Abstract
Background
The invasive mosquito species, Aedes japonicus japonicus, was detected in northeastern Italy for the first time in 2015, at the border with Austria. After this finding, a more intensive monitoring was carried out to assess its distribution and to collect biological data. Herein, we report the results of four years (2015–2018) of activity.
Methods
The presence of Ae. j. japonicus was checked in all possible breeding sites through collections of larvae. The monitoring started from the site of the first detection at the Austrian border and then was extended in all directions. The mosquitoes were identified morphologically and molecularly.
Results
Aedes j. japonicus was found in 58 out of 73 municipalities monitored (79.5%). In total (2015–2018), 238 sampling sites were monitored and 90 were positive for presence of Ae. j. japonicus larvae (37.8%). The mosquito was collected mainly in artificial containers located in small villages and in rural areas. Cohabitation with other mosquito species was observed in 55.6% of the samplings.
Conclusions
Aedes j. japonicus is well established in Italy and in only four years has colonised two Italian Regions, displaying rapid spreading throughout hilly and mountainous areas. Colonization towards the south seems limited by climatic conditions and the occurrence of a large population of the larval competitor, Ae. albopictus. The further spread of Ae. j. japonicus has the potential to pose new threats of zoonotic agents (i.e. Dirofilaria spp. and West Nile virus) within areas at altitudes previously considered at negligible risk in Italy.
Similar content being viewed by others
Background
The Asian bush or rock pool mosquito, Aedes (Finlaya) japonicus japonicus (Theobald, 1901) (syn. Hulecoeteomyia japonica) (Diptera: Culicidae), is one of the most invasive mosquito species (IMS) worldwide and has spread throughout North America and Europe. In its native area of East Asia (Japan, Korea, southern China, southeastern Russia), Ae. j. japonicus occurs in temperate regions [1]. Currently, Ae. j. japonicus is reported from nine European countries, i.e. Belgium [2], the Netherlands [3], Switzerland [4], Germany [5], Austria, Slovenia [6], Hungary [7] and Croatia [8].
Aedes j. japonicus is not considered to be a major vector of pathogens in its native area, but its possible role as a vector of disease agents in other parts of the world is unclear; indeed, it seems able to transmit pathogens like flaviviruses and heartworms in laboratory studies [9, 10].
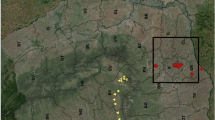
A previous monitoring performed in Austria from 2011 to 2015 detected the mosquito in a village 25 km from the Italian border on July 2015. The researchers hypothesized that the species could spread to Italy and a following survey confirmed the presence of Ae. j. japonicus in three villages along the River Fella, Friuli Venezia Giulia (FVG) Region [11]. In October 2016, another unexpected finding occurred during a local survey in a village located in another area to south, close to the Slovenian border [Cividale del Friuli, 46°04′23.7″N, 13°26′00.4″E, 127 m above sea level (masl)] (Fig. 1).
In this part of northeastern Italy, the occurrence of two other IMS, Aedes albopictus (Skuse, 1895) (syn. Stegomyia albopicta) and Aedes koreicus (Edwards, 1917) (syn. Hulecoeteomyia koreica) was known [12]. Consequently, Ae. j. japonicus is the third Asian mosquito species occurring in Italy.
After the first findings, a more intensive monitoring was carried out to assess the current spreading. In this paper, we report the results of four years of monitoring on the occurrence and spread of Ae. j. japonicus in Italy. In addition, ecological data, such as breeding sites preference and coexistence with other mosquito larvae are also reported.
Methods
Study area
The area monitored is characterized by hills, mountains and valleys typical of the Dolomite Alps with an average elevation of 527.7 masl. The area has a sub-continental climate, characterized by a mild climate, with cold and snowy winters and mild warm summers. The average mean daily temperature ranges between 17–22 °C in the summer and between -2–3 °C in the winter. The annual rainfall is above 1000 mm. The human population density is low compared to other Italian areas (108.4 and 56 inhabitants/km2 in Udine and Belluno Provinces, respectively), and the inhabitants live mainly in small villages; only four have more than 10,000 inhabitants.
