Abstract
Background
Chagas disease (CD) is caused by Trypanosoma cruzi, which is transmitted mainly through the feces/urine of infected triatomine bugs. The acute phase lasts 2–3 months and is characterized by high parasitemia and nonspecific symptoms, whereas the lifelong chronic phase features symptoms affecting the heart and/or digestive tract occurring in 30–40% of infected individuals. As in humans, cardiac abnormalities are observed in T. cruzi-infected dogs and cats. We reviewed the technological advances in the serological diagnosis of CD in dogs and cats.
Methods
A review of the published literature during the last 54 years (1968–2022) on the epidemiology, clinical features, diagnosis, treatment and prevention of CD in dogs and cats was conducted.
Results
Using predefined eligibility criteria for a search of the published literature, we retrieved and screened 436 publications. Of these, 84 original studies were considered for inclusion in this review. Dogs and cats are considered as sentinels, potentially indicating an active T. cruzi transmission and thus the risk for human infection. Although dogs and cats are reputed to be important for maintaining the T. cruzi domestic transmission cycle, there are no commercial tests to detect past or active infections in these animals. Most published research on CD in dogs and cats have used in-house serological tests prepared with native and/or full-length recombinant antigens, resulting in variable diagnostic performance. In recent years, chimeric antigens have been used to improve the diagnosis of chronic CD in humans with encouraging results. Some of them have high performance values (> 95%) and extremely low cross-reactivity rates for Leishmania spp., especially the antigens IBMP-8.1 to IBMP-8.4. The diagnostic performance of IBMP antigens was also investigated in dogs, showing high diagnostic performance with negligible cross-reactivity with anti-Leishmania infantum antibodies.
Conclusions
The development of a commercial immunodiagnostic tool to identify past or active T. cruzi infections in dogs and cats is urgently needed. The use of chimeric recombinant T. cruzi antigens may help to fill this gap and is discussed in this review.
Graphical Abstract

Similar content being viewed by others
Background
Chagas disease (CD) or American trypanosomiasis is a neglected parasitic disease caused by the hemoflagellate protozoan Trypanosoma cruzi. Recent estimates indicate that 6–7 million people are infected worldwide, with 10,000 deaths attributable to CD annually in 21 Latin American countries [1]. Due to the continuous presence of the vector, 70 million people in this region are at risk of contracting the disease via vector transmission [1]. The parasite is primarily transmitted through the feces or urine of infected bloodsucking triatomine bugs also referred to as kissing bugs (Hemiptera: Reduviidae) [1]. Over 130 triatomine species have been identified as potential vectors of T. cruzi [2]. Fifty-two triatomine species have been described in Brazil, of which five are considered of epidemiological importance because of their domestic habitats: Triatoma infestans (Fig. 1A), Panstrongylus megistus (Fig. 1B, C), Triatoma brasiliensis, Triatoma pseudomaculata and Triatoma sordida (Fig. 1D, E, F). Non-vectorial routes of transmission are also important for T. cruzi transmission, such as blood transfusion or the use of blood products, congenital transmission, consumption of contaminated food and beverages, organ donation and laboratory accidents [3]. Increased travel and migration flows have facilitated the spread of T. cruzi-infected individuals, making the disease a global health problem, particularly in non-endemic countries in Europe, North America, Asia and Oceania [4,5,6,7].
Triatomines normally found in endemic areas of South America. Preserved pair of Triatoma infestans A and Panstrongylus megistus B, kindly provided by Dr. Gilmar Jose da Silva Ribeiro Júnior (Fiocruz-Bahia). C shows a live Panstrongylus megistus female captured in the city of Barra do Mendes, Bahia, Brazil. Live triatomines of the species Triatoma sordida found on the floor D and roof E of a chicken house in the rural area of the municipality of Tremedal, Bahia. E shows some T. sordida adult specimens captured for gut content analysis
Clinically, human CD is divided into two phases: acute and chronic. The acute phase begins within 1–2 weeks of infection, lasts 2–3 months and is characterized by high parasitemia and nonspecific symptoms such as fever, tachycardia and lymphadenopathy [8]. The lifelong chronic phase can occur in two forms: an indeterminate form, which is usually a latency period in which individuals show no symptoms but have positive serological results, and a symptomatic form. Approximately 30–40% of chronically infected individuals progress to a symptomatic form, which can be further subdivided into cardiac, digestive, or mixed forms (cardiac and digestive) [8]. As in humans, cardiac disease is also observed in T. cruzi-infected dogs [9,10,11] and cats [12]. Indeed, some animals develop progressive chronic myocarditis with cardiac dilatation and electrocardiogram abnormalities that may lead to sudden death. Clinical signs may include splenomegaly, lymphadenopathy and heart failure [13].
Although CD was discovered more than a century ago, this zoonosis still poses a public health threat [14]. The presence of domestic animals in the environment is a risk factor for human infection because they may attract triatomines to human dwellings. Indeed, triatomines typically feed on chickens, pigs, dogs and cats [15, 16]. Figure 2 shows a typical scenario in poor rural communities in Latin American countries where mud houses are still common (Fig. 2A, E, F) and domestic animals such as dogs, chickens and pigs are present (Fig. 2B, C, D respectively). Among domestic animals, dogs and cats play an important role in maintaining the domestic cycle of T. cruzi, since these animals are susceptible to different forms of infection [17, 18]. They are reputed to be the main reservoirs of T. cruzi in Latin America and some regions of the US [13, 19,20,21]. Dogs and cats are also considered as sentinels for human infection [22, 23], since they can indicate the presence of an active T. cruzi transmission cycle and thus the risk of human infection.
The epidemiologic scenario of poor rural communities in many Latin American countries. Mud house with cracks where triatomines can hide (A-C), with detail of a crack in a rural adobe/brick house (A). B Inside of the house illustrated in (A). Mud house with cracks (C) with presence of domestic animals in the environment: dog (D), chickens (E) and pigs (F). These animals can attract triatomines for a blood meal, thus helping maintain the peridomestic cycle of T. cruzi. Photographs were taken in rural areas of the municipalities of Tremedal (A and B) and Irecê, Bahia, Brazil (E–F)
Despite the public health and veterinary importance, there are no commercially available tests to detect past or active T. cruzi infections in dogs and cats. In this review, we summarize basic information on CD in dogs and cats, with particular emphasis on diagnostic methods.
Search strategy, eligibility and review
An online search was performed in the US National Library of Medicine National Institutes of Health (PubMed, Bethesda MD, USA; https://pubmed.ncbi.nlm.nih.gov/), the Latin American and Caribbean Health Science Literature Database (LILACS; https://lilacs.bvsalud.org/) and the Scientific Electronic Library Online (Scielo Brazil, São Paulo SP—Brazil; https://scileo.br/) databases using Health Sciences Descriptors (DeCS). The descriptors in the different databases were “Chagas disease,” “dogs,” and “cats” in Portuguese, English and Spanish. The Boolean operator “AND” was used to cross descriptors and keywords. Search results were then filtered for the period 1968 to 2022 and extracted into a database in Microsoft Excel (Microsoft Corp., Redmond, WA, USA) in CSV format (comma-separated values).
Inclusion criteria were (1) articles indexed in the previously cited databases; (2) original studies in Portuguese, Spanish, or English; (3) published between 1968 and 2022. The survey took place from January to June 2022, excluding secondary publications such as books, monographs, dissertations and theses.
The extraction and analysis of primary data were conducted by two independent researchers. Exclusion of articles was based first on reading the titles as the first analysis, followed by reading the abstracts (if available) and finally reading the full texts. In case of doubts or discrepancies, a third researcher was consulted. A total of 436 articles were found during the initial search. After an initial analysis of the titles, 191 articles were excluded, and another 86 articles were not included because they were duplicates. Of the remaining 159 articles, the abstracts were read and 106 were considered for conducting the integrative review. Of these, 17 articles were excluded because they were not available in the scientific literature. After the qualitative analysis, 84 of the 89 selected articles were considered for conducting this review. The process of study selection was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Additional file 1: PRISMA) model [24], which is shown in Fig. 3.
Trypanosoma cruzi infection and CD in dogs
Dogs become infected after coming in contact with the feces of triatomine bugs containing T. cruzi trypomastigote (Fig. 4), by ingesting infected triatomines, or congenitally [25]. They are considered the most important domestic reservoirs of T. cruzi in areas where CD is endemic owing to their proximity to humans, high parasitemia in acute phase of the disease and propensity to attract triatomines [17, 20, 22]. Nonetheless, the role of dogs as reservoirs may apparently vary. For instance, a study conducted in Brazil showed that dogs from some regions presented negative blood cultures and fresh blood preparations [26], whereas studies conducted in Argentina [22, 27] and Brazil [28] indicated that vast majority of seropositive dogs had active parasitemia.
A study conducted in 1996 estimated that dogs contribute 13.9 times more than humans to triatomine infection in households [29]. Accordingly, the likelihood of a triatomine bug to become infected by T. cruzi was found 50 times higher after a single blood meal on a dog than on a human [29]. In another study examining the feeding habits of over 1000 domestic T. infestans, it was found that dogs were the most common blood source (49%), followed by cats (39%), humans (38%) and chickens (29%) [17].
