Abstract
Genome editing technology has evolved rather quickly and become accessible to most researchers. It has resulted in far reaching implications and a number of novel designer systems including epigenome editing. Epigenome editing utilizes a combination of nuclease-null genome editing systems and effector domains to modulate gene expression. In particular, Zinc Finger, Transcription-Activator-Like Effector, and CRISPR/Cas9 have emerged as modular systems that can be modified to allow for precision manipulation of epigenetic marks without altering underlying DNA sequence. This review contains a comprehensive catalog of effector domains that can be used with components of genome editing systems to achieve epigenome editing. Ultimately, the evidence-based design of epigenome editing offers a novel improvement to the limited attenuation strategies. There is much potential for editing and/or correcting gene expression in somatic cells toward a new era of functional genomics and personalized medicine.
Similar content being viewed by others
Background
The modulation of gene expression can be achieved by a variety of biotechnologies such as RNA interference, non-precision drugs, and artificial transcription factors (ATFs). Epigenome editing is an emerging field of synthetic biology that falls under the category of ATF [1]. It is distinguished from other gene expression modulation technologies in that it can create precise and long-lasting epigenetic modification without the need to keep or maintain the system after the initial event [2].
The epigenome editing systems that are the focus of this review contain the DNA-binding element of genome editing systems. Thus, in order to gain a full appreciation for epigenome editing one must start with the fundamentals of genome editing as the two share not only components but also obstacles. Genome editing represents a revolution in genetic engineering as it allows for precision targeting and manipulation of genome. Genome editing systems rely on two components, a DNA-binding element, and nuclease, to modify the targeted DNA sequence. Genome editing can be used to study protein function by altering coding sequence or achieve transcriptional control by altering the sequence of regulatory regions. Epigenome editing, on the other hand, uses the same DNA-binding principle but utilizes an effector domain, rather than a nuclease. The effector domain is a fragment of a desired regulatory protein and is used to create a desired epigenetic mark at a targeted locus without altering the underlying sequence.
DNA-binding genome editing systems
The concept of genome editing is not new. What is new is the refinement of methods that make it feasible for most laboratories to undertake the protocol successfully. Today, genome editing allows for precise manipulation of DNA sequences and brings about desired genetic changes at will in vitro and in vivo [3–5]. The principle involves precise targeting of a specific DNA sequence in the genome to create a site-specific double-stranded break using a nuclease. A cell will then attempt to correct this damage by homology-directed repair (HDR), which makes it possible to introduce desired donor sequence(s). Additionally, non-homologous end-joining (NHEJ) can be used to delete desired sequences. The methods available make use of Zinc Fingers (ZFs), Transcription-Activator-Like Effectors (TALEs), and the Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) sequences with CRISPR-Associated Protein 9 (Cas9) detailed below.
ZFN
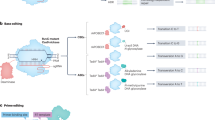
Zinc Finger Nuclease (ZFN) is the oldest genome editing technology [6–8]. It is based on two-modules. The first are Zinc Finger Proteins that recognize and bind to DNA sequences. Zinc finger proteins coordinate zinc ions using a backbone of conjugated Cysteine (Cys) and Histidine (His) residues to achieve their structure. They come in a number of folding groups the most widely used being the Cys2His2 group. This folding group represents the classical zinc finger and is widely used as a natural transcription factor in mammals. Cys2His2 ZFs also have a relatively conserved backbone. ZF specificity for DNA sequence comes from a part of the α helix, known as the recognition domain, which binds to the major groove of DNA. The specificity is determined by amino acids in the recognition domain. Variation in this region, either naturally occurring or synthetic, results in recognition of alternative nucleic acid sequences. Experimentally, a designer zinc finger is fused to a nuclease (FokI), which requires dimerization for double-stranded DNA cleavage. Here, the targeting specificity comes about from the recognition domain, with each ZF recognizing 3–4 bp per amino acid in the domain. Typically, ZFN systems use a combination of 3–6 ZFs fused to a FokI domain. The inverted dimer required for nuclease activity gives additional sequence specificity as there is a required space, known as the spacer. This design approach facilitates a target specificity of ~24 bp, which is enough to target most unique regions in most genomes (Fig. 1a). In terms of practicality, ZFNs are limited by the higher cost and effort of designing the custom proteins, interactions between residues affecting targeting, and altered sequence recognition from the effects of additional genomic and chromatin content surrounding the target sites [9]. However, they have the advantage of being oldest, most studied, and only genome editing system to be in clinical trials.
(Epi)Genome editing systems. a Zinc Finger Nuclease (ZFN), b Transcription-Activator-Like Effector Nuclease (TALEN), c CRISPR/Cas9, d Zinc Finger (ZF) with a DNA methyltransferase effector domain, e Transcription-Activator-Like Effector (TALE) with a histone methyltransferase, and f Catalytically deactivated Cas9 (dCas9) and sgRNA from with a histone acetyltransferase. Components are not to scale as critical features are exaggerated and some non-variable features are removed
TALEN
TALENs represent a fusion of a Transcription-Activator-Like Effector (TALE), which is a viral element evolved to target plant transcription and a designer nuclease [10–12]. TALEs have a central repeat domain that confers its sequence specificity (Fig. 1b). This domain is 33–35 amino acids long and has two highly variable sites at the 12th and 13th amino acids, which are known as the repeat variable di-residues (RVDs). Different combinations at the RVDs allow for recognition of a single base. While both ZFN and TALEN systems theoretically allow for single-base targeted design, TALEs recognize single nucleotides as opposed to the triplet combinations of a ZFPs recognition sequence. Also, this system performs better than ZFNs since they are not as influenced by sequence and chromatin surrounding the target site [13–17]. More importantly, TALEs and TALENs represent a significant improvement in specificity and protocol [18, 19].
