Abstract
Background
The recently discovered Babesia sp. Mymensingh, which causes clinical bovine babesiosis, has a wide geographical distribution. We investigated the phylogenetic position of Babesia sp. Mymensingh using its mitochondrial, plastid, and nuclear genes. Based on morphological and molecular data, Babesia sp. Mymensingh is a unique species and we named it as Babesia naoakii n. sp.
Methods
A blood DNA sample from a Babesia sp. Mymensingh-infected cow was subjected to genome sequencing to obtain the sequences of mitochondrial, plastid, and nuclear genes. Six phylogenetic trees were then constructed with (1) concatenated amino acid sequences of cytochrome oxidase subunit I, cytochrome oxidase subunit III, and cytochrome b genes of the mitochondrial genome; (2) 16S rRNA of the plastid genome; (3) nucleotide sequences of the elongation factor Tu gene of the plastid genome; (4) ITS1-5.8S rRNA-ITS2; (5) concatenated nucleotide sequences of 89 nuclear genes; and (6) concatenated amino acid sequences translated from the 89 nuclear genes.
Results
In all six phylogenetic trees, B. naoakii n. sp. formed a sister clade to the common ancestor of Babesia bigemina and B. ovata. The concatenated nuclear genes of B. naoakii n. sp. and their translated amino acid sequences shared lower identity scores with the sequences from B. bigemina (82.7% and 84.7%, respectively) and B. ovata (83.5% and 85.5%, respectively) compared with the identity scores shared between the B. bigemina and B. ovata sequences (86.3% and 87.9%, respectively).
Conclusions
Our study showed that B. naoakii n. sp. occupies a unique phylogenetic position distinct from existing Babesia species. Our findings, together with morphological differences, identify B. naoakii n. sp. as a distinct parasite species.
Graphical Abstract

Similar content being viewed by others
Background
Babesia species are intraerythrocytic protozoan parasites that are transmitted to vertebrate hosts via tick vectors [1]. Babesia parasites invade, asexually reproduce in, and egress from red blood cells in vertebrate hosts, resulting in intravascular haemolytic anaemia [2]. As a result, babesiosis leads to a clinically serious condition in infected animals. In cattle, Babesia parasite species cause bovine babesiosis, which is a fatal disease that mostly affects cattle in tropical and subtropical regions of the world [3]. The disease results in huge economic losses to the cattle industry due to loss of production, cost of treatment and prevention, mortality, and restrictions to international cattle movement. Several species of Babesia, such as Babesia bovis, B. bigemina, B. divergens, B. ovata, B. major, and B. occultans, infect cattle, but only B. bovis, B. bigemina, and B. divergens are known to cause clinical babesiosis [3].
However, our recent investigation found that Babesia sp. Mymensingh, which was first characterised based on an 18S ribosomal RNA (18S rRNA) sequence in a cow in Bangladesh [4], can cause clinical babesiosis in cattle [5]. Additional studies detected Babesia sp. Mymensingh in cattle, buffalo, sheep, goats, and camels in Asian, African, and American countries [6,7,8]. Despite the clinical significance and wide geographical distribution, the taxonomy of Babesia sp. Mymensingh is still incomplete, because a scientific name for this parasite species has not been assigned yet. Correct taxonomic description is vital for the smooth communication of biological information. Therefore, the incomplete taxonomy of Babesia sp. Mymensingh might create unnecessary confusion. For example, a recent survey detected an 18S rRNA gene closely related to that of Babesia sp. Mymensingh in cattle, but the authors described the organism as B. bigemina [9].
Morphological distinctions demonstrate that Babesia sp. Mymensingh is a separate parasite species (Fig. 1), but its phylogenetic position is unclear [5]. Sequences of only three genes from Babesia sp. Mymensingh, comprising 18S rRNA, cytochrome oxidase subunit III (cox3), and apical membrane antigen 1 (ama-1), exist in the databases. In phylogenetic trees, the 18S rRNA gene and cox3 of Babesia sp. Mymensingh form sister clades to those of B. bigemina [5]. In contrast, Babesia sp. Mymensingh ama-1 sequences form a sister clade to the common ancestor of B. bigemina and B. ovata [5,6,7]. Because of these discrepancies, the phylogenetic position of Babesia sp. Mymensingh is still undetermined.