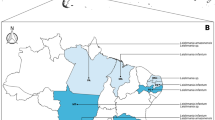
Considering the role of dogs as reservoirs for T. cruzi, the seroprevalence in dogs has also been used in mathematical models for domestic transmission of human CD [30]. However, seroprevalence data from different countries may vary widely (Fig. 5), which may be partly attributed to the variability in terms of transmission risk, but also to sample size and serological tests used by different research groups to detect anti-T. cruzi antibodies in dogs, as well as to the genetic variability of the circulating T. cruzi strains (discrete typing units (DTU). In Mexico, a study conducted in 2010 reported a prevalence of 34% [31], while another study carried out in 2017 detected a prevalence of 4.4% [32]. Other studies conducted in Mexico have reported different prevalence values [33,34,35,36,37,38,39,40,41,42,43]. Recently, anti‑T. cruzi antibodies were detected in 50% (17/34) of dogs from two rural settlements in the Sierra de Los Tuxtlas, Veracruz, Mexico [44]. In Costa Rica, 5.2–27.7% of dogs were seropositive in endemic areas [45,46,47], whereas in Colombia the prevalence ranged from 9.6% to 34% [48,49,50,51,52]. In the US, a study with 86 working dogs reported a seroprevalence of 14.1% [53], whereas other studies reported a lower prevalence [54,55,56,57]. Trypanosoma cruzi infection was reported in 63 (16.8%) of 375 dogs from a teaching hospital in Texas [58] and in 110 (18.1%) of 608 dogs in shelters across this same state [59]. In 2020, an American nationwide study using dog samples from 41 states and Washington DC revealed a seropositivity in 120/1610 animals (7.5%) [60]. In a National Park located along the Texas-Mexico border, 28.6% (4/14) of dogs were reactive on at least two serologic assays [61]. More recently, 26 of 197 (13.2%) shelter dogs from Oklahoma had detectable antibodies against T. cruzi [62]. In Brazil, the seroprevalence in dogs ranged from 0 to 53%, according to research conducted in different regions [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. In other Latin American countries, the seroprevalence varies according to geographic setting, e.g. 1.9% in Peru [84], 4.3% in Grenada [85], 5.2% in French Guiana [86], 10% in Nicaragua [87], 4.6–19.9% in Chile [88,89,90], 11.1–17.6% in Panama [91, 92], 6.4–22.1% in Venezuela [93,94,95,96], 17.5–53% in Argentina [17, 97,98,99], 22% in Bolivia [100] and 57.1% in Ecuador [101].
Regarding predisposing factors, it has been observed that dogs with poor nutritional conditions are 6.3 times more likely to be infected compared to well-nourished dogs in the same endemic area [102]. This is thought to be related to a deficient innate immune response in dogs with poor nutritional conditions, which favors the occurrence of higher parasitemia [102]. Another predisposing factor is keeping dogs in kennels with multiple dogs. In fact, an American study found that the risk of T. cruzi infection in dogs living in kennels is 30.7% per year [103].
During the acute phase of infection, T. cruzi circulates in the bloodstream and trypomastigotes can be observed in most tissues, triggering a systemic inflammatory response with the production of proinflammatory cytokines (Fig. 6).
Clinical signs vary widely according to infection phase (acute versus chronic) and to dog’s age. For instance, the main presenting clinical signs in young puppies are lethargy, generalized lymphadenopathy, slow capillary refill time with pale mucous membranes and in some cases splenomegaly and hepatomegaly [13]. On the other hand, if infection occurs after 6 months of age, dogs may display no signs of acute disease other than slight depression and low-rising parasitemia [13]. In general, the main lesion in young dogs experimentally infected with T. cruzi is acute myocarditis that begins in the atria and spreads through the interventricular septum toward the ventricles [104]. When fully developed, it is located predominantly in the right atrium, the right half of the ventricular septum and the free wall of the right ventricle. Electrocardiogram (ECG) changes are progressive and reflect atrial involvement. Heart block occurs only in the terminal stage and is associated with severe inflammation and necrosis along the A-V conduction tissue. Specific treatment of dogs with severe acute disease often results in regression of histologic and ECG changes [104].
Dogs that survive the acute phase enter the undetermined phase, characterized by the lack of clinical signs and subpatent parasitemia [13]. Some dogs will progress to develop chronic disease, typified by cardiac alterations, including chronic myocarditis with cardiac dilatation [13, 105, 106]. During this phase electrocardiogram abnormalities become more evident [13, 107, 108]. Other lesions are associated with fibrosis and cardiomyocyte necrosis, possibly caused by the inflammatory processes that trigger hyalinization and fibrosis [107,108,109,110]. Right-side and, eventually, left-side chamber failure may occur, inducing pulse deficits, ascites, pleural effusion, hepatomegaly and jugular venous congestion [13, 111, 112]. In general, naturally infected dogs showed hyperproteinemia, low hemoglobin and hematocrit levels, hypoalbuminemia, hyperglobulinemia, high lactate dehydrogenase (LDH) and aspartate transferase (AST) levels, creatine kinase (CK) and creatine kinase myocardial band (CK-MB) and troponin I profiles consistent with active myocarditis [113,114,115,116].
Chagasic megaesophagus and megacolon can be observed in both the acute and chronic phases [117]. In the acute phase, T. cruzi triggers an inflammatory reaction in the esophagus/colon and causes myenteric denervation. Ganglionitis and periganglionitis of the Auerbach's plexus ranged from mild to moderate and resulted in significant neuronal lesions in dogs experimentally infected with T. cruzi strain Berenice-78 [118]. In the chronic phase, persistent myenteric denervation occurs and may lead to impaired digestive function. Glial cell involvement occurs in the acute phase and may lead to a decrease in the glial fibrillary acidic protein immunoreactive area of enteric glial cells in the chronic phase [117].
Prognosis may be unpredictable and the survival rate of chronically infected, untreated dogs is variable. For instance, dogs diagnosed with CD at an older age tend to survive longer than dogs diagnosed at a younger age [13]. A study showed that a combination of amiodarone and itraconazole may increase the survival time of T. cruzi-infected dogs [119]. On the other hand, a recent study showed that two dogs with severe, symptomatic Chagas cardiomyopathy treated with itraconazole and amiodarone died suddenly within 6 months of diagnosis [120]. These findings underscore the need for early recognition of CD in dogs and continued research to develop effective antiparasitic treatment protocols.
Trypanosoma cruzi infection and CD in cats
Chagas disease in cats is not as well studied as in dogs. Risk factors for the development of CD in cats are still unclear, but it appears that free-roaming cats are more susceptible to T. cruzi infection and are an important risk factor for transmission to humans [37]. Indeed, xenodiagnosis data indicated that cats are highly likely to infect peridomestic triatomine vectors [17]. Clinical signs and histologic findings in cats are similar to those described in humans and dogs [121]. Although more common in humans, digestive symptoms such as esophagitis have also been described in T. cruzi-infected cats [12]. Neurological signs associated with CD have been described in several species, including dogs [13], but never in cats.
Some cats can mount an effective immune response to T. cruzi. However, when immunocompromised or coinfected with other infectious agents [such as feline infectious peritonitis, feline leukemia virus (FELV), feline immunodeficiency virus (FIV) and feline herpesvirus type 1], they may be more susceptible to T. cruzi [37], but this is something that requires further study.
There are few studies on the epidemiology of T. cruzi infection in cats (Fig. 5). In a study conducted in three Mexican cities (Mérida, Umán and Tulum) in Yucatán, 7.8% of 95 cats were positive by ELISA and Western blot, using excreted superoxide dismutase as antigen [122]. Interestingly, no infection was observed in other studies conducted in distinct parts of Mexico [32, 38, 39].
A study conducted in Paraná (Brazil) showed 30.8% of 679 cats had anti-T. cruzi antibodies detectable by IFAT and 23.6% by ELISA. Only 7.6% of the cats were simultaneously positive to both tests, showing a large discrepancy between these methods [123]. Recent studies in the US revealed 7.3–11.4% of the cats were seropositive in South Texas [12] and Louisiana [124], respectively. Among studies that investigated T. cruzi infections in cats, the highest prevalence was reported in Trinidad and Mercedes, two rural villages in the province of Santiago del Estero, Argentina [17]. The authors found an overall seroprevalence of 39–40% in cats at baseline and 1 year later, respectively. Seroprevalence was found to increase with age but was not with sex [17].
Diagnostic methods
In endemic areas, the presence of the above-mentioned clinical signs and clinicopathological abnormalities can lead veterinarians to suspect CD in dogs and cats. In the acute phase, parasitological (e.g., fresh or stained blood preparations, hemoculture and xenodiagnosis) [26] or molecular methods [125] may be useful to confirm the infection (Fig. 7). In addition, molecular methods may be useful for monitoring parasitemia during drug treatment of CD in dogs [126]. However, parasitemia in dogs and cats is generally low and intermittent in the chronic phase [106], which reduces the sensitivity of parasitological and molecular methods. On the other hand, during the chronic phase, anti-T. cruzi antibody production reaches detectable titers and can be identified by indirect immunoassays. In dogs, serum immunoglobulin M (IgM) begins to decrease markedly about 3 months after infection, whereas the opposite is true for immunoglobulin G (IgG), which increases up to 15 months and then gradually decreases up to 2 years and then appears to stabilize over the years [127].
Table 1 summarizes serological methods and antigens previously used in studies involving dogs and cats. Some of the tests described were manufactured for the diagnosis of CD in humans, but they have been adapted for dogs and cats. One of the major drawbacks of this adaptation is the lack of phase 1 and phase 2 studies to validate the method in dogs and cats, so the results may not be reproducible. For example, Zecca et al. (2020) used two immunochromatographic tests developed for humans—Chagas Stat-Pak (Chembio Diagnostic Systems, Inc., Medford, NY) and Chagas Detect Plus Rapid Test (InBios International, Inc., Seattle, WA)—to detect anti-T. cruzi antibodies in cats. Although both tests use protein A to detect IgG antibodies, there are no studies validating their use in cats.