TALENs have been successfully used in mice for mitochondrial transfer, which allows for a 3-parent child. This is done in order to prevent an inherited disorder in the mitochondrial genome that would normally be transmitted from the mother. It has recently been approved as a medical procedure in the UK and is currently under serious consideration in USA. Intriguingly, a mitoTALEN system was recently used in mice to overcome mitochondria heteroplasmy by targeting and selectively destroying diseased mitochondria but still allowing for the transmission of wild-type mitochondria in mouse oocytes from the original mother [20].
CRISPR/Cas9
This system also consists of two components. First, the Cas9 protein is a nuclease. Second, the CRISPR/Cas9 system consists of a synthetic guide RNA (sgRNA) [21]. The sgRNA is used for sequence specificity and has a 20 bp target recognition domain. However, the sgRNA contains more information than just targeting specificity and has a complex stem loop structure. The loading of the sgRNA is critical for activating the catalytic activity of Cas9 [22]. The binding and catalytic activity of the Cas9/sgRNA complex on target DNA is also dependent on the presence of an external sequence known as the Protospacer Adjacent Motif (PAM) [23]. Target DNA sequences complementary to the sgRNA are ignored by the Cas9/sgRNA complex if PAM is not present upstream of the target DNA. This is because DNA strand separation and the RNA–DNA heteroduplex are initiated at the PAM site (Fig. 1c). While there are target sequence limitations created by the requirement of PAM before the target sequence, research into overcoming the initial limitations is showing that by using Cas9 orthologs with alternative PAM sequences [24–26] and distinct sgRNA recognition sequences [27] there may be no practical limit to the targetable sites in the genome. Another practical advantage of CRISPR/Cas9 is the relative ease of creating short custom nucleotide (<100 bp) sequences compared to engineering proteins to bind different sequences while also maintaining activity and specificity. The CRISPR/Cas9 protocol [28–32] has undergone numerous improvements, allowing it to become the most widely used genome editing system. The advantage of CRISPR/Cas9 over ZFNs and/or TALENs is its scalability in that multiple sites within the mammalian genome can be modified simultaneously, providing a robust, high-throughput approach for gene editing in mammals. These advantages in this system are largely due to the fact that RNA, instead of designed protein, is used to specify the target.
The CRISPR system has been adapted to target double-strand breaks and modify DNA sequence in the genomes of a number of organisms including humans [33], mice [34], and zebrafish [35]. In fact CRISPR/Cas9 has been adopted to work in species ranging from prokaryote to eukaryote, plant to animal, and vertebrate to invertebrate. Of particular interest is the fact that in its earliest stages of development CRISPR/Cas9 has been used to alter the mouse by using a knockin system [36, 37] and also enabled reverse genetic studies in post-mitotic neurons of the adult brain [38]. CRISPR/Cas9 has also been used to generate one-cell embryos that go on to develop into edited monkeys [39]. Finally, it has been demonstrated to deplete synaptic proteins in rat hippocampal neuron cultures [40] and correct the mutation in the hemoglobin beta gene responsible for sickle cell anemia [41]. This was done in blood cells derived from induced pluripotent stem cells (iPSCs) of patients and with a higher efficiency than possible using ZFNs and TALENs [41, 42].
The CRISPR/Cas9 system can also be used to create gene drives [43]. Gene drives are a synthetic biological system in which a transgene can aggressively propagate independently of natural selection. It can quickly take over a population in a few generations with only just a few founders. This is because the mutation edited into a founder will overwrite the content of the wild-type parent in their offspring, thus it overcomes the diploid genome and makes an inherited heterozygote and homozygote [44].
Genome editing the epigenome
Genome editing systems can also be used to edit the epigenome in a fashion that is distinct from epigenome editing, as it involves altering sequence critical to the epigenome. CRISPR/Cas9 has been utilized to study chromatin architecture and make targeted and unprecedented alterations to the repeat rich regulatory elements. It created deletions, inversions, and duplications that enabled the study of the clustered protocadherins [45], a complex locus that generates individual neuronal identity and is involved in neurodevelopmental disorders [46]. The approach has lead to the discovery of regulatory elements from the protocadherin α cluster that are also involved in the regulation of the γ cluster. Finally, CRISPR/Cas9 has been used to remove CTCF binding sites in the Hox gene clusters during embryonic stem cell differentiation in cervical motor neurons and disrupt the topological chromatin boundaries, turning repressed chromatin into active chromatin by preventing CTCFs targeted function as a genomic insulator [47]. Also, TALENs have been used to study long-range chromatin interactions by altering the sequence of inter- and intra-chromosomal contact points [48].
Furthermore, the cross-species capability of CRISPR/Cas9 has lead to profound insight in mammalian systems that were previously inaccessible at the level of basic research, particularly in monkeys [49] and humans. CRISPR/Cas9 has already been used to investigate DNA methylation machinery. Two genome editing approaches were developed in an in vivo mouse model, one had a single gene approach to target the reader MeCP2 that allowed for visualization and cell sorting and the other had a multi-gene approach to target the DNA methyltransferases (DNMTs) Dnmt1, Dnmt3a, and Dnmt3b [38]. More recently, the CRISPR/Cas9 system was used for experimentation in human embryonic stem cells to create precise knockout deletions in the DNMTs [50]. By creating catalytically inactivating mutations using both multiplex and singleplex approaches the targets of DNMT1, DNMT3A, and DNMT3B were mapped with single-base pair resolution via whole-genome sodium bisulfite sequencing. This study was done in reference to a previous mouse model study of embryonic stem cells [51]. In both humans and mice, ESCs are viable without DNMT3A or DNMT3B, but interestingly only human cells undergo rapid cell death from the removal of DNMT1. This occurred even with an inducible system to control the temporal disruption, where cell death occurs immediately upon inducing repression of the system masking the homozygous mutation. Thus, the CRISPR/Cas9 technology was able to illustrate a fundamental difference between humans and mice.
Epigenome editing
Besides editing genome sequence, genome editing systems have been used in altering the transcription of specific gene(s) without altering the underlying sequence. This modification for transcriptional alterations involves exclusion or inactivation of nuclease activity followed by use of the targeting system fused to a modular effector domain and is known as epigenome editing. A list of effectors and their effects on gene expression (increase or decrease) is summarized in Table 1. However, it should be noted that the effect of epigenetic marks is context dependent and thus the context of this table is the average consequence of depositing these marks in a promoter or enhancer. These systems have been used to target integrated/synthetic as well as endogenous loci, a distinction that is reviewed by de Groote et al. [1].
