Abstract
Introduction
This study aimed to investigate rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) status and levels as predictors of mortality in two large cohorts of patients with early inflammatory arthritis (EIA).
Methods
Data from the Norfolk Arthritis Register (NOAR) and Leiden Early Arthritis Clinic (EAC) cohorts were used. At baseline, patients had demographic data and smoking status recorded; RF, ACPA and inflammatory markers were measured in the local laboratories. Patients were flagged with national death registers until death or censor date. Antibody status was stratified as negative, low or high positive by RF and ACPA levels individually. In addition, patients were grouped as seronegative, RF positive, ACPA positive or double antibody (RF and ACPA) positive. Cox regression models explored associations between antibody status and mortality adjusting for age, sex, smoking status, inflammatory markers and year of enrolment.
Results
A total of 4962 patients were included, 64% were female. Median age at onset was 56 (NOAR) and 54 (EAC) years. In NOAR and EAC respectively, 35% and 42% of patients were ACPA/RF positive. When antibody status was stratified as negative, low or high positive, there were no consistent findings between the two cohorts. Double antibody positivity was associated with excess mortality in both cohorts compared to seronegative patients: NOAR and EAC respective adjusted HR (95% confidence interval) 1.35 (1.09 to 1.68) and 1.58 (1.16 to 2.15).
Conclusions
Patients with EIA who are seropositive for both RF and ACPA have increased mortality compared to those who are single positive or seronegative. Antibody level in seropositive patients was not consistently associated with excess mortality.
Similar content being viewed by others
Introduction
In patients with inflammatory arthritis, the autoantibodies rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) have been associated with poor outcomes, such as increased disease activity, radiographic progression and disability [1]-[5]. However, the utility of antibody level in predicting the prognosis of inflammatory arthritis, in particular rheumatoid arthritis (RA), has not been clearly established. In a recent multicentre prospective study of patients with early inflammatory arthritis (EIA), the presence of RF and/or ACPA was a significant predictor of RA diagnosis within two years, but level did not appear to be important [6]. In contrast, in a study of patients with EIA from Norway in 2010, Mjaavatten et al. found that increasing levels of RF and ACPA were associated with persistent joint inflammation [7]. Other studies have failed to show consistently that either RF or ACPA antibody level is important in predicting poor outcome in patients with EIA and RA [8]-[10]. In addition, recent data from a subset of the Leiden Early Arthritis Clinic have shown that the avidity of ACPA may be prognostically more important than the level itself [11].
Nevertheless, antibody level is included in the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for RA [12], which aim to identify those patients with EIA with poor prognosis sufficient to require intervention with disease modifying therapy. The presence of RF and ACPA are weighted as part of the total score according to their level; patients are said to be low positive if their level is greater than the upper limit of normal (ULN) but less than three times the ULN, and high positive if their level is at least three times the ULN. Thus, patients with high antibody levels are more likely to fulfil the criteria, and it would be interesting to investigate whether these cut-offs are appropriate in predicting other adverse outcomes, such as mortality.
The increased mortality in patients with RA has been long established [13]. It is also well recognised that the presence of RF in sera of patients with inflammatory arthritis (whether or not they meet formal classification criteria for RA) is associated with an increased risk of premature death [14]-[16]. In fact, this association has been demonstrated even in subjects without symptoms of arthritis [17]. ACPA positivity has also been shown to predict premature mortality in the Norfolk Arthritis Register [18]; however this association has yet to be confirmed in other cohorts.
The aims of this study were to investigate the association between mortality and RF and/or ACPA positivity and level in patients with EIA. The term EIA includes all patients with RA early in the disease process, and studying these patients allows additional inclusion of those patients who may later go on to meet formal classification criteria for RA. It has been recognised that significant variability in antibody testing can occur between laboratories [19]. Thus, to strengthen the external validity of the study results, we investigated these questions in two large prospective cohorts of patients with EIA: the Norfolk Arthritis Register (NOAR) in the UK and the Leiden Early Arthritis Clinic (EAC) in the Netherlands.