Micrographs of B. naoakii n. sp. Microscopic examination of a Giemsa-stained thin blood smear (type-material) from an infected cow revealed the presence of different morphological forms, including paired pyriforms (a), ring forms (b), and irregularly shaped single forms (c), within red blood cells. The holotype is marked with an arrow in a
Apicomplexan parasites, including Babesia species, possess three genomes: mitochondrial, plastid, and nuclear genomes. Previous studies found that phylogenetic analyses of genes from all three genomes are useful for identifying congeneric apicomplexan species. The mitochondrial and plastid genes are considered taxonomic markers of closely related species due to their low rates of recombination, relatively rapid rates of evolution, and high phylogenetic signal indexes [10, 11]. In particular, concatenated genes of the mitochondrial genome and 16S ribosomal RNA (16S rRNA) and elongation factor Tu (tufA) of plastid genomes have been widely used for phylogenetic analyses [12, 13]. In addition, phylogenies based on concatenated sequences of nuclear genes have also been widely employed to aid the discovery of novel parasite species [14]. In the present study, therefore, we analysed the concatenated mitochondrial genes, 16S rRNA and tufA of the plastid genome, and concatenated nuclear genes and their translated amino acid sequences to determine the phylogenetic position of Babesia sp. Mymensingh. We also investigated whether Babesia sp. Mymensingh forms a distinct clade in a phylogenetic tree constructed using the internal transcribed spacer 1–5.8S ribosomal RNA-internal transcribed spacer 2 (ITS1-5.8S rRNA-ITS2) region, which has relatively high diversity within a given parasite population compared to other marker genes [15].
Methods
To obtain gene sequences for phylogenetic analyses, the genome of Babesia sp. Mymensingh was sequenced. In brief, 10 ml of whole blood was drawn from a cow infected with Babesia sp. Mymensingh in the Badulla district of Sri Lanka in 2017 using EDTA as an anticoagulant [5]. Polymerase chain reaction (PCR) and sequencing analyses detected only Babesia sp. Mymensingh, not any other Babesia species [5]. The parasitaemia, as determined by the microscopic examination of a Giemsa-stained thin blood smear, was 2.3%. A Plasmodipur filter (EuroProxima, Arnhem, Netherlands) was used to remove leukocytes from the collected blood. The DNA was then extracted using the QIAamp DNA Blood Maxi Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions.
Subsequently, the DNA sample was subjected to whole-genome amplification using the GenomiPhi V2 DNA Amplification Kit (GE Healthcare, Chicago, IL, USA) following the manufacturer’s instructions. A library was constructed with the Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA, USA) and then subjected to 300-bp paired-end sequencing with MiSeq (Illumina). The quality of the reads was controlled with Trimmomatic [16] using the following parameters: LEADING:20, TRAILING:20, SLIDINGWINDOW:4:15, and MINLEN:100. The trimmed reads, which were 1,922,738,761 bp in total, were then assembled with paired-end ABySS [17] using k = 64. The de novo assembled sequence was 13.35 Mbp and consisted of 90,524 contigs. Potential open reading frames and amino acid sequences coded by each contig were specified using getorf in the European Molecular Biology Open Software Suite (EMBOSS) [18]. Amino acid sequences and coding sequences for B. bovis, B. bigemina, B. ovata, B. divergens, and Theileria annulata were retrieved from PiroplasmaDB (https://piroplasmadb.org). Genes homologous to those of Babesia sp. Mymensingh were identified using Blastp, and the corresponding amino acid sequences and coding sequences were then obtained. The Babesia sp. Mymensingh sequences used in this study were registered with the DNA Data Bank of Japan (DDBJ) under Accession numbers LC684678-LC684772.