The function of ATF was the primary purpose for DNA sequence specificity of designer ZFs and TALEs with the goal of precise transcriptional activation, also known as transactivation. Transactivation effector domains are based on viral elements. The original VP16 domain [52] comes from Herpes Simplex Viral Protein 16 and consists of amino acids 437–447 [DALDDFDLDML]. VP16 was later engineered into VP64 domain [53], which is a fusion containing four tandem copies of VP16 connected by glycine-serine linkers [DALDDFDLDML]-GS-[DALDDFDLDML]-GS-[DALDDFDLDML]-GS-[DALDDFDLDML]. It is the most widely used transactivation domain. Interestingly, one effect of using the VP64 transactivation domain is that it recruits p300, which causes activating H3K27Ac to increase at the targeted locus over time and represents an example of transcription driving transcription [54]. Transcriptional repression, on the other hand, utilizes repression domains [55, 56] and is typically achieved by variants of a 45-aa segment from Krüppel-associated boxes (KRAB) [55] or repressive epigenomic modifications [57, 58]. The KRAB repressor domain appears to be the most potent natural repressor in the genome and used by half of zinc fingers, which make up half of the genome’s transcription factors. Interestingly, the KRAB repressor domain recruits histone modifying domains and results in a decrease of activating H3K4me3 and increase of repressive H3K9me3 and H3K27me2 but does not alter DNA methylation [59]. However, these modifications may not reflect the immediate effect of transcriptional repression and could be a later consequence [60]. A bacterial DNA methyltransferase (M.SssI) is also capable of repression and recruiting a heterochromatin protein, H3K9me3, and H3K27me2 [59]. The following are examples of how some of these effector domains have been used with DNA-binding platforms to modulate gene expression.
Zinc Fingers
The ZF system has been extensively used as an artificial transcription factor (Fig. 1d). It was first used to establish epigenome editing in 2002 when an engineered ZF fused to a histone methyltransferase was able to show that H3K9 methylation is causative in gene repression [61]. Since then ZFs have been designed with histone methyltransferases to repress oncogenes [62]. ZFs have also been utilized with DNA methylation machinery. In these cases, the DNA methyltransferases (DNMTs) were fused to designer ZFs to cause targeted DNA methylation and repress related gene expression [63–70]. Such designs have varied from engineered bacterial methyltransferases to select domains of the mammalian DNMT family. Recently, a ZF fused to DNMT3A or the H3K9 methylation writer GLP were delivered by adenoviral delivery system to control the regulation of a cancer gene by targeting its promoter [71]. While DNA methylation repressed longer than H3K9 methylation, the effect was not long-lasting and the authors speculate that multivalent epigenetic modifications must be designed for long-term effects when epigenome editing and to accommodate for large-scale chromatin domains. One promising design of effector domain involves a fusion of the catalytic domain of the de novo methyltransferase DNMT3A and C-terminal domain of (the catalytically inactive) DNMT3L, which naturally stimulates DNMT3A’s activity [67]. Alternatively, ZFs can be used to enhance gene expression by being fused with the DNA demethylase thymidine DNA glycosylase (TDG) [72]. However, epigenetic editing by ZFs is prone to the same problems as genome editing, genome wide off-target effects caused by the nature of ZF recognition being altered by additional (epi)genomic context [60].
TALEs
Taking the modular approach of ZFs, TALEs have been modified to induce transcriptional activation [73] and repression [74] (Fig. 1e). TALE epigenome editing systems have been used to target and modify chromatin at enhancers [75] and regulate gene expression via DNA methylation [76]. Furthermore, using a combination of epigenome editing systems and optogenetics for light induction, it was shown that gene expression, histone acetylation (H3K9ac) and histone methylation (H3K27me3) in the mouse brain can be targeted and modulated in a reversible fashion [57] at will. TALEs have also been efficiently fused to the TET family of active DNA demethylases and drive gene expression in targeted sequences by actively removing DNA methylation [77, 78]. The fusion of the DNMT3A and DNMT3L was replicated in TALEs [76].
dCas9
The CRISPR/Cas9 system has also been used to alter transcription [79–88]. This has been achieved by targeting with sgRNAs and a catalytically inactivated Cas9 (dCas9), which creates a RNA-based targeting system that can be fused to effector domains. Therefore, dCas9 based epigenetic editing gains the target specificity of CRISPR/Cas9 without causing a double-stranded break and while carrying out the function of the effector domain at the target site (Fig. 1f). A dCas9 system fused to the KRAB transcription repressing domain has been used to confirm the function of LRP8-Reelin-regulated neuronal enhancers in cortical neurons and lead to the discovery of a novel synapse-to-nucleus pathway related to glutamatergic signaling [89]. However, in the context of transcriptional control the dCas9 system often achieves low effectiveness, which can be improved by the tiling of multiple sgRNAs. Yet another approach to transcription activation via dCas9 has been to fuse two activation domains per dCas9. This system was used to reprogram the cell lineage of stem cells and drive subsequent phenotypes [90]. Also, the histone acetyltransferase p300 can be used as an effector domain to achieve H3K27 acetylation and induce gene expression by targeting the mammalian β-globin locus control region, which is something that could not be achieved by a VP64 domain [91].
Since each system has its own strengths and weaknesses when it comes to off-target and on-target effects, price, cellular toxicity, and ease of use, a standardized comparison system is needed [92, 93]. In practice, each system may show a unique potential when used on their own or coupled together [94]. This has recently been exemplified in the case of transcriptional activation of pluripotency factors in humans and mice by both CRISPR- and TALE-based editing systems [95]. When comparing the ability to drive gene expression by targeting enhancers, it was found that TALEs could outperform CRISPR/Cas9 and the authors recommended an approach combining both systems for highly efficient transcriptional regulation [54]. However, this comparison used initial and less effective CRISPR activators and not the most enhanced genetically engineered improvements [84, 87] that are described below.