Methods
Patients and setting
Patients in Norfolk, UK, were recruited to NOAR between 1990 and 2009 from primary and secondary care if they were adults (≥16 years) and had ≥2 swollen joints for ≥4 weeks; NOAR has been described in detail elsewhere [20]. Leiden EAC has also been described previously [21]; briefly patients in the region of Leiden, the Netherlands, with synovitis confirmed by a rheumatologist were recruited to the Leiden EAC from 1993 onwards if their symptom duration was less than two years at presentation. In order to make the two cohorts as comparable as possible, patients in NOAR were only included in this study if they had symptom duration of less than two years at presentation.
Assessment and follow up
Patients in NOAR are assessed at baseline by a research nurse who administers a structured questionnaire, including demographic details as well as disease and smoking history (never, past, current), performs a 51 tender and swollen joint count and obtains a blood sample. Sera are stored frozen and tested for C-reactive protein (CRP) and RF (latex test, low positive cut-off 40 units/ml, high positive cut-off 120 units/ml); subsequently ACPA, as defined by anti-CCP2 antibodies, are tested for using the Axis-Shield, Dundee, UK Diastat Anti-CCP kit (low positive cut-off 5 units/ml, high positive cut-off 15 units/ml). The Leiden EAC initial assessment includes medical history, clinical examination and joint counts. Blood samples are taken and tested for erythrocyte sedimentation rate (ESR), RF (IgM-RF in-house ELISA, low positive cut-off 5 units/ml, high positive cut-off 15 unit/ml) and ACPA (AntiCCP-2, Euro-Diagnostica, Malmo, Sweden ImmunoscanRA Mark 2, low positive cut-off 25 units/ml, high positive cut-off 75 units/ml). All cut-offs used are those recommended by the relevant manufacturers. Patients in NOAR are flagged with the NHS Information Centre (NHS IC) from baseline. NHS IC provide copies of death certificates to NOAR with approximately six months lag in reporting. They also provide a date of ‘embarkation’ for patients who leave the UK. Mortality data on patients recruited to the EAC are tracked nationally using the civic registries (Gemeentelijke Basis Administratie) in the Netherlands. NOAR is approved by Norfolk and Norwich University Hospital Local Research Ethics Committee UK, and EAC was approved by the local medical ethics committee LUMC The Netherlands.
Statistical analysis
Antibody levels were divided into negative, low positive and high positive as defined by the 2010 classification criteria [12]. These cut-offs were selected to investigate the ability of this aspect of the criteria to predict mortality. NOAR patients were censored for analysis at date of death, date of embarkation or 30 June 2012, whichever came first. Leiden EAC patients were censored at date of death or 1 May 2012. Analyses were conducted separately in each cohort. Kaplan-Meier survival curves were used to compare survival univariately in patients grouped according to their antibody status. Cox proportional hazard models were used to investigate the association between antibody status, antibody level and subsequent mortality. A number of different models were developed. Firstly, patients were categorised according to antibody status as negative, low positive or high positive, and two models were then developed considering RF and ACPA status separately. A third model investigated whether the presence (above the ULN) of both antibodies, rather than antibody level, was important in predicting mortality by categorising patients as seronegative, RF single antibody positive, ACPA single antibody positive and double antibody positive (that is, both RF and ACPA positive). Univariate models were constructed initially, then age and sex adjusted; finally a multivariate model was developed adjusting for age, gender, baseline smoking status (categorised as current, ever or never smokers), inflammatory marker (ESR in EAC or CRP in NOAR) level, and year of enrolment to the cohort as a proxy for changing treatment strategies over time. All analyses were repeated in the population of patients fulfilling the 2010 ACR/EULAR criteria for RA. We aimed to focus on the predictive properties of the antibodies specifically and were deliberately parsimonious with our variable selection in the multivariate model. Thus, if a variable was not considered a confounder a priori, that is, would not have associations with both antibody status and mortality, it was not included. Similarly, variables that might be on the causal pathway between antibody status and mortality (such as disease activity over time) were also not included, as the relationship between antibody status and disease activity can only occur in one direction.