Whole-cell homogenates or fractionated lysates of T. cruzi epimastigotes have traditionally been used as complex antigen mixtures to detect anti-T. cruzi antibodies. Although these combinations have been shown to provide sufficient sensitivity to detect even low antibody levels [128], difficulties in standardization, cross-reactivity and specificity issues have hindered their use in humans [129,130,131,132]. This is especially true for IFAT and ELISA results used to diagnosis CD in humans and other species, which may vary depending on the circulating T. cruzi strain in the study area and the epimastigote strain used in the tests [65, 133, 134]. Another drawback is the use of different epimastigote strains in IFAT because T. cruzi has a high antigenic variation that can lead to false-negative or false-positive results [65], depending on the geographic region.
In the last 2 decades, advances in DNA recombination technology have enabled the use of recombinant proteins in immunoassays (primarily ELISA and chemiluminescence assays), as large quantities of purified antigens can be produced in transformed prokaryotic cells grown in bioreactors [135]. Approximately 25% of the proteins expressed by T. cruzi contain tandem repeat amino acid sequences consisting of 5–68 amino acids [136,137,138,139]. This improved recognition by antibodies compared to proteins that lack repeated sequences [130, 140, 141] and improved the performance of immunoassays compared to cell extracts or whole epimastigotes [136, 142]. Indeed, sera from infected humans often contain high titers of antibodies against these repeated sequences [143,144,145]. However, it has been observed that assays using recombinant proteins can also lead to false-negative results [130]. The high genetic variability of the parasite may be responsible for these results, as the tandem repeat amino acid sequences contain a limited repertoire of antigenic determinants that are not expressed or only partially expressed in some T. cruzi strains [146]. To overcome this limitation, several studies have described the combined use of two or more recombinant proteins in a single assay to increase sensitivity without losing specificity [147,148,149,150,151]. In theory, this strategy could compromise assay performance due to imbalanced binding of these epitopes to the solid surface, competition for binding and spatial distribution of epitopes in the solid phase. However, immunoassays containing a mixture of fusion proteins showed good performance [136]. More recently, an array of different antigens printed in each well of 96-well plates has been shown potentially useful for the diagnosis of human chronic CD [152].
In recent years, synthetic chimeric recombinant antigens consisting of conserved repetitive amino acid fragments of different antigenic T. cruzi proteins have been proposed to improve the accuracy of immunoassays for the diagnosis of human CD [130, 136, 153]. In 1999, a study investigated the diagnostic potential of a branched synthetic peptide (2/D/E/Lo1.2) and a linear recombinant peptide (r2/D/E/Lo1.2). The results showed that both antigens increased the reactivity of weakly reactive sera [137]. High diagnostic performance was obtained in a study examining antigens CP1, CP2 or a mixture between them. CP1 contains repetitive fragments of flagellar repetitive antigen (FRA) and shed acute phase antigen (SAPA), whereas CP2 consists of amino acid sequences of three antigens: FRA, SAPA and B13 [136]. The discriminative ability values obtained for CP1 and CP2 were 25% and 52% higher, respectively, than those of their individual antigen mixtures. CP2 was the only antigen that showed higher discriminative capability between T. cruzi-positive and -negative samples compared to the homogenate of the whole parasite [136]. Similar results were obtained with a chimeric antigen designated TcBCDE, a 24-kDa fusion protein composed of repetitive sequences of nine T. cruzi proteins (MAP, JL8, CRA, B13, TcD, TcE and SAPA). This antigen was evaluated and proven to be highly sensitive for the diagnosis of human CD [154]. A chimeric protein called CP3, composed of the antigenic determinants microtubule-associated protein (MAP), TcD and trypomastigote small surface antigen (TSSA)-II/V/VI, was 100% sensitive and 90.5% specific [155]. These results not only demonstrate that chimeric recombinant proteins are highly accurate in the diagnosis of chronic CD, but also that they are able to detect anti-T. cruzi antibodies regardless of parasite strain or gene expression intensity. In addition, these findings support the utility of performing immunochemical assays with hybrid, chimeric single-molecule antigens rather than peptide mixtures or recombinant proteins.
Recently, four chimeric recombinant T. cruzi antigens have been proposed for the diagnosis of chronic CD in humans: IBMP-8.1, IBMP-8.2, IBMP-8.3 and IBMP-8.4 (IBMP is the Portuguese acronym for Biology Molecular Instituto of Paraná, where the antigens were expressed and purified). The diagnostic potential of these proteins for the detection of CD in humans has been extensively studied, using different diagnostic methods and platforms such as indirect ELISA [129, 156,157,158,159,160,161], liquid microarray [162], lateral flow assay [163], double-antigen sandwich ELISA [164], Western blot (unpublished data) and immunosensor [165]. IBMP antigens are composed of different epitopes of several T. cruzi proteins, as described in Fig. 8 [156, 159, 166]. In general, this diversity of antigenic determinants is responsible for their high reactivity to anti-T. cruzi antibodies.
The ability of IBMP antigens to discriminate T. cruzi-positive from -negative human samples was evaluated, and the area (AUC) under the receiver-operator curve was determined for each molecule. The determination of AUC values is used as the global accuracy of immunoassays [129] and can be classified as low (51–61%), moderate (62–81%), elevated (82–99%) or outstanding (100%) [167]. Accordingly, AUC values ranged from 98.4% to 100% and from 97.8% to 99.7% when positive and negative samples were assayed with IBMP antigens using indirect ELISA and liquid microarray, respectively, as diagnostic platforms [129]. These results indicate that all four IBMP antigens have high discriminatory capability. Considering the high overall accuracy values, the IBMP antigens were used to participate in a phase II study with T. cruzi human positive and -negative samples from different geographic endemic regions of Brazil and other endemic countries using indirect ELISA [156,157,158] and liquid microarray [162]. Sensitivity, specificity and diagnostic odds ratio values were obtained that were higher than those obtained with commercial tests [156,157,158, 162, 168]. Cross-reactivity with Leishmania spp. was extremely low in patients with American cutaneous and visceral leishmaniasis [160]. In light of the negligible cross-reactivity, the authors recommend the use of IBMP antigens in regions where T. cruzi and Leishmania spp. are co-endemic [160]. In 2020, Silva et al. [163] proposed a lateral flow assay using IBMP-8.1 and IBMP-8.4 chimeric antigens for the diagnosis of CD in humans. The study showed that the assay can correctly diagnose both T. cruzi-positive and -negative individuals regardless of geographic origin or clinical presentation. AUC values were 100%, demonstrating an outstanding diagnostic accuracy. The study showed that the lateral flow assay based on these antigens is a promising method for screening CD [163]. In 2020, this device was licensed by the Brazilian Health Regulatory Agency to form the portfolio of diagnostic products of the Brazilian Ministry of Health for use in the Unified Health System: the TR-Chagas Bio-Manguinhos (Oswaldo Cruz Foundation, Rio de Janeiro, RJ, Brazil) [169]. Recently, all four IBMP antigens have shown promising results in a phase 3 study with more than 5000 samples from a Brazilian blood bank, especially the IBMP-8.3 and IBMP-8.4 antigens [161].
With the exception of IBMP antigens, all chimeric recombinant proteins discussed here (CP1, CP2, CP3, 2/D/E/Lo1.2, r2/D/E/Lo1.2, TcBCDE) have been evaluated only for human diagnostics. As mentioned previously, there are no commercial tests for the diagnosis of CD in dogs and cats. In 2019, a phase I study investigated the diagnostic performance of IBMP antigens in dogs [170]. AUC values ranged from 91–100%, demonstrating good diagnostic performance of these molecules also for the diagnosis of CD in dogs. Cordeiro et al. [165] reached the same conclusion by showing that IBMP-8.1 reached a maximum AUC value for both human and canine samples using an impedimetric immunosensor for rapid detection of anti-T. cruzi antibodies. Recently, two recombinant T. cruzi proteins (IBMP-8.1 and IBMP-8.4) were tested as diagnostic platforms using a rapid immunochromatographic assay (TR Chagas, Bio-Manguinhos, Rio de Janeiro, Brazil) [171]. Recombinant antigens were formatted in a rapid immunochromatographic assay using either Staphylococcus aureus protein A or Streptococcus pyogenes protein G as gold-labeled reagents to visualize the precipitin band formed between immunoglobulin (Ig) G-specific antibodies and the recombinant antigen immobilized on the nitrocellulose strip used in the assay. Protein A and protein G were based on the fact that these microbial molecules bind with different affinity and specificity to immunoglobulins of different species, including dogs. The authors found that the intensity pattern of the bands was directly proportional to the serological titer in IFAT. The sensitivity was 94% and the specificity was 91%. The agreement obtained was considered substantial by kappa analysis (84%). Of the T. cruzi-positive hemoculture samples, 88.9% were positive with TR-Chagas Bio-Manguinhos. The assay was efficient in detecting infections with five of the six T. cruzi discrete typing units (DTU; TcI, n = 8; TcII, n = 1; TcI/TcII, n = 2; TcIII, n = 2; TcIV, n = 1; TcIII/TcV, n = 6). Cross-reactions were not observed in infections with Leishmania infantum, Trypanosoma rangeli, Trypanosoma caninum and Dirofilaria immitis, but were observed in sera from dogs infected with Crithidia mellificae, Anaplasma spp. and Erlichia spp. However, the authors used a convenient serum panel for cross-reactivity analysis, many of which had only a single sample per disease. Therefore, further studies should be conducted to confirm or refute these results. This test provides rapid preventive measures in areas at high risk for Chagas disease occurrence in a safe, reliable, cost-effective and immediate manner without the need for more complex laboratory testing. In 2020, the diagnostic performance of a rapid test based on trypomastigote small surface antigen (TSSA) was evaluated (namely Chagas Sero K-SeT). However, low sensitivity for the diagnosis of Chagas disease in dogs was observed (28%; 16/57), indicating the need for further studies to improve test performance [172].