Mosquito sampling and identification
At the time of the first findings of Ae. j. japonicus in the FVG Region there was no specific monitoring for invasive mosquitoes, which was activated only after the first report. Conversely, in Belluno Province a monitoring for IMS has been ongoing since 2011. The survey in FVG started in September 2015 near the most western site where the first Ae. j. japonicus mosquitoes were found (Pontebba; 46°30′16.9740″N, 13°18′10.8324″E; 561 masl). The monitoring was extended towards the west in 2016, following the Dolomites and their valleys with samplings in March, July and October. Since no further expansion in the area bordering Austria was found, in 2017 the monitoring focused on the area of the second finding (October 2016) bordering Slovenia, with samplings in June, July and September. In 2018, the surveillance was intensified throughout north FVG and in the bordering area of Veneto Region (Belluno Province) performing monthly samplings from April to November.
The detection of IMS should be performed with different methods and traps; however, due to budget and personnel constraints, we decided to focus the survey on the larval stage. Surveillance of larvae is considered one of the best methods in terms of targeted and rapid IMS detection and optimal for the cost-benefit ratio [13]. It is also well known that IMS develop mainly in artificial breeding containers; therefore, the surveillance of presence and availability of breeding sites was focused mostly at human settlements.
Larval collection was made using a standard larval dipper (500 ml, 10 cm diameter), checking all potential breeding sites present in each site, i.e. artificial containers, catch basins, tires and natural mosquito larval habitats. All the collection sites were georeferenced. The areas monitored included private and public places. When Ae. j. japonicus was found in an area, the surrounding environment was explored up to places with no longer positive for the species. Several sites negative for the presence of the species in 2015 were checked again in 2016 and 2018 as well as some positive sites to confirm its occurrence. The extention of the area colonized by Ae. j. japonicus was then estimated adding the surface of the municipalities where Aedes japonicus was recorded.
The larvae collected were identified morphologically as described in Montarsi et al. [14]. In case of the detection of Ae. j. japonicus for the first time in a municipality, at least one larval stage and eventually adults reared in the laboratory were confirmed through molecular biology. DNA was amplified using an in-house real-time SYBR green PCR, targeting two mitochondrial loci, nicotinamide adenine dinucleotide dehydrogenase subunit 4 gene (nad4, 480 bp) [15] and cytochrome c oxidase subunit 1 gene (cox1, 590/600 bp) [16], and one nuclear locus, β tubulin gene (BTUB, 370 bp) [17]. Briefly, the reactions were carried out in a total volume of 20 μl, containing, 5.8 μl of RNase free water, 10 μl of QuantiFast SYBR Green PCR Master Mix 2× (Qiagen GmbH, Hilden, Germany), 0.3 μM of sense and reverse primer and 3 μl of extracted DNA. Amplifications were performed in a StepOnePlus™ instrument (Applied Biosystems, Foster City, CA, USA). The thermal profile consisted of 5 min at 95 °C, followed by 40 cycles at 95 °C for 15 s, 55 °C for 30 s (for nad4 and BUTB primers), 58 °C for 30 s (for cox1 primers) and 60 °C for 30 s. Following amplification, dissociation was performed by slowly raising the temperature of the thermal chamber from 60 to 95 °C. Negative and positive controls were included in each run.
The amplicons were sequenced, and the sequences obtained compared with GenBank entries. Representative sequences were submitted to GenBank.
Statistical analysis
The differences of Ae. j. japonicus prevalence (only place/breeding sites monitored more than 10 times) according to the municipality of collection and type of larval breeding sites were tested using the Chi-square test or Fisher’s exact test when appropriate using the free software WinEpi [18].
Maps were created using the GIS software ESRI® ArcMap™ version 10.5.1 offered by ArcGIS™ Desktop [19].
Results
The current distribution of Ae. j. japonicus in northern Italy is reported in Fig. 1. To date, Ae. j. japonicus has been found in 58 municipalities out of 73 monitored (79.5%): 51/62 (82.3%) in FVG Region and 7/11 (63.6%) in Veneto Region (Table 1). The prevalence of positive municipalities in FVG Region increased every year, from 21.4% in 2016, to 41.7% in 2017 and 87.8% in 2018 (χ2 = 26.567, df = 2, P < 0.0001). In total (2015–2018), 238 sampling sites were monitored and 90 were positive for presence of Ae. j. japonicus larvae (37.8%) (Fig. 2, see Additional file 1: Table S1). In 2016, the species was found in one already positive municipality (Pontebba), in another one monitored but negative the previous year (Resiutta), and in a new site far away (Cividale del Friuli) by local authorities. In 2017, 5 out of 12 municipalities (41.7%), one of them already positive the previous year (Cividale del Friuli), and 8 out of 16 (50%) sampling sites were found infested. In 2018, most of the municipalities and sites monitored were positive to Ae. j. japonicus larvae (87.8 and 63.6% in FVG and Belluno Province, respectively) (Table 1). Notably, nine municipalities that were found positive in FVG in 2018 were negative in 2016. In Belluno Province, Ae. j. japonicus was found in a survey carried out in September 2018, while previously the same sites were negative. Notably, three positive municipalities in September were negative in samplings performed in May and July (see Additional file 1: Table S1). The mosquito spread from the valleys to the hilly and mountainous areas in the range of altitude between 99 masl. (Torreano, Province of Udine; 46°07′57″N, 13°25′56″E) and 1263 m a.s.l. (Sappada, Province of Udine; 46°34′13″N, 12°42′17.8″E).