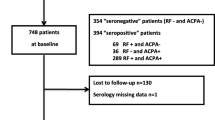
In the model in which the presence of both antibodies was compared to single antibody positivity and seronegativity, only patients who had been tested for both antibodies were included. In NOAR, 2,195 (72%) patients had data on both antibodies; data were more complete for the EAC, where 1,663 (87%) had both antibodies measured. In NOAR, therefore, baseline characteristics of patients with and without complete antibody data were assessed for differences. In addition, in order to ensure that the reported results were representative, multiple imputation using chained equations was performed to impute the antibody status of those patients with missing data. A subsequent sensitivity analysis was performed using the imputed dataset and these results were compared with those from the complete case analysis. Data from NOAR were analysed using the Stata 11 software package (Stata, College Station, TX, USA), data from EAC were analysed using SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA).
Results
A total of 4,962 patients with EIA were included in the study (3,053 from NOAR, 1,909 from Leiden EAC). The cohorts had similar age and gender distributions, 65% (1,970) female in NOAR, 63% (1,205) female in the EAC, respective median (interquartile range) age at symptom onset 56 (44 to 68) and age at inclusion 54 (42 to 67) years. In NOAR, 63% of patients fulfilled the 2010 ACR/EULAR classification criteria for RA, in the EAC this proportion was 57% of patients. Baseline characteristics of patients from the two cohorts are shown in Table 1. The mean (standard deviation) follow up in each study was 11.8 (5.8) years in NOAR and 8.5 (5.2) years in EAC. There were 787 deaths during 36,109 person years follow up in NOAR, and 275 deaths during 16,187 person years follow up in the EAC; this resulted in crude death rates of 21.8 and 17.0 deaths per 1,000 person years in each cohort, respectively. The number of deaths in each of the antibody subgroups are shown in Table 2.
Antibody levels
The first Cox proportional hazards models (univariate and adjusted) examined RF and ACPA levels separately (Table 3). There appeared to be a marked difference in RF high and low positivity in the NOAR cohort: low positive RF adjusted hazard ratio (HR) (95% confidence interval (CI)) 0.80 (0.59 to 1.08), high positive RF adjusted HR (95% CI) 1.49 (1.25 to 1.77). However, this was not replicated in the EAC cohort: low positive RF adjusted HR (95% CI) 1.62 (1.16 to 2.26), high positive RF adjusted HR (95% CI) 1.63 (1.19 to 2.24). Differences between the two cohorts were also seen with ACPA (Table 3). In the EAC, low positive ACPA status was associated with increased mortality, but high positive ACPA was not, respective adjusted HR (95% CI) 2.21 (1.31 to 3.72) and 1.25 (0.93 to 1.69). Conversely, in NOAR there was a trend towards increased mortality in the low positive ACPA group, and high positive ACPA status was significantly associated, adjusted HR (95% CI) 1.32 (1.08 to 1.61). Of note, there were only a small number of patients and, therefore, deaths in the ACPA low positive group in either cohort: 21 deaths in NOAR and 17 in the EAC. Similar findings were observed in the population of patients fulfilling the 2010 ACR/EULAR criteria for RA, although not always reaching statistical significance, probably due to smaller group sizes. Data on the full multivariate models are available as part of Additional file 1. The Additional file 1 also includes a model comparing patients negative for both antibodies to those with low and high levels of either antibody and models dividing RF and ACPA levels into tertiles rather than using the predefined cut-offs of the 2010 criteria. These additional models demonstrated similar results to those reported here.