Conclusion
Although the detection of anti-T. cruzi antibodies is possible in any mammalian species, serological tests may give discrepant results in different situations. This is mainly due to the high genetic and phenotypic intraspecific diversity of T. cruzi [173, 174], the selection of antigens used to sensitize the solid phase of immunoassays [133], the variable prevalence of the disease [175, 176] and the variable immune responses in T. cruzi-infected individuals [177]. The development of commercial diagnostic tools to detect past exposure to T. cruzi in dogs and cats would be useful from both veterinary and public health perspectives. Such a test should be able to detect antibodies regardless of the geographical region and the circulating DTU, with high sensitivity, specificity and accuracy, as well as with low risk of cross-reactivity (especially with Leishmania spp.). Furthermore, the test should be rapid (rapid diagnostic test), inexpensive and easy-to-use under field conditions (point-of-care test). Data show that the chimeric recombinant antigens combine all the necessary characteristics for a test with good applicability for epidemiological surveillance in veterinary clinical practice and in animal blood centers.
Availability of data and materials
All the data generated or analyzed during this study are included in this published article.
Abbreviations
- A:
-
Armadillos
- AST:
-
Aspartate transferase
- AUC:
-
Area under the receiver-operator curve
- C:
-
Cats
- CD:
-
Chagas disease
- CFT:
-
Complement fixation test
- D:
-
Dogs
- DAT:
-
Direct agglutination test
- DeCS:
-
Health sciences descriptors
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase myocardial band
- DNA:
-
Deoxyribonucleic acid
- DTU:
-
Discrete typing units
- ECG:
-
Electrocardiogram
- ELISA:
-
Enzyme-linked immunosorbent assay
- FELV:
-
Feline leukemia virus
- FIV:
-
Feline immunodeficiency virus
- FRA:
-
Flagellar repetitive antigen
- IBMP:
-
Portuguese acronym for Biology Molecular Instituto of Paraná
- IBT:
-
Immunoblot test
- IFAT:
-
Immunofluorescence antibody test
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- MABA:
-
Microplate Alamar Blue Assay
- MAP:
-
Microtubule-associated protein
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RDT:
-
Rapid diagnostic test/immunochromatography
- Scielo:
-
Scientific electronic library online
- SAPA:
-
Shed acute phase antigen
- TSSA:
-
Mucin trypomastigote small surface antigen
- WB:
-
Western blot
- WM:
-
Wild mammals
References
World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;90:33–43.
Carod-Artal FJ. American trypanosomiasis. Handb Clin Neurol. 1st ed. Amsterdam: Elsevier; 2013
Santos EF, Silva ÂAO, Leony LM, Freitas NEM, Daltro RT, Regis-Silva CG, et al. Acute Chagas disease in Brazil from 2001 to 2018: a nationwide spatiotemporal analysis. PLoS Negl Trop Dis. 2020;14:e0008445.
Lidani KCF, Andrade FA, Bavia L, Damasceno FS, Beltrame MH, Messias-Reason IJ, et al. Chagas disease: from discovery to a worldwide health problem. Front Public Health. 2019;7:166.
Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21.
Manne-Goehler J, Reich MR, Wirtz VJ. Access to care for Chagas disease in the United States: a health systems analysis. Am J Trop Med Hyg. 2015;93:108–13.
Conners EE, Vinetz JM, Weeks JR, Brouwer KC. A global systematic review of Chagas disease prevalence among migrants. Acta Trop. 2016;156:68–78.
Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100.
Carvalho EB, Ramos IPR, Nascimento AFS, Brasil GV, Mello DB, Oti M, et al. Echocardiographic measurements in a preclinical model of chronic chagasic cardiomyopathy in dogs: validation and reproducibility. Front Cell Infect Microbiol. 2019;9:1–8.
Guedes PMM, Veloso VM, Caliari MV, Carneiro CM, Souza SM, de Lana M, et al. Trypanosoma cruzi high infectivity in vitro is related to cardiac lesions during long-term infection in Beagle dogs. Mem Inst Oswaldo Cruz. 2007;102:141–7.
Tafuri WL, de Lana M, Chiari E, Caliari MV, Bambirra EA, Rios-Leite VH, et al. Dogs as experimental models for the study of the natural course of Chagas disease. Rev Soc Bras Med Trop. 1988;21:77.
Zecca IB, Hodo CL, Slack S, Auckland L, Rodgers S, Killets KC, et al. Prevalence of Trypanosoma cruzi infection and associated histologic findings in domestic cats (Felis catus). Vet Parasitol. 2020;278:109014.
Barr SC. Canine Chagas’ disease (American trypanosomiasis) in North America. Vet Clin North Am Small Anim Pract. 2009;39:1055–64.
Maggi RG, Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasites Vectors. 2019;12:1–37.
Lima AF, Jeraldo VD, Silveira MS, Madi RR, Santana TB, Melo CM. Triatomines in dwellings and outbuildings in an endemic area of Chagas disease in northeastern Brazil. Rev Soc Bras Med Trop. 2012;45:701–6.
Gürtler RE, Ceballos LA, Ordóñez-Krasnowski P, Lanati LA, Stariolo R, Kitron U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: implications for the epidemiology of Chagas disease. PLoS Negl Trop Dis. 2009;3:e447.
Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82.
Wisnivesky-Colli C, Gürtler RE, Solarz ND, Lauricella MA, Segura EL. Epidemiological role of humans, dogs and cats in the transmission of Trypanosoma cruzi in a central area of Argentina. Rev Inst Med Trop Sao Paulo. 1985;27:346–52.
Travi BL. Considering dogs as complementary targets of Chagas disease control. Vector borne zoonotic Dis. 2019;19:90–4.
Gürtler RE, Cardinal MV. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32–50.
Blandon R, Leandro IM. Johnson CM [Clinical, electrocardiographic and angiographic evaluation of natural reservoirs of Chagas’ disease in the Republic of Panama]. Rev Med Panama. 1995;20:108–15.
Enriquez GF, Cardinal MV, Orozco MM, Schijman AG, Gürtler RE. Detection of Trypanosoma cruzi infection in naturally infected dogs and cats using serological, parasitological and molecular methods. Acta Trop. 2013;126:211–7.
Castillo-Neyra R, Chou Chu L, Quispe-Machaca V, Ancca-Juarez J, Malaga Chavez FS, Bastos Mazuelos M, et al. The potential of canine sentinels for reemerging Trypanosoma cruzi transmission. Prev Vet Med. 2015;120:349–56.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:1–15.
Roellig DM, Ellis AE, Yabsley MJ. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J Parasitol. 2009;95:360–4.
Jansen AM, Luiz A, Roque R, Maria A, Jansen AM. Importância dos animais domésticos sentinelas na identificação de áreas de risco de emergência de doença de Chagas. Rev Soc Bras Med Trop. 2008;41:191–3.
Enriquez GFF, Bua J, Orozco MMM, Wirth S, Schijman AGG, Gürtler REE, et al. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites for domestic transmission. Infect Genet Evol. 2014;25:36–43.
Coura JR, Petana WB. Additional data on the epidemiology of Chagas disease in the municipality of Caxias, Rio de Janeiro state Brazil. Rev Soc Bras Med Trop. 1975;9:83–9.
Gurtler RE, Cecere MC, Castanera MB, Canale D, Lauricella MA, Chuit R, et al. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am J Trop Med Hyg. 1996;55:24–31.
Coffield DJ, Spagnuolo AM, Shillor M, Mema E, Pell B, Pruzinsky A, et al. A model for Chagas disease with oral and congenital transmission. PLoS ONE. 2013;8:e67267.
Jiménez-Coello M, Guzmán-Marín E, Ortega-Pacheco A, Acosta-Viana KY. Serological survey of American trypanosomiasis in dogs and their owners from an urban area of Mérida Yucatàn México. Transbound Emerg Dis. 2010;57:33–6.
Quijano-Hernández IA, Castro-Barcena A, Barbabosa-Pliego A, Ochoa-García L, Del Ángel-Caraza J, Vázquez-Chagoyán JC. Seroprevalence survey of American trypanosomiasis in Central Valley of Toluca. Sci World J. 2012;2012:1–3.
Balan LU, Yerbes IM, Piña MAN, Balmes J, Pascual A, Hernández O, et al. Higher seroprevalence of Trypanosoma cruzi infection in dogs than in humans in an urban area of Campeche Mexico. Vector Borne Zoonotic Dis. 2011;11:843–4.
Barbabosa-Pliego A, Gil PC, Hernández DO, Aparicio-Burgos JE, de Oca-Jiménez RM, Martínez-Castañeda JS, et al. Prevalence of Trypanosoma cruzi in dogs (Canis familiaris) and triatomines during 2008 in a sanitary region of the State of Mexico Mexico. Vector Borne Zoonotic Dis. 2011;11:151–6.
Mejía A, Portugal-García C, Chávez-López V, García-Vázquez Z, Ramos C. Evidencia serológica de infección por Trypanosoma cruzi en perros atendidos en clínicas veterinarias del área conurbada de Cuernavaca. Morelos Salud Publica Mex. 2017;59:205–6.
Estrada-Franco JG, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbabosa A, Vazquez-Chagoyan JC, et al. Human Trypanosoma cruzi infection and seropositivity in dogs. Mexico Emerg Infect Dis. 2006;12:624–30.
Jiménez-Coello M, Acosta-Viana KY, Guzman-Marin E, Gomez-Rios A, Ortega-Pacheco A. Epidemiological survey of Trypanosoma cruzi infection in domestic owned cats from the tropical southeast of Mexico. Zoonoses Public Health. 2012;59:102–9.
García-Vazquez Z, Rosario-Cruz R, Miranda-Miranda E, Dominguez-Marquez A. A serological survey of Trypanosoma cruzi infection in dogs of two urban areas of Mexico. Prev Vet Med. 1995;25:1–6.