Engineered improvements
Several laboratories have begun to utilize the genetically engineered Cas9 proteins and sgRNAs (Fig. 2). One alterative involves a versatile scaffolding platform to attach multiple VP64 domains to dCas9 [96] and a different approach uses the tripartite activator VP64-p65-Rta (VPR) [97]. Another variation is the use of second-generation sgRNAs (sgRNA 2.0) for multi-effector programming [88], where scRNAs (extended sgRNAs) have effector domain recruitment sites added into their sequence after the targeting site. This approach further creates modularity in that there are layers of variation created in Cas9 orthologs having different sgRNA recognition sites. Additional layers are then created by the fact that each sgRNA can be programmed to have two recognizable loops that can be bound in homogenous or heterogeneous configurations by a unique binding protein being fused to unique effector domains [88]. One example of this system is the Synergistic Activation Mediator (SAM), which is a potent transcription activation system [84]. In SAM, the exposed and engineered RNA loops from the Cas9–sgRNA–DNA complex are used as anchoring points for the RNA-binding protein (MS2) that is also fused to a p65-HSF1 fusion effector domain. This allows for a synergistic combination in activating gene expression at levels much higher than a single effector domain. Another approach involves further enhancing the sgRNA to create a system known as CRISPR-Display [98]. CRISPR-Display allows for functional RNA domains (~4.8 kb) to be inserted into the sgRNA loops at multiple positions, including the same loop as sgRNA 2.0 as well as 5′ and 3′ positions. This approach uses functional motifs like the protein-binding cassettes of earlier sgRNA 2.0 approaches but also enables long non-coding RNA to be inserted into the dCas9/sgRNA complex. Ultimately, the CRISPR-Display system enables precision ectopic targeting of RNA and ribonucleoprotein to loci of interest in order to fully characterize the functionality of the RNA.
Designer epigenome editing systems based on dCas9. VPR refers to the effector domain, which is a tripartite design. sgRNA 2.0 refers to a scaffolding system that allows for modular effectors to be added to the sgRNAs that have been modified to contain protein-binding sites for RNA recognizing proteins. SAM refers to a synergistic activator that contains an effector domain fused to the dCas9 protein as well as the sgRNA 2.0 design to add additional designer activators. Components are not to scale as critical features are exaggerated and some non-variable features are removed
Combinatorial biotechnology
(Epi)genome editing systems allow not only for fusing a genome editing system to an effector but they can also add additional genetic changes and facilitating methodologies. For example, Konermann et al. [57] used transcriptional control or histone modifying (acetylation and methylation) effector domains (Table 1) along with a light inducible (optogenetic) element. The optogenetic induction system involves light-sensitive cryptochrome 2 (CRY2) and CIB1, its binding partner [99]. These two proteins only heterodimerize upon exposure to blue light, a process that is rapid and reversible, and can be applied to study neurons in mammalian brains [100, 101]. They can then be separately fused to an epigenome editing system, with one attached to the targeting system and the other to the effector. This can be utilized in epigenome editing by allowing for the editing to be induced when and where it is desired in a rapid and reversible manner. The optogenetic approach has also been combined with CRISPR/Cas9 transactivation systems [102, 103]. Alternate inducible transactivation systems use steroids that has been created using TALEs [104]. Finally, cell imaging can also be achieved using an EGFP effector domain [105] that can visualize pericentric, centric, and telomeric repeats [106]. This allows for the visualization of repetitive sequences using a single sgRNA or an array of sgRNAs for non-repetitive sequences to enable visualization and tracking through cellular processes. This technique was demonstrated by imaging telomere dynamics and the dynamic sub-nuclear localization of a single gene through mitosis. Another visualization system has been developed that allows for multicolor analysis. It was initially successful in TALEs [107] and was recently adopted for dCas9 orthologs with three spectral systems [108]. The spectral systems were used to target telomeres, several target loci, and also determine the intranuclear distance between loci on different chromosomes, which allowed for the assessment of DNA compaction in live cells.
Furthermore, epigenome editing systems have been used for enrichment and purification of proteins interacting with target loci. They have the potential to allow for an examination of all the proteins and histone PTMs associated with a single genomic locus, the epiproteome [109]. These techniques couple chromatin immunoprecipitation and the target specificity of genome editing systems (without the catalytic activity) by using an effector domain to allow for enrichment that can then be coupled to analysis by mass spectrometry. One variant of this approach is known as engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP). It has been developed using TALEs [110] and CRISPR/Cas9 [111] to study telomeres. Another variant involving the CRISPR/Cas9 system has also been developed and termed Chromatin Affinity Purification with Mass Spectrometry (CRISPR-ChAP-MS) [112]. This approach was able to reveal the changes during the activation of transcription although it had difficulty with repressive contexts, including when using TALEs [113]. The developments identified above represent some selected issues that will be further enabled using epigenome editing technology. More importantly, we anticipate additional future modifications and applications of this system to provide insights into other biological problems that have remained difficult to investigate.
Future challenges
A flurry of publications in recent years have established that (epi)genome editing may hold the key to the next generation of genomic revolution; the alteration of gene sequences as well as its expression in designated tissues at will. To date, most of this research has focused on understanding biological complexities including correction of gene defects that may be associated with diseases. It is also apparent that genome editing systems have met a number of challenges in providing the needed proof of principle for epigenome editing systems. These include off-target effects, editing efficiency, delivery [114–116], and cytotoxicity [117]. In particular given the complexity of the genome sequence and organization, it may not be easy to avoid off-target effects in most if not all cases, particularly in the highly open and dynamic chromatin of embryos that is not well characterized. These limitations mean that only some of the cells in question will have the desired outcome. This level of correction and expected somatic mosaicism may be sufficient in some but not all cases.