Number of antibodies
This Cox model stratified patients by the number of antibodies present (negative, RF positive, ACPA positive, and double antibody positive if both RF and ACPA were positive). The results were more consistent between the two cohorts (Table 4 and Figure 1) and between the total EIA population and the 2010 ACR/EULAR RA population. In both NOAR and the EAC there was a trend towards increased mortality in patients who had a single positive antibody compared to no positive antibodies, other than single ACPA positivity in the Leiden EAC, where the number of deaths was small. In both cohorts the presence of two positive antibodies was significantly associated with increased mortality, adjusted HRs (95% CI) NOAR: 1.35 (1.09 to 1.68), EAC: 1.57 (1.15 to 2.14). No differences were identified in the baseline characteristics of patients with missing data in NOAR for this model, and the sensitivity analysis using imputed data produced similar results to the complete case analysis [see Additional file 1].
Discussion
In two well established observational cohorts of EIA and its sub-population of patients with RA, we have shown that RF and ACPA positivity are predictors of excess mortality, and that the presence of both antibodies was a stronger predictor of mortality than single antibody positivity. However, in this first large study to investigate the association between antibody levels and mortality, the influence of increasing antibody level was not consistent between the two cohorts.
Our data have once again demonstrated the known relationship between RF positivity and early mortality [14], and confirmed that a similar association exists in patients who are ACPA positive. This has previously been described in NOAR [18] but only reported elsewhere by two other groups of investigators. The first study was in a subset of 299 patients in the Rochester epidemiology project [22], half of whom had RA. The second small study, by Sihvonen et al. [23] used logistic regression (which does not allow for censoring) rather than Cox models to analyse the data. It was, therefore, important to corroborate this association in another large EIA cohort, such as the Leiden EAC.
The results of our study are concordant with the findings of Ursum et al., who studied 545 patients with early arthritis in the Netherlands [10]. They found no association after two years between antibody levels and early disease outcomes, including disease activity measured by DAS28, functional status measured by the Health Assessment Questionnaire (HAQ) and radiographic progression. Similarly, a number of other small studies have reinforced the association between ACPA positivity and other poor outcomes, such as increased disease activity and radiographic damage, but have failed to identify an association with increasing ACPA levels [8],[24]. By contrast, Syversen et al. conducted a study of 125 patients who met the 1987 ACR classification criteria for RA [25] in a subpopulation of the European Research on Incapacitating Disease and Social Support (EURODISS) project [26]. They found that 10 year radiographic progression was increased in patients with low-moderate ACPA levels (>ULN and ≤8 times ULN), but this appeared to be further increased in patients with very high levels of ACPA (>8 times the ULN). However, they also demonstrated that the highest probability of radiographic progression occurred in patients who were positive for both RF and ACPA. A recent study in Italy examined progression from EIA to RA in 192 patients [6]. In accordance with our findings, they demonstrated the presence of both antibodies predicted RA, but antibody high or low positivity had no influence. In the Norwegian Very Early Arthritis Clinic (NOR-VEAC) study, Mjaavatten et al. showed additive value in testing for both antibodies in order to predict disease persistence [7]. They also demonstrated an association between antibody level and persistent arthritis, however the number of patients per group was small (<30). In addition, their analysis employed last observation carried forward to account for patients who did not have complete follow up. It is possible, therefore, that their results were influenced by attrition bias; that is, patients whose arthritis resolved may not have attended further follow up, and at their last recorded visit, their arthritis appeared to be persistent even though it subsequently resolved. It is possible that the different characteristics and follow up of these cohorts account for the different findings; in addition the different cut-offs of the commercially available assays may not correspond. Nevertheless, this emphasises that the role of antibody levels in predicting outcomes for patients with inflammatory arthritis has not been robustly established.
There are limitations to our study. We decided not to perform a pooled analysis of data from both cohorts because the different inclusion criteria of the two cohorts could potentially produce misleading conclusions. We did not aim to develop a full predictive model for mortality in RA, but focussed specifically on the association between antibody status and level, and mortality. Therefore, the number of confounders included in the multivariate model was small, and the final model does not account for all predictors of mortality in RA. As in all observational studies, there remains potential for residual confounding for which we have not adjusted. Further, in our analyses we did make the assumption that antibody status is fixed. This assumption seemed reasonable as the majority of studies have shown for both RF and, particularly, ACPA, that few patients convert from seropositive to negative over time [27]-[29], and when this does occur, risk of poor outcome may be maintained [30].
