Imputations were carried out by IMPUTE V2.2 software based on the combined 1000 Genomes V3 population reference set. Individuals and SNPs with a call rate of <0.98 were excluded from analysis, as were monomorphic markers and those with a minor allele frequency of <0.01, and a post-imputation quality threshold of 0.30 was used. Imputations were successful for all eleven SNPs and all imputed SNPs were in Hardy-Weinberg equilibrium (P >0.005) with the exception of NLRP3 rs7512998 in ARIC (P HWE = 1.3 × 10-27) - these data were excluded from analysis. Where necessary, imputed data were strand-adjusted to match the strand interrogated by the Sequenom data prior to analysis.
Association testing
Allelic association analysis was carried out using R software and adjusting for age and sex. For Polynesian samples, a genetically estimated proportion of Polynesian ancestry, calculated as previously described [27], was included as an additional covariate. All odds ratios are reported relative to the minor allele present in the genotyped NZ European control sample set. Meta-analyses were carried out using METAL [28] with weighting based on standard error and log (odds ratio (OR)) as the effect variable. Significant association was declared if P was <0.05 in the European and Polynesian meta-analysis. No correction for multiple testing was applied because there is prior functional evidence for each variant tested.
Power curves are shown in Additional file 1. The Polynesian sample set was adequately powered to detect common effects (minor allele frequency (MAF) >0.1) of OR ≥1.4, whereas the European sample set was adequately powered to detect common effects of MAF >0.2 at OR >1.2 and stronger effects (OR >1.4) at lower MAF (>0.05).
Interaction analysis
As reviewed by Cordell [29] there are a number of ways to test for interaction or a departure from additivity. We used Stata 13.1 software to carry out logistic regression analysis comparing the disease risk for heterozygosity and minor allele homozygosity for each locus individually and in combination. Previously we reported a large allele frequency difference at ABCG2 rs2231142 and heterogeneity in association with gout between Eastern (EP) and Western (WP) Polynesian sample sets [30]. Similarly, there are differences in allele and genotype frequencies between the two groups (Additional file 2), and heterogeneity will be magnified in combined genotypes. Therefore, in the interaction analysis adjustment was made separately for EP and WP, along with European data. An interaction term was included and a P value <0.017 (Bonferroni-adjusted for number of interaction analyses performed) was considered to indicate significant multiplicative interaction between genetic variants.
Results
Eleven functional genetic variants in NLRP3, CARD8, IL1B, DAPK1, TXNIP, TLR2, P2XR7, MYD88 and CD14 were selected from the literature (Table 2) and genotyped; genotype distributions are presented in Additional file 2. There was nominal allelic association (P <0.05) for three variants in the combined European and Polynesian analysis (Table 3) - IL1B, CARD8 and CD14 (OR = 1.10, 1.12 and 1.08, respectively). CARD8 rs2043211 was also associated with gout in Europeans (OR = 1.11).
Because of reported interactions between NLRP3 and CARD8 in other auto-inflammatory conditions [30, 31], and the biological interaction between the inflammasome and IL-1β we tested for pairwise multiplicative interaction between NLRP3/CARD8, NLRP3/IL1B and CARD8/IL1B (Table 4), using only rs10754558 of NLRP3 owing to the low MAF of rs35829419 in both Europeans and Polynesians and the low MAF of rs7512998 in Polynesians. There was evidence for interaction between CARD8 and IL1B (P = 0.005, P c = 0.015), driven by amplification of the risk conferred by the CARD8 rs2043211 T allele in the presence of the rs1143623 IL1B minor allele homozygous (GG) genotype. Nominally significant interaction between NLRP3/IL1B (P Nominal = 0.048) was also observed, although this was not significant after adjustment for multiple testing (P c = 0.144).