Aedes j. japonicus larvae were found mainly in tires and in any kind of artificial containers, often located in private gardens (Table 2). In general, 52.2% of breeding sites checked, located in semi-urban areas (usually small villages), were positive to Ae. j. japonicus larvae. Compared to the other invasive mosquitoes occurring in the same area (Ae. albopictus and Ae. koreicus), Ae. j. japonicus was less present in catch basins and cemeteries, the last being positive only in two cases out of 17 monitored [14]. Over the period of sampling, the first larvae were observed in March 2016 and the last one in November 2018.
During the survey, other mosquito larvae were collected belonging to 11 species: Culex pipiens, Cx. hortensis, Anopheles maculipennis (s.l.), An. plumbeus, An. claviger/petragnani, Culiseta longiareolata, Cs. annulata, Aedes albopictus, Oc. geniculatus, Ae. koreicus and Oc. communis. Cohabitation with other mosquito species was observed in 55.6% of the positive larval samplings. Aedes j. japonicus was associated with Cx. hortensis (27 times), Cx. pipiens (27 times) and with Ae. albopictus (13 times) (Fig. 3). Interestingly, Ae. j. japonicus was never found to share breeding sites with Ae. koreicus, even if their distribution partially overlapped (Fig. 1). Coexistence with at least one species was observed 29 times (58.0%), with another two species 15 times (30.0%), with three species twice (10.0%) and with four species once (2.0%). The breeding sites shared by Ae. j. japonicus and other mosquito larvae were mainly large and small artificial containers (Fig. 4). Large water containers were significantly more positive to cohabitation (42.6%) than tires (13.0%) (χ2 = 11.815, df = 1, P = 0.0006), catch basins and vases/saucers (7.4%) (χ2 = 17.827, df = 1, P < 0.0001) and basins of fountains (1.8%) (χ2 = 25.929, df = 1, P < 0.0001).
In total, 83 samples were submitted to PCR and 50 larvae and 11 adults were confirmed as Ae. j. japonicus with the three genes and with a similarity to GenBank sequences ranging between 98–100%. Other mosquitoes identified by molecular analysis were Ae. koreicus (n = 17), Oc. communis (n = 3), Ae. albopictus (n = 1) and Cx. hortensis (n = 1). Sequences of Ae. j. japonicus obtained by the three genes were submitted to the GenBank database under the accession numbers MK265679-MK265696.
Discussion
After the first finding of Ae. japonicus in Italy in 2015 [10] this invasive mosquito spread throughout north Italy in the Provinces of Udine, bordering with Austria and Slovenia, and in the Province of Belluno and it is currently established in an area of approximately 3273 km2.
During 2016, the spread of Ae. j. japonicus seemed limited because it was absent around the municipalities positive in 2015 except for some collections in a municipality (Resiutta) 20 km to the southwest. In 2018, Ae. j. japonicus spread far, 40 km towards the west around the Alps and Alpine foothills infesting sites in FVG which were negative in 2016 and reaching the Belluno Province in September. In this part of Italy (Belluno Province) a well-organized IMS surveillance programme is ongoing and targeted to determine the distribution of Ae. koreicus [12] with frequent larval surveys; the species had not been found previously. Conversely, the spread towards the south was limited and the species did not reach the plain area of FVG Region. The rapid colonization observed in Italy is faster than in other European countries [7, 20, 21], suggesting environmental conditions particularly favorable to the development of this species. Indeed, it has been reported that in suitable habitats Ae. j. japonicus is able to increase its population within three years after the initial colonization [1, 7, 8, 22, 23].