Conclusions
In conclusion, in this large study investigating the relationship between antibody levels and mortality in EIA, we have shown that patients with both RF and ACPA, rather than the higher levels of the antibodies, had increased rates of early death. We have also confirmed the association between ACPA positivity and excess mortality in a second large EIA cohort. Therefore, in patients presenting with early rheumatoid arthritis, the number of positive antibodies may be more important than the antibody levels in assessing the mortality risk in clinical practice.
Additional file
Abbreviations
- ACPA:
-
anti-citrullinated protein antibody
- ACR:
-
American College of Rheumatology
- DAS28:
-
Disease Activity Score based on 28 joint count
- CRP:
-
C-reactive protein
- EAC:
-
Leiden Early Arthritis Clinic
- EIA:
-
early inflammatory arthritis
- ELISA:
-
enzyme-linked immunosorbent assay
- ESR:
-
erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- EURODISS:
-
European Research on Incapacitating Disease and Social Support
- HR:
-
hazards ratio
- NHS-IC:
-
NHS Information Centre
- IQR:
-
inter-quartile range
- NOAR:
-
Norfolk Arthritis Register
- RA:
-
rheumatoid arthritis
- RF:
-
rheumatoid factor
- ULN:
-
upper limit of normal
- 95% CI:
-
95% confidence interval
References
Quinn MA, Gough AK, Green MJ, Devlin J, Hensor EM, Greenstein A, Fraser A, Emery P: Anti-CCP antibodies measured at disease onset help identify seronegative rheumatoid arthritis and predict radiological and functional outcome. Rheumatology. 2006, 45: 478-480. 10.1093/rheumatology/kei203.
De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, Lebeer K, Wyns B, Vincent C, Mielants H, Boullart L, Serre G, Veys EM, De Keyser F: Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis. 2004, 63: 1587-1593. 10.1136/ard.2003.017574.
Ronnelid J, Wick MC, Lampa J, Lindblad S, Nordmark B, Klareskog L, van Vollenhoven RF: Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis. 2005, 64: 1744-1749. 10.1136/ard.2004.033571.
Kudo-Tanaka E, Ohshima S, Ishii M, Mima T, Matsushita M, Azuma N, Harada Y, Katada Y, Ikeue H, Umeshita-Sasai M, Miyatake K, Saeki Y: Autoantibodies to cyclic citrullinated peptide 2 (CCP2) are superior to other potential diagnostic biomarkers for predicting rheumatoid arthritis in early undifferentiated arthritis. Clin Rheumatol. 2007, 26: 1627-1633. 10.1007/s10067-007-0558-5.
Bos WH, Bartelds GM, Vis M, van der Horst AR, Wolbink GJ, Van De Stadt RJ, Van Schaardenburg D, Dijkmans BA, Lems WF, Nurmohamed MT, Aarden L, Hamann D: Preferential decrease in IgG4 anti-citrullinated protein antibodies during treatment with tumour necrosis factor blocking agents in patients with rheumatoid arthritis. Ann Rheum Dis. 2009, 68: 558-563. 10.1136/ard.2008.088401.
Bizzaro N, Bartoloni E, Morozzi G, Manganelli S, Riccieri V, Sabatini P, Filippini M, Tampoia M, Afeltra A, Sebastiani G, Alpini C, Bini V, Bistoni O, Alunno A, Gerli R: Anti-cyclic citrullinated peptide antibody titer predicts time to rheumatoid arthritis onset in patients with undifferentiated arthritis: results from a 2-year prospective study. Arthritis Res Ther. 2013, 15: R16-10.1186/ar4148.
Mjaavatten MD, van der Heijde D, Uhlig T, Haugen AJ, Nygaard H, Sidenvall G, Helgetveit K, Kvien TK: The likelihood of persistent arthritis increases with the level of anti-citrullinated peptide antibody and immunoglobulin M rheumatoid factor: a longitudinal study of 376 patients with very early undifferentiated arthritis. Arthritis Res Ther. 2010, 12: R76-10.1186/ar2995.