Ortega-Pacheco A, Guzmán-Marín E, Acosta-Viana KY, Vado-Solís I, Jiménez-Delgadillo B, Cárdenas-Marrufo M, et al. Serological survey of Leptospira interrogans, Toxoplasma gondii and Trypanosoma cruzi in free roaming domestic dogs and cats from a marginated rural area of Yucatan Mexico. Vet Med Sci. 2017;3:40–7.
Jimenez-Coello M, Poot-Cob M, Ortega-Pacheco A, Guzman-Marin E, Ramos-Ligonio A, Sauri-Arceo CH, et al. American trypanosomiasis in dogs from an urban and rural area of Yucatan Mexico. Vector Borne Zoonotic Dis. 2008;8:755–61.
Zamora-Ledesma S, Hernández-Camacho N, Sánchez-Moreno M, Ruiz-Piña H, Villagrán-Herrera ME, Marín-Sánchez C, et al. Seropositivity for Trypanosoma cruzi and Leishmania mexicana in dogs from a metropolitan region of Central Mexico. Vet Parasitol Reg Stud Reports. 2020;22:100459.
Martínez I, Martínez-Ibarra A, Arce-Fonseca M, Rodríguez-Morales O, Pérez-Morales D, Reyes López PA, et al. Seroprevalence and major antigens recognized by sera from Trypanosoma cruzi-infected dogs from Jalisco México. Rev Argent Microbiol. 2014;46:85–90.
Arce-Fonseca M, Carrillo-Sánchez SC, Molina-Barrios RM, Martínez-Cruz M, Cedillo-Cobián JR, Henao-Díaz YA, et al. Seropositivity for Trypanosoma cruzi in domestic dogs from Sonora. Mexico Infect Dis Poverty. 2017;6:120.
Benítez-Villa GE, López-Monteon A, Waleckx E, Dumonteil E, Márquez-Fernández AJ, Rovirosa-Hernández MJ, et al. Presence of Anti-T cruzi antibodies in inhabitants and dogs of two rural settlements in the Sierra de Los Tuxtlas, Veracruz Mexico. Acta Parasitol. 2022. https://doi.org/10.1007/s11686-022-00557-x.
Reyes L, Silesky E, Cerdas C, Chinchilla M, Guerrero O. Presencia de anticuerpos contra Trypanosoma cruzi en perros de Costa Rica. Parasitol Latinoam. 2002;57:66–8.
Bonilla MC, Castro-Vásquez RM, Herrero-Acosta MV, Urbina-Villalobos A, Dolz G. Canine trypanosomiasis in an endemic Costa Rican community: Demonstration of the active infection cycle. Vet Parasitol Reg Stud reports. 2019;17:100307.
Montenegro VM, Jimenez M, Dias JCP, Zeledon R. Chagas disease in dogs from endemic areas of Costa Rica. Mem Inst Oswaldo Cruz. 2002;97:491–4.
Mesa-Arciniegas P, Parra-Henao G, Carrión-Bonifacio Á, Casas-Cruz A, Patiño-Cuellar A, Díaz-Rodríguez K, et al. Trypanosoma cruzi infection in naturally infected dogs from an endemic region of Cundinamarca Colombia. Vet Parasitol Reg Stud reports. 2018;14:212–6.
Jaimes-Dueñez J, Triana-Chávez O, Cantillo-Barraza O, Hernández C, Ramírez JD, Góngora-Orjuela A. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev Vet Med. 2017;141:1–6.
Cantillo-Barraza O, Torres J, Hernández C, Romero Y, Zuluaga S, Correa-Cárdenas CA, et al. The potential risk of enzootic Trypanosoma cruzi transmission inside four training and re-training military battalions (BITER) in Colombia. Parasites Vectors. 2021;14:1–13.
Cantillo-Barraza O, Bedoya SC, Xavier SCC, Zuluaga S, Salazar B, Vélez-Mira A, et al. Trypanosoma cruzi infection in domestic and synanthropic mammals such as potential risk of sylvatic transmission in a rural area from north of Antioquia Colombia. Parasite Epidemiol Control. 2020. https://doi.org/10.1016/j.parepi.2020.e00171.
Manrique-Abril D, Manrique-Abril F, Lorca HM, Ospina DJ. Prevalencia de anticuerpos para Trypanosoma cruzi en caninos de dos municipios endémicos de Boyacá. Rev MVZ Córdoba. 2012;17:2916–23.
Curtis-Robles R, Snowden KF, Dominguez B, Dinges L, Rodgers S, Mays G, et al. Epidemiology and molecular typing of Trypanosoma cruzi in naturally-infected hound dogs and associated Triatomine vectors in Texas, USA. PLoS Negl Trop Dis. 2017;11:e0005298.
Shadomy SV, Waring SC, Martins-Filho OA, Oliveira RC, Chappell CL. Combined use of enzyme-linked immunosorbent assay and flow cytometry to detect antibodies to Trypanosoma cruzi in domestic canines in Texas. Clin Diagn Lab Immunol. 2004;11:313–9.
Tenney TD, Curtis-Robles R, Snowden KF, Hamer SA. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas. Emerg Infect Dis. 2014;20:1323–6.
Elmayan A, Tu W, Duhon B, Marx P, Wolfson W, Balsamo G, et al. High prevalence of Trypanosoma cruzi infection in shelter dogs from southern Louisiana, USA. Parasit Vectors. 2019;12:322.
Bradley KK, Bergman DK, Woods JP, Crutcher JM, Kirchhoff LV. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J Am Vet Med Assoc. 2000;217:1853–7.
Meyers AC, Hamer SA, Matthews D, Gordon SG, Saunders AB. Risk factors and select cardiac characteristics in dogs naturally infected with Trypanosoma cruzi presenting to a teaching hospital in Texas. J Vet Intern Med. 2019;33:1695–706.
Hodo CL, Rodriguez JY, Curtis-Robles R, Zecca IB, Snowden KF, Cummings KJ, et al. Repeated cross-sectional study of Trypanosoma cruzi in shelter dogs in Texas, in the context of Dirofilaria immitis and tick-borne pathogen prevalence. J Vet Intern Med. 2019;33:158–66.
Meyers AC, Purnell JC, Ellis MM, Auckland LD, Meinders M, Hamer SA. Nationwide exposure of U.S. working dogs to the chagas disease parasite, Trypanosoma cruzi. Am J Trop Med Hyg. 2020;102:1078–85.
Curtis-Robles R, Meyers AC, Auckland LD, Zecca IB, Skiles R, Hamer SA. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop. 2018;188:225–33.
Allen KE, Lineberry MW. Trypanosoma cruzi and other vector-borne infections in shelter dogs in two counties of Oklahoma United States. Vector-Borne Zoonotic Dis. 2022;22:273–80.
Constantino C, Pellizzaro M, de Paula EFE, Vieira TSWJ, Brandão APD, Ferreira F, et al. Serosurvey for Leishmania spp, Toxoplasma gondii, Trypanosoma cruzi and Neospora caninum in neighborhood dogs in Curitiba-Paraná Brazil. Rev Bras Parasitol Veterinária. 2016;25:504–10.
de Oliveira Porfirio GE, Santos FM, de Macedo GC, Barreto WT, Campos JB, Meyers AC, et al. Maintenance of Trypanosoma cruzi, T evansi and Leishmania spp by domestic dogs and wild mammals in a rural settlement in Brazil-Bolivian border. Int J Parasitol Parasites Wildl. 2018;7:398–404.
Costa TF, Rocha AVVO, Miranda LM, Lima LFS, Santos FLN, Silva ÂAO, et al. Seroprevalence and detection of Trypanosoma cruzi in dogs living in a non-endemic area for Chagas disease in the legal Amazon region. Brazil Vet Parasitol Reg Stud Reports. 2021;26:100648.
Dario MA, Rodrigues MS, Barros JH, Xavier SC, D’Andrea PS, Roque AL, et al. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espírito Santo state, Brazil). Parasit Vectors. 2016;9:477.
Bezerra CM, Cavalcanti LP, Souza RD, Barbosa SE, Xavier SC, Jansen AM, et al. Domestic, peridomestic and wild hosts in the transmission of Trypanosoma cruzi in the Caatinga area colonised by Triatoma brasiliensis. Mem Inst Oswaldo Cruz. 2014;109:887–98.
Lima MM, Sarquis O, de Oliveira TG, Gomes TF, Coutinho C, Daflon-Teixeira NF, et al. Investigation of Chagas disease in four periurban areas in northeastern Brazil: Epidemiologic survey in man, vectors, non-human hosts and reservoirs. Trans R Soc Trop Med Hyg. 2012;106:143–9.
Santos F, Magalhães-Junior JT, de Carneiro IO, Santos FLN, Silva ÂAO, Novais JMCB, et al. Eco-epidemiology of vectorial Trypanosoma cruzi transmission in a region of Northeast Brazil. Acta Trop. 2022;225:106184.
Malavazi PFNS, Daudt C, Melchior LAK, Meneguetti DUO, Xavier SCC, Jansen AM, et al. Trypanosomes of vectors and domestic dogs in Trypanosoma cruzi transmission areas from Brazilian southwestern amazon: New mammalian host for Trypanosoma janseni. Acta Trop. 2020;210:105504.
Leça Júnior NF, dos Almeida VA, Carvalho FS, Albuquerque GR, Silva FL. First report of Trypanosoma cruzi infection in naturally infected dogs from southern Bahia Brazil. Rev Bras Parasitol Veterinária. 2013;22:182–5.
de Souza GB, Guedes PEB, Oliveira TNDA, Carvalho FS, Albuquerque GR, Silva FL. Natural infection by Trypanosoma cruzi in dogs located in Ituberá, Southern Bahia Brazil. Semin Ciências Agrárias. 2018;39:881.