The spread of Ae. j. japonicus for long distances is likely due to the transportation of eggs, larvae and adults by human activities through vehicles, while spread in close municipalities is due to the active expansion of the locally established population [1].
Aedes j. japonicus is a mosquito species adapted to tolerate cold temperatures. In a recent study based on predictive models of potential distribution species [24], the southernmost limits of this species in Europe have been indicated as within “a small region in northern Italy”, perfectly matching the area currently colonised. The expansion southwards seems to be limited by high mean temperatures (the average of mean temperature is over 32 °C for the warmest quarter in the plain area of the Region, [25]) and by the high density of the competitor species Ae. albopictus in the plain area [26, 27].
Aedes j. japonicus is confirmed to utilize artificial containers as main breeding sites [1, 27] and to be more common in natural and rural areas than in urban sites [28]. The capacity to establish in early spring and to be active until autumn is characteristic of this species, which is able to tolerate cold temperature [26]. Compared to other invasive mosquito species, the seasonal activity period is longer, lasting at least seven months (April-October); in our monitoring the first larvae were found on 29 March 2016 and on 26 April 2018 and the last on 12 October 2016 and on 08 November 2018. Aedes j. japonicus larvae pre-empted the finding of Ae. albopictus larvae of two months and remained active at least one more month, therefore decreasing the possibility of larval competition [29]. This phenology seems to require several generations per year, which occurs in areas with certain climatic characteristics, such as the winter not being extremely cold [26].
The ability of an invasive mosquito to establish in a new area is not only dependent on climate but also on the availability of empty ecological niches [27]. In our study, Ae. j. japonicus larvae were found mainly in areas too cold for Ae. albopictus, which occurs in approximately one-third of the area invaded by Ae. j. japonicus (Fig. 1).
Larval coexistence with other species was observed with other container-breeding mosquitoes. Aedes j. japonicus seemed not negatively affected by the presence of Culex spp. species, as reported elsewhere [30, 31] and multiple larval cohabitation was also possible, contrasting with reports of Ae. j. japonicus displacing native mosquitoes [20, 32, 33].
The rapid expansion of a new invasive mosquito and potential vector of pathogens may pose new threats to animals and humans. The vectorial role of Ae. j. japonicus has been assessed in laboratory studies which need confirmation in the field [1]. A potential vector competence for several viruses and nematodes (Dirofilaria immitis and D. repens) of medical and veterinary relevance has been suggested [10, 34, 35]. Notably, recent studies report that populations of Ae. j. japonicus collected in Switzerland are susceptible to West Nile virus (WNV) lineage 2 [36, 37]. As this mosquito species is an opportunistic feeder on mammals and birds [38] it could act as a bridge vector of WNV in Europe in case of considerable abundance. In the same geographical area of FVG Region colonised by Ae. j. japonicus, a high prevalence of D. immitis in stray dogs was reported [39], as well as circulation of WNV [40] in the lowland, an area not yet overlapping with the area colonised by Ae. j. japonicus. In the case of further spreading of Ae. j. japonicus, the risk of exposure to D. immitis and WNV could increase both for animals and humans, within areas previously considered at negligible risk in Italy, especially at high altitudes.
Conclusions
This study shows that Ae. j. japonicus is well established in Italy and in only four years has rapidly colonised two Italian Regions throughout hilly and mountainous areas. According to these findings, northern Italy has a high probability of being invaded by Ae. j. japonicus in the future, possibly limited towards south by climatic conditions and occurrence of the larval competitor Ae. albopictus. The establishment of Ae. j. japonicus in an area where other invasive species occur has complicated the current entomological monitoring system, due to the similar biology and morphology. Therefore, a long-term surveillance and an early detection are needed to limit the further spread and plan control actions against this invasive mosquito.
Abbreviations
- FVG:
-
Friuli Venezia Giulia
- IMS:
-
invasive mosquito species
- nad4:
-
nicotinamide adenine dinucleotide dehydrogenase subunit 4
- cox1:
-
cytochrome c oxidase subunit 1
- BTUB:
-
β tubulin
- masl:
-
meters above sea level
- WNV:
-
West Nile virus
- PCR:
-
polymerase chain reaction
References
Kampen H, Werner D. Out of the bush: The Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasit Vectors. 2014;7:59.
Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol. 2009;46:1464–7.
Ibañez-Justicia A, Stroo A, Dik M, Beeuwkes J, Scholte EJ. National mosquito (Diptera: Culicidae) survey in the Netherlands 2010–2013. J Med Entomol. 2015;52:185–98.
Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in central Europe. Med Vet Entomol. 2009;23:448–51.
Werner D, Kampen H. The further spread of Aedes japonicus japonicus (Diptera, Culicidae) towards northern Germany. Parasitol Res. 2013;112:3665–8.
Seidel B, Duh D, Nowotny N, Allerberger F. First record of the mosquitoes Aedes (Ochlerotatus) japonicus japonicus (Theobald, 1901) in Austria and Slovenia 2011 and for Aedes (Stegomyia) albopictus (Skuse, 1895) in Austria. Entomol Zeitschrift. 2012;122:223–6.
Seidel B, Nowotny N, Bakonyi T, Allerberger F, Schaffner F. Spread of Aedes japonicus japonicus (Theobald, 1901) in Austria, 2011–2015, and first records of the subspecies for Hungary, 2012, and the principality of Liechtenstein, 2015. Parasit Vectors. 2016;9:356.
Klobučar A, Lipovac I, Žagar N, Mitrović-Hamzić S, Tešić V, Vilibić-Čavlek T, et al. First record and spreading of the invasive mosquito Aedes japonicus japonicus (Theobald, 1901) in Croatia. Med Vet Entomol. 2018; https://doi.org/10.1111/mve.12337.
Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013;19:685–92.
Silaghi C, Beck R, Capelli G, Montarsi F, Mathis A. Development of Dirofilaria immitis and Dirofilaria repens in Aedes japonicus and Aedes geniculatus. Parasit Vectors. 2017;10:94.
Seidel B, Montarsi F, Huemer HP, Indra A, Capelli G, Allerberger F, et al. First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: invasion from an established Austrian population. Parasit Vectors. 2016;9:284.
Montarsi F, Drago A, Martini S, Calzolari M, De Filippo F, Bianchi A, et al. Current distribution of the invasive mosquito species, Aedes koreicus [Hulecoeteomyia koreica] in northern Italy. Parasit Vectors. 2015;8:614.
European Centre for Disease Prevention and Control. Guidelines for the surveillance of invasive mosquitoes in Europe. Stockholm: ECDC; 2012.
Montarsi F, Martini S, Dal Pont M, Delai N, Ferro Milone N, Mazzucato M, et al. Distribution and habitat characterization of the recently introduced invasive mosquito Aedes koreicus [Hulecoeteomyia koreica], a new potential vector and pest in north-eastern Italy. Parasit Vectors. 2013;6:292.
Cameron EC, Wilkerson RC, Mogi M, Miyagi I, Toma T, Kim H-C, et al. Molecular phylogenetics of Aedes japonicus, a disease vector that recently invaded western Europe, North America, and the Hawaiian Islands. Evol J Med Entomol. 2010;47:527–35.
Jalali SK, Rakshit O, Venkatesan T. DNA barcoding for identification of agriculturally important insects. In: Chakravarthy AK, editor. New Horizons in Insect Science: Towards Sustainable Pest Management. New Delhi: Springer; 2015.
Demirci B, Lee Y, Lanzaro GC, Alten B. Identification and characterization of single nucleotide polymorphisms (SNPs) in Culex theileri (Diptera: Culicidae). J Med Entomol. 2012;49:581–8.
Working in Epidemiology. http://www.winepi.net/uk/index.htm. Accessed 27 Jan 2019.
Esri. http://www.esri.com/. Accessed 28 Jan 2019.
Zielke DE, Walther D, Kampen H. Newly discovered population of Aedes japonicus japonicus (Diptera: Culicidae) in Upper Bavaria, Germany, and Salzburg, Austria, is closely related to the Austrian/Slovenian bush mosquito population. Parasit Vectors. 2016;9:163.
Damiens D, Ayrinhac A, Van Bortel W, Versteirt V, Dekoninck W, Hance T. Invasive process and repeated cross-sectional surveys of the mosquito Aedes japonicus japonicus establishment in Belgium. PLoS One. 2014;9:e89358.
Kampen H, Zielke D, Werner D. A new focus of Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) distribution in western Germany: Rapid spread or a further introduction event? Parasit Vectors. 2012;5:284.
Kaufman MG, Stanuszek WW, Brouhard EA, Knepper RG, Walker ED. Establishment of Aedes japonicus japonicus and its colonization of container habitats in Michigan. J Med Entomol. 2012;49:1307–17.