The following sequences from Babesia sp. Mymensingh, together with homologous sequences from other Babesia species, were used to construct six phylogenetic trees: (1) concatenated amino acid sequences of cytochrome oxidase subunit I (cox1), cox3, and cytochrome b (cob) genes of the mitochondrial genome; (2) 16S rRNA of the plastid genome; (3) nucleotide sequences of tufA gene of the plastid genome; (4) ITS1-5.8S rRNA-ITS2; (5) concatenated nucleotide sequences of 89 nuclear genes (Additional file 1: Table S1); and (6) concatenated amino acid sequences translated from the nuclear genes. The 89 nuclear-encoded gene sequences had lengths comparable to those of other bovine Babesia species, including B. bovis (T2Bo strain) [19], B. bigemina (BBOND strain) [20], B. ovata (Miyake strain) [21], B. divergens (Rouen 1987 strain) [22], and T. annulata (Ankara strain) [23]. The Babesia sp. Mymensingh sequences and those of other parasite species were processed for multiple alignment using fast Fourier transform software (https://mafft.cbrc.jp/alignment/server/) [24]. The alignments of 16S rRNA, tufA, ITS1-5.8S rRNA-ITS2, and concatenated nuclear genes consisted of 877, 934, 421, and 103,646 nucleotides, respectively. The alignment of the concatenated amino acid sequences translated from the mitochondrial and nuclear genes consisted of 986 and 34,846 residues, respectively. The alignments were analysed with Molecular Evolutionary Genetics Analysis, version X, software (MEGA X) [25], and best-fitting substitution models were predicted based on the lowest Akaike information criterion value. Maximum likelihood phylogenetic trees rooted with either Plasmodium falciparum, Toxoplasma gondii, or T. annulata sequences were then constructed based on the general time reversible model (nucleotide sequences) [26], the Le–Gascuel 2008 model (concatenated amino acid sequences of nuclear genes) [27], and the general reversible mitochondrial model (concatenated amino acid sequences of mitochondrial genes) [28] using MEGA X.
The concatenated nuclear genes of Babesia sp. Mymensingh, B. bigemina, and B. ovata were analysed for single-nucleotide polymorphisms (SNPs) using DNA Sequence Polymorphism software [29], as well as for nucleotide and amino acid identity scores using EMBOSS Stretcher (https://www.ebi.ac.uk/Tools/psa/emboss_stretcher/).
Results
Order Piroplasmida Wenyon, 1926
Family Babesiidae Poche, 1913
Genus Babesia Starcovici, 1893
Babesia naoakii n. sp.
Type host: Cattle (Bos taurus).
Other hosts: The parasite’s DNA has been detected in water buffalo, goats, sheep, and Bactrian camels.
Type locality: Badulla district (6°59ʹ31.2″N, 81°03ʹ00.3″E), Sri Lanka.
Other localities: Bangladesh [4], India [9], Mongolia [7, 8], Vietnam [6], Uganda [6], and Argentina [6]
Type material: A blood DNA sample and a stained thin blood smear containing the holotype (Fig. 1) prepared from an infected cow have been registered with the Material Management Center, Ministry of Education, Culture, Sports, Science, and Technology, Japan with the Accession number OUMR-2021-00077 and OUMR-2022-00010, respectively.
Vector: Unknown.
Representative DNA sequences: Gene sequences with the following Accession numbers have been submitted to DDBJ: LC684678-LC684772.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN) [30], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:4DBE1F9C-4F97-4655-89F8-CF1DEE1BEC9C. The LSID for the new name Babesia naoakii n. sp. is urn:lsid:zoobank.org:act:842EB419-FBB5-4C1B-ABF8-74DC947A02BE.
Description: Microscopically, B. naoakii n. sp. appears as paired pyriforms, elongated or irregularly shaped single forms, and ring forms within infected erythrocytes [5]. The paired pyriforms form an obtuse angle. The length and width of the paired pyriforms are 2.25 μm to 3.04 μm and 1.58 μm to 2.20 μm, respectively, while the ring forms are 1.52 μm to 1.97 μm in diameter [5]. A PCR assay using a forward (TGGCGCCGACTTCCTGGAGCCCATCTCCAA) and reverse (AGCTGGGGCCCTCCTTCGATGAACCGTCGG) primer targeting the ama-1 gene specifically detects B. naoakii n. sp. infection in host animals [5].
Etymology: Babesia naoakii n. sp. was named after Naoaki Yokoyama, who first observed this species under a microscope and identified the morphological distinctions.