Fernandesda ARF, Pimenta CLRM, Vidal IF, Oliveira GC, Sartori RS, Araújo RB, et al. Risk factors associated with seropositivity for Leishmania spp and Trypanosoma cruzi in dogs in the state of Paraiba Brazil. Rev Bras Parasitol Veterinária. 2016;25:90–8.
Freitas YBN, de Souzada CSF, Magalhães JME, de Sousa MLR, D’Escoffier LN, Valledo TZ, et al. Natural infection by Trypanosoma cruzi in triatomines and seropositivity for Chagas disease of dogs in rural areas of Rio Grande do Norte Brazil. Rev Soc Bras Med Trop. 2018;51:190–7.
de Araújo-Neto VT, Honorato NRM, de SantanaO R, Barbosa-Silva AN, Guedesda PMM, Chiari E, et al. Trypanosoma cruzi circulating among dogs and triatomines in the endemic countryside of the State of Rio Grande do Norte Brazil. Acta Trop. 2019;200:105067.
Da Paz GS, Da Silva CB, Anton MM, De Souza RK, Da Silva DB, De Moraes CCG, et al. Infection by Toxoplasma gondii, Neospora caninum, Leishmania major and Trypanosoma cruzi in dogs from the state of Pará. Cienc Anim Bras. 2019;20:1–10.
Tome RO, Gaio FC, Generoso D, Menozzi BD, Langoni H. Active surveillance of canine visceral leishmaniasis and American trypanossomiasis in rural dogs from non endemic area. Rev Bras Parasitol Veterinária. 2011;20:64–6.
Perez TD, Figueiredo FB, Junior AAMV, Silva VL, de MadeiraF M, Brazil RP, et al. Prevalence of American trypanosomiasis and leishmaniasis in domestic dogs in a rural area ofthe municipality of São João do Piauí, Piauí state, Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:79.
Xavierdas SCC, Roque ALR, Bilac D, de Araújo VAL, Netoda SFC, Lorosa ES, et al. Distantiae transmission of Trypanosoma cruzi: A new epidemiological feature of acute Chagas disease in Brazil. PLoS Negl Trop Dis. 2014;8:e2878.
Mendes RS, Santana VL, Jansen AM, Xavier SCC, Vidal IF, Rotondano TEF, et al. Aspectos epidemiológicos da doença de Chagas canina no semiárido paraibano. Pesqui Vet Bras. 2013;33:1459–65.
Rocha FL, Roque ALR, Arrais RC, Santos JP, dos LimaS V, XavierdasC SC, et al. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas Brazil. Parasitology. 2013;140:160–70.
Cruz ACFG, Santos NAF, de JeraldoLS V, Madi RR, Rosada JA, de Melo CM. Shelter dogs as indicators for Trypanosoma cruzi infection in an urban area of Aracaju. Brazil Acta Trop. 2020;210:105577.
Morais AN, Sousa MG, Meireles LR, Kesper N Jr, Umezawa ES. Canine visceral leishmaniasis and Chagas disease among dogs in Araguaína. Tocantins Rev Bras Parasitol Veterinária. 2013;22:225–9.
Norma U, Edgar M. Huapaya J. Rev Horiz [Seroprevalence in animals, possible reservoir of chagas disease in Nazca, department of Ica - Peru]. Médico. 2006;6:80–3.
Rosypal AC, Tripp S, Kinlaw C, Sharma RN, Stone D, Dubey JP. Seroprevalence of canine leishmaniasis and American trypanosomiasis in dogs from Grenada. West Indies J Parasitol. 2010;96:228–9.
Tahir D, Davoust B, Heu K, Lamour T, Demar M, Lou M-L, et al. Molecular and serological investigation of Trypanosoma cruzi infection in dogs in French Guiana. Vet Parasitol Reg Stud reports. 2018;12:106–9.
Roegner AF, Daniels ME, Smith WA, Gottdenker N, Schwartz LM, Liu J, et al. Giardia infection and Trypanosoma cruzi exposure in dogs in the Bosawás Biosphere Reserve. Nicaragua Ecohealth. 2019;16:512–22.
González CR, Reyes C, Canals A, Parra A, Muñoz X, Rodríguez K. An entomological and seroepidemiological study of the vectorial-transmission risk of Chagas disease in the coast of northern Chile. Med Vet Entomol. 2015;29:387–92.
Ríos A, Alcaíno H, Apt W. Enfermedad de Chagas en caninos, bovinos y equidos sinantrópicos de la Provincia del Limarí. Chile Parasitol al día. 1986;10:40–5.
Burchard L, Cáceres J, Sagua H, Inés Bahamonde M, Neira I, Araya J, et al. Current human and canine seroprevalence of Chagasic infection in San Pedro de Atacama County, II Region of Antofagasta, Chile, 1995. Bol Chil Parasitol. 1996;51:76–9.
Saldaña A, Calzada JE, Pineda V, Perea M, Rigg C, González K, et al. Risk factors associated with Trypanosoma cruzi exposure in domestic dogs from a rural community in Panama. Mem Inst Oswaldo Cruz. 2015;110:936–44.
Pineda V, Saldaña A, Monfante I, Santamaría A, Gottdenker NL, Yabsley MJ, et al. Prevalence of trypanosome infections in dogs from Chagas disease endemic regions in Panama Central America. Vet Parasitol. 2011;178:360–3.
Bonfante-Cabarcas R, Rodríguez-Bonfante C, Vielma BO, García D, Saldivia AM, Aldana E, et al. Seroprevalencia de la infección por Trypanosoma cruzi y factores asociados en un área endémica de Venezuela. Cad Saude Publica. 2011;27:1917–29.
Rodríguez-bonfante CC, Rojas ME, Aldana E, Concepción JL, Bonfante-cabarcas RA. Persistence of Chagas disease active transmission among dogs in Venezuela rural community. Rev Costarric Salud Publica. 2011;20:97–101.
Rojas ME, Várquez P, Villarreal MF, Velandia C, Vergara L, Morán-Borges YH, et al. Estudio seroepidemiológico y entomológico sobre la enfermedad de Chagas en un área infestada por Triatoma maculata (Erichson 1848) en el centro-occidente de Venezuela. Cad Saude Publica. 2008;24:2323–33.
Berrizbeitia M, Concepción JL, Carzola V, Rodríguez J. Seroprevalencia de la infección por Trypanosoma cruzi en Canis familiaris del estado Sucre. Venezuela Biomédica. 2013;33:214–25.
Monje-Rumi MM, Brandán CP, Ragone PG, Tomasini N, Lauthier JJ, Alberti D’Amato AM, et al. Trypanosoma cruzi diversity in the Gran Chaco: mixed infections and differential host distribution of TcV and TcVI. Infect Genet Evol. 2015;29:53–9.
Gorodner OL, Mendivil GT, Risso A, Risso J, Petraglia G, De Francesco C, et al. Enfermedad de Chagas natural en perros: estudios serológicos anatomopatológicos y electrocardiográficos de la fase crónica indeterminada de la infección. Med. 1985;45:535–8.
Lauricella MA, Sinagra AJ, Paulone I, Riarte AR, Segura EL. Natural Trypanosoma cruzi infection in dogs of endemic areas of the Argentine Republic. Rev Inst Med Trop Sao Paulo. 1989;31:63–70.
Gabrielli S, Spinicci M, Macchioni F, Rojo D, Totino V, Rojas P, et al. Canine Trypanosoma cruzi infection in the Bolivian Chaco. Parasit Vectors. 2018;11:632.
Rivadeneira-Barreiro PE, Montes de Oca-Jiménez R, Vázquez-Chagoyán JC, Martínez-Subiela S, Morán-Loor A, Ochoa-García L, et al. Trypanosoma cruzi co-infections with other vector borne diseases are frequent in dogs from the pacific coast of Ecuador. Microb Pathog. 2021;155:104884.
Petersen RM, Gürtler RE, Cecere MC, Rubel DN, Lauricella MA, Hansen D, et al. Association between nutritional indicators and infectivity of dogs seroreactive for Trypanosoma cruzi in a rural area of northwestern Argentina. Parasitol Res. 2001;87:208–14.
Busselman RE, Meyers AC, Zecca IB, Auckland LD, Castro AH, Dowd RE, et al. High incidence of Trypanosoma cruzi infections in dogs directly detected through longitudinal tracking at 10 multi-dog kennels, Texas, USA. PLoS Negl Trop Dis. 2021;15:e0009935.
Andrade ZA, Andrade SG. Pathology of experimental Chagas disease in dogs. Mem Inst Oswaldo Cruz. 1980;75:77–95.
Pellegrino J. O eletrocardiograma na fase crônica da doença de Chagas experimental no cão. Mem Inst Oswaldo Cruz. 1946;44:615–47.
Eloy L, Lucheis S. Canine trypanosomiasis: etiology of infection and implications for public health. J Venom Anim Toxins incl Trop Dis. 2009;15:589–611.
Barbabosa-Pliego A, Díaz-Albiter HM, Ochoa-García L, Aparicio-Burgos E, López-Heydeck SM, Velásquez-Ordoñez V, et al. Trypanosoma cruzi circulating in the southern region of the State of Mexico (Zumpahuacan) are pathogenic: a dog model. Am J Trop Med Hyg. 2009;81:390–5.
Andrade ZA, Andrade SG, Sadigursky M. Enhancement of chronic Trypanosoma cruzi myocarditis in dogs treated with low doses of cyclophosphamide. Am J Pathol. 1987;127:467–73.
de Lana M, Chiari E, Tafuri WL. Experimental Chagas’ disease in dogs. Mem Inst Oswaldo Cruz. 1992;87:59–71.