Cunze S, Koch LK, Kochmann J, Klimpel S. Aedes albopictus and Aedes japonicus - two invasive mosquito species with different temperature niches in Europe. Parasit Vectors. 2016;9:573.
Agenzia regionale per la protezione ambientale (ARPA) della regione Friuli Venezia Giulia (FVG) (ARPA FVG-Meteo). Clima of FVG. http://www.osmer.fvg.it/clima.php. Accessed 23 Jan 2019.
Reuss F, Wieser A, Niamir A, Bálint M, Kuch U, Pfenninger M, et al. Thermal experiments with the Asian bush mosquito (Aedes japonicus japonicus) (Diptera: Culicidae) and implications for its distribution in Germany. Parasit Vectors. 2018;11:81.
Kaufman MG, Fonseca DM. Invasion Biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu Rev Entomol. 2014;59:31–49.
Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, et al. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J Med Entomol. 2012;49:813–24.
Murrell EG, Noden BH, Juliano SA. Contributions of temporal segregation, oviposition choice, and non-additive effects of competitors to invasion success of Aedes japonicus (Diptera: Culicidae) in North America. Biol Invasions. 2015;17:1669–81.
Hardstone MC, Andreadis TG. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med Entomol. 2012;49:277–85.
Andreadis TG, Wolfe RJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. J Med Entomol. 2010;47:43–52.
Rochlin I, Gaugler R, Williges E, Farajollahi A. The rise of the invasives and decline of the natives: Insights revealed from adult populations of container-inhabiting Aedes mosquitoes (Diptera: Culicidae) in temperate North America. Biol Invasions. 2013;15:991–1003.
Murrell EG, Juliano SA. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia. 2013;173:1033–42.
Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera: Culicidae). J Med Entomol. 1989;26:454–8.
Schaffner F, Vazeille M, Kaufmann C, Failloux AB, Mathis A. Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosq Bull. 2011;29:141–2.
Wagner S, Mathis A, Schönenberger AC, Becker S, Schmidt-Chanasit J, Silaghi C, et al. Vector competence of field populations of the mosquito species Aedes japonicus japonicus and Culex pipiens from Switzerland for two West Nile virus strains. Med Vet Entomol. 2018;32:121–4.
Veronesi E, Paslaru A, Silaghi C, Tobler K, Glavinic U, Torgerson P, et al. Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol Res. 2018;117:1925–32.
Schönenberger AC, Wagner S, Tuten HC, Schaffner F, Torgerson P, Furrer S, et al. Host preferences in host-seeking and blood-fed mosquitoes in Switzerland. Med Vet Entomol. 2016;30:39–52.
Pietrobelli M, Soldano F, Frangipane di Regalbono A, Bandera C. Cardio-pulmonary filariosis of the dog in Friuli-Venezia Giulia Region. O&DV. 1998;1:63–67 (In Italian).
Riccardo F, Monaco F, Bella A, Savini G, Russo F, Cagarelli R, et al. An early start of West Nile virus seasonal transmission: the added value of one heath surveillance in detecting early circulation and triggering timely response in Italy, June to July 2018. Euro Surveill. 2018. https://doi.org/10.2807/1560-7917.es.2018.23.32.1800427.
Acknowledgments
The authors would like to thank Sara Carlin (IZSVe) and Patrizia Visentin (Entostudio) for their help in sampling and Silvia Ravagnan (IZSVe) for molecular identification of mosquitoes. Publication of this paper has been sponsored by Bayer Animal Health in the framework of the 14th CVBD World Forum Symposium.
Funding
This work was funded by the Friuli Venezia Giulia Region.
Availability of data and materials
The data supporting the conclusions of this article are included within the article and its additional file. The newly generated sequences were submitted to the GenBank database under the accession numbers MK265679-MK265696.
Authors’ contributions
FM and SM conceived the study. FM, SM, DQ and DDG carried out the sampling. MDF and MDP helped with the local organization of the sampling. GDR performed molecular analysis. MM made the maps. MP partially funded this study. FM wrote the first draft of the manuscript. AM and GC reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Data, georeferenced position and description of sampling sites for Ae. j. japonicus surveillance.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Montarsi, F., Martini, S., Michelutti, A. et al. The invasive mosquito Aedes japonicus japonicus is spreading in northeastern Italy. Parasites Vectors 12, 120 (2019). https://doi.org/10.1186/s13071-019-3387-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-019-3387-x