Molecular phylogeny
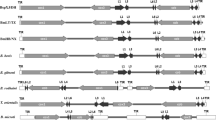
In all six phylogenetic trees, B. naoakii n. sp. formed a sister clade to the common ancestor of B. ovata and B. bigemina (Figs. 2 and 3). The position of Babesia sp. Mymensingh was further confirmed in additional phylogenetic trees constructed with concatenated nucleotide sequences of nuclear genes (n = 30; Additional file 2: Table S2) and their translated amino acid sequences from B. naoakii n. sp., B. bovis [19], B. bigemina [20], B. ovata [21], B. divergens [22], Babesia sp. Xinjiang [31], T. annulata [23], Theileria parva [32], Theileria orientalis [33], and P. falciparum [34] (Additional file 3: Fig. S1). Apart from the 16S rRNA and tufA phylogenies (Fig. 2b and c), the separation of B. naoakii n. sp. was supported by high bootstrap values (Figs. 2a and 3). A reason for the relatively low bootstrap values in the 16S rRNA gene and tufA phylogenies could be the partial fragments used in their construction. Additionally, our sequencing analyses found more SNPs between B. naoakii n. sp. and B. bigemina and between B. naoakii n. sp. and B. ovata than between B. bigemina and B. ovata (Table 1). Similarly, the nucleotide and amino acid identity scores between B. naoakii n. sp. and B. bigemina and between B. naoakii n. sp. and B. ovata were lower than those between B. bigemina and B. ovata (Table 1).
Phylogenetic analyses of mitochondrial and plastid genes. Maximum likelihood phylogenetic trees were constructed with concatenated amino acid sequences of cytochrome oxidase subunit I (cox1), cox3, and cytochrome b genes of the mitochondrial genome (a), and the nucleotide sequences of 16S rRNA (b) and tufa genes (c) of the plastid genome. In all three phylogenetic trees, Babesia naoakii n. sp. formed a sister clade to the common ancestor of B. bigemina and B. ovata
Phylogenetic analyses of ITS1-5.8S rRNA-ITS2 and concatenated nuclear genes. Maximum likelihood phylogenetic trees were constructed with ITS1-5.8S rRNA-ITS2 (a), concatenated nucleotide sequences encoding 89 nuclear genes (b), and concatenated amino acid sequences translated from 89 nuclear genes (c). In all three phylogenetic trees, Babesia naoakii n. sp. formed a sister clade to the common ancestor of B. bigemina and B. ovata
Discussion
Taken together, our present findings on the phylogenetic positions, SNPs, and sequence identity scores indicate that B. naoakii n. sp. is a distinct Babesia species.
Babesia naoakii n. sp. formed a sister clade to the common ancestor of B. ovata and B. bigemina. Babesia bigemina and B. ovata are two distinct Babesia species: B. bigemina is a virulent species capable of causing clinical babesiosis in cattle [3], whereas B. ovata is a relatively benign species that may cause clinical babesiosis in immunocompromised or T. orientalis-infected cattle [35, 36]. Babesia bigemina is transmitted by Rhipicephalus (Boophilus) ticks, while B. ovata is transmitted by Haemaphysalis ticks [3]. The distribution of B. bigemina and B. ovata also differs: B. bigemina has a worldwide distribution, whereas B. ovata is limited to a few Asian countries [3, 37]. In addition, recent genomic analyses have demonstrated that these two parasite species differ genetically from each other [21]. Therefore, the finding that B. naoakii n. sp. forms a sister clade to the common ancestor of two different parasite species, B. bigemina and B. ovata, convincingly distinguishes it as a distinct Babesia species.
Similar to B. bigemina, B. naoakii n. sp. may apparently cause clinical disease in cattle. For instance, fever, anaemia, and haemoglobinuria were observed in a naturally infected cow from Sri Lanka. The animal red blood cell counts, haemoglobin concentration, and haematocrit values fell below the normal range. Further research is needed to gain more information on the clinical picture of B. naoakii n. sp. infection in cattle.
Conclusion
Based on morphology and data from mitochondrial, plastid, and nuclear genes, Babesia sp. Mymensingh is named B. naoakii n. sp.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Abbreviations
- ama-1 :
-
Apical membrane antigen 1
- cob :
-
Cytochrome b
- cox1 :
-
Cytochrome oxidase subunit I
- cox3 :
-
Cytochrome oxidase subunit III
- DDBJ:
-
DNA Data Bank of Japan
- EMBOSS:
-
European Molecular Biology Open Software Suite
- ITS1-5.8S rRNA-ITS2:
-
Internal transcribed spacer 1–5.8S ribosomal RNA-internal transcribed spacer 2
- MEGA X:
-
Molecular Evolutionary Genetics Analysis version X software
- PCR:
-
Polymerase chain reaction
- tufA :
-
Elongation factor Tu
- 16S rRNA:
-
16S ribosomal RNA
- 18S rRNA:
-
18S ribosomal RNA
References
Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–69.
Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37.
Bock R, Jackson L, de Vos A, Jorgensen W. Babesiosis of cattle. Parasitol. 2004;129:247–69.
Roy BC, Krücken J, Ahmed JS, Majumder S, Baumann MP, Clausen PH, et al. Molecular identification of tick-borne pathogens infecting cattle in Mymensingh district of Bangladesh reveals emerging species of Anaplasma and Babesia. Transbound Emerg Dis. 2018;65:e231–42.
Sivakumar T, Tuvshintulga B, Zhyldyz A, Kothalawala H, Yapa PR, Kanagaratnam R, et al. Genetic analysis of Babesia isolates from cattle with clinical babesiosis in Sri Lanka. J Clin Microbiol. 2018;56:e00895-e918.
Sivakumar T, Tuvshintulga B, Kothalawala H, Silva SSP, Lan DTB, Long PT, et al. Host range and geographical distribution of Babesia sp. Mymensingh Transbound Emerg Dis. 2020;67:2233–9.
Otgonsuren D, Sivakumar T, Amgalanbaatar T, Enkhtaivan B, Narantsatsral S, Tuvshintulga B, et al. Molecular epidemiological survey of Babesia bovis, Babesia bigemina, and Babesia sp Mymensingh infections in Mongolian cattle. Parasitol Int. 2020;77:102107.
Otgonsuren D, Sivakumar T, Amgalanbaatar T, Enkhtaivan B, Narantsatsral S, Davaasuren B, et al. Molecular survey of bovine Babesia species in Bactrian camels (Camelus bactrianus) in Mongolia. Ticks Tick Borne Dis. 2022;13:101871.
Pradeep RK, Nimisha M, Sruthi MK, Vidya P, Amrutha BM, Kurbet PS, et al. Molecular characterization of South Indian field isolates of bovine babesia spp and Anaplasma spp. Parasitol Res. 2019;118:617–30.
Rubinoff D, Holland BS. Between two extremes: mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Syst Biol. 2005;54:952–61.
Mitsui H, Arisue N, Sakihama N, Inagaki Y, Horii T, Hasegawa M, et al. Phylogeny of Asian primate malaria parasites inferred from apicoplast genome encoded genes with special emphasis on the positions of Plasmodium vivax and P. fragile. Gene. 2010;450:32–8.
Janouškovec J, Paskerova GG, Miroliubova TS, Mikhailov KV, Birley T, Aleoshin VV, et al. Apicomplexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. Elife. 2019;8:e49662.
Vohsen SA, Anderson KE, Gade AM, Gruber-Vodicka HR, Dannenberg RP, Osman EO, et al. Deep-sea corals provide new insight into the ecology, evolution, and the role of plastids in widespread apicomplexan symbionts of anthozoans. Microbiome. 2020;8:34.
Knowles DP, Kappmeyer LS, Haney D, Herndon DR, Fry LM, Munro JB, et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria implications for apicomplexan parasite surveillance. Int J Parasitol. 2018;48:679–90.
Jirapattharasate C, Adjou Moumouni PF, Cao S, Iguchi A, Liu M, Wang G, et al. Molecular detection and genetic diversity of bovine babesia spp Theileria orientalis, and anaplasma marginale in beef cattle in Thailand. Parasitol Res. 2017;116:751–62.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol İ. ABySS: a parallel assembler for short read sequence data. Genome Res. 2009;19:1117–23.
Rice P, Longden I, Bleasby A. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16:276–7.
Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ, et al. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–13.
Jackson AP, Otto TD, Darby A, Ramaprasad A, Xia D, Echaide IE, et al. The evolutionary dynamics of variant antigen genes in Babesia reveal a history of genomic innovation underlying host-parasite interaction. Nucl Acids Res. 2014;42:7113–31.
Yamagishi J, Asada M, Hakimi H, Tanaka TQ, Sugimoto C, Kawazu SI. Whole-genome assembly of Babesia ovata and comparative genomics between closely related pathogens. BMC Genom. 2017;18:832.