It is apparent that most research on genomic correction in humans will involve ex vivo methods [118]. The ex vivo approach involves harvesting appropriate cells from the patient, correcting them in culture, and then returning the corrected cells via autologous transformation. The in vivo approach involves directly transforming somatic cells in the patient. On the other hand, ZFs can cross cell membranes and induce genome editing in human cells [119, 120]. Furthermore, incorporating tandem nuclear localization signal repeats into the ZFN protein backbone may improve cell permeability to ~13-fold and allow for genome modification success rates of 26 % in CD4+ T cells and 17 % in CD34+ hematopoietic progenitor cells [121]. TALENs can also be modified for enhanced cell penetrating abilities by conjugating with peptides that allow for optimized protein machinery delivery. It may allow for effective parallel viral transfection [122]. Cas9 and sgRNAs have also been utilized for effective genome editing that does not require transformation of the editing system into host by using common cationic lipid nucleic acid transfection reagents to deliver the system [123] or by using electroporation [124]. As it stands, there are still key obstacles to overcome with epigenome editing but given the exponential rate of advancement most technological limitations will shortly be overcome.
Conclusion
It is apparent from the examples listed above that future application of epigenetic correction using the current and evolving technologies is only limited by imagination. Besides monogenic diseases, epigenome editing may apply in cases of complex traits, such as the long-term effects on neuroplasticity from stress and drug exposure. A recent example showed that a locus-specific epigenetic remodeling may control cocaine addiction- and depression-related behaviors [125]. This study used ZFs and TALEs to target histone methylation (H3K9me2 via G9a) or acetylation (correlated with transcriptional activator; p65) at transcription factor binding sites (SRF and CREB) of the Fosb promoter in the nucleus accumbens, a brain region involved in reward and addiction.
There is every reason to argue that cell-type-specific epigenome editing systems should be used to modify cells in vivo and/or in vitro to further basic science as well as correct a variety of diseases. Ultimately, epigenome editing represents a much-needed tool for the advancement of functional genomics and personalized medicine. Yet, until the trans-generational and population level consequences are fully understood and debated it must remain limited to somatic cells and not cross to the human germ-line.
Abbreviations
- ncRNA:
-
non-coding RNA
- DNMT:
-
DNA MethylTransferase
- 5mC:
-
5-MethylCtyosine
- 5hmC:
-
5-HydroxyMethylCtyosine
- PTM:
-
post-translational modification
- ZF (or ZFP):
-
Zinc Finger (Protein)
- ZFN:
-
Zinc Finger Nuclease
- TALE:
-
Transcription-Activator-Like Effector
- TALEN:
-
TALE + Nuclease
- RVD:
-
repeat variable di-residue
- CRISPR/Cas9:
-
Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR)-Associated 9 (Cas9)
- sgRNA:
-
short guide RNA
- PAM:
-
Protospacer Adjacent Motif
- ATF:
-
artificial transcription factor
- TDG:
-
thymidine DNA glycosylase (DNA Demethylase)
- TET:
-
Ten-Eleven Translocation (DNA Demethylase)
- dCas9:
-
deactivated Cas9
- VPR:
-
VP64-p65-Rta
- scRNA:
-
scaffolding RNA (sgRNA 2.0)
- SAM:
-
Synergistic Activation Mediator
References
de Groote ML, Verschure PJ, Rots MG. Epigenetic Editing: targeted rewriting of epigenetic marks to modulate expression of selected target genes. Nucleic Acids Res. 2012;40(21):10596–613.
Jurkowski TP, Ravichandran M, Stepper P. Synthetic epigenetics-towards intelligent control of epigenetic states and cell identity. Clin Epigenetics. 2015;7(1):1–12.
Ramalingam S, Annaluru N, Chandrasegaran S. A CRISPR way to engineer the human genome. Genome Biol. 2013;14(2):107.
Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405.
Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Development. 2014;141(21):4042–54.
Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA. 1996;93(3):1156–60.
Bibikova M, Carroll D, Segal DJ, Trautman JK, Smith J, Kim YG, Chandrasegaran S. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 2001;21(1):289–97.
Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–46.
Liu J, Stormo GD. Context-dependent DNA recognition code for C2H2 zinc-finger transcription factors. Bioinformatics. 2008;24(17):1850–7.
Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F. A transcription activator-like effector toolbox for genome engineering. Nat Protoc. 2012;7(1):171–92.
Sun N, Zhao H. Transcription activator-like effector nucleases (TALENs): a highly efficient and versatile tool for genome editing. Biotechnol Bioeng. 2013;110(7):1811–21.
Deng D, Yan C, Wu J, Pan X, Yan N. Revisiting the TALE repeat. Protein Cell. 2014;5(4):297–306.
Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–12.
Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326(5959):1501.
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186(2):757–61.
Morbitzer R, Romer P, Boch J, Lahaye T. Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc Natl Acad Sci USA. 2010;107(50):21617–22.
Cong L, Zhou R, Kuo YC, Cunniff M, Zhang F. Comprehensive interrogation of natural TALE DNA-binding modules and transcriptional repressor domains. Nat Commun. 2012;3:968.
Reyon D, Maeder ML, Khayter C, Tsai SQ, Foley JE, Sander JD, Joung JK. Engineering customized TALE nucleases (TALENs) and TALE transcription factors by fast ligation-based automatable solid-phase high-throughput (FLASH) assembly. Curr Protoc Mol Biol. 2013;Chapter 12:Unit 12.16.
Maeder ML, Thibodeau-Beganny S, Sander JD, Voytas DF, Joung JK. Oligomerized pool engineering (OPEN): an ‘open-source’ protocol for making customized zinc-finger arrays. Nat Protoc. 2009;4(10):1471–501.
Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, Lam D, Xiong X, Montserrat N, Esteban CR, Liu GH, Sancho-Martinez I, Manau D, Civico S, Cardellach F, Del Mar O’Callaghan M, Campistol J, Zhao H, Campistol JM, Moraes CT, Izpisua Belmonte JC. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161(3):459–69.
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013;31(9):827–32.
Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, Anders C, Hauer M, Zhou K, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343(6176):1247997.
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507(7490):62–7.