Papadopoulos NG, Tsiaousis GZ, Pavlitou-Tsiontsi A, Giannakou A, Galanopoulou VK: Does the presence of anti-CCP autoantibodies and their serum levels influence the severity and activity in rheumatoid arthritis patients?. Clin Rev Allergy Immunol. 2008, 34: 11-15. 10.1007/s12016-007-8018-1.
Shiozawa K, Kawasaki Y, Yamane T, Yoshihara R, Tanaka Y, Uto K, Shiozawa S: Anticitrullinated protein antibody, but not its titer, is a predictor of radiographic progression and disease activity in rheumatoid arthritis. J Rheumatol. 2012, 39: 694-700. 10.3899/jrheum.111152.
Ursum J, Bos WH, van Dillen N, Dijkmans BA, Van Schaardenburg D: Levels of anti-citrullinated protein antibodies and IgM rheumatoid factor are not associated with outcome in early arthritis patients: a cohort study. Arthritis Res Ther. 2010, 12: R8-10.1186/ar2907.
Suwannalai P, Britsemmer K, Knevel R, Scherer HU, Levarht EW, van der Helm-van Mil AH, Van Schaardenburg D, Huizinga TW, Toes RE, Trouw LA: Low-avidity anticitrullinated protein antibodies (ACPA) are associated with a higher rate of joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2014, 73: 720-726. 10.1136/annrheumdis-2012-202615.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D: 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010, 69: 1580-1588. 10.1136/ard.2010.138461.
Sokka T, Abelson B, Pincus T: Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008, 26: S35-S61.
Goodson NJ, Wiles NJ, Lunt M, Barrett EM, Silman AJ, Symmons DP: Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002, 46: 2010-2019. 10.1002/art.10419.
Sokka T, Hakkinen A, Krishnan E, Hannonen P: Similar prediction of mortality by the health assessment questionnaire in patients with rheumatoid arthritis and the general population. Ann Rheum Dis. 2004, 63: 494-497. 10.1136/ard.2003.009530.
Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC: Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003, 107: 1303-1307. 10.1161/01.CIR.0000054612.26458.B2.
Tomasson G, Aspelund T, Jonsson T, Valdimarsson H, Felson DT, Gudnason V: Effect of rheumatoid factor on mortality and coronary heart disease. Ann Rheum Dis. 2010, 69: 1649-1654. 10.1136/ard.2009.110536.
Farragher TM, Goodson NJ, Naseem H, Silman AJ, Thomson W, Symmons D, Barton A: Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum. 2008, 58: 359-369. 10.1002/art.23149.
van der Linden MP, Batstra MR, Bakker-Jonges LE, Detert J, Bastian H, Scherer HU, Toes RE, Burmester GR, Mjaavatten MD, Kvien TK, Huizinga TW, van der Helm-van Mil AH: Toward a data-driven evaluation of the 2010 American College of Rheumatology/European League against Rheumatism criteria for rheumatoid arthritis: is it sensible to look at levels of rheumatoid factor?. Arthritis Rheum. 2011, 63: 1190-1199. 10.1002/art.30200.
Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ: The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol. 1994, 33: 735-739. 10.1093/rheumatology/33.8.735.
de Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH: Predicting arthritis outcomes–what can be learned from the Leiden Early Arthritis Clinic?. Rheumatology (Oxford). 2011, 50: 93-100. 10.1093/rheumatology/keq230.
Liang KP, Kremers HM, Crowson CS, Snyder MR, Therneau TM, Roger VL, Gabriel SE: Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009, 36: 2462-2469. 10.3899/jrheum.090188.
Sihvonen S, Korpela M, Mustila A, Mustonen J: The predictive value of rheumatoid factor isotypes, anti-cyclic citrullinated peptide antibodies, and antineutrophil cytoplasmic antibodies for mortality in patients with rheumatoid arthritis. J Rheumatol. 2005, 32: 2089-2094.