Discussion
TLR signalling via the NLRP3 inflammasome has been implicated in gout susceptibility and pathology in vivo and in vitro [10]; for example, MSU uptake and IL-1β production by bone marrow-derived macrophages derived from TLR2, TLR4 or Myd88 knockout mice is significantly reduced, as is neutrophil influx, in response to subcutaneous injection of MSU in whole animals [18]. To further elucidate the role of the TLR-inflammasome-IL-1β cascade in gout pathogenesis, eleven candidate genetic variants that functionally impact on this pathway (reviewed in [10]) were tested for association with gout in a sample set of 2,357 cases, adequately powered to detect association with common variants having an effect size of odds ratio 1.4 or greater (Additional file 1). As discussed below, the nominal evidence for association between gout and CD14, CARD8 and IL1B, and the multiplicative interaction between CARD8 and IL1B in determining the risk of gout, support the considerable evidence that TLR-mediated activation of the inflammasome and subsequent release of active IL-1β is a central causal pathogenic pathway of gout [10, 32].
Variants rs2043211 (CARD8), rs1143623 (IL1B) and rs2569190 (CD14), which were associated with gout, are functional variations in genes directly involved in the NLRP3 signaling pathway, and as such are likely to represent genuine disease-susceptibility loci. CARD8 is an adaptor protein that regulates IL-1β secretion by inhibiting NFKβ signaling (required for the expression of pro-IL-1β) and/or interacting with caspase 1 or NLRP3 to inhibit the generation of active IL-1β from inactive pro-IL-1β [13, 15]. The effect size of CARD8 SNP rs2043211 was consistent between the European and NZ Polynesian samples sets (OR = 1.11, P = 0.023 and OR = 1.15, P = 0.078, respectively; combined OR = 1.12, P = 0.007) and the Chinese sample set reported by Chen et al. (OR = 1.19, P = 0.08) [17]. Collectively our results and the study by Chen et al. [17] do suggest that the association of the minor allele of rs2043211 with gout is not a false positive one. SNP rs2043211 encodes a missense protein variation (C10X or F52I depending on transcript, [33]) with the minor allele increasing the risk of gout. Although the functional effect of this variation has not been specifically evaluated, the SNP is within an expression quantitative trait locus peak and carriage of the minor allele is associated with decreased CARD8 expression [34]. It has been inconsistently associated with other auto-inflammatory phenotypes, with some evidence for epistatic interaction with NLRP3 rs35829419 in determining risk of inflammatory bowel disease and abdominal aortic aneurysms [31, 35]. However, we found no evidence of interaction between NLRP3 and CARD8 in determining risk of gout (Table 4) (although we did not specifically analyse rs35829419), and it has also been suggested that under certain conditions, ASC-dependent IL1-β production in response to MSU stimulation can occur in the absence of NLRP3 [36].
IL1B SNP rs1143623 is within a promoter GATA transcription factor family binding site. Although the minor allele exhibits enhanced protein-binding [37] and decreased expression in vitro and in vivo, the effect of this variation seems to be influenced both by the wider promoter haplotype [38–40], and the identity of the stimulatory signal; the minor allele shows increased rather than decreased expression in vitro in response to TNF-α [40], and has also been associated with increased post-prandial triglyceride and IL6 (an effector of IL-1β) levels [41]. The minor allele is over-represented in gout cases compared to controls (OR = 1.10, P = 0.020). If this is replicated it would be consistent with an etiological role for increased IL1B expression in gout. There was evidence of multiplicative interaction with CARD8 rs2043211 in which the IL1B rs1143623 minor (risk) allele homozygous genotype appeared to amplify the effect of the minor allele of rs2043211. This would be consistent with a synergy of greater inflammasome activity (resulting from reduced CARD8) combined with higher levels of pre-IL-1β expression leading to increased production of mature IL-1β in gout.
The final variation nominally associated with gout was CD14 SNP rs2569190. This SNP is in the 5’UTR of one of the two CD14 splice variants, with the minor allele (that increases risk of gout) increasing expression in monocytes by decreasing affinity for the inhibitory Sp3 transcription factor [42], enhancing the loading of RNA polymerase II [43] and is associated with increased soluble CD14 levels in healthy individuals [44, 45]. Membrane-bound CD14 forms functional complexes with TLR2 or TLR4 and leukocyte β2-integrins, which could mediate TLR dimerization and optimize the innate immune response to MSU crystals [10].
Conclusions
In conclusion we provide evidence for association of gout with functional innate immune system variants in CARD8, IL1B and CD14, and multiplicative interaction between IL1B and CARD8. The findings involving IL1B are consistent with genetically determined levels of IL-1β being important in gout.