Ranganathan V, Wahlin K, Maruotti J, Zack DJ. Expansion of the CRISPR–Cas9 genome targeting space through the use of H1 promoter-expressed guide RNAs. Nat Commun. 2014;5:4516.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu X, Makarova KS, Koonin EV, Sharp PA, Zhang F. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546):186–91.
Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng Z, Gonzales APW, Li Z, Peterson RT, Yeh JJ, Aryee MJ, Joung JK. Engineered CRISPR–Cas9 nucleases with altered PAM specificities. Nature. 2015;523(7561):481–5.
Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, Church GM. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods. 2013;10(11):1116–21.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR–Cas9 system. Nat Protoc. 2013;8(11):2281–308.
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc. 2013;8(11):2180–96.
Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, Polstein LR, Thakore PI, Glass KA, Ousterout DG, Leong KW, Guilak F, Crawford GE, Reddy TE, Gersbach CA. RNA-guided gene activation by CRISPR–Cas9-based transcription factors. Nat Methods. 2013;10(10):973–6.
Xie S, Shen B, Zhang C, Huang X, Zhang Y. sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS One. 2014;9(6):e100448.
Cong L, Zhang F. Genome engineering using CRISPR–Cas9 system. Methods Mol Biol. 2015;1239:197–217.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–8.
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK. Efficient genome editing in zebrafish using a CRISPR–Cas system. Nat Biotechnol. 2013;31(3):227–9.
Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng G, Sharp PA, Zhang F. CRISPR–Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014;159(2):440–55.
Aida T, Chiyo K, Usami T, Ishikubo H, Imahashi R, Wada Y, Tanaka KF, Sakuma T, Yamamoto T, Tanaka K. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol. 2015;16(1):87.
Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F. In vivo interrogation of gene function in the mammalian brain using CRISPR–Cas9. Nat Biotechnol. 2015;33(1):102–6.
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156(4):836–43.
Incontro S, Asensio CS, Edwards RH, Nicoll RA. Efficient, complete deletion of synaptic proteins using CRISPR. Neuron. 2014;83(5):1051–7.
Huang X, Wang Y, Yan W, Smith C, Ye Z, Wang J, Gao Y, Mendelsohn L, Cheng L. Production of gene-corrected adult beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells. 2015;33(5):1470–9.
Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, Kan YW. Seamless gene correction of beta-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24(9):1526–33.
Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci. 2003;270(1518):921–8.
Esvelt KM, Smidler AL, Catteruccia F, Church GM. Concerning RNA-guided gene drives for the alteration of wild populations. Elife. 2014;3:e03401.
Li J, Shou J, Guo Y, Tang Y, Wu Y, Jia Z, Zhai Y, Chen Z, Xu Q, Wu Q. Efficient inversions and duplications of mammalian regulatory DNA elements and gene clusters by CRISPR/Cas9. J Mol Cell Biol. 2015;7(4):284–98.
Chen WV, Maniatis T. Clustered protocadherins. Development. 2013;140(16):3297–302.
Narendra V, Rocha PP, An D, Raviram R, Skok JA, Mazzoni EO, Reinberg D. Transcription. CTCF establishes discrete functional chromatin domains at the Hox clusters during differentiation. Science. 2015;347(6225):1017–21.
Fanucchi S, Shibayama Y, Burd S, Weinberg MS, Mhlanga MM. Chromosomal contact permits transcription between coregulated genes. Cell. 2013;155(3):606–20.
Belmonte JC, Callaway EM, Churchland P, Caddick SJ, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J, Zhang F. Brains, genes, and primates. Neuron. 2015;86(3):617–31.
Liao J, Karnik R, Gu H, Ziller MJ, Clement K, Tsankov AM, Akopian V, Gifford CA, Donaghey J, Galonska C, Pop R, Reyon D, Tsai SQ, Mallard W, Joung JK, Rinn JL, Gnirke A, Meissner A. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat Genet. 2015;47(5):469–78.
Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, Matsuoka C, Shimotohno K, Ishikawa F, Li E, Ueda HR, Nakayama J, Okano M. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11(7):805–14.
Seipel K, Georgiev O, Schaffner W. Different activation domains stimulate transcription from remote (‘enhancer’) and proximal (‘promoter’) positions. EMBO J. 1992;11(13):4961–8.
Beerli RR, Segal DJ, Dreier B, Barbas CF 3rd. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc Natl Acad Sci USA. 1998;95(25):14628–33.
Gao X, Tsang JC, Gaba F, Wu D, Lu L, Liu P. Comparison of TALE designer transcription factors and the CRISPR/dCas9 in regulation of gene expression by targeting enhancers. Nucleic Acids Res. 2014;42(20):e155.
Margolin JF, Friedman JR, Meyer WK, Vissing H, Thiesen HJ, Thiesen FJ 3rd. Kruppel-associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA. 1994;91(10):4509–13.
Ayer DE, Laherty CD, Lawrence QA, Armstrong AP, Eisenman RN. Mad proteins contain a dominant transcription repression domain. Mol Cell Biol. 1996;16(10):5772–81.
Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500(7463):472–6.
Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, Maehr R. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat Methods. 2015;12(5):401–3.
Ma AN, Wang H, Guo R, Wang YX, Li W, Cui J, Wang G, Hoffman AR, Hu JF. Targeted gene suppression by inducing de novo DNA methylation in the gene promoter. Epigenetics Chromatin. 2014;7:20.
Grimmer MR, Stolzenburg S, Ford E, Lister R, Blancafort P, Farnham PJ. Analysis of an artificial zinc finger epigenetic modulator: widespread binding but limited regulation. Nucleic Acids Res. 2014;42(16):10856–68.
Snowden AW, Gregory PD, Case CC, Pabo CO. Gene-specific targeting of H3K9 methylation is sufficient for initiating repression in vivo. Curr Biol. 2002;12(24):2159–66.
Falahi F, Huisman C, Kazemier HG, van der Viles P, Kok K, Hospers GAP, Rots MG. Towards sustained silencing of HER2/neu in cancer by epigenetic editing. Mol Cancer Res. 2013;11(9):1029–39.