Landmann T, Kehl G, Bergner R: The continuous measurement of anti-CCP-antibodies does not help to evaluate the disease activity in anti-CCP-antibody-positive patients with rheumatoid arthritis. Clin Rheumatol. 2010, 29: 1449-1453. 10.1007/s10067-010-1557-5.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS: The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31: 315-324. 10.1002/art.1780310302.
Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P, van der Heijde D, Landewe R, Kvien TK: High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis. 2008, 67: 212-217. 10.1136/ard.2006.068247.
Barra L, Pope J, Bessette L, Haraoui B, Bykerk V: Lack of seroconversion of rheumatoid factor and anti-cyclic citrullinated peptide in patients with early inflammatory arthritis: a systematic literature review. Rheumatology (Oxford). 2011, 50: 311-316. 10.1093/rheumatology/keq190.
Mjaavatten MD, van der Heijde DM, Uhlig T, Haugen AJ, Nygaard H, Bjorneboe O, Kvien TK: Should anti-citrullinated protein antibody and rheumatoid factor status be reassessed during the first year of followup in recent-onset arthritis? A longitudinal study. J Rheumatol. 2011, 38: 2336-2341. 10.3899/jrheum.110234.
Burr ML, Viatte S, Bukhari M, Plant D, Symmons DP, Thomson W, Barton A: Long-term stability of anti-cyclic citrullinated peptide antibody status in patients with early inflammatory polyarthritis. Arthritis Res Ther. 2012, 14: R109-10.1186/ar3834.
Nell-Duxneuner V, Machold K, Stamm T, Eberl G, Heinzl H, Hoefler E, Smolen JS, Steiner G: Autoantibody profiling in patients with very early rheumatoid arthritis: a follow-up study. Ann Rheum Dis. 2010, 69: 169-174. 10.1136/ard.2008.100677.
Acknowledgements
The authors would like to acknowledge ‘BeTheCure’ for its support of collaborative work between European cohort studies. They also gratefully acknowledge the support of clinical staff in Norfolk, the NOAR research nurses, as well as the Leiden EAC clinical and research staff. NOAR is funded by Arthritis Research UK grant reference numbers: 20380 and 20385. JH Humphreys is funded by Arthritis Research UK grant reference number: 19743. This research has also been funded by The European Community Seventh Framework Program FP7 Health-F2-2008-223404 (Masterswitch) and a Vidi grant of the Netherlands Organization for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
All authors declare that they have no competing interests.
Authors’ contributions
JH participated in conception and design of the study, data analysis of NOAR data, manuscript writing and final approval of the manuscript. JN participated in conception and design of the study, data analysis of Leiden data, help drafting and final approval of the manuscript. JC participated in data collection, drafting of manuscript and final approval of the manuscript. TM participated in data collection, drafting of manuscript and final approval of the manuscript. AH participated in conception and design of the study, revising the manuscript and final approval of the manuscript. DS participated in conception and design of the study, revising the manuscript and final approval of the manuscript. SV participated in conception and design of the study, revising the manuscript and final approval of the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
13075_2014_483_MOESM1_ESM.pdf
Additional file 1: Table S1.: Univariate and multivariate Cox proportional hazard models comparing RF/ACPA high/low positive versus negative. Table S2. Univariate and multivariate Cox proportional hazard models comparing RF and ACPA high/low positive versus negative. Table S3. Comparison of RF or ACPA levels in tertiles. Table S4. Univariate and multivariate Cox proportional hazard models comparing RFand ACPA positive versus single positive and both antibodies negative. Table S5. Sensitivity analysis with imputed data. (PDF 418 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Humphreys, J.H., van Nies, J.A., Chipping, J. et al. Rheumatoid factor and anti-citrullinated protein antibody positivity, but not level, are associated with increased mortality in patients with rheumatoid arthritis: results from two large independent cohorts. Arthritis Res Ther 16, 483 (2014). https://doi.org/10.1186/s13075-014-0483-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-014-0483-3