Abbreviations
- ARA:
-
American Rheumatology Association
- ARIC:
-
Atherosclerosis Risk in Communities
- ASC:
-
apoptosis-associated speck-like protein containing a CARDD
- CARD8:
-
caspase recruitment domain-containing protein 8
- DAMP:
-
damage-associated molecular pattern
- EP:
-
Eastern Polynesian
- FHS:
-
Framingham Heart Study
- IL:
-
interleukin
- MAF:
-
minor allele frequency
- MSU:
-
monosodium urate
- NALP3:
-
NACHT, LRR and PYD domains-containing protein 3
- NLRP3:
-
NOD-like Receptor Pyrin containing 3
- NOD:
-
nucleotide-binding oligomerisation domain
- NZ:
-
New Zealand
- OR:
-
odds ratio
- PAMP:
-
pathogen-associated molecular pattern
- UTR:
-
untranslated region
- RNA:
-
ribonucleic acid
- SNP:
-
single nucleotide polymorphism
- TLR:
-
toll-like receptor
- TNF:
-
tumour necrosis factor
- WP:
-
Western Polynesian
References
Dalbeth N, Haskard D. Mechanisms of inflammation in gout. Rheumatol. 2005;44:1090–6.
Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis. 1992;51:375–7.
Gibson T, Waterworth R, Hatfield P, Robinson G, Bremner K. Hyperuricaemia, gout and kidney function in New Zealand Maori men. Br J Rheumatol. 1984;23:276–82.
Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45:145–54.
Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheum Dis Clin North Am. 2014;40:279–90.
Phipps-Green A, Merriman M, Topless R, Altaf S, Montgomery G, Franklin C, et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Ann Rheum Dis. 2014. doi:10.1136/annrheumdis-2014-205877.
Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–6.
Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunological Rev. 2010;233:203–17.
Ryan JG, Goldbach-Mansky R. The spectrum of autoinflammatory diseases: recent bench to bedside observations. Curr Op Rheumatol. 2008;20:66–75.
Liu-Bryan R, Terkeltaub R. Tophus biology and pathogenesis of monosodium urate crystal–induced inflammation. Gout and Other Crystal Arthropathies. 2011;2011:59.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61.
Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Ann Rev Immunol. 2009;27:229–65.
Razmara M, Srinivasula SM, Wang L, Poyet J-L, Geddes BJ, DiStefano PS, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277:13952–8.
Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–25.
Ito S, Hara Y, Kubota T. CARD8 is a negative regulator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes escapes the restriction. Arthritis Res Ther. 2014;16:R52.
Paramel G, Sirsjö A, Fransén K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Inflamm. 2015;2015:846782.
Chen Y, Ren X, Li C, Xing S, Fu Z, Yuan Y, et al. CARD8 rs2043211 polymorphism is associated with gout in a Chinese male population. Cell Physiol Biochem. 2015;35:1394–400.
Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005;52:2936–46.
Qing Y-F, Zhou J-G, Zhang Q-B, Wang D-S, Li M, Yang Q-B, et al. Association of TLR4 gene rs2149356 polymorphism with primary gouty arthritis in a case-control study. PLoS One. 2013;8:64845.
Meng D-M, Zhou Y-J, Wang L, Ren W, Cui L-L, Han L, et al. Polymorphisms in the NLRP3 gene and risk of primary gouty arthritis. Mol Med Rep. 2013;7:1761–6.
Winnard D, Wright C, Taylor WJ, Jackson G, Te Karu L, Gow PJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology (Oxford). 2012;51:901–9.
Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nature Rev Rheumatol. 2015. doi:10.1038/nrrhuem.2015.91.
Liote F, Merriman T, Nasi S, So A. 4th European Crystal Netrowk meeting, Paris 8-9th March 2013. Arthritis Res Ther. 2013;15:304.
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900.
Stocker SL, McLachlan AJ, Savic RM, Kirkpatrick CM, Graham GG, Williams KM, et al. The pharmacokinetics of oxypurinol in people with gout. Br J Clin Pharmacol. 2012;74:477–89.
www.ncbi.nim.nih/gov/dbgap. The database of Genotypes and Phenotypes.
Hollis-Moffatt JE, Phipps-Green AJ, Chapman B, Jones GT, van Rij A, Gow PJ, et al. The renal urate transporter SLC17A1 locus: confirmation of association with gout. Arthritis Res Ther. 2012;14:R92.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1.
Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nature Rev Genet. 2009;10:392–404.
Phipps-Green AJH-MJ, Dalbeth N, Merriman ME, Topless R, Gow PJ, Harrison AA, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010;19:4813–9.
Roberts R, Topless R, Phipps-Green A, Gearry R, Barclay M, Merriman T. Evidence of interaction of CARD8 rs2043211 with NALP3 rs35829419 in Crohn's disease. Genes Immun. 2010;11:351–6.
Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206.
Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Eyre S, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014;73:1202–10.
Roberts RL, Van Rij AM, Phillips LV, Young S, McCormick S, Merriman TR, et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis. 2011;218:123–6.
Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate crystal-induced gouty arthritis. Arthritis Rheum. 2010;62:3237–48.
Lee K-A, Ki C-S, Kim H-J, Sohn K-M, Kim J-W, Kang WK, et al. Novel interleukin 1β polymorphism increased the risk of gastric cancer in a Korean population. J Gastoenterol. 2004;39:429–33.
Chen H, Wilkins LM, Aziz N, Cannings C, Wyllie DH, Bingle C, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–29.
Landvik NE, Hart K, Haugen A, Zienolddiny S. Functional analysis of a lung cancer risk haplotype in the IL1B gene regulatory region. J Hum Genet. 2012;57:747–52.
Landvik NE, Hart K, Skaug V, Stangeland LB, Haugen A, Zienolddiny S. A specific interleukin-1B haplotype correlates with high levels of IL1B mRNA in the lung and increased risk of non-small cell lung cancer. Carcinogenesis. 2009;30:1186–92.
Delgado-Lista J, Garcia-Rios A, Perez-Martinez P, Solivera J, Yubero-Serrano EM, Fuentes F, et al. Interleukin 1B variant-1473G/C (rs1143623) influences triglyceride and interleukin 6 metabolism. J Clin Endocrinol Metab. 2011;96:E816–20.
LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, Martinez FD, et al. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol. 2001;167:5838–44.
Mertens J, Bregadze R, Mansur A, Askar E, Bickeböller H, Ramadori G, et al. Functional impact of endotoxin receptor CD14 polymorphisms on transcriptional activity. J Mol Med. 2009;87:815–24.
LeVan TD, Michel O, Dentener M, Thorn J, Vertongen F, Beijer L, et al. Association between CD14 polymorphisms and serum soluble CD14 levels: Effect of atopy and endotoxin inhalation. J Allergy Clin Immunol. 2008;121:434–40.
Baldini M, Carla Lohman I, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Resp Cell Mol Biol. 1999;20:976–83.
Hitomi Y, Ebisawa M, Tomikawa M, Imai T, Komata T, Hirota T, et al. Associations of functional NLRP3 polymorphisms with susceptibility to food-induced anaphylaxis and aspirin-induced asthma. J Allergy Clin Immunol. 2009;124:779–85.
Verma D, Lerm M, Blomgran Julinder R, Eriksson P, Söderkvist P, Särndahl E. Gene polymorphisms in the NALP3 inflammasome are associated with interleukin-1 production and severe inflammation: relation to common inflammatory diseases? Arthritis Rheum. 2008;58:888–94.
Verma D, Särndahl E, Andersson H, Eriksson P, Fredrikson M, Jönsson J-I, et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1β and IL-18 production. PLoS One. 2012;7:e34977.
Pontillo A, Vendramin A, Catamo E, Fabris A, Crovella S. The missense variation Q705K in CIAS1/NALP3/NLRP3 gene and an NLRP1 haplotype are associated with celiac disease. Am J Gastroenterol. 2011;106:539–44.
Fontalba AM-TV, Gutierrez O, Pipaon C, Benito N, Balsa A, Blanco R, et al. Deficiency of the NF-kappaB inhibitor caspase activating and recruitment domain 8 in patients with rheumatoid arthritis is associated with disease severity. J Immunol. 2007;179:4867–73.
Bagnall RD, Roberts RG, Mirza MM, Torigoe T, Prescott NJ, Mathew CG. Novel isoforms of the CARD8 (TUCAN) gene evade a nonsense mutation. Eur J Hum Genet. 2008;16:619–25.