González LM, Estrada K, Grande R, Jiménez-Jacinto V, Vega-Alvarado L, Sevilla E, et al. Comparative and functional genomics of the protozoan parasite Babesia divergens highlighting the invasion and egress processes. PLoS Negl Trop Dis. 2019;13:e0007680.
Pain A, Renauld H, Berriman M, Murphy L, Yeats CA, Weir W, et al. Genome of the host-cell transforming parasite theileria annulata compared with T. parva. Science. 2005;309:131–3.
Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20:1160–6.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Nei M, Kumar S. Molecular evolution and phylogenetics. 1st ed. Oxford: Oxford University Press; 2000.
Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25:1307–20.
Adachi J, Hasegawa M. Model of amino acid substitution in proteins encoded by mitochondrial DNA. J Mol Evol. 1996;42:459–68.
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302.
ICZN. International commission on zoological nomenclature: amendment of articles 8, 9, 10, 21 and 78 of the international code of zoological nomenclature to expand and refine methods of publication. Bull Zool Nomencl. 2012;69:161–9.
Guan G, Korhonen PK, Young ND, Koehler AV, Wang T, Li Y, et al. Genomic resources for a unique, low-virulence Babesia taxon from China. Parasit Vectors. 2016;9:564.
Gardner MJ, Bishop R, Shah T, de Villiers EP, Carlton JM, Hall N, et al. Genome sequence of Theileria parva, a bovine pathogen that transforms lymphocytes. Science. 2005;309:134–7.
Hayashida K, Hara Y, Abe T, Yamasaki C, Toyoda A, Kosuge T, et al. Comparative genome analysis of three eukaryotic parasites with differing abilities to transform leukocytes reveals key mediators of Theileria induced leukocyte transformation. Mbio. 2012;3:e00204-12.
Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511.
Fujinaga T. Bovine babesiosis in Japan: clinical and clinico-pathological studies on cattle experimentally infected with Babesia ovata. Nihon Juigaku Zassh. 1981;43:803–13.
Sivakumar T, Tagawa M, Yoshinari T, Ybanez AP, Igarashi I, Ikehara Y, et al. PCR detection of Babesia ovata from cattle reared in Japan and clinical significance of coinfection with Theileria orientalis. J Clin Microbiol. 2012;50:2111–3.
Sivakumar T, Igarashi I, Yokoyama N. Babesia ovata: Taxonomy, phylogeny and epidemiology. Vet Parasitol. 2016;229:99–106.
Acknowledgements
We thank the veterinary staff in Sri Lanka for their assistance in sampling and DNA extraction. We also thank Ms. Hiroko Yamamoto of the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Japan, for her outstanding technical assistance.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS KAKENHI nos. 19KK0174 and 19K23704) and the Open Partnership Joint Project of the JSPS Bilateral Joint Research Projects.
Author information
Authors and Affiliations
Contributions
TS, JY, and NY conceived the study, conducted the experiments, and wrote the manuscript. TS, BT, DO, EB, BA, JY, and NY analysed the data. HK, SC, and SSPS conducted the cattle survey. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Animal Care and Use Committee of Obihiro University of Agriculture and Veterinary Medicine, Japan, approved all the animal procedures (Approval Number 29-53). All experiments were carried out in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. List of nuclear genes (n = 89) used for concatenated phylogenetic trees in Fig. 3.
Additional file 2: Table S2
. List of nuclear genes (n = 30) used for concatenated phylogenetic trees in Fig. S1.
Additional file 3: Figure S1
. Phylogenetic trees constructed with concatenated nuclear genes. Nucleotide sequences of 30 nuclear genes (panel a) and their translated amino acid sequences (panel b) were concatenated and used to construct maximum likelihood phylogenetic trees based on general time reversible and Le–Gascuel 2008 models, respectively. In both phylogenetic trees, Babesia naoakii n. sp. formed a sister clade to the common ancestor of B. bigemina and B. ovata.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sivakumar, T., Tuvshintulga, B., Otgonsuren, D. et al. Phylogenetic analyses of the mitochondrial, plastid, and nuclear genes of Babesia sp. Mymensingh and its naming as Babesia naoakii n. sp.. Parasites Vectors 15, 299 (2022). https://doi.org/10.1186/s13071-022-05374-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05374-9