Li F, Papworth M, Minczuk M, Rohde C, Zhang Y, Ragozin S, Jeltsch A. Chimeric DNA methyltransferases target DNA methylation to specific DNA sequences and repress expression of target genes. Nucleic Acids Res. 2007;35(1):100–12.
Meister GE, Chandrasegaran S, Ostermeier M. Heterodimeric DNA methyltransferases as a platform for creating designer zinc finger methyltransferases for targeted DNA methylation in cells. Nucleic Acids Res. 2010;38(5):1749–59.
Rivenbark AG, Stolzenburg S, Beltran AS, Yuan X, Rots MG, Strahl BD, Blancafort P. Epigenetic reprogramming of cancer cells via targeted DNA methylation. Epigenetics. 2012;7(4):350–60.
Chaikind B, Kilambi KP, Gray JJ, Ostermeier M. Targeted DNA methylation using an artificially bisected M. HhaI fused to zinc fingers. PLoS One. 2012;7(9):e44852.
Siddique AN, Nunna S, Rajavelu A, Zhang Y, Jurkowska RZ, Reinhardt R, Rots MG, Ragozin S, Jurkowski TP, Jeltsch A. Targeted methylation and gene silencing of VEGF-A in human cells by using a designed Dnmt3a–Dnmt3L single-chain fusion protein with increased DNA methylation activity. J Mol Biol. 2013;425(3):479–91.
Nunna S, Reinhardt R, Ragozin S, Jeltsch A. Targeted methylation of the epithelial cell adhesion molecule (EpCAM) promoter to silence its expression in ovarian cancer cells. PLoS One. 2014;9(1):e87703.
Chaikind B, Ostermeier M. Directed evolution of improved zinc finger methyltransferases. PLoS One. 2014;9(5):e96931.
Stolzenburg S, Beltran AS, Swift-Scanlan T, Rivenbark AG, Rashwan R, Blancafort P. Stable oncogenic silencing in vivo by programmable and targeted de novo DNA methylation in breast cancer. Oncogene. 2015. doi:10.1038/onc.2014.470.
Kungulovski G, Nunna S, Thomas M, Zanger UM, Reinhardt R, Jeltsch A. Targeted epigenome editing of an endogenous locus with chromatin modifiers is not stably maintained. Epigenetics Chromatin. 2015;8:12.
Gregory DJ, Zhang Y, Kobzik L, Fedulov AV. Specific transcriptional enhancement of inducible nitric oxide synthase by targeted promoter demethylation. Epigenetics. 2013;8(11):1205–12.
Maeder ML, Linder SJ, Reyon D, Angstman JF, Fu Y, Sander JD, Joung JK. Robust, synergistic regulation of human gene expression using TALE activators. Nat Methods. 2013;10(3):243–5.
Cho H, Kang JG, Lee J, Lee J, Jeon SK, Ko J, Kim D, Park K, Kim Y, Kim N. Direct regulation of E-cadherin by targeted histone methylation of TALE-SET fusion protein in cancer cells. Oncotarget. 2015 [Epub ahead of print].
Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31(12):1133–6.
Bernstein DL, Le Lay JE, Ruano EG, Kaestner KH. TALE-mediated epigenetic suppression of CDKN2A increases replication in human fibroblasts. J Clin Invest. 2015;125(5):1998–2006.
Maeder ML, Angstman JF, Richardson ME, Linder SJ, Cascio VM, Tsai SQ, Ho QH, Sander JD, Reyon D, Bernstein BE, Costello JF, Wilkinson MF, Joung JK. Targeted DNA demethylation and activation of endogenous genes using programmable TALE-TET1 fusion proteins. Nat Biotechnol. 2013;31(12):1137–42.
Chen H, Kazemier HG, de Groote ML, Ruiters MH, Xu GL, Rots MG. Induced DNA demethylation by targeting Ten-Eleven Translocation 2 to the human ICAM-1 promoter. Nucleic Acids Res. 2014;42(3):1563–74.
Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10(10):977–9.
Cheng AW, Wang H, Yang H, Shi L, Katz Y, Theunissen TW, Rangarajan S, Shivalila CS, Dadon DB, Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23(10):1163–71.
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–51.
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152(5):1173–83.
Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31(9):833–8.
Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR–Cas9 complex. Nature. 2015;517(7536):583–8.
Kearns NA, Genga RM, Enuameh MS, Garber M, Wolfe SA, Maehr R. Cas9 effector-mediated regulation of transcription and differentiation in human pluripotent stem cells. Development. 2014;141(1):219–23.
Sander JD, Joung JK. CRISPR–Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32(4):347–55.
Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159(3):647–61.
Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, La Russa M, Tsai JC, Weissman JS, Dueber JE, Qi LS, Lim WA. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1–2):339–50.
Telese F, Ma Q, Perez P, Notani D, Oh S, Li W, Comoletti D, Ohgi K, Taylor H, Rosenfeld M. LRP8-Reelin-regulated neuronal enhancer signature underlying learning and memory formation. Neuron. 2015;86(3):696–710.
Chakraborty S, Ji H, Kabadi AM, Gersbach CA, Christoforou N, Leong KW. A CRISPR/Cas9-based system for reprogramming cell lineage specification. Stem Cell Rep. 2014;3(6):940–7.
Hilton IB, D'Ippolito AM, Vockley CM, Thakore PI, Crawford GE, Reddy TE, Gersbach CA. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat Biotech. 2015;33(5):510–7.
Carroll D. Staying on target with CRISPR–Cas. Nat Biotechnol. 2013;31(9):807–9.
Hendel A, Fine EJ, Bao G, Porteus MH. Quantifying on- and off-target genome editing. Trends Biotechnol. 2015;33(2):132–40.
Wei C, Liu J, Yu Z, Zhang B, Gao G, Jiao R. TALEN or Cas9—rapid, efficient and specific choices for genome modifications. J Genet Genom. 2013;40(6):281–9.