Meyers AC, Ellis MM, Purnell JC, Auckland LD, Meinders M, Saunders AB, et al. Selected cardiac abnormalities in Trypanosoma cruzi serologically positive, discordant, and negative working dogs along the Texas-Mexico border. BMC Vet Res. 2020;16:101.
Matthews DJ, Saunders AB, Meyers AC, Gordon SG, Hamer SA. Cardiac diagnostic test results and outcomes in 44 dogs naturally infected with Trypanosoma cruzi. J Vet Intern Med. 2021;35:1800–9.
Stoner CH, Saunders AB. Cardiac manifestations of Trypanosoma cruzi infection in a domestic dog. Case Reports. 2020;4:410–4.
de Souza AI, Paulino-Junior D, Sousa MG, Camacho AA. Aspectos clínico-laboratoriais da infecção natural por Trypanosoma cruzi em cães de Mato Grosso do Sul. Ciência Rural. 2008;38:1351–6.
Santana VL, Souza AP, Lima DASD, Araújo AL, Justiniano SV, Dantas RP, et al. Caracterização clínica e laboratorial de cães naturalmente infectados com Trypanosoma cruzi no semiárido nordestino. Pesqui Vet Bras. 2012;32:536–41.
Pasconda JPE, Neto GBP, Sousa MG, Paulino D, Camacho AA. Clinical characterization of chronic chagasic cardiomyopathy in dogs. Pesqui Vet Bras. 2010;30:115–20.
Meyers AC, Edwards EE, Sanders JP, Saunders AB, Hamer SA. Fatal Chagas myocarditis in government working dogs in the southern United States: Cross-reactivity and differential diagnoses in five cases across 6 months. Vet Parasitol Reg Stud Reports. 2021;24:100545.
Nogueira-Paiva NC, Fonseca KS, Vieira PMA, Diniz LF, Caldas IS, Moura SAL, et al. Myenteric plexus is differentially affected by infection with distinct Trypanosoma cruzi strains in Beagle dogs. Mem Inst Oswaldo Cruz. 2014;109:51–60.
Bahia MT, Tafuri WL, Caliari MV, Veloso VM, Martins Carneiro C, Machado Coelho GLL, et al. Comparison of Trypanosoma cruzi infection in dogs inoculated with blood or metacyclic trypomastigotes of Berenice-62 and Berenice-78 strains via intraperitoneal and conjunctival routes. Rev Soc Bras Med Trop. 2002;35:339–45.
Madigan R, Majoy S, Ritter K, Luis Concepción J, Márquez ME, Silva SC, et al. Investigation of a combination of amiodarone and itraconazole for treatment of American trypanosomiasis (Chagas disease) in dogs. J Am Vet Med Assoc. 2019;255:317–29.
Malcolm EL, Saunders AB, Vitt JP, Boutet BG, Hamer SA. Antiparasitic treatment with itraconazole and amiodarone in 2 dogs with severe, symptomatic Chagas cardiomyopathy. J Vet Intern Med. 2022. https://doi.org/10.1111/jvim.16422.
de Higuchi ML, De Brito T, Martins Reis M, Barbosa A, Bellotti G, Pereira-Barreto AC, et al. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: Light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2:101–6.
Longoni SS, López-Cespedes A, Sánchez-Moreno M, Bolio-Gonzalez ME, Sauri-Arceo CH, Rodríguez-Vivas RI, et al. Detection of different Leishmania spp. and Trypanosoma cruzi antibodies in cats from the Yucatan Peninsula (Mexico) using an iron superoxide dismutase excreted as antigen. Comp Immunol Microbiol Infect Dis. 2012;35:469–76.
de Matos AMRN, Caldart ET, Ferreira FP, Monteiro KC, de Souza M, Brunieri DTSC, et al. Antibodies anti-trypanosomatides in domestic cats in Paraná: who is at highest risk of infection? Rev Bras Parasitol Veterinária. 2018;27:232–6.
Dumonteil E, Desale H, Tu W, Duhon B, Wolfson W, Balsamo G, et al. Shelter cats host infections with multiple Trypanosoma cruzi discrete typing units in southern Louisiana. Vet Res. 2021;52:53.
Curtis-Robles R, Zecca IB, Roman-Cruz V, Carbajal ES, Auckland LD, Flores I, et al. Trypanosoma cruzi (agent of Chagas Disease) in sympatric human and dog populations in “colonias” of the Lower Rio Grande Valley of Texas. Am J Trop Med Hyg. 2017;96:805–14.
Zao C, Yang Y, Tomanek L, Cooke A, Berger R, Chien L, et al. PCR monitoring of parasitemia during drug treatment for canine Chagas disease. J Vet Diagn Invest. 2019. https://doi.org/10.1177/1040638719868508.
Lana M, Vieira LM, Machado-Coelho GL, Chiari E, Veloso VM, Tafuri WL. Humoral immune response in dogs experimentally infected with Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1991;86:471–3.
Guhl F, Jaramillo C, Carranza JC, Vallejo GA. Molecular characterization and diagnosis of Trypanosoma cruzi and T. rangeli. Arch Med Res. 2002;33:362–70.
Santos FLN, Celedon PAF, Zanchin NIT, Brasil TAC, Foti L, Souza WV, et al. Performance assessment of four chimeric Trypanosoma cruzi antigens based on antigen-antibody detection for diagnosis of chronic Chagas disease. PLoS ONE. 2016;11:e0161100.
Da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 2001;17:286–91.
Saez-Alquézar A, Sabino EC, Salles N, Chamone DF, Hulstaert F, Pottel H, et al. Serological confirmation of Chagas’ disease by a recombinant and peptide antigen line immunoassay: INNO-LIA Chagas. J Clin Microbiol. 2000;38:851–4.
Gomes YM, Lorena VMB, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009;104:115–21.
Santos FLN, Souza WV, Barros MS, Nakazawa M, Krieger MA, Gomes YM. Chronic Chagas disease diagnosis: a comparative performance of commercial enzyme immunoassay tests. Am J Trop Med Hyg. 2016;94:1034–9.
Luciano RM, Lucheis SB, Troncarelli MZ, Luciano DM, Langoni H. Cross reaction evaluation of Leishmania spp and Trypanosoma cruzi antigens in dogs’ serologic response by indirect immunofluorescence test (IIF). Brazilian J Vet Res Anim Sci. 2009;46:181.
Tripathi NK, Shrivastava A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front Bioeng Biotechnol. 2019;7:420.
Camussone C, Gonzalez VV, Belluzo MSMS, Pujato N, Ribone MEME, Lagier CM, et al. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin Vaccine Immunol. 2009;16:899–905.
Houghton RL, Benson DR, Reynolds LD, McNeill PD, Sleath PR, Lodes MJ, et al. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J Infect Dis. 1999;179:1226–34.
Lafaille JJ, Linss J, Krieger Ma, Souto-Padrón T, de Souza W, Goldenberg S. Structure and expression of two Trypanosoma cruzi genes encoding antigenic proteins bearing repetitive epitopes. Mol Biochem Parasitol. 1989;35:127–36.
Araujo FG. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related trypanosomatids. Infect Immun. 1986;53:179–85.
Goto Y, Carter D, Reed SG. Immunological dominance of Trypanosoma cruzi tandem repeat proteins. Infect Immun. 2008;76:3967–74.
Buscaglia Ca, Campetella O, Leguizamón MS, Frasch Ca. The repetitive domain of Trypanosoma cruzi trans-sialidase enhances the immune response against the catalytic domain. J Infect Dis. 1998;177:431–6.
Valiente-Gabioud Aa, Veaute C, Perrig M, Galan-Romano FS, Sferco SJ, Marcipar IS. Effect of repetitiveness on the immunogenicity and antigenicity of Trypanosoma cruzi FRA protein. Exp Parasitol. 2011;127:672–9.
Frasch AC, Cazzulo JJ, Aslund L, Pettersson U. Comparison of genes encoding Trypanosoma cruzi antigens. Parasitol Today. 1991;7:148–51.
Hoft DF, Kim KS, Otsu K, Moser DR, Yost WJ, Blumin JH, et al. Trypanosoma cruzi expresses diverse repetitive protein antigens. Infect Immun. 1989;57:1959–67.
Umezawa ES, Luquetti AO, Levitus G, Ponce C, Ponce E, Henriquez D, et al. Serodiagnosis of chronic and acute Chagas’ disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol. 2004;42:449–52.
De Pablos LM, Osuna A. Multigene families in Trypanosoma cruzi and their role in infectivity. Infect Immun. 2012;80:2258–64.
Almeida E, Krieger MA, Carvalho MR, Oelemann W, Goldenberg S. Use of recombinant antigens for the diagnosis of Chagas disease and blood bank screening. Mem Inst Oswaldo Cruz. 1990;85:513–7.
Krieger MA, Almeida E, Oelemann W, Lafaille JJ, Pereira JB, Krieger H, et al. Use of recombinant antigens for the accurate immunodiagnosis of Chagas’ disease. Am J Trop Med Hyg. 1992;46:427–34.
Carvalho MR, Krieger MA, Almeida E, Oelemann W, Shikanai-Yassuda MA, Ferreira AW, et al. Chagas’ disease diagnosis: evaluation of several tests in blood bank screening. Transfusion. 1993;33:830–4.
Pastini AC, Iglesias SR, Carricarte VC, Guerin ME, Sánchez DO, Frasch AC. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas disease. Clin Chem. 1994;40:1893–7.
Peralta JM, Teixeirada MGGM, Shreffler WG, Pereira JB, Burns JM, Sleath PR, et al. Serodiagnosis of Chagas’ disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J Clin Microbiol. 1994;32:971–4.
Granjon E, Dichtel-Danjoy M-L, Saba E, Sabino E, Campos de Oliveira L, Zrein M. Development of a novel multiplex immunoassay multi-cruzi for the serological confirmation of Chagas disease. PLoS Negl Trop Dis. 2016;10:e0004596.