Lacruz-Guzmán D, Torres-Moreno D, Pedrero F, Romero-Cara P, García-Tercero I, Trujillo-Santos J, et al. Influence of polymorphisms and TNF and IL1β serum concentration on the infliximab response in Crohn’s disease and ulcerative colitis. Eur J Clin Pharmacol. 2013;69:431–8.
Harrison P, Pointon J, Chapman K, Roddam A, Wordsworth B. Interleukin-1 promoter region polymorphism role in rheumatoid arthritis: a meta-analysis of IL-1B-511A/G variant reveals association with rheumatoid arthritis. Rheumatol. 2008;47:1768–70.
Potaczek D, Nastalek M, Okumura K, Wojas‐Pelc A, Undas A, Nishiyama C. An association of TLR2–16934A > T polymorphism and severity/phenotype of atopic dermatitis. J Eur Acad Dermatol Venerol. 2011;25:715–21.
Wang Z, Hu J, Fan R, Zhou J, Zhong J. Association between CD14 gene C-260 T polymorphism and inflammatory bowel disease: a meta-analysis. PLoS One. 2012;7:e45144.
Zhao L, Bracken MB. Association of CD14-260 (-159) C > T and asthma: a systematic review and meta-analysis. BMC Med genet. 2011;12:93.
Wang Y, Sun C, Li T, Xu H, Zhou Y, Dan H, et al. Integrative approach detected association between genetic variants of microRNA binding sites of TLRs pathway genes and OSCC susceptibility in Chinese Han population. PLoS One. 2014;7:e101965.
Roger S, Mei Z-Z, Baldwin JM, Dong L, Bradley H, Baldwin SA, et al. Single nucleotide polymorphisms that were identified in affective mood disorders affect ATP-activated P2X 7 receptor functions. J Psychiatric Res. 2010;44:347–55.
Li Y, Grupe A, Rowland C, Nowotny P, Kauwe JS, Smemo S, et al. DAPK1 variants are associated with Alzheimer's disease and allele-specific expression. Hum Mol Genet. 2006;15:2560–8.
Ferreira NE, Omae S, Pereira A, Rodrigues MV, Miyakawa AA, Campos LC, et al. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis. 2012;221:131–6.
Meng D-M, Zhou Y-J, Wang L, Ren W, Cui L-L, Han L, et al. Erratum: Polymorphisms in the NLRP3 gene and risk of primary gouty arthritis. Mol Med Rep. 2013;8:1888.
Acknowledgements
This work was supported by the Health Research Council of New Zealand, Arthritis New Zealand, New Zealand Lottery Health and the University of Otago. The authors would like to thank Jill Drake (Canterbury District Health Board), Roddi Laurence, Chris Franklin, Meaghan House (all University of Auckland) and Gabrielle Sexton (University of Otago) for recruitment. Matthew Brown, Linda Bradbury and The Arthritis Genomics Recruitment Initiative in Australia network are acknowledged. The European Crystal Network was formed after the first European Crystal Workshop in Paris, March 2010 (Prof Frédéric Lioté, Paris, and Prof Alexander So, Lausanne, convenors). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The Framingham Heart Study and the Framingham SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University. The Framingham SHARe data used for the analyses described in this manuscript were obtained through dbGaP. This manuscript was not prepared in collaboration with investigators of the Framingham Heart Study and does not necessarily reflect the opinions or views of the Framingham Heart Study, Boston University, or the NHLBI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CM, RKT and TRM helped to design the study, oversee its execution, and prepare the manuscript. LKS, ND, ROD, DRWK, KMW, MJ, TLJ, LAJ, TRR, PLR, A-KT, FL and AS helped to provide clinical recruitment and prepare the manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1:
Association power curves. Figure presenting the power of the genetic association analysis. (PPTX 161 kb)
Additional file 2:
Genotype distributions. Excel file with genotype distributions in the European and Polynesian sample sets for each of the single nucleotide polymorphisms (SNPs) studied. (XLSX 20 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
McKinney, C., Stamp, L.K., Dalbeth, N. et al. Multiplicative interaction of functional inflammasome genetic variants in determining the risk of gout. Arthritis Res Ther 17, 288 (2015). https://doi.org/10.1186/s13075-015-0802-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-015-0802-3