Hu J, Lei Y, Wong WK, Liu S, Lee KC, He X, You W, Zhou R, Guo JT, Chen X, Peng X, Sun H, Huang H, Zhao H, Feng B. Direct activation of human and mouse Oct4 genes using engineered TALE and Cas9 transcription factors. Nucleic Acids Res. 2014;42(7):4375–90.
Tanenbaum ME, Gilbert LA, Qi LS, Weissman JS, Vale RD. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159(3):635–46.
Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, P R Iyer E, Lin S, Kiani S, Guzman CD, Wiegand DJ, Ter-Ovanesyan D, Braff JL, Davidsohn N, Housden BE, Perrimon N, Weiss R, Aach J, Collins JJ, Church GM. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12(4):326–8.
Shechner DM, Hacisuleyman E, Younger ST, Rinn JL. Multiplexable, locus-specific targeting of long RNAs with CRISPR-Display. Nat Methods. 2015;12(7):664–70.
Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, Lin C. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science. 2008;322(5907):1535–9.
Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–8.
Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31(30):10829–35.
Nihongaki Y, Yamamoto S, Kawano F, Suzuki H, Sato M. CRISPR–Cas9-based photoactivatable transcription system. Chem Biol. 2015;22(2):169–74.
Polstein LR, Gersbach CA. A light-inducible CRISPR–Cas9 system for control of endogenous gene activation. Nat Chem Biol. 2015;11(3):198–200.
Mercer AC, Gaj T, Sirk SJ, Lamb BM, Barbas CF 3rd. Regulation of endogenous human gene expression by ligand-inducible TALE transcription factors. ACS Synth Biol. 2014;3(10):723–30.
Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, Huang B. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell. 2013;155(7):1479–91.
Anton T, Bultmann S, Leonhardt H, Markaki Y. Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system. Nucleus. 2014;5(2):163–72.
Pederson T. Repeated TALEs: visualizing DNA sequence localization and chromosome dynamics in live cells. Nucleus. 2014;5(1):28–31.
Ma H, Naseri A, Reyes-Gutierrez P, Wolfe SA, Zhang S, Pederson T. Multicolor CRISPR labeling of chromosomal loci in human cells. Proc Natl Acad Sci USA. 2015;112(10):3002–7.
Byrum SD, Raman A, Taverna SD, Tackett AJ. ChAP-MS: a method for identification of proteins and histone posttranslational modifications at a single genomic locus. Cell Rep. 2012;2(1):198–205.
Fujita T, Asano Y, Ohtsuka J, Takada Y, Saito K, Ohki R, Fujii H. Identification of telomere-associated molecules by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP). Sci Rep. 2013;3:3171.
Fujita T, Fujii H. Efficient isolation of specific genomic regions and identification of associated proteins by engineered DNA-binding molecule-mediated chromatin immunoprecipitation (enChIP) using CRISPR. Biochem Biophys Res Commun. 2013;439(1):132–6.
Waldrip ZJ, Byrum SD, Storey AJ, Gao J, Byrd AK, Mackintosh SG, Wahls WP, Taverna SD, Raney KD, Tackett AJ. A CRISPR-based approach for proteomic analysis of a single genomic locus. Epigenetics. 2014;9(9):1207–11.
Byrum SD, Taverna SD, Tackett AJ. Purification of a specific native genomic locus for proteomic analysis. Nucleic Acids Res. 2013;41(20):e195.
Li L, He Z, Wei X, Gao G, Wei Y. Challenges in CRISPR/CAS9 delivery: potential roles of non-viral vectors. Hum Gene Ther. 2015;26(7):452–62.
Gori JL, Hsu PD, Maeder ML, Shen S, Welstead GG, Bumcrot D. Delivery and specificity of CRISPR–Cas9 genome editing technologies for human gene therapy. Hum Gene Ther. 2015;26(7):443–51.
Liu DR. Delivery of negatively charged proteins using cationic lipids. 2015 (20150118216).
Wang D, Mou H, Li S, Li Y, Hough S, Tran K, Li J, Yin H, Anderson DG, Sontheimer E. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther. 2015;26(7):432–42.
Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–31.
Gaj T, Guo J, Kato Y, Sirk SJ, Barbas CF 3rd. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat Methods. 2012;9(8):805–7.
Gaj T, Liu J, Anderson KE, Sirk SJ, Barbas CF 3rd. Protein delivery using Cys2-His2 zinc-finger domains. ACS Chem Biol. 2014;9(8):1662–7.
Liu J, Gaj T, Wallen MC, Barbas CF 3rd. Improved cell-penetrating zinc-finger nuclease proteins for precision genome engineering. Mol Ther Nucleic Acids. 2015;4:e232.
Liu J, Gaj T, Patterson JT, Sirk SJ, Barbas CF 3rd. Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS One. 2014;9(1):e85755.
Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80.
Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, Gate RE, Haliburton GE, Ye CJ, Bluestone JA, Doudna JA, Marson A. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci. 2015;12(33):10437–42.
Heller EA, Cates HM, Pena CJ, Sun H, Shao N, Feng J, Golden SA, Herman JP, Walsh JJ, Mazei-Robison M, Ferguson D, Knight S, Gerber MA, Nievera C, Han MH, Russo SJ, Tamminga CS, Neve RL, Shen L, Zhang HS, Zhang F, Nestler EJ. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat Neurosci. 2014;17(12):1720–7.
Authors’ contributions
BIL and SMS wrote the manuscript and created the figures. Both authors read and approved the final manuscript.
Acknowledgements
This review was written with the support of a Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to SMS and a research scholarship from NSERC to BIL. Finally, the authors would like to thank EpiGenie, LLC (http://www.EpiGenie.com) and contributors for highlighting some of the biotechnology breakthroughs as they happened.
Compliance with ethical guidelines
Competing interests The authors would like to disclose that BIL has a non-financial dual interest as a freelance writer and editor contributing to EpiGenie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Laufer, B.I., Singh, S.M. Strategies for precision modulation of gene expression by epigenome editing: an overview. Epigenetics & Chromatin 8, 34 (2015). https://doi.org/10.1186/s13072-015-0023-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13072-015-0023-7