Houghton RL, Benson DR, Reynolds L, McNeill P, Sleath P, Lodes M, et al. Multiepitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in patients with treated or untreated Chagas’ disease. J Infect Dis. 2000;181:325–30.
Hernández P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, et al. Highly effective serodiagnosis for Chagas’ disease. Clin Vaccine Immunol. 2010;17:1598–604.
Peverengo LM, Garcia V, Rodeles LM, Mendicino D, Vicco M, Lagier C, et al. Development and assessment of an improved recombinant multiepitope antigen-based immunoassay to diagnose chronic Chagas disease. Parasitology. 2018;145:1594–9.
Santos FLN, Celedon PA, Zanchin NI, Souza WV, Silva ED, Foti L, et al. Accuracy of chimeric proteins in the serological diagnosis of chronic Chagas disease—a Phase II study. PLoS Negl Trop Dis. 2017;11:e0005433.
Dopico E, Del-Rei RP, Espinoza B, Ubillos I, Zanchin NIT, Sulleiro E, et al. Immune reactivity to Trypanosoma cruzi chimeric proteins for Chagas disease diagnosis in immigrants living in a non-endemic setting. BMC Infect Dis. 2019;19:251.
Del-Rei RP, Leony LM, Celedon PAF, Zanchin NIT, Reis MG, Gomes YDM, et al. Detection of anti-Trypanosoma cruzi antibodies by chimeric antigens in chronic Chagas disease-individuals from endemic South American countries. PLoS ONE. 2019;14:e0215623.
Santos FLN, Campos ACP, Amorim LDAF, Silva ED, Zanchin NIT, Celedon PAF, et al. Highly accurate chimeric proteins for the serological diagnosis of chronic Chagas disease: a latent class analysis. Am J Trop Med Hyg. 2018;99:1174–9.
Daltro RT, Leony LM, Freitas NEM, Silva ÂAO, Santos EF, Del-Rei RP, et al. Cross-reactivity using chimeric Trypanosoma cruzi antigens: diagnostic performance in settings co-endemic for Chagas disease and American cutaneous or visceral leishmaniasis. J Clin Microbiol. 2019. https://doi.org/10.1128/JCM.00762-19.
Dos Santos EF, Silva ÂA, Freitas NE, Leony LM, Daltro RT, Santos CA, et al. Performance of chimeric Trypanosoma cruzi antigens in serological screening for Chagas disease in blood banks. Front Med. 2022;9:852864.
Santos FLN, Celedon PAF, Zanchin NIT, Leitolis A, Crestani S, Foti L, et al. Performance assessment of a Trypanosoma cruzi chimeric antigen in multiplex liquid microarray assays. J Clin Microbiol. 2017;55:2934–45.
Silva ED, Silva ÂAO, Santos EF, Leony LM, Freitas NEM, Daltro RT, et al. Development of a new lateral flow assay based on IBMP-8.1 and IBMP-8.4 chimeric antigens to diagnose Chagas disease. Biomed Res Int. 2020. https://doi.org/10.1155/2020/1803515.
Freitas NEM, dos Santos EF, Leony LM, Silva ÂAO, Daltro RT, de MedradoCV L, et al. Double-antigen sandwich ELISA based on chimeric antigens for detection of antibodies to Trypanosoma cruzi in human sera. PLoS Negl Trop Dis. 2022;16:e0010290.
Cordeiro TAR, Martins HR, Franco DL, Santos FLN, Celedon PAF, Cantuária VL, et al. Impedimetric immunosensor for rapid and simultaneous detection of Chagas and visceral leishmaniasis for point of care diagnosis. Biosens Bioelectron. 2020;169:112573.
Celedon PAF, Leony LM, Oliveira UD, Freitas NEM, Silva ÂAO, Daltro RT, et al. Stability assessment of four chimeric proteins for human Chagas disease immunodiagnosis. Biosensors. 2021;11:289.
Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93.
Santos EF, Leony LM, Silva ÂAO, Daltro RT, Freitas NEM, Vasconcelos LCM, et al. Assessment of Liaison XL Murex Chagas diagnostic performance in blood screening for Chagas disease using a reference array of chimeric antigens. Transfusion. 2021;61:2701–9.
Brasil. Resolução-RE No 2.281, de 2 de julho de 2020. Diário Oficial da União. Brazil: Diário Oficial da União; 2020. p. 69. https://pesquisa.in.gov.br/imprensa/servlet/INPDFViewer?jornal=515&pagina=69&data=06/07/2020&captchafield=firstAccess. Accessed 15 Jan 2022.
Leony LM, Freitas NEM, Del-Rei RP, Carneiro CM, Reis AB, Jansen AM, et al. Performance of recombinant chimeric proteins in the serological diagnosis of Trypanosoma cruzi infection in dogs. PLoS Negl Trop Dis. 2019;13:e0007545.
Rodrigues ES, Santos GQ, da Silva MV, Barros JHS, Bernardo AR, Diniz RL, et al. Chagas immunochromatographic rapid test in the serological diagnosis of Trypanosoma cruzi infection in wild and domestic canids. Front Cell Infect Microbiol. 2022;12:1–14.
McClean MCW, Bhattacharyya T, Mertens P, Murphy N, Gilleman Q, Gustin Y, et al. A lineage-specific rapid diagnostic test (Chagas Sero K-SeT) identifies Brazilian Trypanosoma cruzi II/V/VI reservoir hosts among diverse mammalian orders. PLoS ONE. 2020;15:1–13.
Zingales B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52.
Truyens C, Dumonteil E, Alger J, Cafferata ML, Ciganda A, Gibbons L, et al. Geographic variations in test reactivity for the serological diagnosis of Trypanosoma cruzi infection. J Clin Microbiol. 2021. https://doi.org/10.1128/JCM.01062-21.
Leeflang MMG, Bossuyt PMM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. J Clin Epidemiol. 2009;62:5–12.
Leeflang MMG, Rutjes AWS, Reitsma JB, Hooft L, Bossuyt PMM. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ. 2013;185:E537–44.
Martin DL, Marks M, Galdos-Cardenas G, Gilman RH, Goodhew B, Ferrufino L, et al. Regional variation in the correlation of antibody and T-cell responses to Trypanosoma cruzi. Am J Trop Med Hyg. 2014;90:1074–81.
Jaimes-Dueñez J, Jiménez-Leaño ÁP, Esteban-Mendoza M, Moreno-Salcedo LA, Triana-Chávez O, Cantillo-Barraza O. Epidemiological and clinical characteristics of Trypanosoma cruzi infection in dogs (Canis lupus familiaris) from a Chagas Disease-Endemic Urban Area in Colombia. Prev Vet Med. 2020;182:105093.
Murphy N, Macchiaverna NP, Victoria Cardinal M, Bhattacharyya T, Mertens P, Zeippen N, et al. Lineage-specific rapid diagnostic tests can resolve Trypanosoma cruzi TcII/V/VI ecological and epidemiological associations in the Argentine Chaco. Parasit Vectors. 2019;12:424.
Alvarez M, Cerisola J, Rohwedde R. Test de inmunofluorescencia para el diagnóstico de la enfermedad de Chagas. Bol Chil Parasitol. 1968;23:4–9.
Lauricella M, Castañera M, Gürtler R, Segura E. Immunodiagnosis of Trypanosoma cruzi (Chagas’ disease) infection in naturally infected dogs. Mem Inst Oswaldo Cruz. 1998;93:501–7.
Rodríguez-Morales O, Ballinas-Verdugo MA, Alejandre-Aguilar R, Reyes PA, Arce-Fonseca M. Trypanosoma cruzi connatal transmission in dogs with Chagas disease: experimental case report. Vector Borne Zoonotic Dis. 2011;11:1365–70.
Lauricella MA, Wisnivesky-Colli C, Gürtler R, Petersen R, Bujas M, Segura EL. Standardization of serological tests for detecting anti-Trypanosoma cruzi antibodies in dogs. Mem Inst Oswaldo Cruz. 1993;88:413–7.
Lauricella M, AR R, JO L, A B, EL S. Enfermidad de Chagas en perros experimentalmente infectados con T. cruzi. Medicina. 1986;46:195–200.
Sartor PA, Cardinal MV, Orozco MM, Gürtler RE, Leguizamón MS. Trans-sialidase neutralizing antibody detection in Trypanosoma cruzi-infected domestic reservoirs. Clin Vaccine Immunol. 2011;18:984–9.
Acknowledgements
Figures were created with biorender.com.
Funding
This research was supported by the Coordination of Superior Level Staff Improvement-Brazil (CAPES; Finance Code 001), the Research Support Foundation of the State of Bahia (FAPESB); and Inova Fiocruz/VPPCB (grant number VPPCB-008-FIO-18–2-20). FLNS is CNPq research fellows (grant number 309263/2020–4). Funders had no role on the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception and design of the study. The systematic review was conducted by NEMF, FLH and FLNS. NEMF, FLH, EFS, ÂAOS, NDF, LML, DDS and MCA were responsible for writing the manuscript. Images were provided by DDS, MCA and FLNS. The figures were prepared by FLNS. All authors contributed to changes made to subsequent versions from the first version. FDT was responsible for reviewing the final version of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Filipe Dantas-Torres is Editor-in-Chief of Parasites & Vectors, but the peer review process and final decision was handled independently by the Professor Anna Bajer, Subject Editor of the section Protozoa and protozoan diseases.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Freitas, N.E.M., Habib, F.L., Santos, E.F. et al. Technological advances in the serological diagnosis of Chagas disease in dogs and cats: a systematic review. Parasites Vectors 15, 343 (2022). https://doi.org/10.1186/s13071-022-05476-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05476-4